Method for detecting small polar impurities in pazufloxacin mesylate bulk drug
A technology for pazufloxacin mesylate and an API is applied in the field of quality control of the pazufloxacin mesylate API, which can solve problems such as ineffective detection and achieve the effect of scientific quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
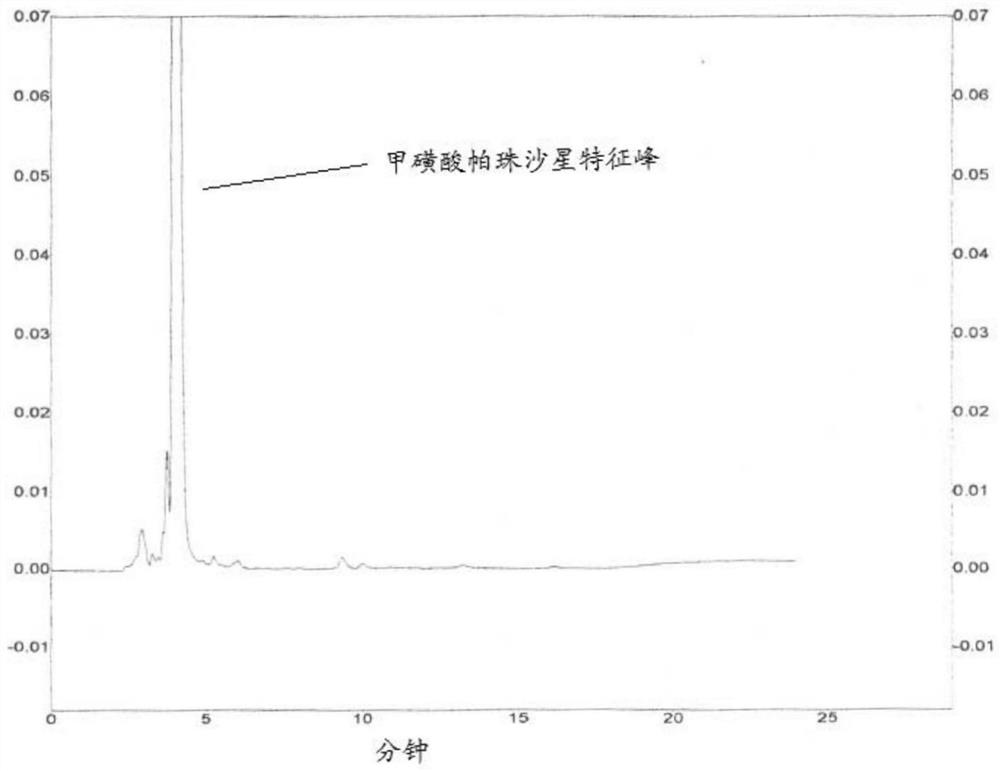
[0035] Embodiment 1 is used to detect the screening of the mobile phase of small polar impurity
[0036] The mobile phase pazufloxacin mesylate raw materials in Table 1 below were used for detection.
[0037] Table 1
[0038] mobile phase 1 Acetonitrile-10% triethylamine methanesulfonate-1M dipotassium hydrogen phosphate-water (30:10:7:170) mobile phase 2 Acetonitrile-10% triethylamine methanesulfonate-1M dipotassium hydrogen phosphate-water (30:10:7:153) mobile phase 3 Acetonitrile-10% triethylamine methanesulfonate-1M dipotassium hydrogen phosphate-water (45:10:7:138) mobile phase 4 Acetonitrile-10% triethylamine methanesulfonate-1M dipotassium hydrogen phosphate-water (70:10:7:113)
[0039] Wherein, the preparation of 10% triethylamine methanesulfonate solution is as follows:
[0040] Under the condition of ice bath, slowly add 30ml of methanesulfonic acid and 30ml of triethylamine into 200ml of water, after the dissolution is complete,...
Embodiment 2
[0052] Example 2 HPLC qualitative detection of Pazufloxacin mesylate bulk drug after photodegradation
[0053] Using mobile phase 3, other chromatographic conditions are the same as in Example 1.
[0054] The detection steps are as follows:
[0055] (1) Preparation of photodegradation test solution: take 30 mg of this product, put it in a 100 ml measuring bottle, use mobile phase to make a solution containing 300 μg of pazufloxacin mesylate per 1 ml, put it under ultraviolet light for 7 days, and use it as a Test solution.
