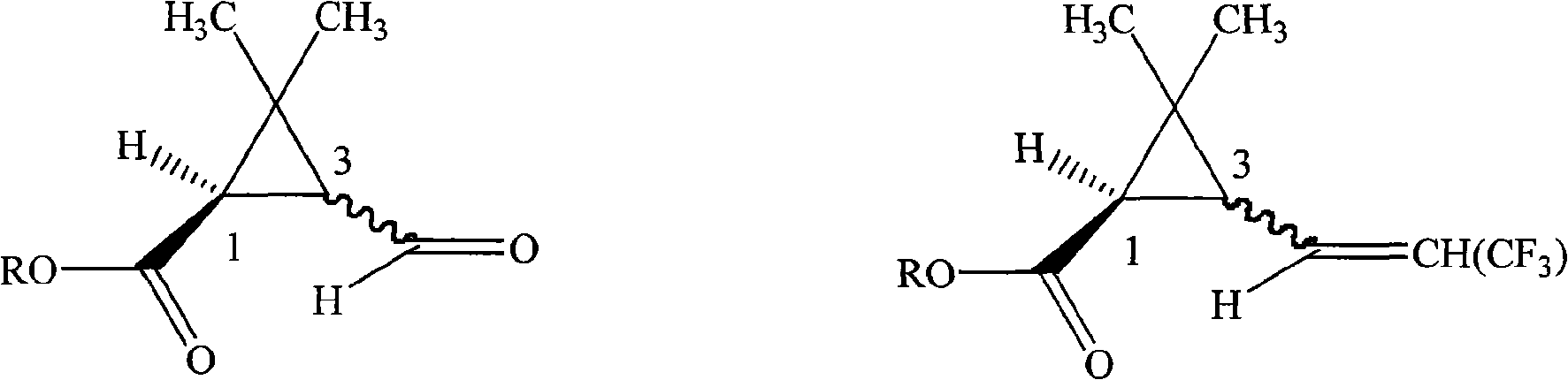New pyrethroid compound and preparation method and application
A technology of pyrethroids and compounds, applied in the field of pyrethroids, can solve the problems of high lethality and fast knockdown, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
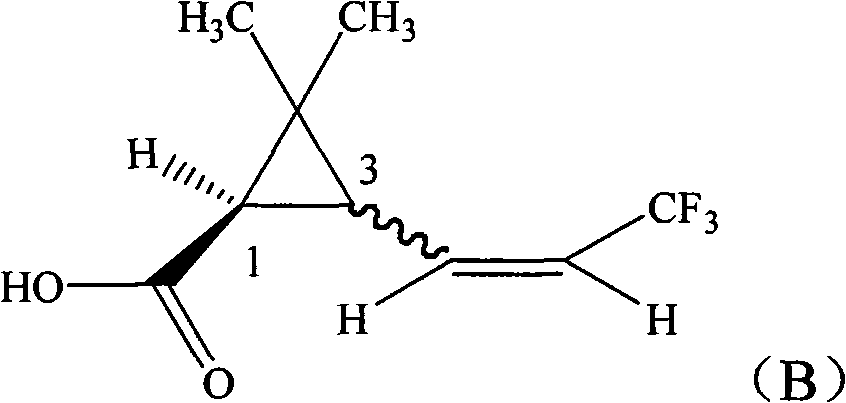
[0023] Synthesis of 1R-3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid with 1:1 ratio of cis to trans
[0024] In a 250ml four-necked bottle, 15.6g (0.1mol) of methyl 1R-3-formyl-2,2-dimethylcyclopropanecarboxylate, 50ml anhydrous tetrahydrofuran, was dropped into with a cis-to-trans ratio of 1:1, Then add 0.5g potassium tert-butoxide, and add 34.5g (0.1mol) (C 6 h 5 ) 3 P + -CH 2 CF 3 - Dissolve the suspension in 80ml tetrahydrofuran, drop it within 2 hours, then raise the temperature to 20°C for 8 hours reaction. Remove tetrahydrofuran under 50mmHg negative pressure, add 100ml of toluene, wash twice with 200ml of water respectively, separate the toluene layer and add 100g of 10% sodium hydroxide solution, heat to 80°C under stirring for 2 hours, cool to room temperature, separate Remove the toluene layer. Transfer the water layer to a 250ml three-neck flask, add 100g of 30% sulfuric acid solution dropwise in an ice-water bath, keep the temperat...
