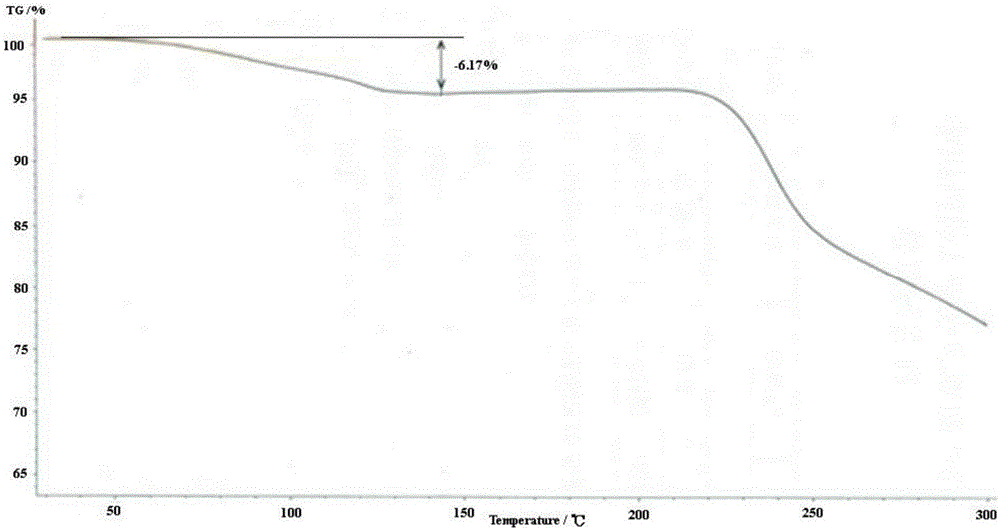
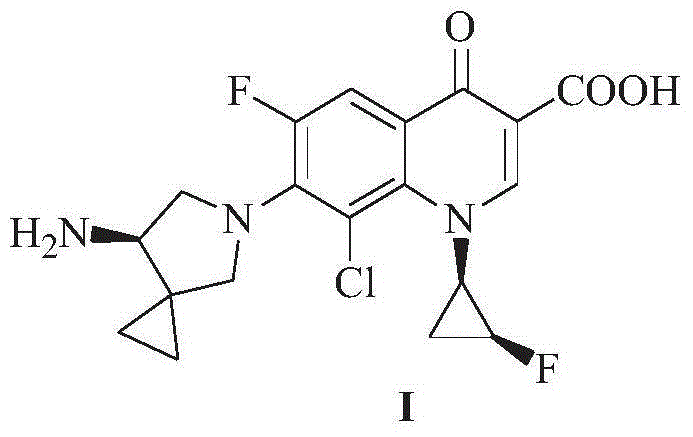
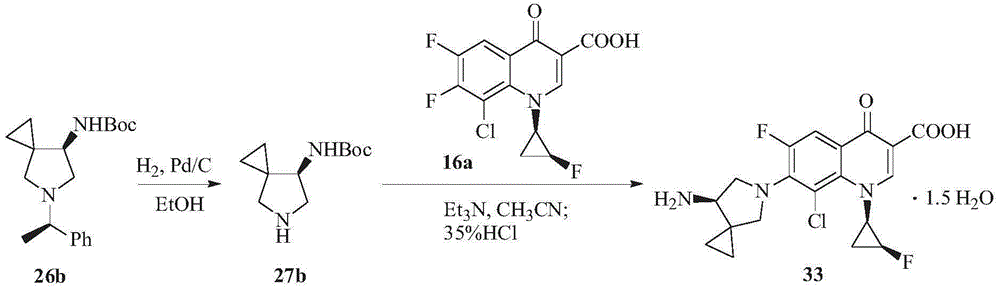
Preparation method for sitafloxacin hydrate
A technology for sitafloxacin hydrate and compounds, which is applied in the field of preparation of sitafloxacin hydrate, can solve the problems of cumbersome operation, poor safety, and high cost, and achieve simplified process operation, reduced dosage, and simplified separation and purification operations Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation method of sitafloxacin hydrate described in the present embodiment comprises the following steps:
[0031] (1) Put 3.09g of compound II and 30.9mL of ethanol into a 50ml reaction flask, add 0.31g of 10% Pd / C and 1.93g of ammonium acetate under stirring, and reflux for 1 hour. After the reaction system is cooled, filter and wash with ethanol The filter cake was filtered three times, and the filtrate was collected and concentrated under reduced pressure to obtain 2.12 g of an off-white solid, namely compound III, with a yield of 97.7%;
[0032] (2) Under stirring, add 24.0mL of acetonitrile, 2.40g of compound IV, 1.89g of triethylamine and 2.10g of compound III to the 100mL reaction flask in sequence, and heat to reflux for reaction. After the reaction is completed, the reaction solution is cooled to room temperature , add 72.0mL of purified water to it, stir evenly, filter, the obtained filter cake is washed with water, and vacuum-dried at 40°C to obtain ...
Embodiment 2
[0042] The preparation method of sitafloxacin hydrate described in the present embodiment comprises the following steps:
[0043] (1) Put 3.09g of compound II and 30.9mL of methanol into a 50ml reaction flask, add 0.11g of 10% Pd / C and 4.81g of ammonium acetate under stirring, and react at 50°C. After the reaction is completed, cool and filter. The filter cake was washed three times with methanol, the filtrate was collected, and concentrated under reduced pressure to obtain 2.17 g of off-white solid, namely compound III, with a yield of 100%;
[0044] (2) Under stirring, add 24.0mL of acetonitrile, 2.83g of compound IV, 2.58g of N,N-diisopropylethylamine and 2.10g of compound III to a 100mL reaction flask in sequence, and react at 25°C. After the reaction is completed, , adding 96.0 mL of purified water to it, stirring evenly, filtering, washing the obtained filter cake with water, and drying in vacuum at 40°C to obtain 4.37 g of off-white solid, which is Compound V, with a yi...
Embodiment 3
[0048] The preparation method of sitafloxacin hydrate described in the present embodiment comprises the following steps:
[0049] (1) 3.09g of compound II and 30mL of isopropanol were dropped into a 50ml reaction flask, and 0.58g of 10% Pd / C and 1.89g of ammonium formate were added under stirring, and the reaction was carried out at 20°C. After the reaction was completed, filter and use The filter cake was washed three times with isopropanol, the filtrate was collected, and concentrated under reduced pressure to obtain 2.17 g of off-white solid, namely compound III, with a yield of 100%;
[0050] (2) Under stirring, add 24.0mL of acetonitrile, 1.42g of Compound IV, 2.04g of aqueous ammonia and 2.10g of Compound III with an ammonia content of 25wt% to a 100mL reaction flask, and react at 55°C. After the reaction is complete, cool , adding 24.0 mL of purified water to it, stirring evenly, filtering, washing the obtained filter cake with water, and drying in vacuum at 40°C to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com