Fluorinated quinolylamide foldamer, preparation method, chirality recognition method and application
A kind of fluoroquinoline amide, quinoline amide technology, applied in the field of fluoroquinoline amide folded body, can solve the problem of consuming a lot of time and energy and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0057] The preparation method of fluorine-containing quinoline amide folding body shown in above-mentioned formula (1) of the present invention, comprises the steps:
[0058]
[0059] 1) As shown in formula (3), compound Q F Soluble in concentrated H 2 SO 4 and CH 3 In COOH (v / v=1 / 1), heat and stir at 115-125°C for 10-15h; TLC monitors the reaction progress, after the reaction is completely completed, cool to room temperature; pour the above reaction solution into ice water, extract three times with DCM , combine the organic phases and wash with anhydrous Na 2 SO 4 Dry and spin dry to obtain compound Q F COOH;
[0060] 2) Put Q F COOH is dissolved in anhydrous DCM (abbreviation of dichloromethane) under the protection of argon, and 1.8-2.2 equivalents of oxalyl chloride and catalytic amount of DMF (abbreviation of dimethylformamide) are added to it; the above solution is reacted at room temperature 1.8-2.2h, after the reaction is over, drain the above solution and c...
Embodiment 1
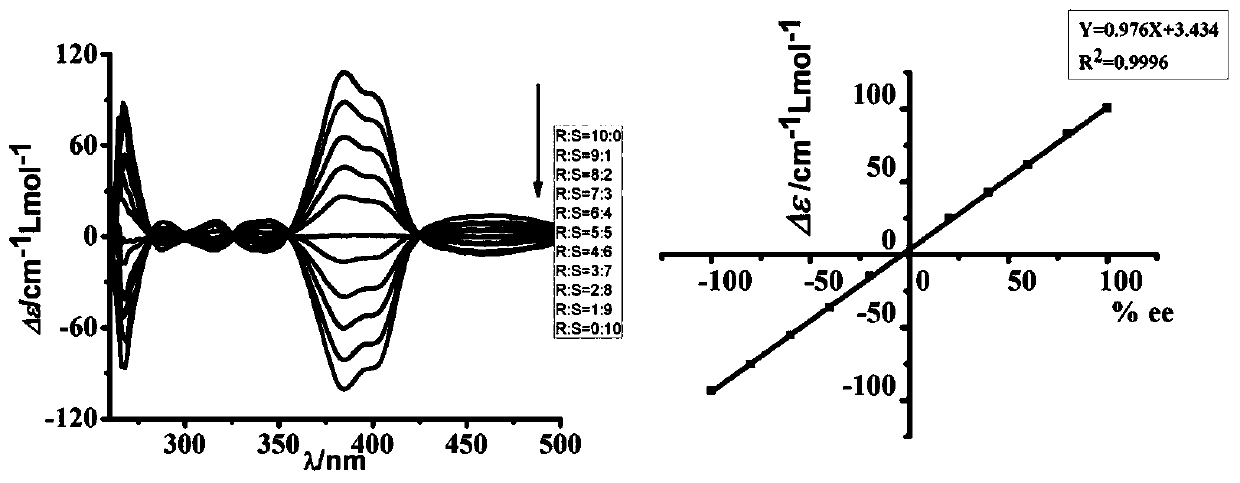
[0080] Fluorine-containing quinoline amide fold Q F Q 4 The absolute configuration of chiral phenylethylamine and the determination of ee value comprise the steps:
[0081] 1) Configure 7.5mmol / L of DMF-d of R-phenethylamine and S-phenethylamine 7 Solution. Weigh 4.5 mg each of R-phenethylamine and S-phenethylamine and add them to two 5mL volumetric flasks, add DMF-d 7 to the tick mark;
[0082] 2) Accurately weigh the fluoroquinolinamide folded body Q F Q 4 3.23 mg, K 2 CO 3 0.52mg is placed in an NMR tube, and the DMF-d of R-phenethylamine and S-phenethylamine is extracted separately with a micro-injector 7 Solution 500μL / 0μL, 450μL / 50μL, 400μL / 100μL, 350μL / 150μL, 300μL / 200μL, 250μL / 250μL, 200μL / 300μL, 150μL / 350μL, 100μL / 400μL, 50μL / 450μL, 0μL / 500μL tube, heated to 95 °C. Use 1H NMR to monitor the reaction until complete;
[0083] 3) Use a micro-injector to take 10 μL of the above reaction solution and add it to a 10mm*10mm cuvette, then add 2mL of spectrally pure D...
Embodiment 2
[0086] Fluorine-containing quinoline amide fold Q F Q 4 The determination of the absolute configuration of 1-naphthylethylamine and the ee value comprises the steps:
[0087] 1) Configure 7.5mmol / L of DMF-d of R-1-naphthylethylamine and S-1-naphthylethylamine 7 Solution. Weigh 6.4 mg each of R-1-naphthylethylamine and S-1-naphthylethylamine and add to two 5mL volumetric flasks, add DMF-d 7 to the tick mark;
[0088] 2) Accurately weigh the fluoroquinolinamide folded body Q F Q 4 3.23 mg, K 2 CO 30.52mg is placed in the NMR tube, and the DMF-d of R-1-naphthylethylamine and S-1-naphthylethylamine are respectively extracted with a micro-injector 7 500μL / 0μL, 450μL / 50μL, 400μL / 100μL, 350μL / 150μL, 300μL / 200μL, 250μL / 250μL, 200μL / 300μL, 150μL / 350μL, 100μL / 400μL, 50μL / 450μL, 0μL / 500μL, respectively In an NMR tube, heat to 85°C; use 1H NMR to detect the reaction until complete;
[0089] 3) Take 10 μL of the above reaction solution and add 2 mL of spectroscopically pure DCM fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com