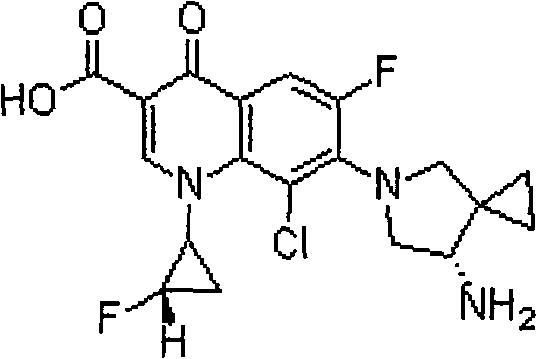
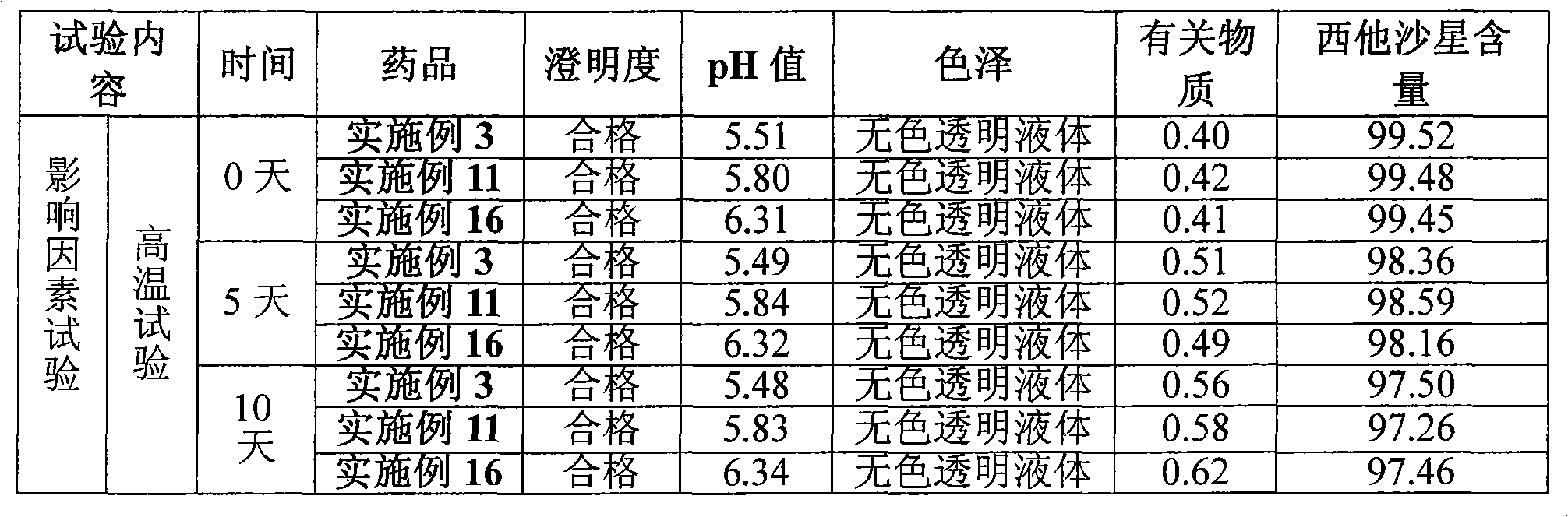
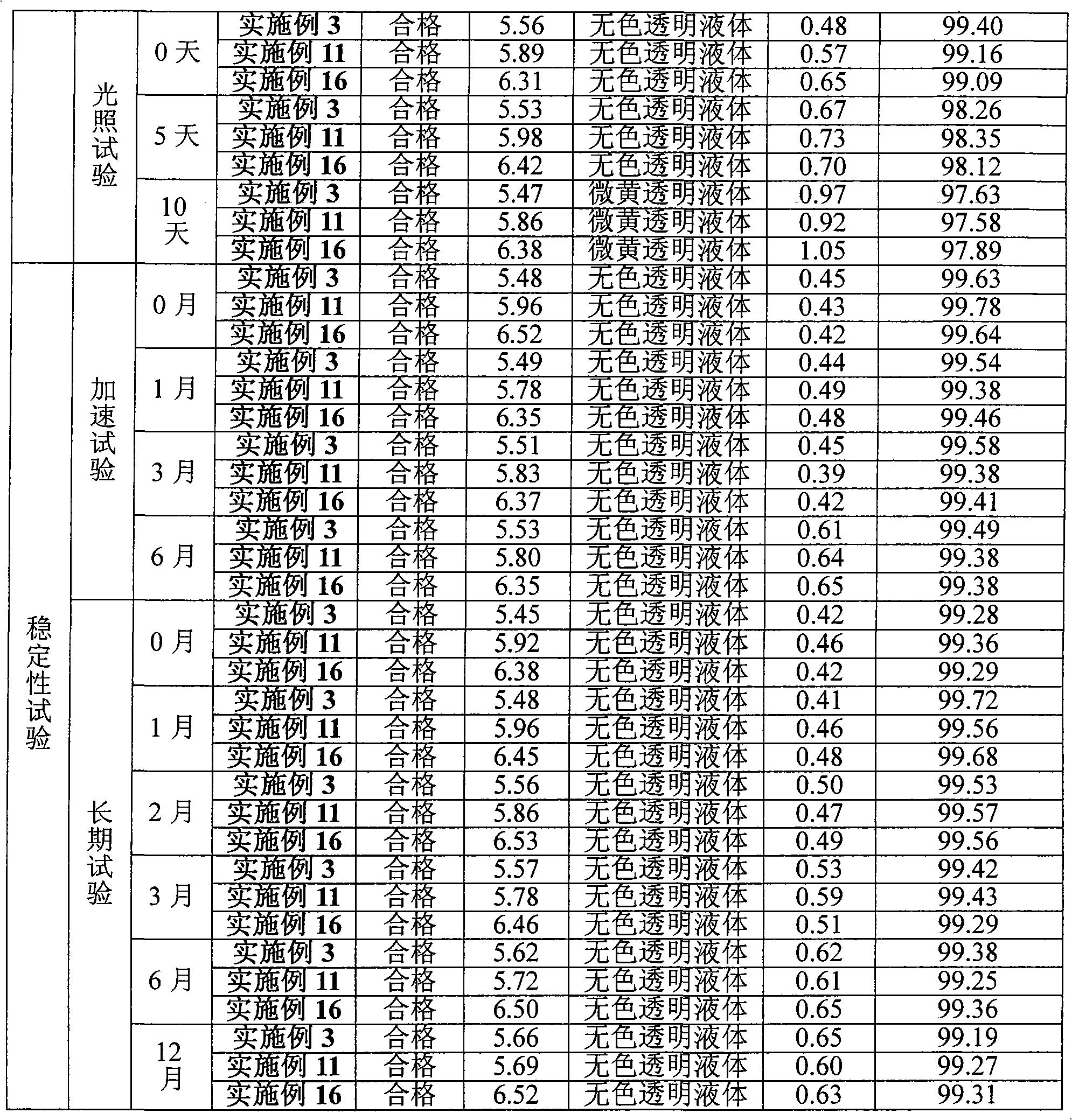
Sitafloxacin hydrate injection and preparation method thereof
A technology for sitafloxacin and injection, applied in the field of medicine, can solve the problems of technical difficulties of injection, unstable aqueous solution, low solubility, etc., and achieve the effects of reducing impurities, increasing stability and improving curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0028] prescription:
[0029] Sitafloxacin 50g
[0030] Sodium Benzoate 40g
[0032] Polyvinyl alcohol 0.4g
[0033] Sodium metabisulfite 10g
[0034] EDTA-2Na 0.75g
[0035] Phosphate buffer saline
[0036] Add water for injection to 1000ml
[0037]
[0038] Made into 500 pieces, 2ml / piece, 100mg / piece
example 2
[0040] prescription:
[0041] Sitasalate 50g
[0042] Sodium Salicylate 15g
[0043] Propylene glycol alginate 0.5g
[0044] Glucose 100g
[0045] EDTA-NaCa 0.75g
[0046] Vitamin C 1g
[0047] Add water for injection to 1000ml
[0048]
[0049] Made into 500 pieces, 2ml / piece, 100mg / piece
example 3
[0051] prescription:
[0052] Sitafloxacin 40g
[0053] Sodium Salicylate 12g
[0054] Sodium chloride 9g
[0055] Cremophor RH40 1.3g
[0056] Glutathione 3g
[0057] EDTA-2Na 0.68g
[0058] Phosphate buffer saline
[0059] Add water for injection to 1000ml
[0060]
[0061] Made into 200 pieces, 5ml / piece, 200mg / piece
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com