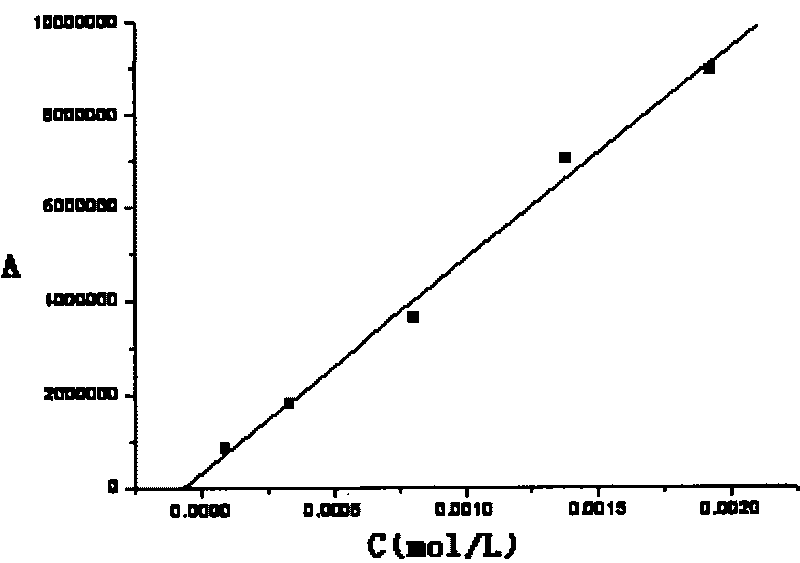
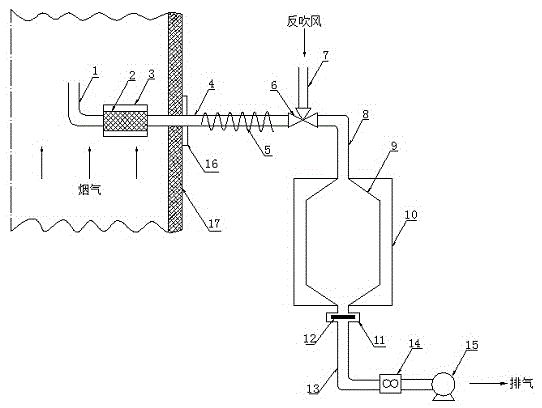
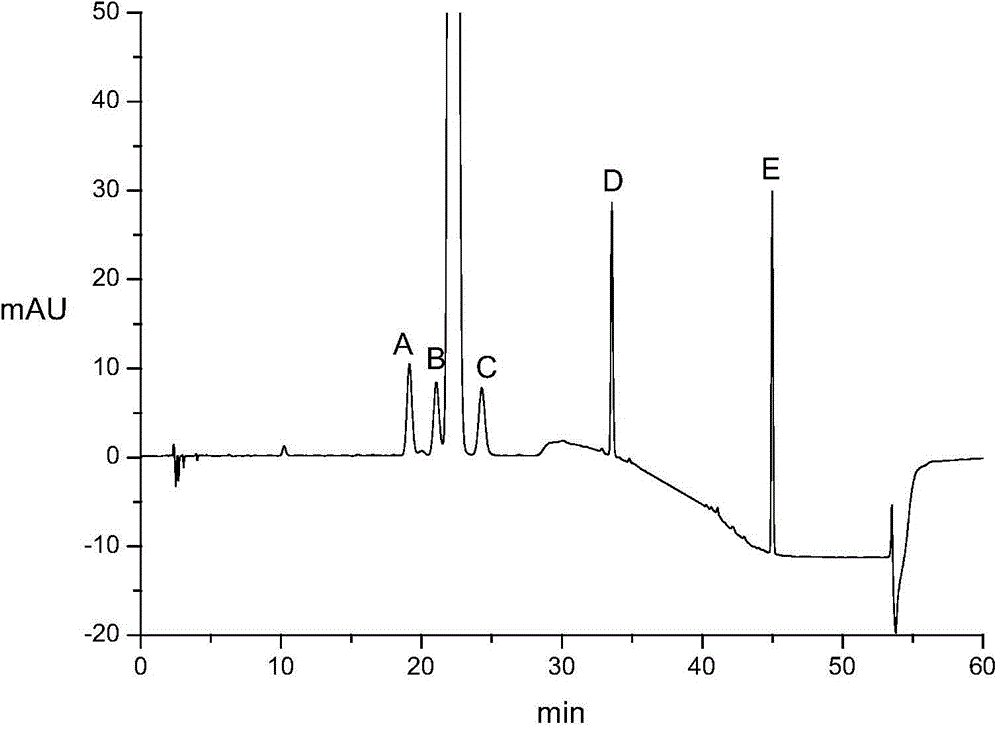
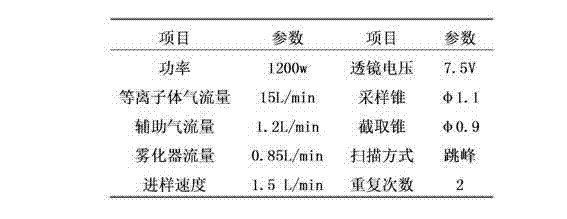
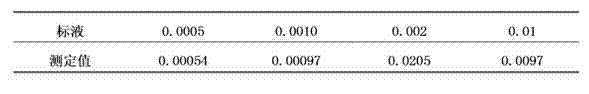
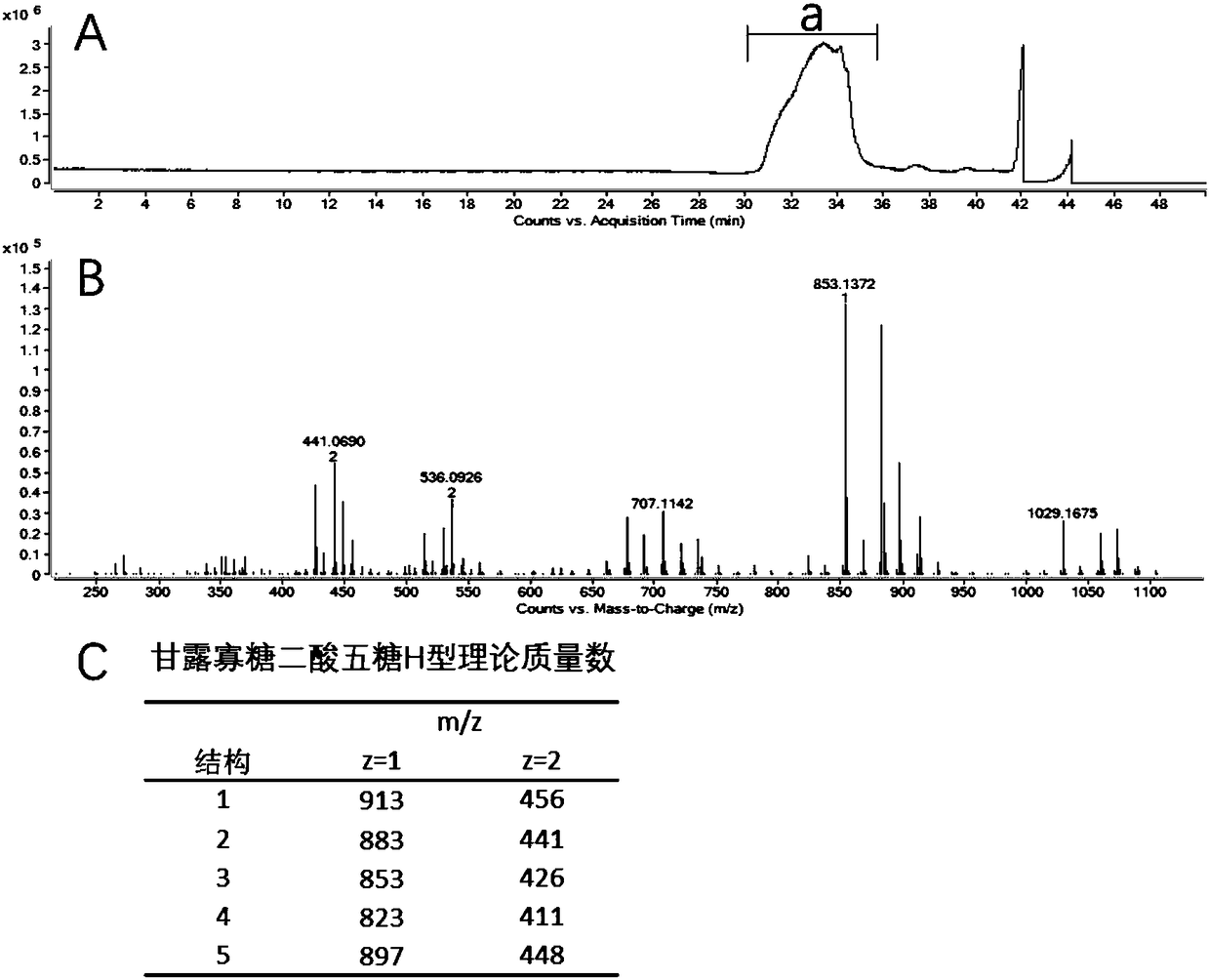
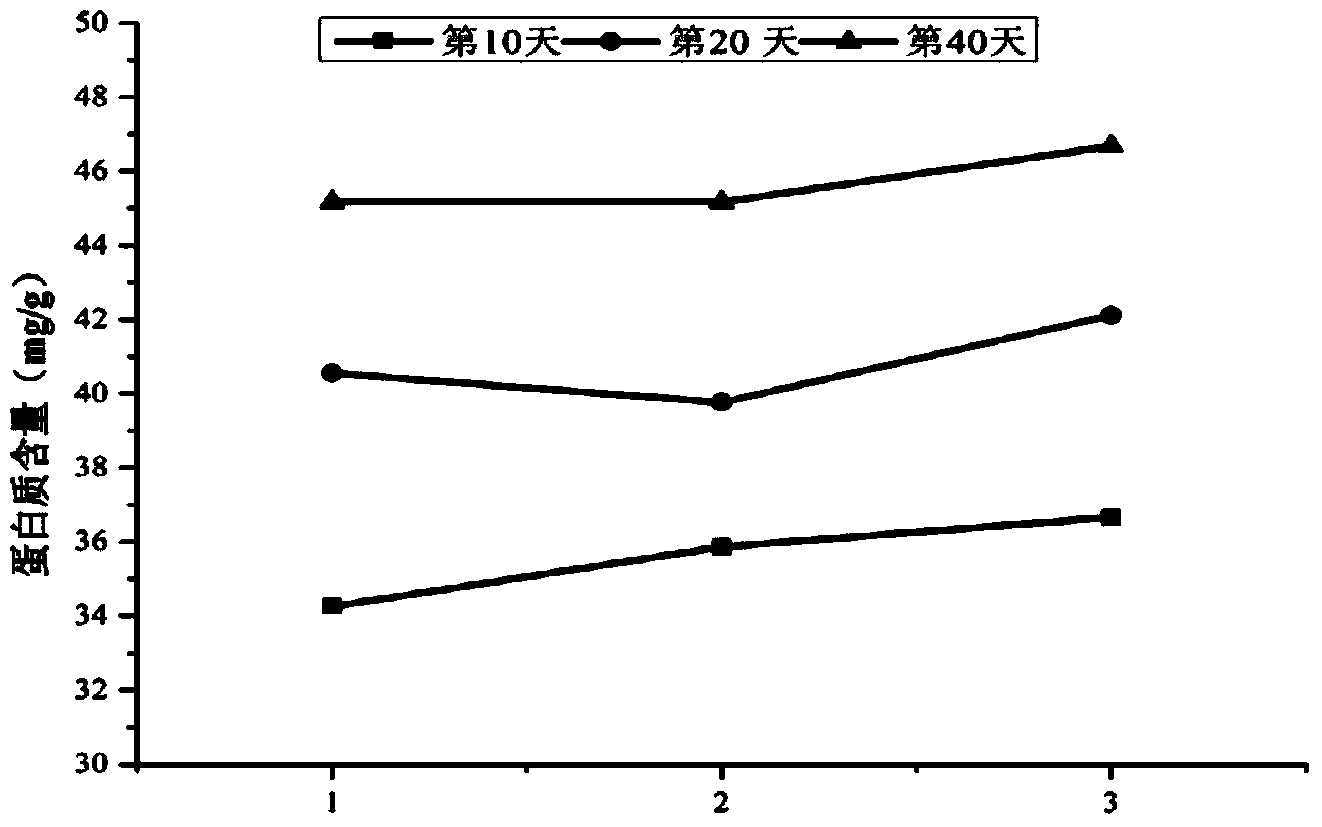
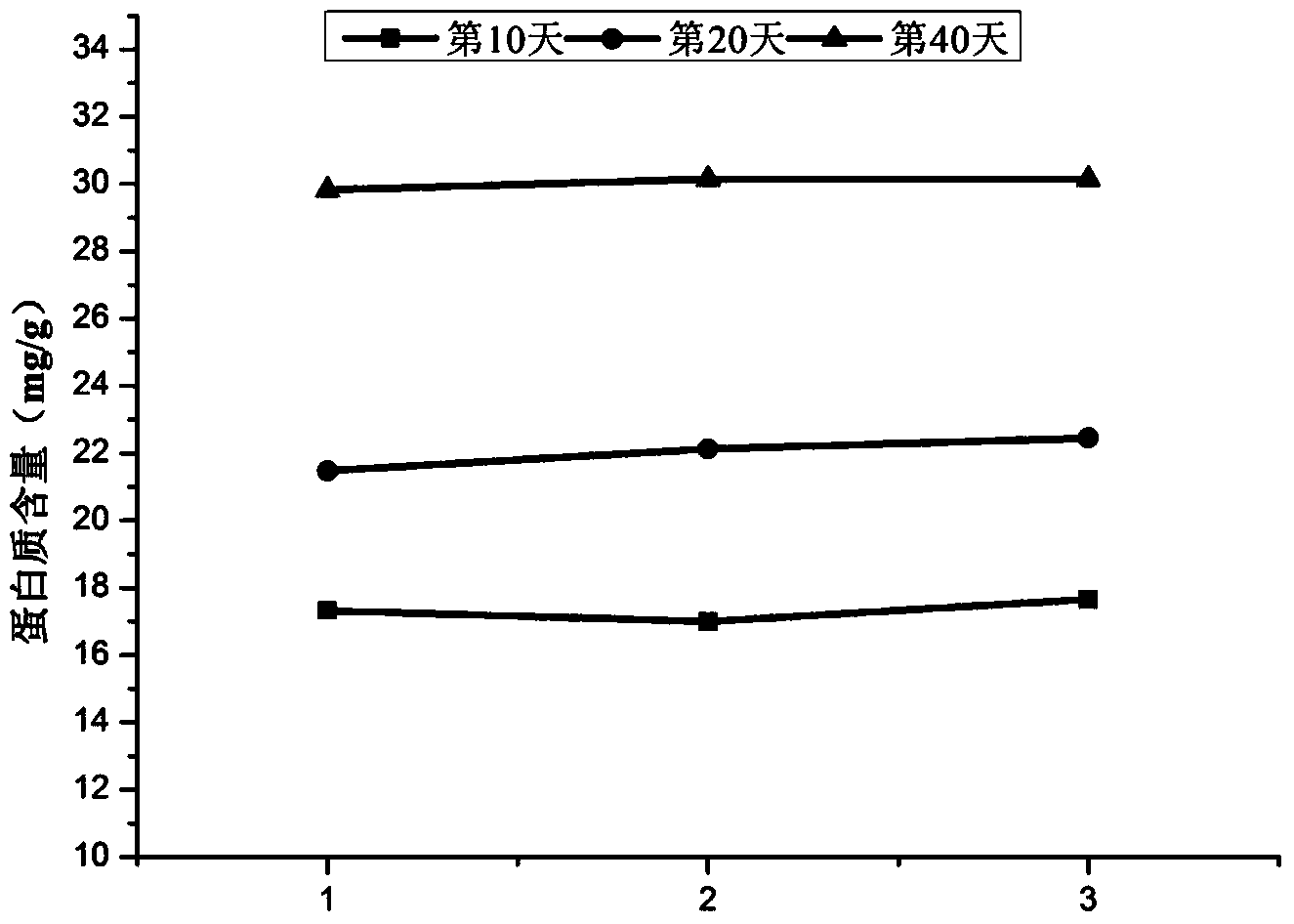
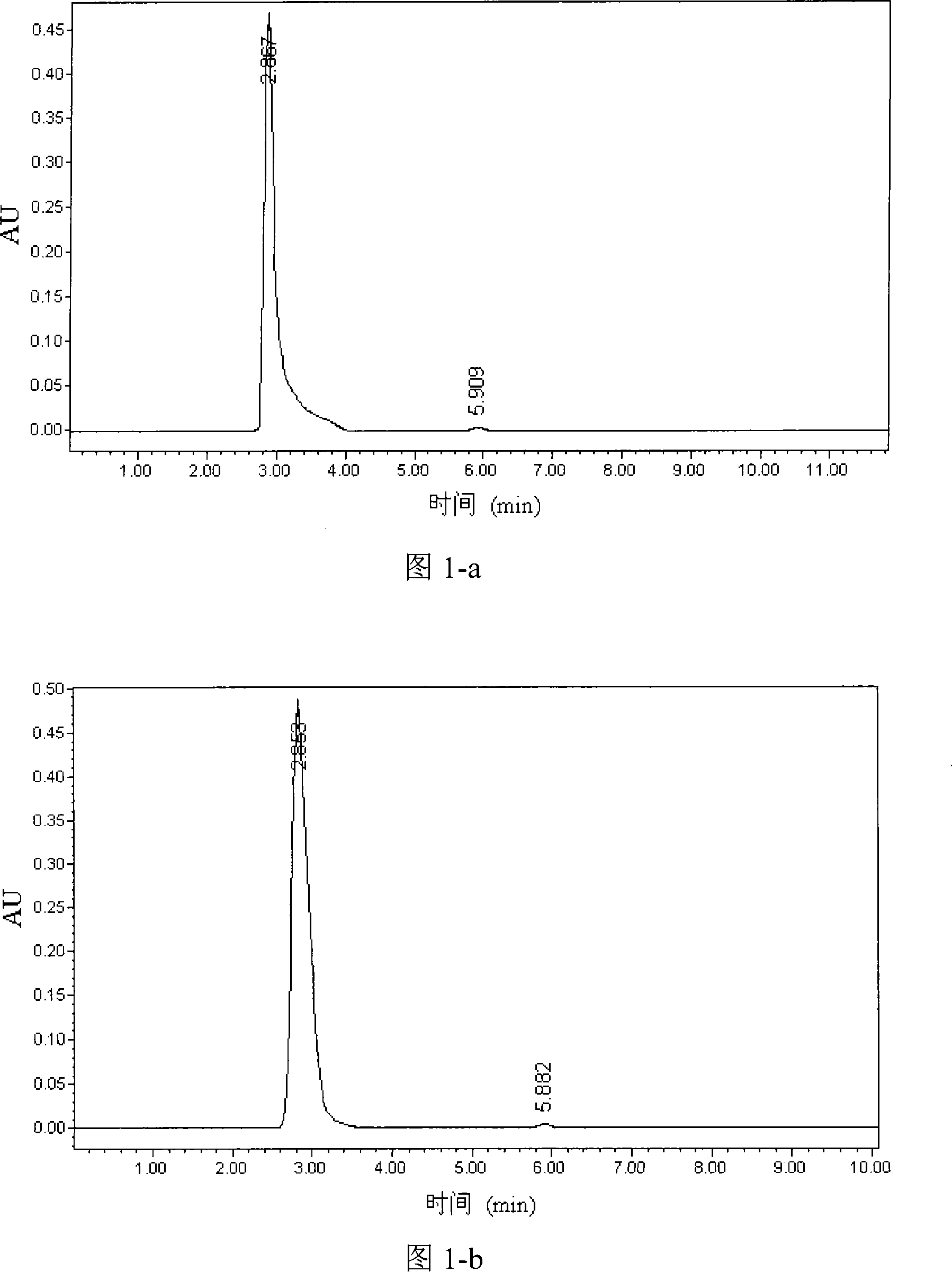
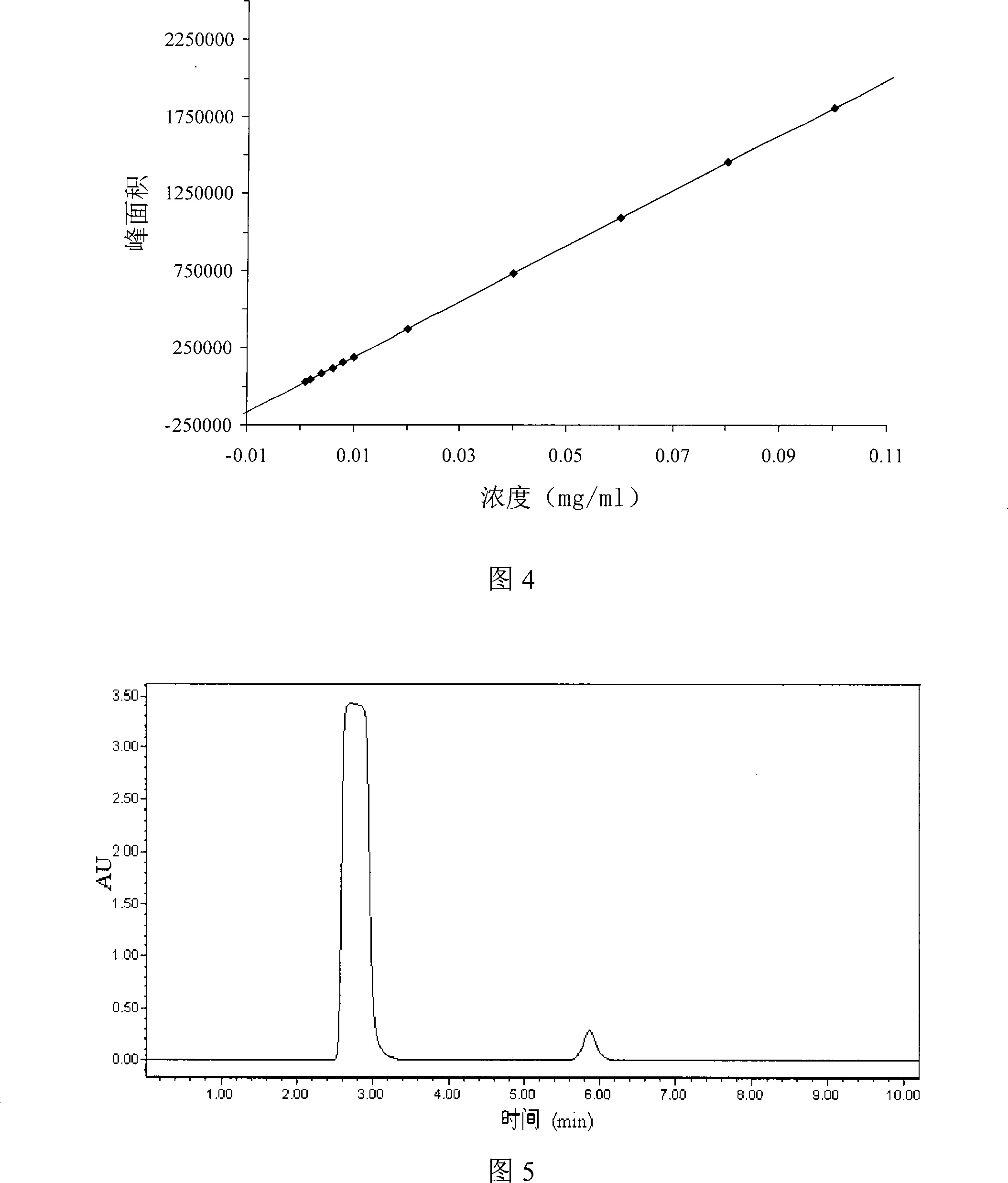
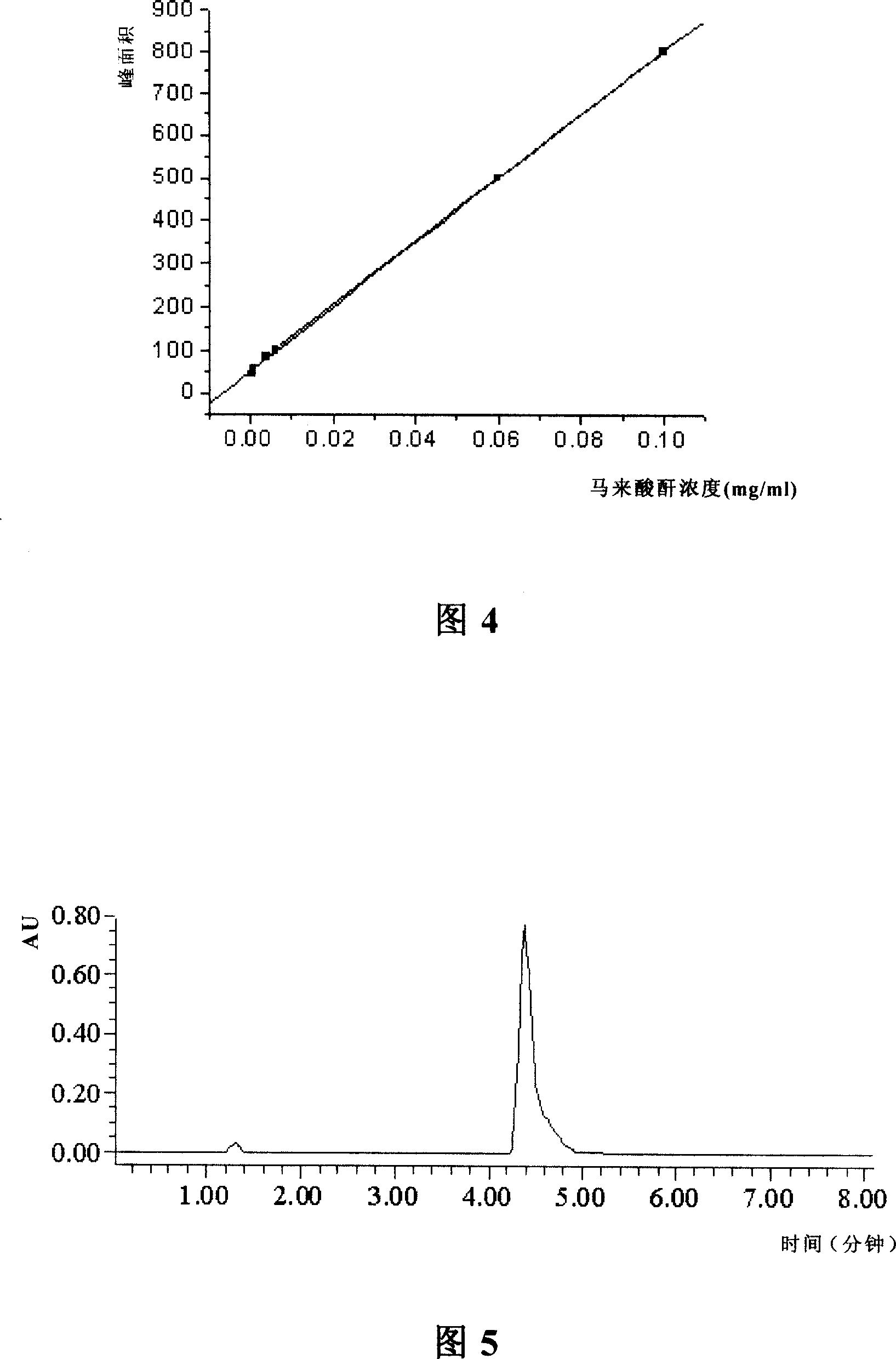
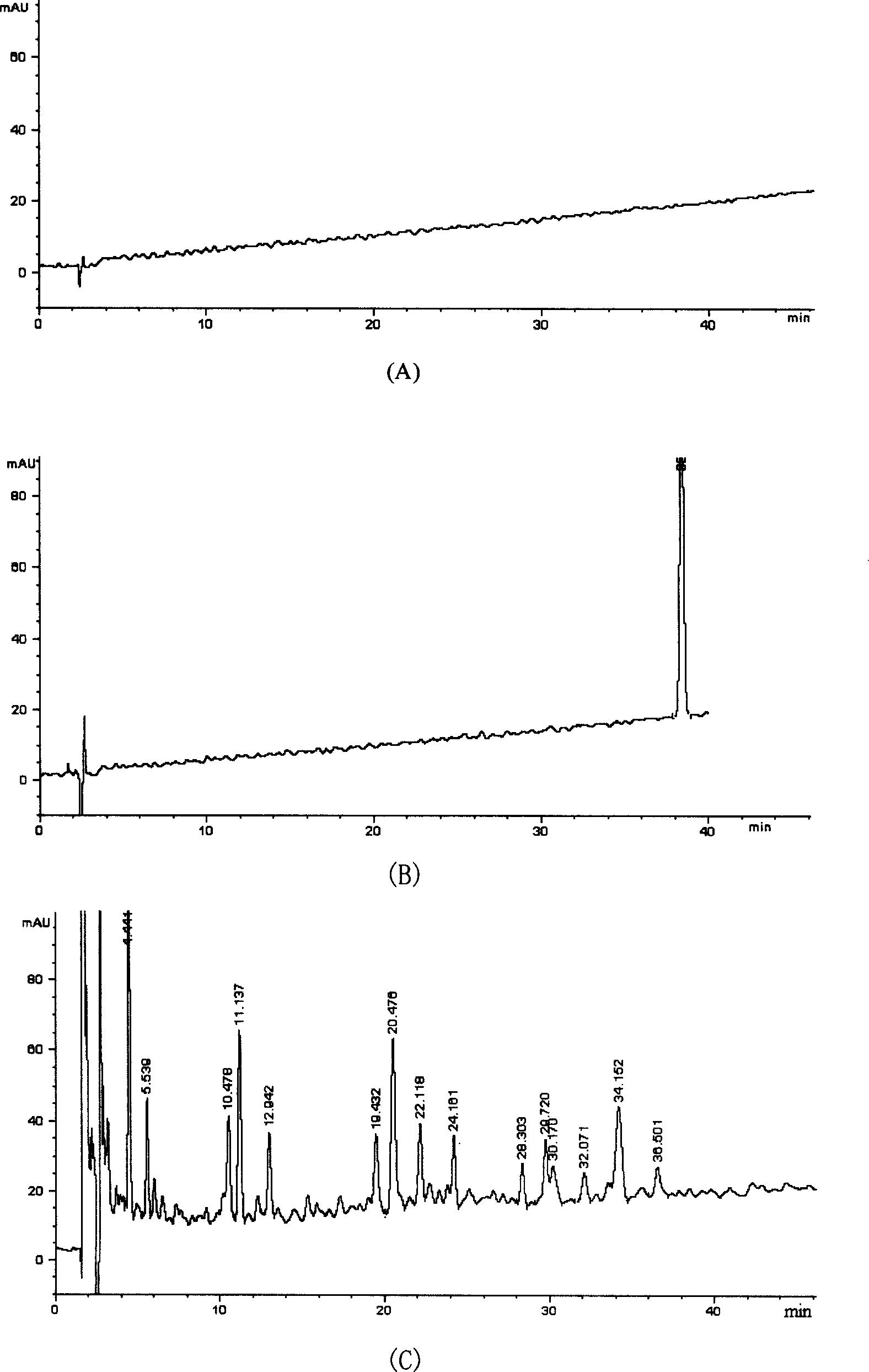
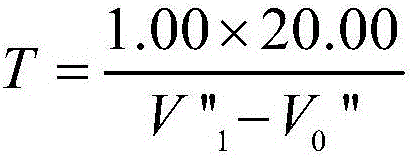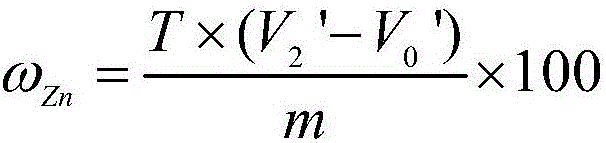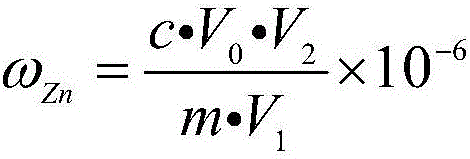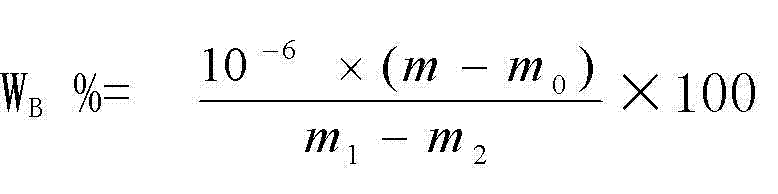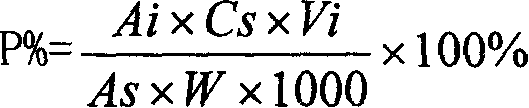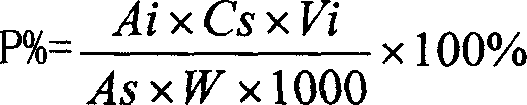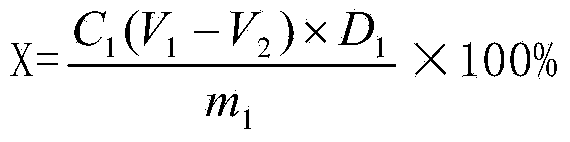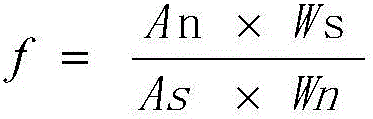Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
195results about How to "Accurate determination of content" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
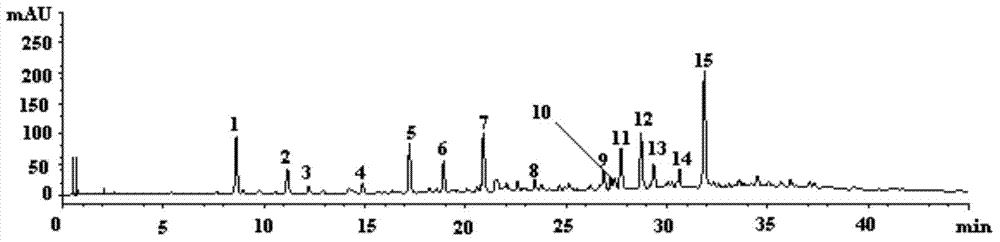
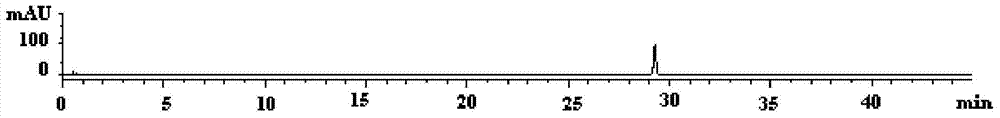
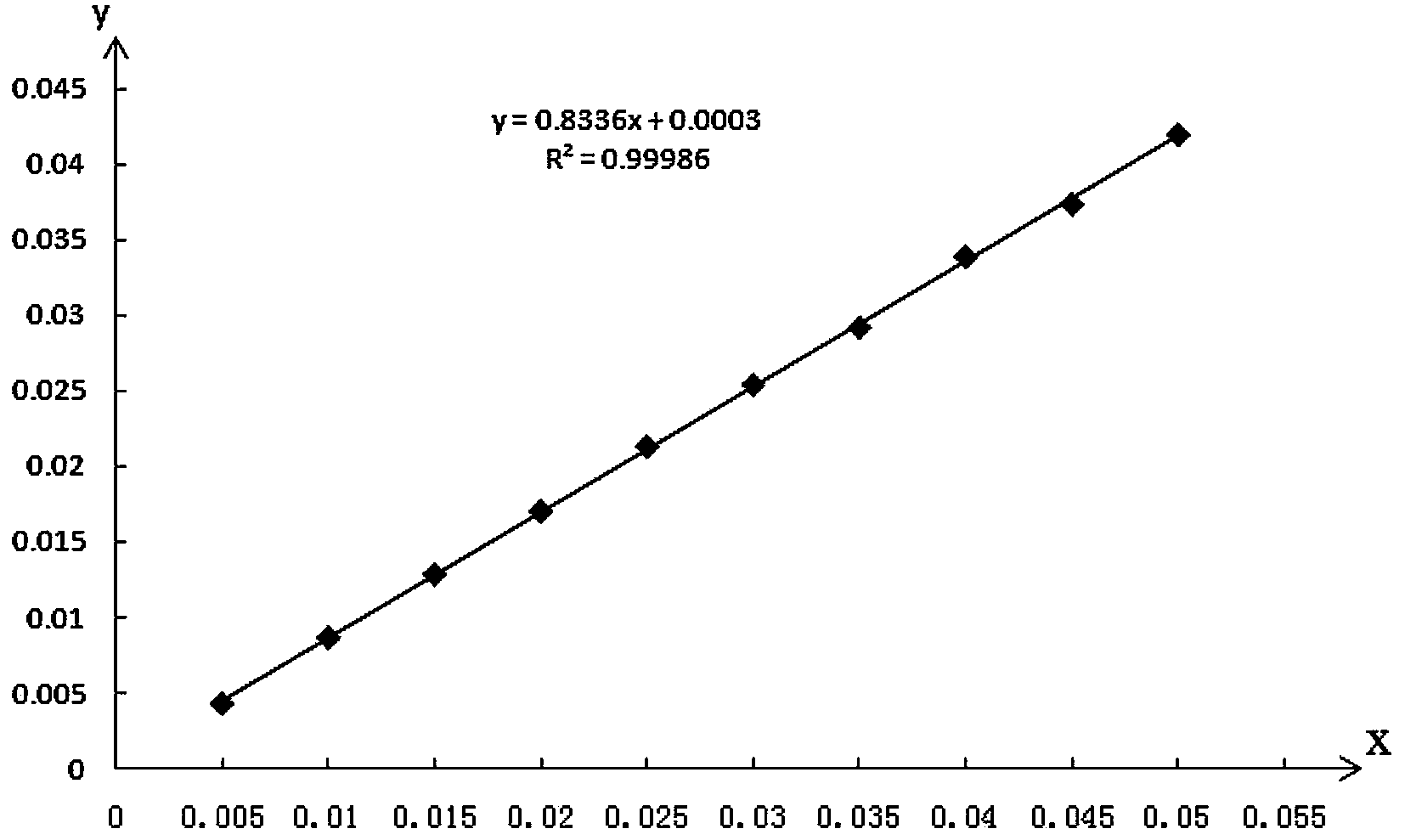
Quality control method of total glycosides single preparation of white paeony roots
ActiveCN102138985AQuality assuranceGuaranteed curative effectComponent separationPlant ingredientsClinical efficacyCurative effect
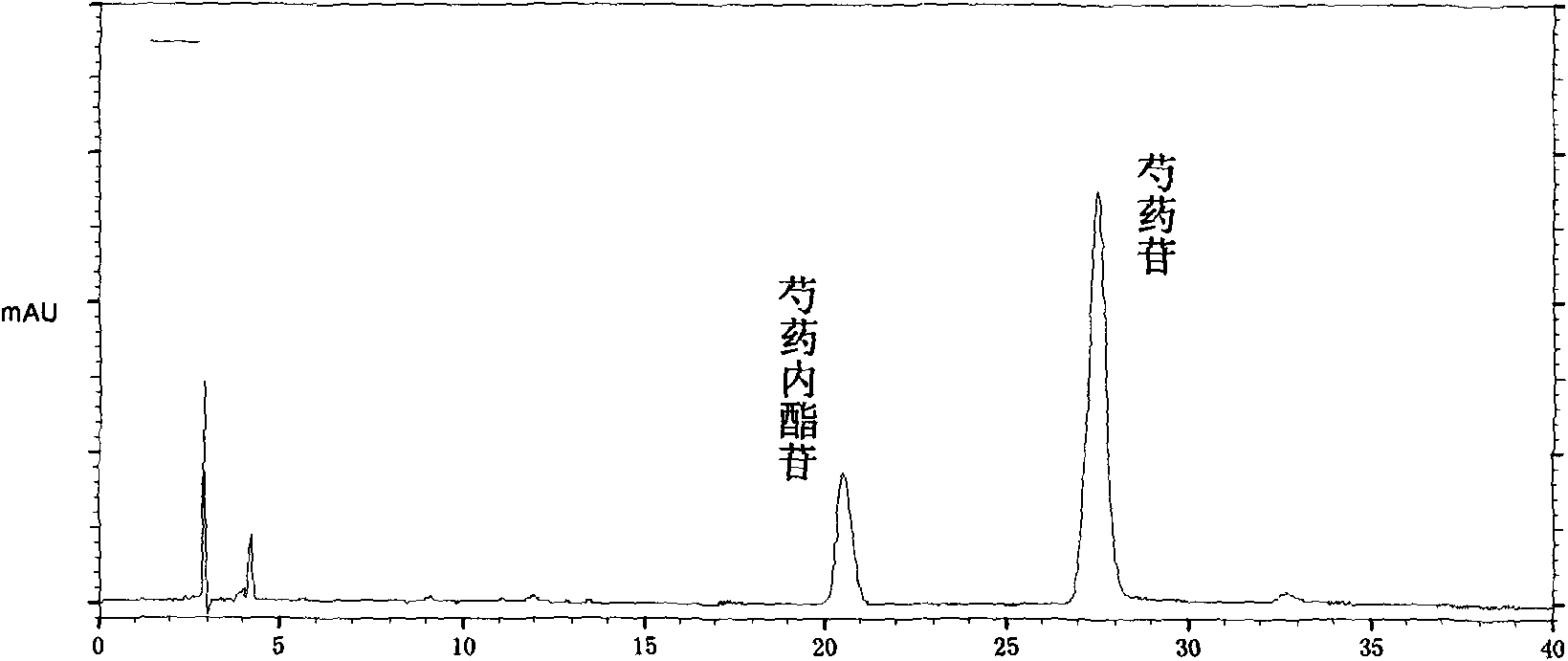
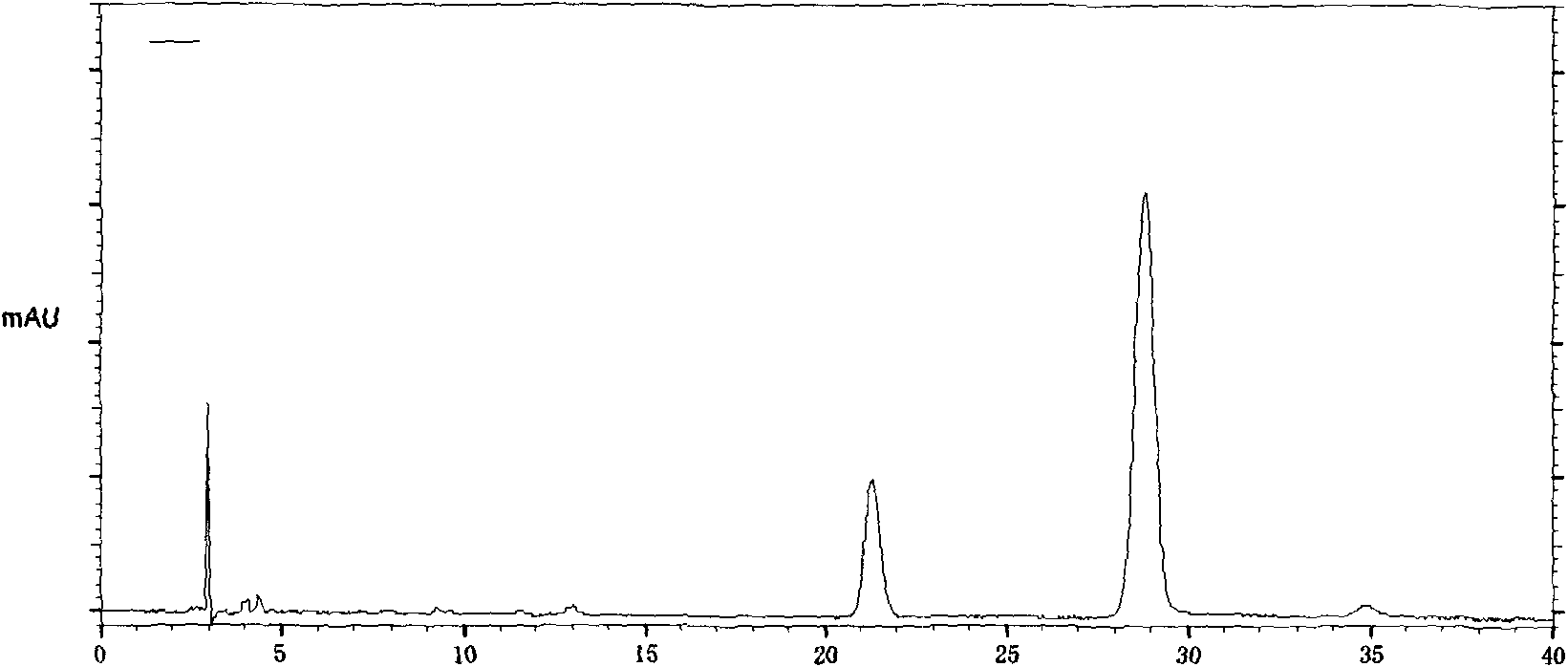
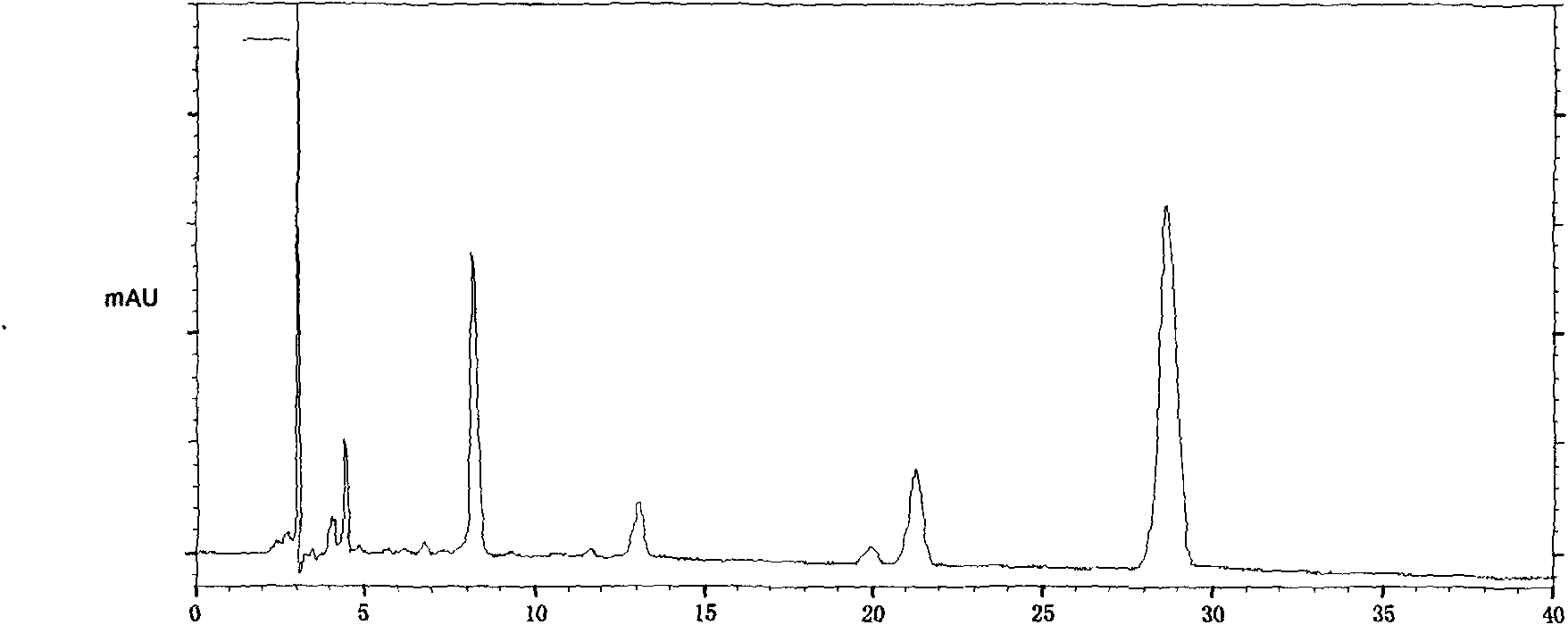
