Novel sitafloxacin crystal form and preparation method thereof
A sitafloxacin and crystal form technology, which is applied in the field of pharmaceutical inventions, can solve the problems of complex desolvation and high solvent residue, and achieve the effects of simple operation, safety and drug quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1 Preparation of sitafloxacin raw material
[0035] The sitafloxacin raw material is prepared according to the method disclosed in the literature [J.Med.Chem.1994, 37, 3344-3352], that is, 5.6 g of ethyl 3-chloro-2,4,5-trifluoro-benzoylacetate , condensed with 15g triethyl orthoformate to generate 3-ethoxy-2-(3-chloro-2,4,5-trifluorobenzoyl)ethyl acrylate, and the solvent was evaporated under reduced pressure for direct use in the following One step, then react with 2.25g (1R,2S)-2-fluoro-cyclopropylamine hydrochloride to synthesize 8-chloro-6,7-difluoro-1-[(1R,2S)-2-fluorocyclopropyl ]-4-oxo-1,4-dihydroxyquinoline-3-carboxylic acid ethyl ester 5.5g, two-step yield 75%, take 3.65g of the solid from the previous step and then cyclize to generate 8-chloro-6,7 -Difluoro-1-[(1R,2S)-2-fluorocyclopropyl]-4-oxo-1,4-dihydroxyquinoline-3-carboxylic acid 3.2g, yield 92.5%, and then passed Hydrochloric acid hydrolysis gives 7-[7-(s)-tert-butoxycarbonyl-5-azaspiro[2,4]...
Embodiment 2
[0036] Example 2 Preparation of new crystal form of sitafloxacin
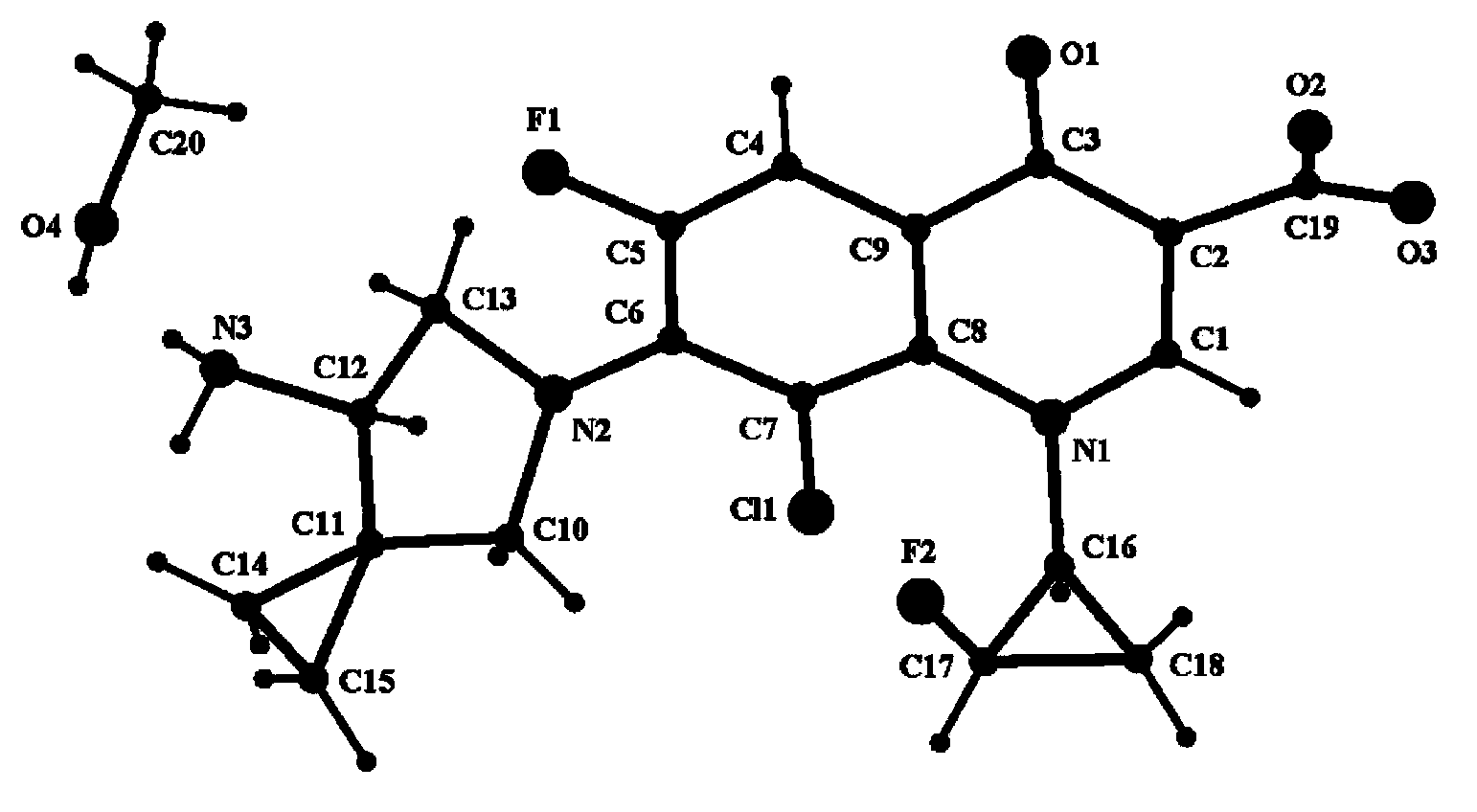
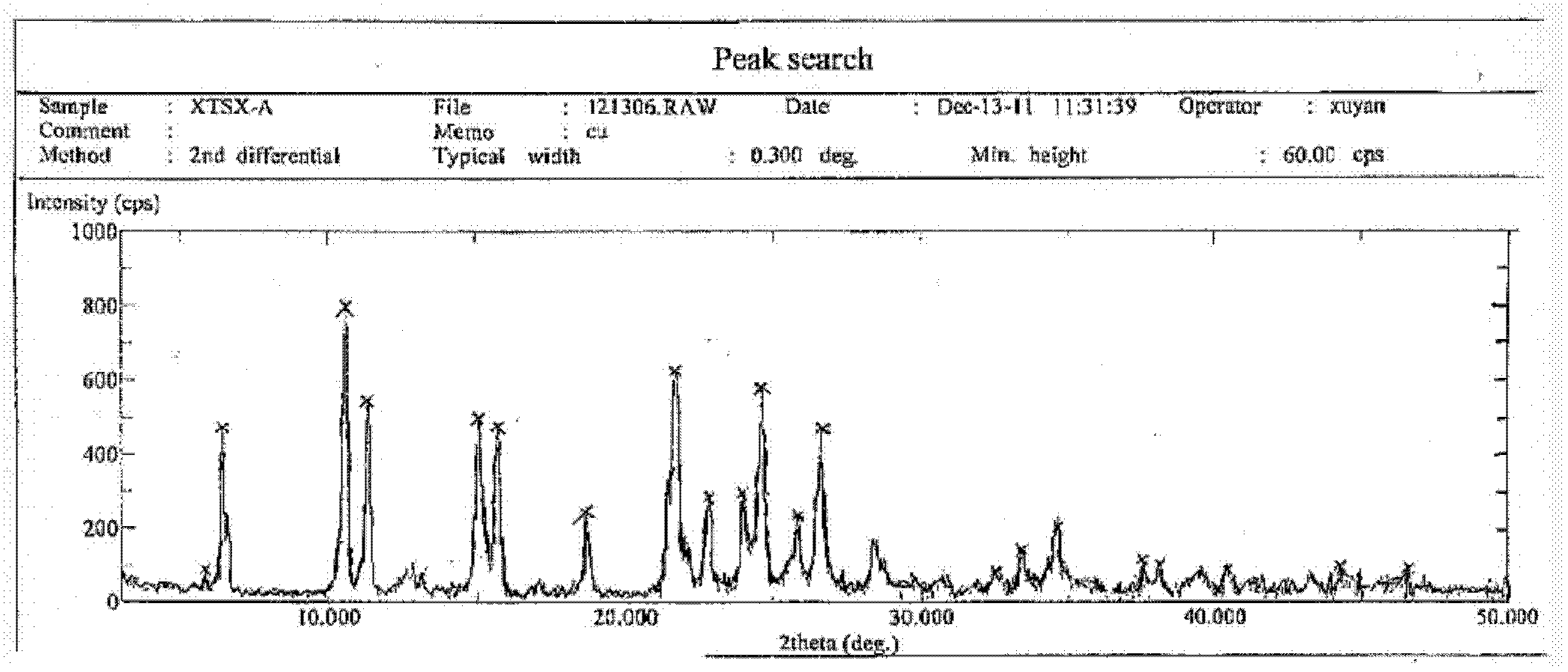
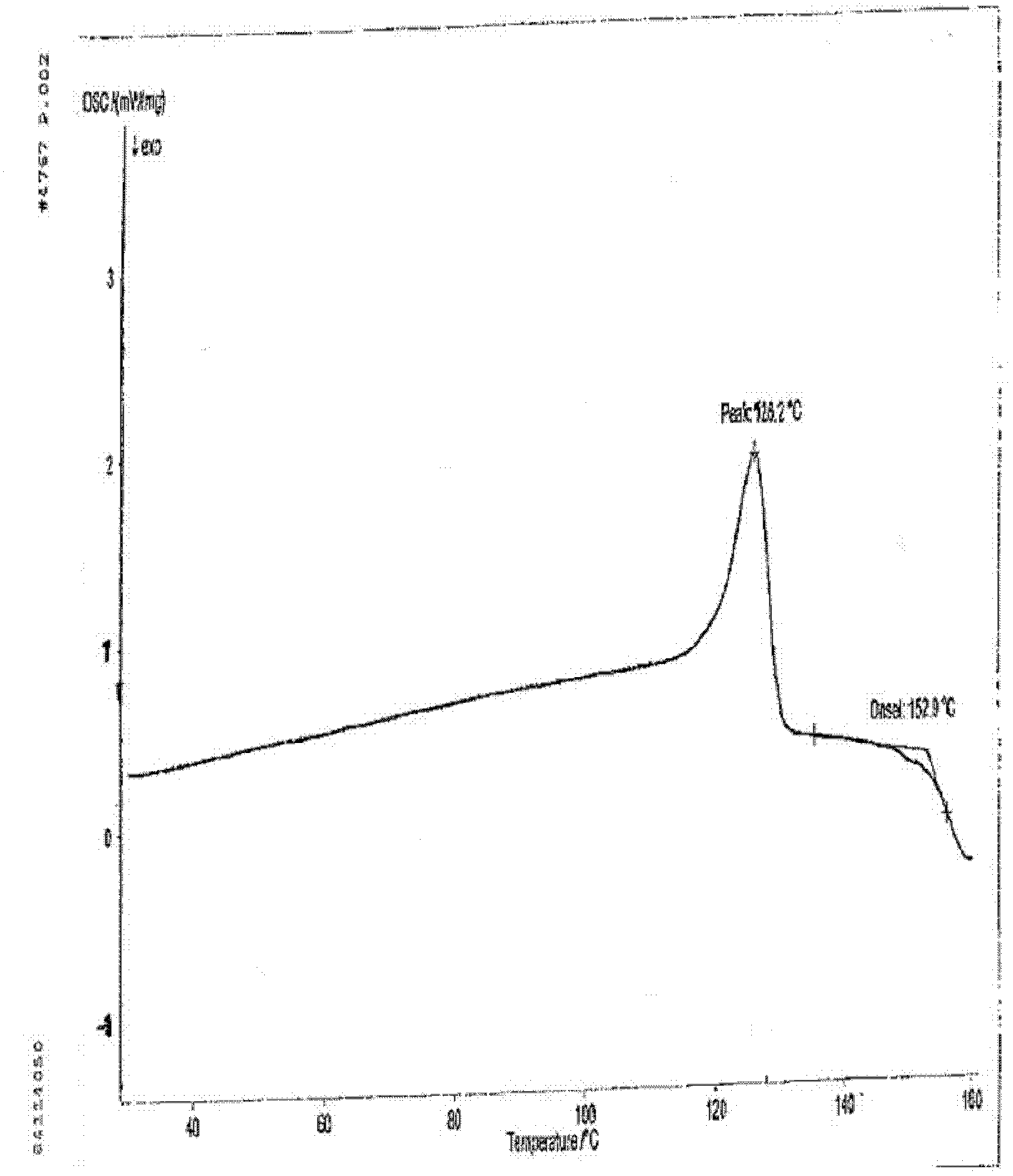
[0037] Take 0.5 g of the crude anhydrous sitafloxacin prepared in Example 1, dissolve it in 25 ml of methanol solution, heat to dissolve at 50° C., place the solution at room temperature, slowly evaporate the solvent, and crystallize to obtain 0.35 g of needle-like crystals. The X-ray powder diffraction spectrum of gained new crystal form product, differential scanning calorimetry (DSC) collection of illustrative plates and the molecular space three-dimensional structure figure obtained by single crystal diffraction are respectively as follows figure 1 , figure 2 , image 3As shown, the single crystal diffractometer uses Mo-kα as the radiation source to measure, and the results of crystallographic parameters are as follows:
[0038]
[0039]
Embodiment 3
[0040] Example 3 Preparation of new crystal form of sitafloxacin
[0041] Take 0.4 g of the anhydrous sitafloxacin crude product prepared in Example 1, dissolve it in 35 ml of methanol solution, heat to dissolve at 40° C., place the solution at room temperature, slowly evaporate the solvent, and crystallize to obtain 0.27 g of needle-like crystals.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com