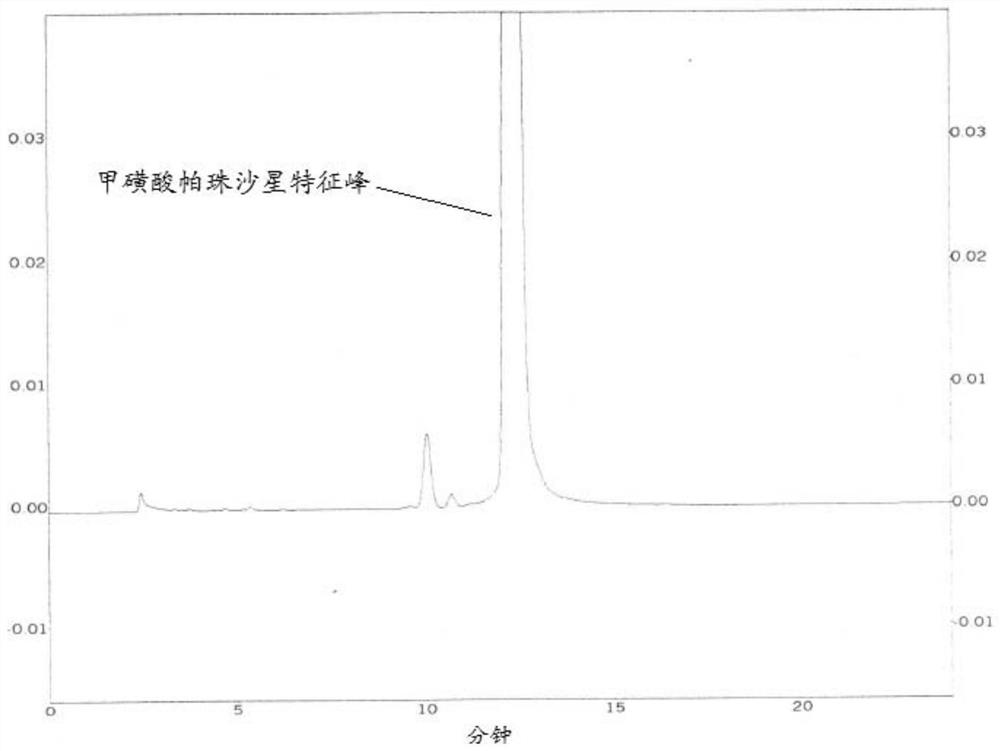
[0056] (2) Perform HPLC detection according to the same method as in Example 1. Chromatograms such as figure 2 shown.
[0057] from figure 2 It can be seen from the figure that for the bulk drug after photodegradation, the small polar impurities produced by the degradation can be comprehensively and effectively removed by using the mobile phase provided by the application within the retention time of the characteristic peak of pazufloxacin mesylate ...
Embodiment 3
[0058] Example 3 The HPLC qualitative detection of the acid-degraded pazufloxacin mesylate crude drug uses mobile phase 3, and other chromatographic conditions are the same as in Example 1.
[0059] The detection steps are as follows:
[0060](1) Prepare the test solution for acid degradation: take 30 mg of pazufloxacin mesylate crude drug, put it in a 50 ml measuring bottle, add 25 ml of 1.0 mol / L hydrochloric acid and heat it in a boiling water bath for 12 hours in the dark, and add 1.0 mol / L sodium hydroxide was diluted and neutralized to the mark as the test solution.
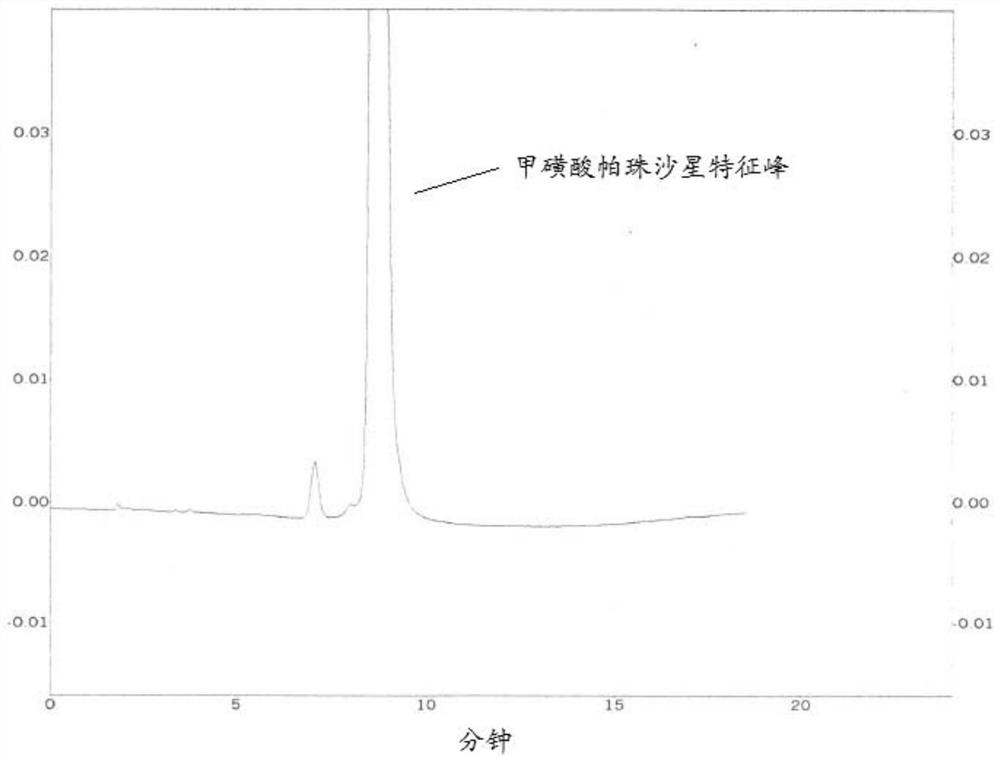
[0061] (2) Perform HPLC detection according to the same method as in Example 1. Chromatograms such as image 3 shown. from image 3 It can be seen from the figure that for the bulk drug after acid degradation, the small polar impurities produced by the degradation can be comprehensively and effectively removed by using the mobile phase provided by the application within the retention time of the charact...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com