The invention provides a quality control method of a preparation of white paeony roots. The method comprises the following steps of: establishing a synchronous content measuring method of white paeony root herbs, white paeony root total glycosides and paeoniflorin and albiflorin in the white paeony root preparation by adopting the same liquid-phase chromatography condition; and accurately measuring the contents of the paeoniflorin and the albiflorin. The method is simple to preprocess a sample, keeps complete characteristic components and provides a stable sample solution and has higher accuracy, favorable reproduction and a certain specificity; characteristic peaks in the obtained fingerprint map have favorable separation effect, the fingerprint maps of the white paeony root herbs, the white paeony root total glycosides and the preparation have favorable relativity; the standard fingerprint map of the white paeony root herbs is established at a new angle by utilizing the relativity research of the fingerprint maps, can be used for identifying the qualities of the white paeony root herbs and improving the controllability of the production process of the white paeony root preparation and is favorable to ensuring the quality stability and the clinical curative effect of the white paeony root preparation.
Owner:NINGBO LIWAH PHARM CO LTD
Method for detecting free radical ration in coal tar
InactiveCN103105409AOptimizing Assay ConditionsSolve the problem of low free radical contentAnalysis using electron paramagnetic resonanaceDPPHTar
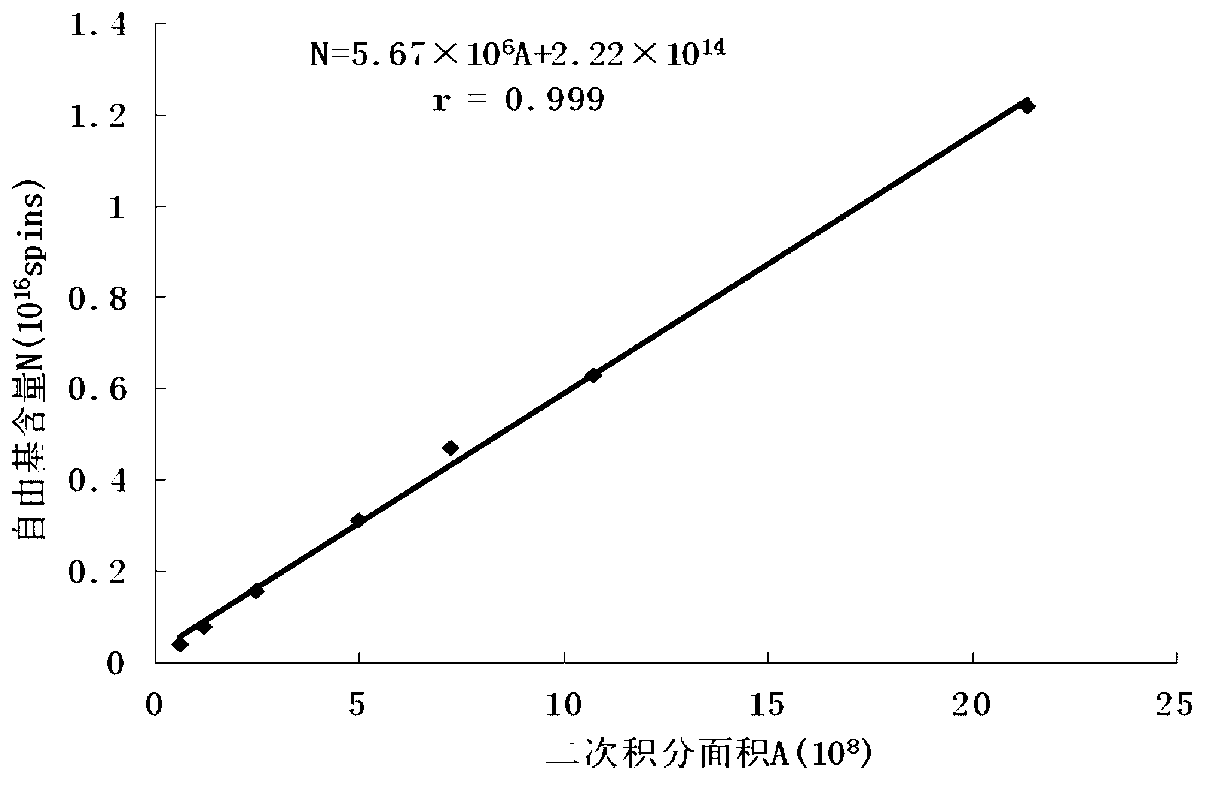
