Method for asymmetrically synthesizing glabridin with optical purity under catalysis of ruthenium compound
A technology of optical purity and ruthenium complex, applied in organic chemistry methods, organic chemistry, bulk chemical production, etc., to achieve the effects of easy product purification, high stereoselectivity, and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] A method for asymmetrically synthesizing glabridin with optical purity, comprising the steps of:
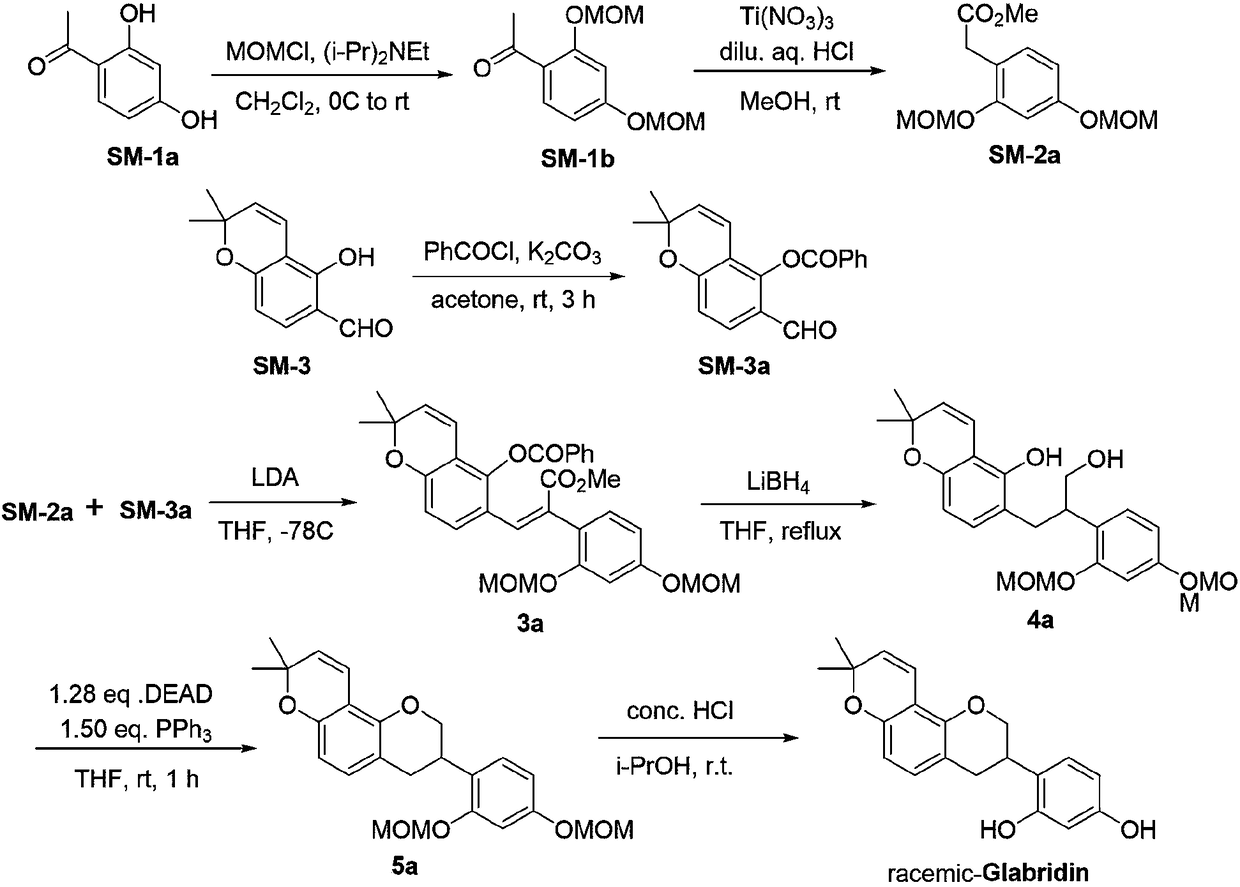
[0047] 1) Synthesis of compound II-a(3R,4R)-3-(2,4-dimethoxyphenyl)-8,8-dimethyl-3,4-dihydro-2H,8H-pyrano[2,3-f]chromen-4 -ol, the chemical equation is:
[0048]
[0049] The specific experimental steps and operations are as follows: at room temperature, 1.25 mmol of dichloro(p-methylisopropylphenyl) ruthenium (II) dimer (CAS number: 52462-29-0, provided by Shanghai Neutron Star Chemical Technology Co., Ltd. company) and 2.5 mmol (R,R)-N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine [(R,R)-TsDPEN, CAS#144222-34-4, supplied by TCI Purchased from Shanghai branch] were added to 150mL of stirred ethyl acetate in sequence; then, the triethylamine / formic acid buffered proton system that was previously mixed with 60mL of triethylamine and 20mL of formic acid and cooled to room temperature was added. The entire mixed system was stirred at room temperature for 30 minutes to ...
