Production method of hexahydrofurofuranol derivative, intermediate therefor and production method thereof
A compound and hydrogen atom technology, applied in organic chemistry and other fields, to achieve excellent results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
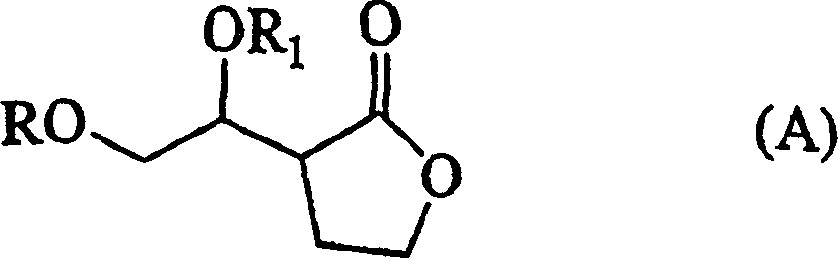
[0288] Compound (XIX) can usually be prepared in THF, hexane, di-n-butyl ether, MTBE, ethylene glycol dimethyl ether, diethylene glycol dimethyl ether, 1,3-dioxolane, 1, 4 -Carry out in reaction solvents such as dioxane and toluene, preferably in THF or hexane. The amount of the reaction solvent used is usually 1-100L, preferably 3-30L, relative to 1kg of compound (XIII).
[0289] The reaction temperature is usually -100°C to 70°C, preferably -80°C to 40°C; the reaction time is usually 0.5-48 hours, preferably 3-24 hours.
[0290] Formula: R 3 O-CH 2 CH 2 The compound represented by -X can be prepared by a known method. For example, 2-benzyloxyethyl iodide can react 2-benzyloxyethanol with methanesulfonyl chloride in the presence of a catalyst such as triethylamine to obtain 2-benzyloxyethyl methanesulfonate, which can be reacted with sodium iodide To prepare by reaction, 2-tert-butoxyethyl iodide can be prepared by using 2-tert-butoxyethanol instead of the aforementioned 2-benzyl...
Embodiment 1
[0399] Example 1 (1’R * , 2S * )-2-[2'-(1,1-Dimethylethoxy)-1'-hydroxyethyl]-4-butyrolactone synthesis (compound with relative stereo configuration shown in formula (VII) )
[0400] Dissolve 4-tert-butoxy ethyl acetoacetate (20.0 g), which can be synthesized according to the method described in Heterocycles 26, 2841 (1987), in methanol (150 mL), and then add sodium borohydride (1.68 g), stir for 1 hour, and then add water (100 mL). Most of the solvent was distilled off, extracted twice with MTBE (150 mL), the organic layer was washed thoroughly with water, and then MTBE was distilled off to obtain ethyl (±)-4-tert-butoxy-3-hydroxybutyrate (16.9 g) . A 1.5M cyclohexane solution (73mL) of lithium diisopropylamide was added to THF (100mL), and (±)-4-tert-butoxy was added to the resulting solution at -58°C to -48°C A solution of ethyl-3-hydroxybutyrate (10.66 g) in THF (30 mL) was then raised to -20°C. In addition, in the presence of p-toluenesulfonic acid monohydrate (10mg), 2-iodoet...
Embodiment 2
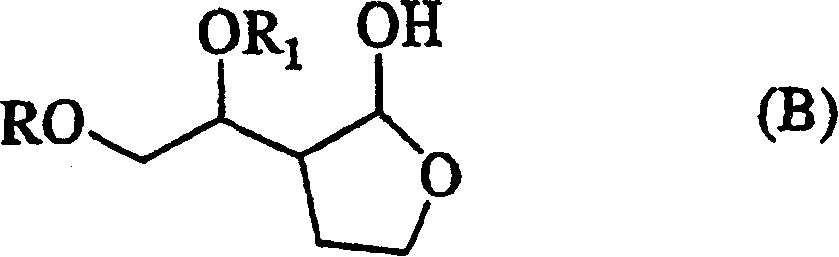
[0402] Example 2 (1’R * , 2S * )-2-(1',2'-Dihydroxyethyl)-4-butyrolactone (compound with relative stereo configuration shown in formula (VIII))
[0403] Will (1’R * , 2S * )-2-[2'-(1,1-Dimethylethoxy)-1'-hydroxyethyl]-4-butyrolactone (1.3g) was added to trifluoroacetic acid (4mL) and placed on ice After stirring in the bath for 90 minutes, the trifluoroacetic acid was directly distilled off under reduced pressure to obtain the title compound (1.0 g).
[0404] 1 H-NMR(CDCl 3 , Δppm): 2.10-2.20(1H, m), 2.37-2.44(1H, m), 2.80-2.87(1H, m), 3.6-3.8(1H(-OH), br), 3.67(1H, dd, J=12Hz, J=6Hz), 3.75 (1H, dd, J=12Hz, J=3Hz), 3.86-3.90 (1H, m), 4.24-4.30 (1H, m), 4.43 (1H, dt, J= 9Hz, J=3Hz), 4.4-4.5(1H(-OH), br).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com