The invention discloses a method for detecting free radical ration in coal tar. The method comprises the steps of removing moisture in the tar in a high speed centrifuging manner, and preserving a tar sample under low temperature or inert atmosphere; adopting a diamagnetism substance to deliquate DPPH(1,1-Diphenyl-2-picrylhydrazyl radical 2,2-Diphenyl-1-(2,4,6-trinitrophenyl) hydrazyl) to prepare a plurality of DPPH standard samples with different free radical concentrations, carrying out EPR (electron paramagnetic resonance) measurement, carrying out secondary integration on an EPR spectrogram, and recording the corresponding secondary integration area; establishing a standard curve according to the free radical contents of the DPPH standard sample with different free radical concentrations and the secondary integration area of the corresponding EPR spectrogram; and filling the tar sample into a pipe, measuring according to the same EPR measuring condition, carrying out secondary integration on the measured EPR spectrogram, and substituting the secondary integration area into the standard curve so as to obtain the free radical content N. The method provided by the invention can exactly measure free radical content in coal tar, meanwhile, the change of the content of the free radical in the coal tar along with the preservation condition is exactly measured, and the method for detecting ration is provided for the research of the free radical in the coal tar and is very simple and easy.
Owner:EAST CHINA UNIV OF SCI & TECH
Impregnating resin with ionic liquid film containing extractive agent on surface, producing method and application thereof
The invention relates to a dipping resin with the surface covered by an ionic liquid film containing extractant, and a fabrication method as well as application thereof. The ionic liquid containing the extractant is fixed in adsorption resin XAD-7; acid or alcohol or heat is not required to be added by using a direct dipping method, the stability of the ionic liquid is hardly affected, and the loading of ionic liquid matrix system in the material is large. Solid-liquid extraction is carried out by using the dipping resin, therefore, the contact area of ion liquid phase and water phase can be effectively increased, the mass transfer efficiency of the system can be improved, clamp strap loss of the ionic liquid can be reduced, and the phenomena of emulsion, phase splitting, etc. in the extraction process can be improved. In an extraction restriction complexation method, complexation agent is added in the water phase, and the extraction efficiency of the ionic liquid phase on the target metallic ions and selection among different metallic ions are improved by the difference of stability constants between the complexation agent and the metallic ions, thus effectively avoiding the phenomena of extraction efficiency reduction and ionic liquid decomposition in the separation process by adjusting the pH value. The two methods can be combined to be applied to imetallic ion separation with excellent separation effect.
