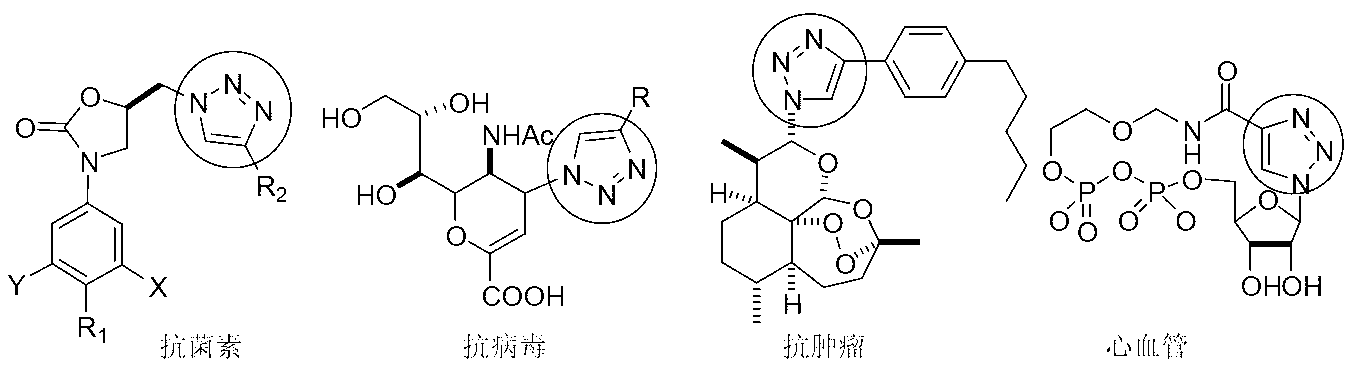
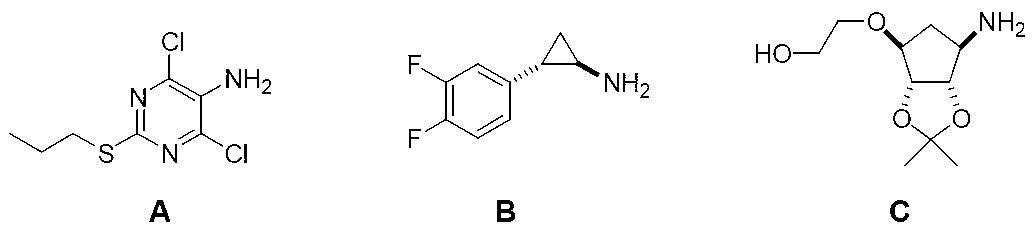
Azide and preparation method thereof
An azide compound and azidation technology, applied in the direction of organic chemistry, to achieve the effects of high product yield and chiral purity, mild and easy-to-control reaction conditions, and simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Add [3aR-(3aα, 4α, 6α, 6aα)]-6-amino-2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxane in the reaction flask Pentan-4-ol (II) (1.73g, 10mmol), potassium carbonate (2.76g, 20mmol), copper sulfate (32mg, 2%eq) and anhydrous methanol 25mL, add imidazolesulfonyl azide at 0°C under nitrogen atmosphere Nitrogen (III) (2.1 g, 12 mmol) in methanol was stirred at room temperature and reacted for 5 hours. TLC detected that the reaction was complete. Concentrate under reduced pressure, and recrystallize the residue with n-hexane and ethyl acetate to obtain [3aR-(3aα, 4α, 6α, 6aα)]-6-azido-2,2-dimethyl-tetrahydro-4H - Cyclopenta-1,3-dioxol-4-ol (I) 1.8 g, yield 90.4%.
Embodiment 2
[0028] Add [3aR-(3aα, 4α, 6α, 6aα)]-[6-amino-2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxa to the reaction flask Cyclopent-4-oxyl]ethanol (II) (2.17g, 10mmol), potassium carbonate (2.76g, 20mmol), copper sulfate (32mg, 2%eq) and acetonitrile 25mL, added trifluoro A solution of methanesulfonyl azide (III) (2.1 g, 12 mmol) in acetonitrile was stirred at room temperature and reacted for 3 hours. TLC detected that the reaction was complete. Concentrate under reduced pressure, and recrystallize the residue with n-hexane and ethyl acetate to obtain [3aR-(3aα, 4α, 6α, 6aα)]-[6-azido-2,2-dimethyl-tetrahydro- 4H-Cyclopenta-1,3-dioxol-4-oxyl]ethanol (I) 2.25 g, yield 92.6%.
Embodiment 3
[0030] Add [3aR-(3aα, 4α, 6α, 6aα)]-[6-amino-2,2-dimethyl-tetrahydro-4H-cyclopentadiene-1,3-dioxa to the reaction flask Cyclopent-4-oxyl]methyl acetate (II) (2.45g, 10mmol) and dry tetrahydrofuran 30mL, cooled to 0°C and added dropwise sodium hydride (6g, 60% mineral oil) in tetrahydrofuran under nitrogen atmosphere solution and p-toluenesulfonyl azide (III) (3.0 g, 15 mmol) in tetrahydrofuran. After the dropwise addition was completed, the temperature was raised to room temperature, and the reaction was stirred for 30 hours, and TLC detected that the reaction was complete. The reaction was quenched with methanol in an ice bath, poured into water, adjusted to weakly acidic pH with hydrochloric acid, extracted three times with ethyl acetate, and dried over anhydrous sodium sulfate. Concentrate to dryness under reduced pressure, recrystallize from n-hexane / ethyl acetate to obtain off-white solid [3aR-(3aα, 4α, 6α, 6aα)]-[6-azido-2,2-dimethyl-tetra Hydrogen-4H-cyclopentadieno-1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com