preparation Embodiment 2
[0026] Preparation of 1R-(Z)-3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarboxylic acid with a cis-trans ratio of 1:1
[0027]In a 100ml beaker, 16.4g (92.3 %), then add 1:1 weight ratio of methanol-water solution 30g, stir at 40°C for 30 minutes, filter, and the gained solid is stirred in 15g of 1:2 weight ratio of methanol-water solution for 30 minutes at 30°C, and again After filtration, the resulting solid was washed once with 5ml of water, and air-dried to obtain 1R-(Z)-3-(3,3,3-trifluoro-1-propenyl)-2,2 with a cis-to-trans ratio of 1:1. - 5.8 g of dimethylcyclopropanecarboxylic acid, content 98.7%. Combine the mother liquors filtered twice, concentrate on a thin-film evaporator at 40°C and 100mmHg negative pressure until solids are precipitated, take back the concentrated solution and cool to 5°C, some solids precipitate again, filter, and air-dry to obtain a cis-trans ratio of 1 : 4.2 g of 1R-(E)-3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanecarbo...
preparation Embodiment 3
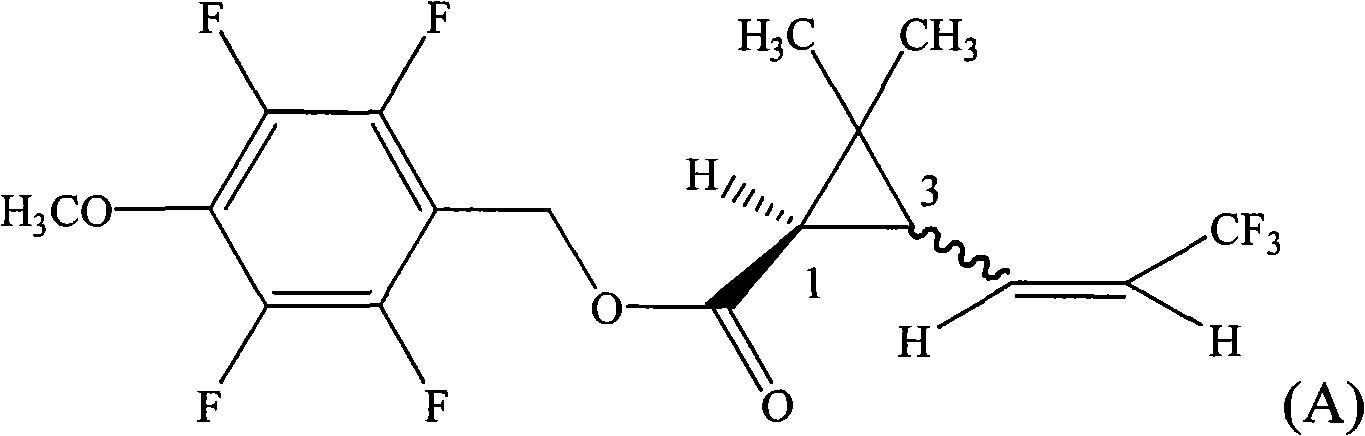
[0029] 2,3,5,6-tetrafluoro-4-methoxybenzyl-1R-(Z)-3-(3,3,3-trifluoro-1-propenyl)- 2, the preparation of 2-dimethyl cyclopropane carboxylate (compound 1)
[0030] In a 500ml flask, the 1R-(Z)-3-(3,3,3-trifluoro-1-propenyl)-2,2 -20.8g (0.1mol) of dimethylcyclopropanecarboxylic acid, 2,3,5,21.0g (0.1mol) of 6-tetrafluoro-4-methoxybenzyl alcohol, 180ml toluene, install the water trap, and Add 0.1g of p-toluenesulfonic acid, heat to reflux, react with water for 6 hours, add 20ml of toluene in the middle, cool to room temperature after the reaction, wash once with 100g water, wash once with 100g 5% dilute hydrochloric acid, wash once with 100g 5% dilute hydrochloric acid Wash once with sodium bicarbonate solution, and finally wash once again with 100g of water, collect the toluene layer and heat it to 100°C under a negative pressure of 10mmHg to remove the solvent toluene to obtain 2,3,5,6-tetrafluoro-4-methoxybenzyl- 1R-(Z)-3-(3,3,3-trifluoro-1-propenyl)-2,2-dimethylcyclopropanec...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com