Owner:CHANGCHUN INST OF APPLIED CHEMISTRY - CHINESE ACAD OF SCI
High performance liquid chromatographic analysis method for antioxidant 1076 content
ActiveCN101710108AAccurate contentAccurate measurementComponent separationColumn temperatureMethyl propionate
The invention discloses a high performance liquid chromatographic analysis method for antioxidant 1076, which adopts a reserved phase high performance liquid chromatography method. The reserved phase high performance liquid chromatography comprises the following conditions that: the chromatographic column is a C18 column and is of 5 mu m and 150*4.6 mm (I.D.); the mobile phase comprises the following components in a volume ratio with gradient conditions: 90 percent of methanol and 10 percent of water in 0 minute, 90 percent of methanol and 10 percent of water in 5 minutes, and 100 percent of methanol and 0 percent of water in 10 minutes; and the flow rate is 0.8 milliliter per minute, the column temperature is 25 DEG C, the detection wavelength is 220 nanometers, and the sampling volume is 20 microliters. The chromatographic analysis method of the invention accurately measures the main body content of the antioxidant 1076, further measures the contents of an intermediate beta-(3,5-tertbutyl-4-hydroxyphenyl) methyl propionate and a raw material 2,6-ditertbutyl phenol at the same time, and is suitable for quality control of the antioxidant 1076 in the industrialized production process.
Owner:SHANGHAI CHEM REAGENT RES INST
Condensable particle sampling device
ActiveCN104406826AAccurate determination of contentReduce blocking lossWithdrawing sample devicesWeighing by absorbing componentExhaust pipeRefrigerated temperature
The invention provides a condensable particle sampling device. The condensable particle sampling device comprises a sampling head, a filter, a heater, a sampling pipe, a heating band, a three-way ball valve, a reverse blowing pipe, a gas-in pipe, a condenser, a refrigerator, a filter membrane clamp, a filter membrane, an exhaust pipe, a flowmeter and a vacuum pump, wherein the sampling head, the filter, the sampling pipe, the three-way ball valve, the gas-in pipe, the condenser, the filter membrane clamp, the exhaust pipe, the flowmeter and the vacuum pump are sequentially connected; the sampling head and the filter are positioned in a flue, and the heater is arranged on the filter; the sampling pipe is fixed on the flue by a flange, one end of the sampling pipe is positioned in the flue, the other end of the sampling pipe is positioned outside the flue, and the heating band is arranged on the sampling pipe; the two ends of the three-way ball valve are respectively connected with the sampling pipe and the gas-in pipe, and the other end of the three-way ball valve is connected with the reverse blowing pipe; the refrigerator is arranged on the condenser; the filter membrane clamp is positioned outside an outlet of the condenser, and the filter membrane is positioned in the filter membrane clamp.
Owner:深圳睿境环保科技有限公司
Determining method of atorvastatin calcium related substance
InactiveCN104931599AEasy to separateImprove separation rateComponent separationGradient elutionSolvent
The invention relates to a determining method of an atorvastatin calcium related substance. The method comprises the following steps of 1, preparing a test solution, i.e. taking a proper amount of atorvastatin calcium, adding a solvent to dissolve and quantitatively dilute the atorvastatin calcium until about 1mg of atorvastatin calcium is contained in 1ml of solution, and taking the solution as the test solution; 2, preparing a mixed reference substance solution, i.e. weighing a proper amount of reference substances of an impurity A, an impurity B, an impurity C, an impurity D and an impurity E, and a proper amount of reference substance of the atorvastatin calcium, adding the solvent to dissolve and quantitatively dilute the reference substances until about 3 micrograms of impurity A, 2 micrograms of impurity B, 2 micrograms of impurity C, 2 micrograms of impurity D, 2 micrograms of impurity E and 10 micrograms of atorvastatin calcium solution are contained in 1ml of solution, and taking the solution as the mixed reference substance solution; 3, performing HPLC (High Performance Liquid Chromatography) analysis, i.e. taking 20 microliters of test solution and 20 microliters of mixed reference substance soulution, respectively filling the test solution and the mixed reference substance into a liquid chromatograph, recording a chromatogram until a gradient elution program is finished, and according to the peak area of the chromatogram, calculating the content of each component.
Owner:BEIJING JIALIN PHARM INC
Separation method of salidroside and impurity therein and RP-HPLC analytical method of salidroside and impurity therein
InactiveCN101357932AAccurate determination of contentThe method is simple and reliableSugar derivativesComponent separationSalidrosideColumn temperature
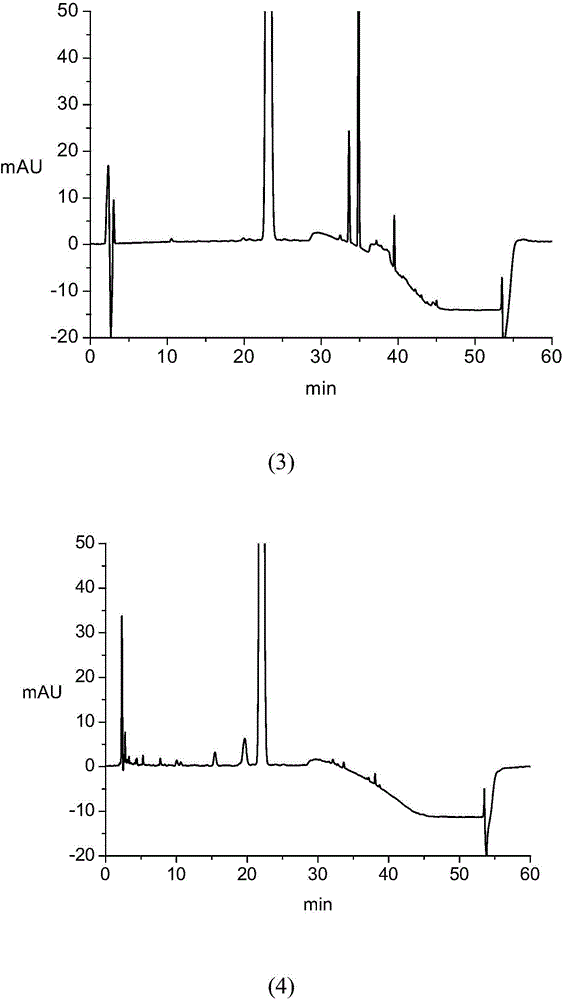
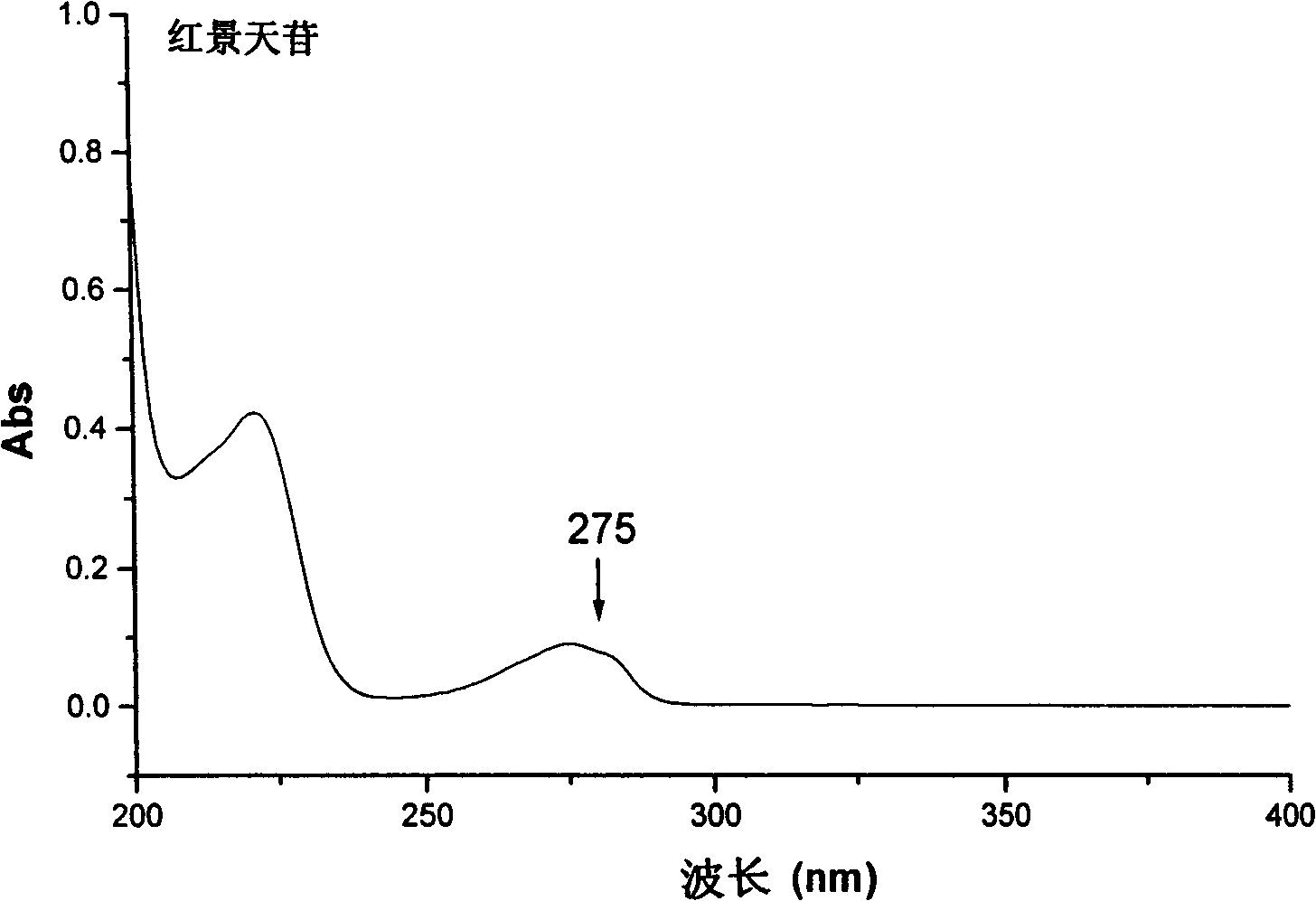
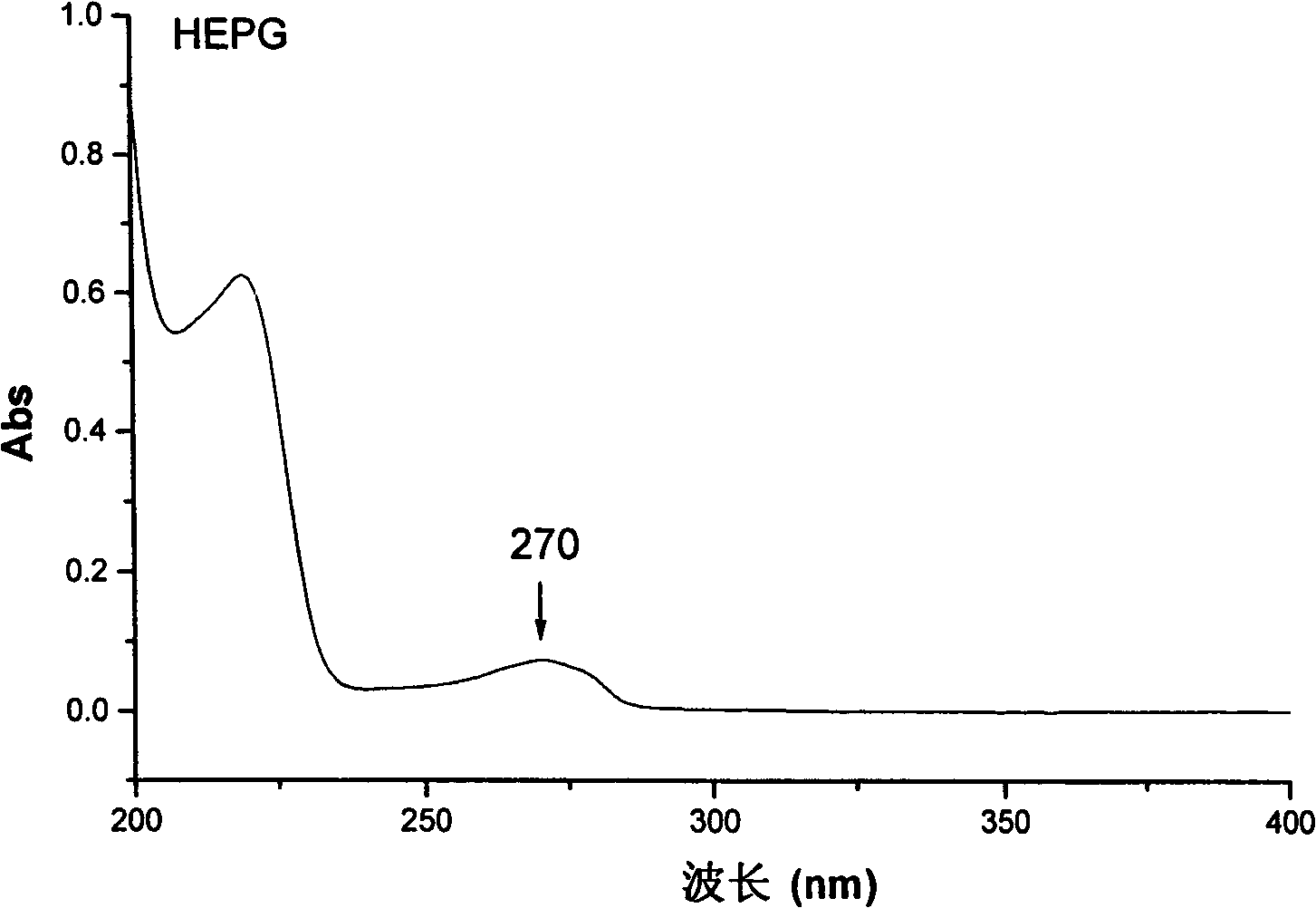
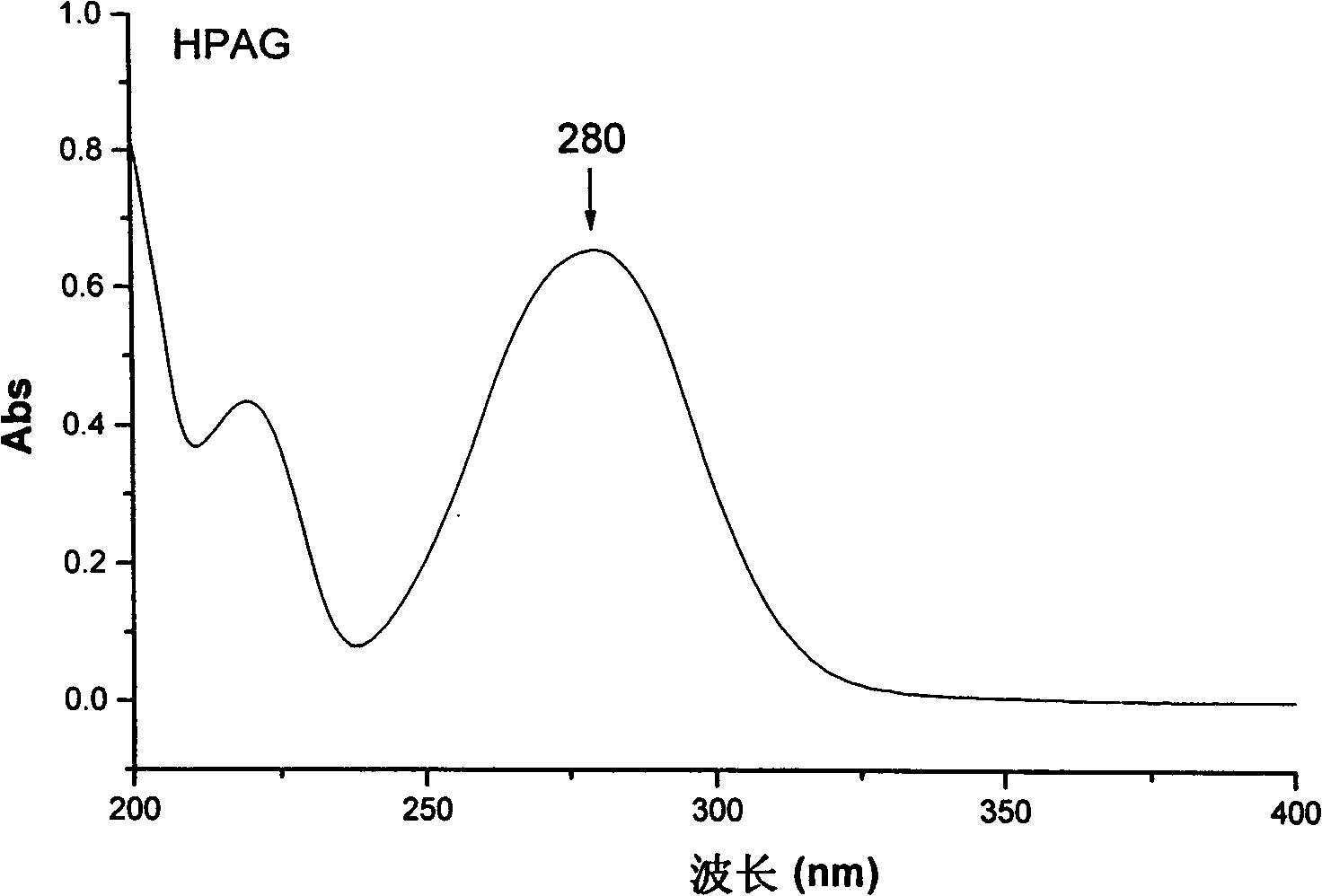
The invention discloses a separation method of salidroside impurities which includes the steps of first separation with silica gel column chromatography, elution with chloroform-ethanol gradients, collection by four sections and the separation and purification with semi-preparative high-performance liquid chromatography; wherein, the semi-preparative high-performance liquid chromatography is provided with the conditions as follows: a chromatographic column: ODS C18, 5mum and 250 * 10.0mm (I. D.); volume ratios of components of mobile phases: methanol to water equals to 11 to 89; flow rate: 3.0 mL / min; column temperature: 25 DEG C; detection wavelength: UV275nm; sample size: 50 muL; effluent liquid collection by section. The high-performance liquid chromatography of the salidroside and impurities thereof adopt the reversed-phase high-performance liquid chromatography with the following chromatographic conditions: a chromatographic column: ODS C18, 5mum and 150 * 4.6mm (I. D.); mobile phases: methanol to water equals to 15 to 85; flow rate: 1.0 mL / min; column temperature: 25 DEG C; detection wavelength: UV275nm; sample size: 20 muL. The contents of the salidroside and the impurities HEPG, HPAG and PAPG of the salidroside samples can be determined accurately and simultaneously by adopting the high performance liquid chromatography of the salidroside and impurities thereof, which provides a simple and reliable method for the quality control analysis in the production process.
Owner:NANJING MEDICAL UNIV
Method for determining form of trace selenium, and applications of method in detection of selenium-rich feed
PendingCN107727758AAccurate determination of contentQuick and safe selectionComponent separationFluorescenceAtomic fluorescence spectrometry
The invention belongs to the technical field of feed quality detection, and particularly to a method for determining the selenium form in a selenium-rich feed, more particularly to a method for determining the selenium form in a selenium-rich feed by combining an ultrasonic probe-assisted enzymolysis technology and high-performance liquid chromatography-hydride generation atomic fluorescence spectrometry (HPLC-HG-AFS). According to the present invention, with the method, the content of the extracted selenium can be accurately determined, and the contents of the five forms of the selenium substances commonly contained in the selenium-rich feed can be effectively separated, such that the selenium form in the selenium-rich feed can be accurately analyzed, and the selenium-rich feed product can be rapidly and safely selected and used.