Embodiment 2
[0069] A method for asymmetrically synthesizing glabridin with optical purity, comprising the steps of:
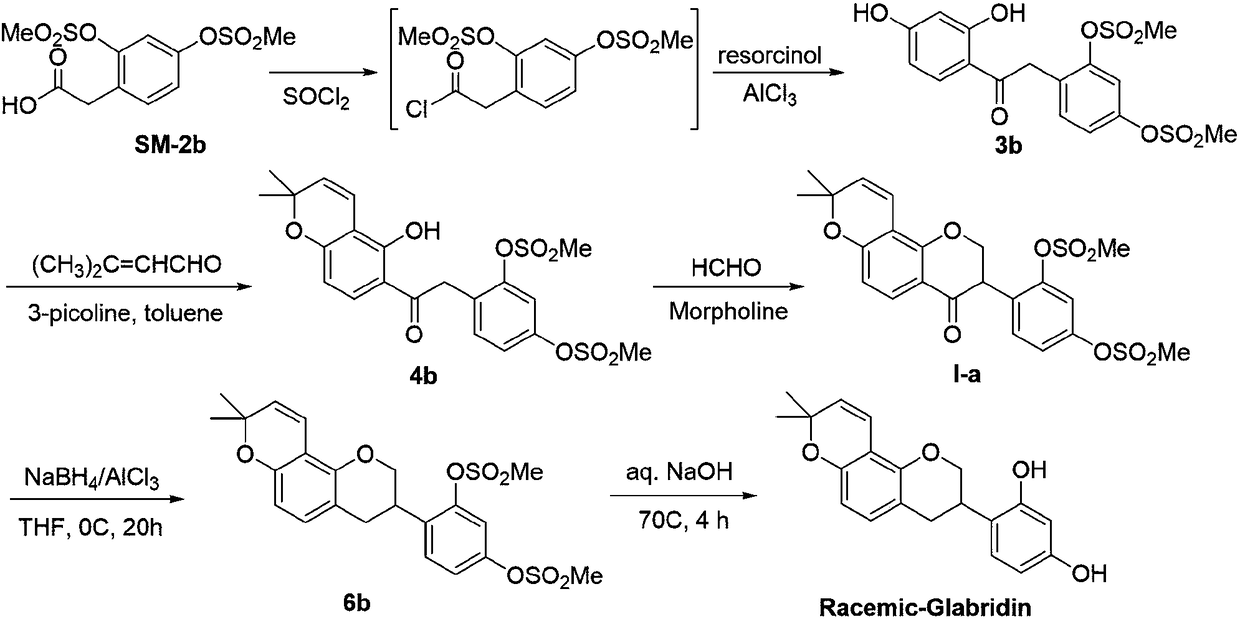
[0070] 1) synthesis of compound II-b,
[0071] 4-((3R,4R)-4-hydroxy-8,8-dimethyl-3,4-dihydro-2H,8H-pyrano[2,3-f]chromen-3-yl)-1,3-phenylene diacetate :
[0072] The chemical formula is:
[0073]
[0074] The experimental steps and operations are as follows: at room temperature (25° C.), 2.50 mmol of dichloro(p-methylisopropylphenyl) ruthenium (II) dimer (CAS number: 52462-29-0) and 5.0 mmol ( R,R)-N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine [(R,R)-TsDPEN, CAS#144222-34-4] was sequentially added to 300 mL of stirred ethyl acetate Then, add the triethylamine / propionic acid buffered proton system which was previously mixed with 120mL triethylamine and 40mL propionic acid and cooled to room temperature. The entire mixed system was stirred at room temperature for 30 minutes to form a uniform LC solution of the active metal catalyst; set aside. Install a mechanical...
Embodiment 3
[0091] A method for asymmetrically synthesizing glabridin with optical purity, comprising the steps of:
[0092] 1) synthesis of compound II-c,
[0093] 4-((3R,4R)-4-hydroxy-8,8-dimethyl-3,4-dihydro-2H,8H-pyrano[2,3-f]chromen-3-yl)-1,3-phenylene dimethylsulfonate :
[0094] The chemical formula is:
[0095]
[0096] The experimental steps and operations are as follows: at room temperature (25°C), mix 0.05 mmol of dichloro(p-methylisopropylphenyl) ruthenium (II) dimer (CAS number: 52462-29-0) and 0.1 mmol ( R,R)-N-(p-toluenesulfonyl)-1,2-diphenylethylenediamine [(R,R)-TsDPEN, CAS#144222-34-4] was sequentially added to 200 mL of stirred ethyl acetate In; then add the diisopropylethylamine / formic acid buffer proton system that was previously mixed with 96mL diisopropylethylamine and 32mL formic acid and cooled to room temperature. The entire mixed system was stirred at room temperature for 30 minutes to form a uniform LC solution of the active metal catalyst; set aside. I...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com