Owner:INST OF QUALITY STANDARD & TESTING TECH FOR AGRO PROD OF CAAS
Determination method of zinc in gold alloy
ActiveCN106198854AAccurate determination of contentSuitable for large batch analysisChemical analysis using titrationColor/spectral properties measurementsSodium acetateHydrazine compound
The invention relates to a determination method of zinc in gold alloy, and belongs to a determination method of amount of zinc in gold alloy. The method comprises the following steps: aqua regia is used for dissolving a sample material, hydrazine hydrate is used for deposition and separation gold, filtering is carried out, and filter residues are used for recycling gold; sodium hydroxide and a hydrogen peroxide solution are added into a filtrate for deposition and separation of nickel and copper, filtering is carried out, and an acetic acid-sodium acetate buffer solution is added into the filtrate; xylenol orange is used as an indicator, EDTA is used for titration till the color of the solution changes into luminous yellow from wine red, and the titration volume is used for calculating the content of zinc; hydrochloric acid is added into the filter residue for dissolving, volumetric flask is prepared, the amount of zinc is determined by an atomic absorption spectrometer at the wavelength of 213.8nm, and sum of the zinc contents which are determined by the two determination methods is the content of the zinc in the alloy. The method can be used for accurately determining the content of zinc in the gold alloy, and is suitable for analysis on a large scale, at the same time the method fills the blank of determination methods of zinc in gold alloy.
Owner:SHENZHEN JINZHI GOLD&SILVER JEWELLERY INSPECTION RES CENT CO LTD
Measuring method of solid-solution boron content in steel
InactiveCN102928501AAccurate determination of contentMaterial analysis by electric/magnetic meansPower flowInstrumentation
The invention relates to a measuring method of solid-solution boron content in steel, and belongs to the technical field of analytical chemistry. The method comprises the steps of: using a constant current electrolysis method to extract solid-solution boron at low temperature; transferring electrolyte with a certain volume, and diluting the electrolyte to be in a ruled volume; using an inductively coupled plasma source mass spectrometer to measure the solid-solution boron; and using a matching method to eliminate the influence of the components of the electrolyte on the measurement of the inductively coupled plasma source mass spectrometer. The measuring method has the advantage that the solid-solution boron content in the steel can be accurately measured.
Owner:INNER MONGOLIA BAOTOU STEEL UNION
Method for measuring weight-average molecular weight and content of mannuronic acid substances
ActiveCN109459505ASolve the large deviation of weight average molecular weight determinationSolve the problem of inaccurate content quantificationComponent separationIon contentQuality control
The invention belongs to the field of natural medicine chemistry and quality control, and relates to a method for measuring the weight-average molecular weight and the content of soluble salt of mannuronic acid substances. Weight-average molecular weight and content results of acid sugar measured by an SEC-MALS (size exclusion chromatography with multi-angle laser scattering detector) are corrected by the aid of the metal ion content of soluble salt samples of the mannuronic acid substances. The weight-average molecular weight and the content of the soluble salt w of the mannuronic acid substances can be rapidly and accurately measured by the method.
Owner:GREEN VALLEY (SHANGHAI) PHARM CO LTD
Method for measuring protein in sludge according to coomassie brilliant blue method
InactiveCN103674942AMild extractionEfficient extractionMaterial analysis by observing effect on chemical indicatorMunicipal sewagePre treatment
A method for measuring protein in sludge according to the coomassie brilliant blue method belongs to the field of influencing factors in municipal sewage treatment. After being combined with protein, coomassie brilliant blue G-250 becomes cyan, the protein-pigment combined material is highest in light absorption value under a 595nm wavelength condition, and the light absorption value is in direct proportion to protein content, the method can be used for quantitative measurement of protein. The method particularly relates to the following steps: reagent preparation, standard curve measuring, sludge pretreatment, protein extraction and measuring, and the like. The method is a simple and efficient method for measuring the protein content in sewage.
Owner:BEIJING UNIV OF TECH
2-methoxymethyl-4-aminophenol and its impurity highly effective liquid phase chromatography analytical method
InactiveCN101216468AAccurate determination of contentSimple and reliable analytical methodComponent separationPhosphatePhosphoric acid
The invention discloses a high-performance liquid chromatographic analysis method of 2-methoxymethyl-4-aminophenol and impurities thereof. The method comprises the following steps of: selecting reverse high-performance liquid chromatography; setting chromatographic conditions, wherein the chromatographic column is C18, 5 mum, 150*4.6 mm (I.D.), the mobile phase includes acetonitrile: phosphoric acid -phosphate buffer solution (pH 7.0) at a volume ratio of 45:55 with a flow rate of 0.8 ml / min, the column temperature is 30 DEG C, the detection wavelength is 232 nm, the injection dose is 5 to 10 mul, and (NH4)2HPO4 20 mmol / l is added into the mobile phase; adding phosphoric acid to adjust pH to 7.0; diluting the concentration of the injection sample to 1 / 50; and determining the contents of 2-methoxymethyl-4-aminophenol and the impurities thereof. The invention provides a simple and reliable method for quality control and analysis of 2-methoxymethyl-4-aminophenol.
Owner:SHANGHAI CHEM REAGENT RES INST
Method for detecting 3-amino-2-azepanone through high performance liquid chromatography
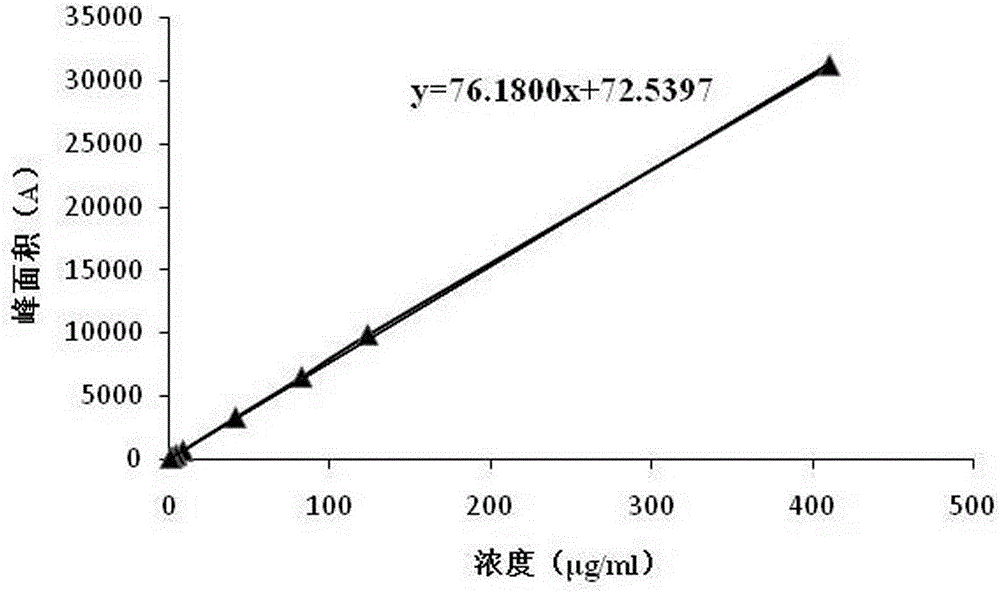
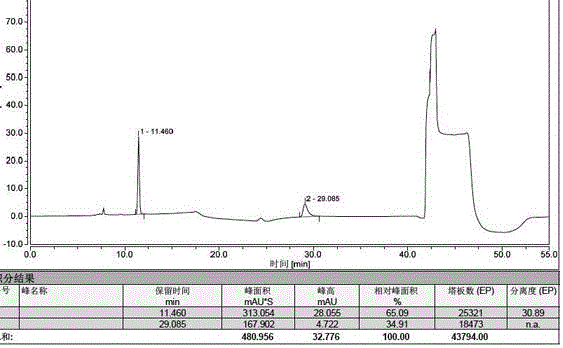
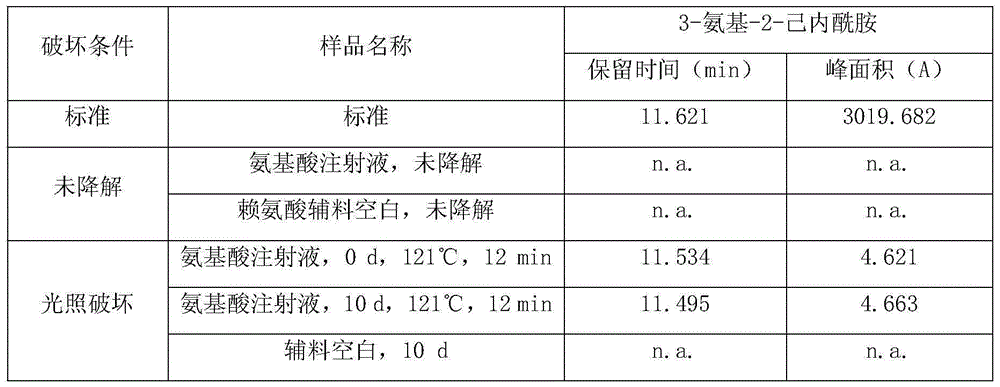
ActiveCN105092741AAccurate determination of contentStrong specificityComponent separationAcetonitrileMonopotassium phosphate
The invention discloses a method for detecting 3-amino-2-azepanone through a high performance liquid chromatography. According to the method, the following chromatographic conditions are adopted: a chromatographic column is an ODS column; a mobile phase A is a 0.01-0.09 mol / L monopotassium phosphate solution; a mobile phase B is a mixed water solution of methanol and acetonitrile in which the volume ratio of methanol, acetonitrile and water is (1-5):(1-5):1; the flow velocity is 0.4-1.2 ml / min; the detection wavelength is 100-300 nm; the column temperature is 20-60 DEG C; the sample size is 5-20 [mu]l. Through adoption of the method, the content of the impurity of 3-amino-2-azepanone degraded by lysine can be accurately measured, and the specificity is good; the quantification limit repeatability is good, the recovery rate is high, and a solution is high in stability; when the column temperature, the detection wavelength and the flow velocity change slightly, the difference between a detected recovery rate of 3-amino-2-azepanone and a result detected at a basic condition is very small, so that the durability of the method is high.
Owner:SICHUAN KELUN PHARMA CO LTD
Kit for quantitatively detecting EGFR mutation
InactiveCN102021232AAccurate determination of contentEasy to operateMicrobiological testing/measurementVector-based foreign material introductionEGFR Tyrosine Kinase InhibitorsEGFR Gene Mutation
Owner:BEIJING ACCB BIOTECH
Correction method for determination of trace element in sample by isotope dilution mass spectrometry
ActiveCN104897766AAccurate determination of contentMaterial analysis by electric/magnetic meansTest sampleTrace element
The present invention provides a correction method for determination of trace element in a sample by isotope dilution mass spectrometry, and belongs to the field of isotope dilution mass spectrometry determination. During value determination of the trace element in a to-be-tested sample by isotope dilution mass spectrometry, when isotopic abundance ratios of a to-be-tested element in a standard material solution and a to-be-tested sample solution are not equal due to matrix interference, the isotopic abundance ratio of the to-be-tested element in the standard material solution and the isotopic abundance ratio of the to-be-tested element in the to-be-tested sample solution are respectively measured, and then according to the correction method, correction coefficient K is calculated; and measurement value associated with the to-be-tested sample can be calibrated by use of the K value for finally realizing the accurate determination of the content of the trace element in the to-be-tested sample by isotope dilution mass spectrometry. The method solves the to-be-tested sample matrix interference effect on the value determination accuracy during determination of the content of the trace element in the to-be-tested sample by isotope dilution mass spectrometry.
Owner:BEIJING INST OF MEDICAL DEVICE TESTING
Antibacterial anti-inflammatory capsule quality detection method
InactiveCN105606756AQuality improvementAdd TLC DiscriminationComponent separationTest sampleMedicine
The invention discloses an antibacterial anti-inflammatory capsule quality detection method. Antibacterial anti-inflammatory capsules are prepared from honeysuckle flowers, radix stemonae, Chinese rhubarb, folium isatidis, radix scutellariae, rhizoma anemarrhenae and a christina loosestrife herb. The antibacterial anti-inflammatory capsule quality detection method comprises the four steps of suitability test on chromatographic conditions and a system, preparation of a reference substance solution, preparation of a test sample solution and determination. In addition, the radix stemonae, the Chinese rhubarb, the radix scutellariae, the rhizoma anemarrhenae and the honeysuckle flowers are subjected to thin-layer identification. The antibacterial anti-inflammatory capsule quality detection method has the advantages of being simple and easy to operate and capable of effectively controlling the product quality.
Owner:贵州省科晖制药有限公司
Method for measuring content of amino acid in black-bone chicken milk increasing preparation
InactiveCN105223280AAccurate determination of contentControl internal qualityComponent separationHydrolysateTotal nitrogen
The invention discloses a method for measuring the content of amino acid in a black-bone chicken milk increasing preparation. The method comprises the following steps: subjecting a black-bone chicken milk increasing preparation to a protein hydrolysis treatment; and then using an amino acid analyzer to analyze the hydrolysate so as to measure the amino acid content. In the conventional standards, the protein content can be only represented by total nitrogen content, the provided method can overcome the shortage, moreover, the method can precisely measure contents of 17 amino acids in the preparation, has the advantages of simple pretreatment, strong specificity, good repeatability, and high precision, can well control the quality of the black-bone chicken milk increasing preparation, and improves the safety and effectiveness of the preparation.
Owner:天津飞鹰玉川药业有限公司
Pre-treating and detecting method for sample containing dithiocarbamate compound
ActiveCN106124279AHigh extraction rateSimple and fast operationMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationChlorideDithiocarbamate
The invention belongs to the field of detection analysis and relates to a pre-treating method for a sample containing a dithiocarbamate compound. The method comprises the following steps: mixing a sample with an acidic stannous chloride ethanol solution and separating, thereby acquiring a solution containing carbon disulfide; optionally, standing by after the mixing and before separating. The invention further relates to a method for detecting the dithiocarbamate compound in the sample. According to the method provided by the invention, the content of dithiocarbamate compound in the sample can be accurately determined, the stability is high, the operation is simple and convenient and the method is clean and environmentally friendly.
Owner:CHINA TOBACCO FUJIAN IND
Method for measuring content of luteolin in lamiophlomis rotata pharmaceutical preparation by liquid chromatography
ActiveCN101446573AAccurate determination of contentEasy to separateComponent separationPhosphoric acidColumn temperature
The invention provides a method for measuring the content of luteolin in lamiophlomis rotata by liquid chromatography. The method comprises the following steps that the octadecylsilane chemically bonded silica is taken as a filling agent and the acetonitrile-tetrahydrofuran-0.5 percent of phosphoric acid is taken as the mobile phase; under the chromatographic conditions that the detected wavelength is 350 plus or minus 2 nm, the column temperature is 25 DEG C to 50 DEG C, the flow velocity is 0.5 ml / min to 1.5ml / min and the sample size is 5 mul to 20 mul, the liquid chromatography analysis is carried out on the prepared test solution, the chromatographic peak area of the corresponding position of the reference solution is measured, and the corresponding content is calculated according to the standard curve. The measuring method has the advantages of high precision, good reproducibility, high recovery ratio and accurate measuring result, improves the quality standard of the pharmaceutical preparation containing the lamiophlomis rotata herb, facilitates the quality stability and the quality control of the industrial production and ensures the security and the validity on medication use by people.
Owner:GANSU CHEEZHENG TIBETAN MEDICINE CO LTD
High-efficient liquid phase chromatographic analysing method for regulating pyrurine and its impurity
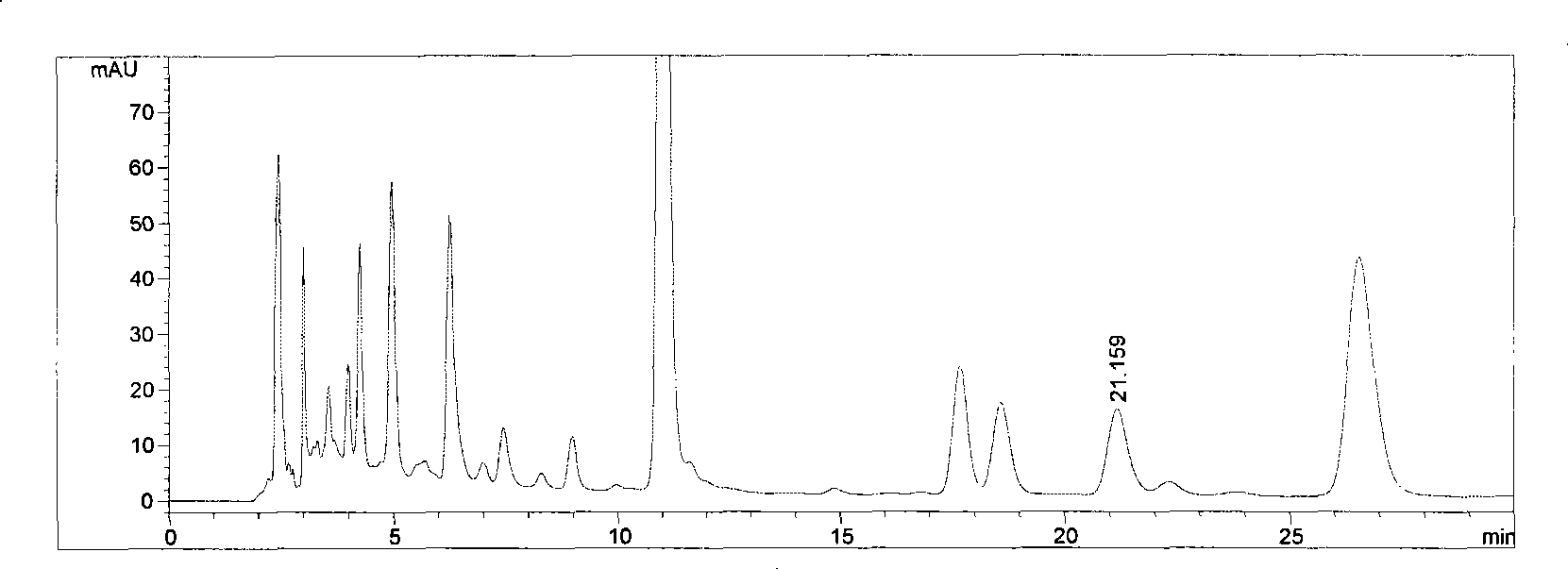
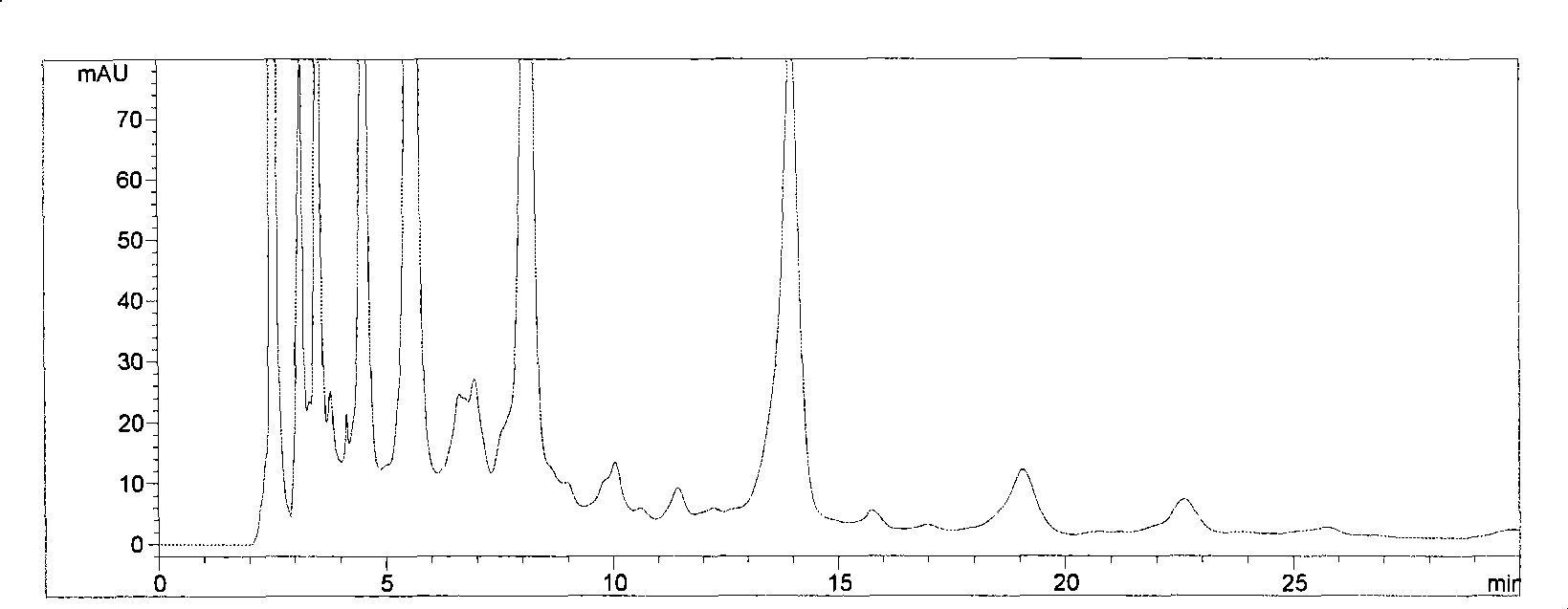
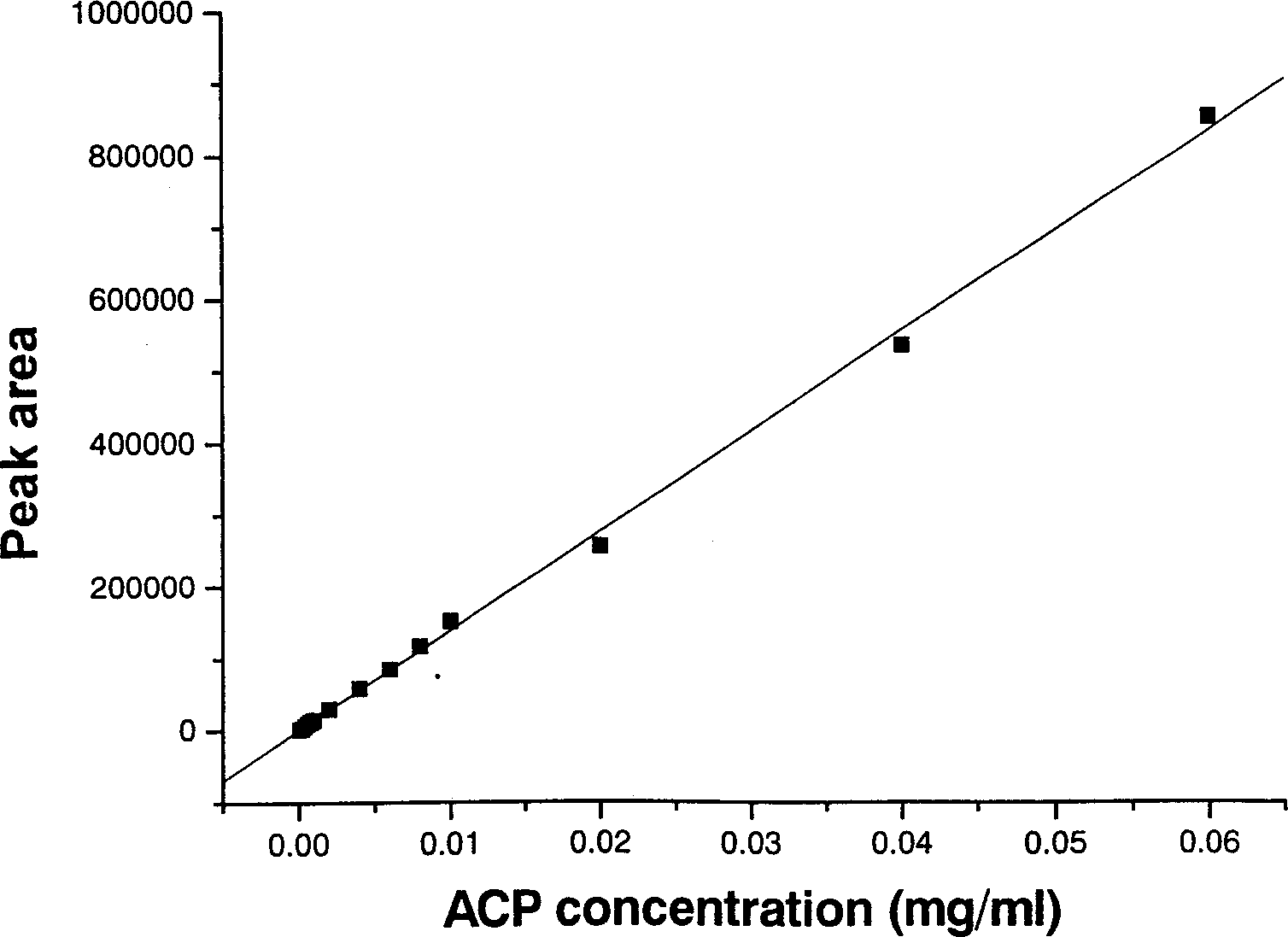
The invention is a high efficiency liquid phase chromatogram analysis method for forchlorfenuron and its impurities, the high efficiency liquid phase chromatogram condition is: chromatogram pile: C18, 5 micrometer, 150X4.6mm (I.D.): flow phase composition volume proportion: carbinol: water=60:40: flow speed: 1.0mL / min; pile temperature: 30deg.C; the detecting wavelength: UV 254nm; the sample taking quantity: 5-10 micro litre. The method in the invention also can measure the content of CPPU and the impurities ACP and DPU in the CPPU sample. It provides a simple and reliable method for the quality control analysis in the producing process.
Owner:NANJING UNIV
Highly effective liquid phase chromatogram analyzing method of N-phenyl maleimide
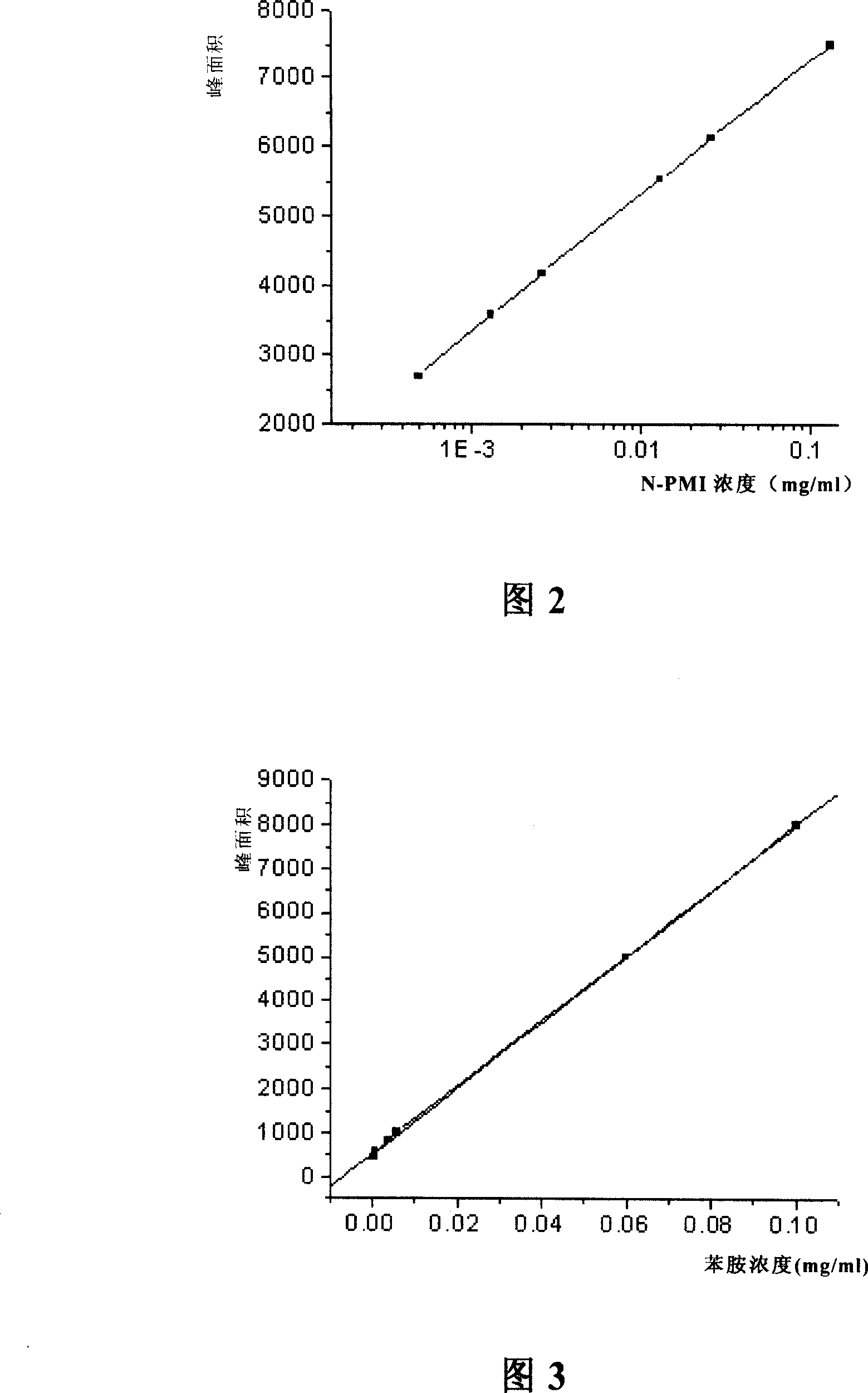
InactiveCN101013113AAccurate contentAccurate determination of contentComponent separationHplc methodColumn temperature
The invention discloses an N-phenyl maleimide HPLC analysis method. The invention uses RP-HPLC method, and HPLC conditions are: chromatography column: C18, 5mum, 150X4.6mm (I.D), the volume ratio of the mobile phase: acetonitrile: water = 40-60:60-40, flow rate: 0.8ml / min, column temperature: 30 deg.C, detection wavelength: 217nm, sample volume: 3-5mul, diluting the determined content sample concentration 50 times, and determining N-PMI and impurity content. The HPLC analysis method of the invention can accurately determine N-PMI main content, and meanwhile, it can accurately determine impurity maleic anhydride and aniline content, providing a simple and reliable analysis method for quality control of N-PMI production process.
Owner:SHANGHAI CHEM REAGENT RES INST
Determination method of content of zirconium in uranium-zirconium alloy
ActiveCN106645122AThe detection data is accurateAccurate determination of zirconium contentMaterial analysis by observing effect on chemical indicatorPreparing sample for investigationZirconium alloyTitration
The invention belongs to the technical field of a chemical detection method and particularly relates to a specific method for determining the content of zirconium in a uranium-zirconium alloy by adopting an EDTA (Ethylene Diamine Tetraacetic Acid) titration method. The invention establishes a detection method of the content of the zirconium in the uranium-zirconium alloy by adopting the EDTA titration method. By carrying out experiments including selection of a sample dissolving temperature, an adding amount of hydrofluoric acid, an interference experiment of uranium, titration acidity, a titration temperature and the like and taking 0.2g of a uranium-zirconium alloy sample as the standard, the precision density of the method is better than 1%; and the method is accurate and reliable, and index requirements of a project analysis technology are met.
Owner:CHINA NORTH NUCLEAR FUEL
Assaying method of Chinese medicine laying particle active principle and content
InactiveCN1833670AThe method is simpleEliminate distractionsAntipyreticComponent separationActive principleChemistry
A method for discriminating the active component of Chinese elder flower particle and measuring its content features that the HPLC / MS method is used to discriminating the polypeptide as its active component and the efficient liquid-phase chromatography is used to measure the content of polypeptide.
Owner:FUDAN UNIV
Measuring method of content of rare earth elements in thorium dioxide
InactiveCN105784683AAccurate determination of contentGood precisionPreparing sample for investigationAnalysis by thermal excitationRare-earth elementSubstrate concentration
The invention relates to a novel chemical detection method, and more specifically relates to a method used for measuring the content of 15 rare earth elements in thorium dioxide powder and blocks via inductively coupled plasma-atomic emission spectrometry. The method comprises following steps: sample dissolving, wherein a 0.2-0.5% hydrofluoric acid solution is added in dissolving of thorium dioxide, and adding amount is controlled to be 0.1ml or less; separation of thorium and the rear earth elements, wherein 3mol / L nitric acid is taken as a medium, an extraction agent composed of carbon tetrachloride and tributyl phosphate at 1:1 is used for three times of extraction so as to separate thorium and the rear earth elements; and sample measuring. According to the method, analytical line of the measured elements, sample substrate concentration, sample dissolving method, thorium and the rear earth elements separation method and conditions, and instrument parameters are determined, and inter element measuring interference test is carried out at the same time; an inductive coupling plasma emission spectrometer is used for simultaneous determination of the contents of the elements; method precision is high than 10%; and average recovery rate ranges from 91 to 110%. Operation of the method is simple; and the method is accurate and reliable.
Owner:CHINA NUCLEAR BAOTOU GUANGHUA CHEM IND
Resina draconis detecting method
The invention provides a resina draconis detecting method. The resina draconis detecting method comprises the following steps: (1) mixing a resina draconis sample to be detected and an alcohol component to obtain a solution to be detected; (2) carrying out a liquid chromatography on the solution to be detected to obtain a fingerprint of the solution to be detected, wherein an elution program of the liquid chromatography is gradient elution; and (3) obtaining contents of included components in the resina draconis sample to be detected according to the fingerprint and a pre-set standard curve of the solution to be detected and the quality of the resina draconis sample to be detected. The resina draconis detecting method adopts the gradient elution for carrying out the liquid chromatography to separate active components in the resina draconis. Furthermore, a chromatography peak form and a resolution of the active components in the obtained fingerprint are good and are good for calculation of a peak area of the chromatography peak, so as to obtain the contents of the components in the resina draconis sample to be detected. According to the method provided by the invention, the active components in the resina draconis are quantitatively determined, so as to more accurately reflect the quality of the resina draconis.
Owner:JIANGSU KANION PHARMA CO LTD
Kit for quantificationally detecting BRAF (Block Repeat Active Flag) mutation
InactiveCN102161990AAccurate determination of contentEasy to operateMicrobiological testing/measurementFluorescence/phosphorescenceBRAF Gene MutationEGFR Tyrosine Kinase Inhibitors
The invention relates to a method and kit for detecting BRAF (Block Repeat Active Flag) mutation relevant to the curative effect of a molecular-targeting anti-cancer medicament, in particular relating to a fluorescence quantificational PCR (Polymerase Chain Reaction) detecting method and kit for detecting mutation in BRAF gene mutation hot spot regions and application thereof. According to the invention, mutation at special positions of BRAF genes is detected, the curative effect of the molecular-targeting anti-cancer medicament such as an EGFR (Epidermal Growth Factor Receptor) tyrosine kinase inhibitor, and the like can be forecasted, and furthermore, the individual medicament use schemes of patients with tumors are directed.
Owner:BEIJING ACCB BIOTECH
Determination method of content of fulvic acid in fermentation liquor
ActiveCN103543240AAccurate determination of contentChemical analysis using titrationLinear relationshipGlucose polymers
The invention discloses a determination method of content of fulvic acid in fermentation liquor. The method comprises the following steps of 1) determining sugar in the fermentation liquor; and 2) determining the total fulvic acid in the fermentation liquor; 3) calculating the content of pure fulvic acid in the fermentation liquor: a, calculating the content of glucose equivalent to fulvic acid according to the linear relationship that glucose is corresponding to fulvic acid; and b, mapping by taking the glucose mass as an x-coordinate and the corresponding fulvic acid mass as a y-coordinate, thereby obtaining a working curve and an equation of linear regression as Y=aX+b, wherein a refers to slope, and b refers to intercept, converting the content of sugar in a sample into the content of fulvic acid, and then subtracting the content of sugar which is converted into fulvic acid by the content of the total fulvic acid, and then dividing the total amount of the fermentation liquor sample, thereby obtaining the percentage composition of pure fulvic acid in the fermentation liquor. The method provided by the invention can accurately determine the content of fulvic acid in the fermentation liquor.
Owner:SAAS BIOTECH & NUCLEAR TECH RES INST +1
Freeze-dried powder preparation of kuh-seng native, preparation and detection method thereof
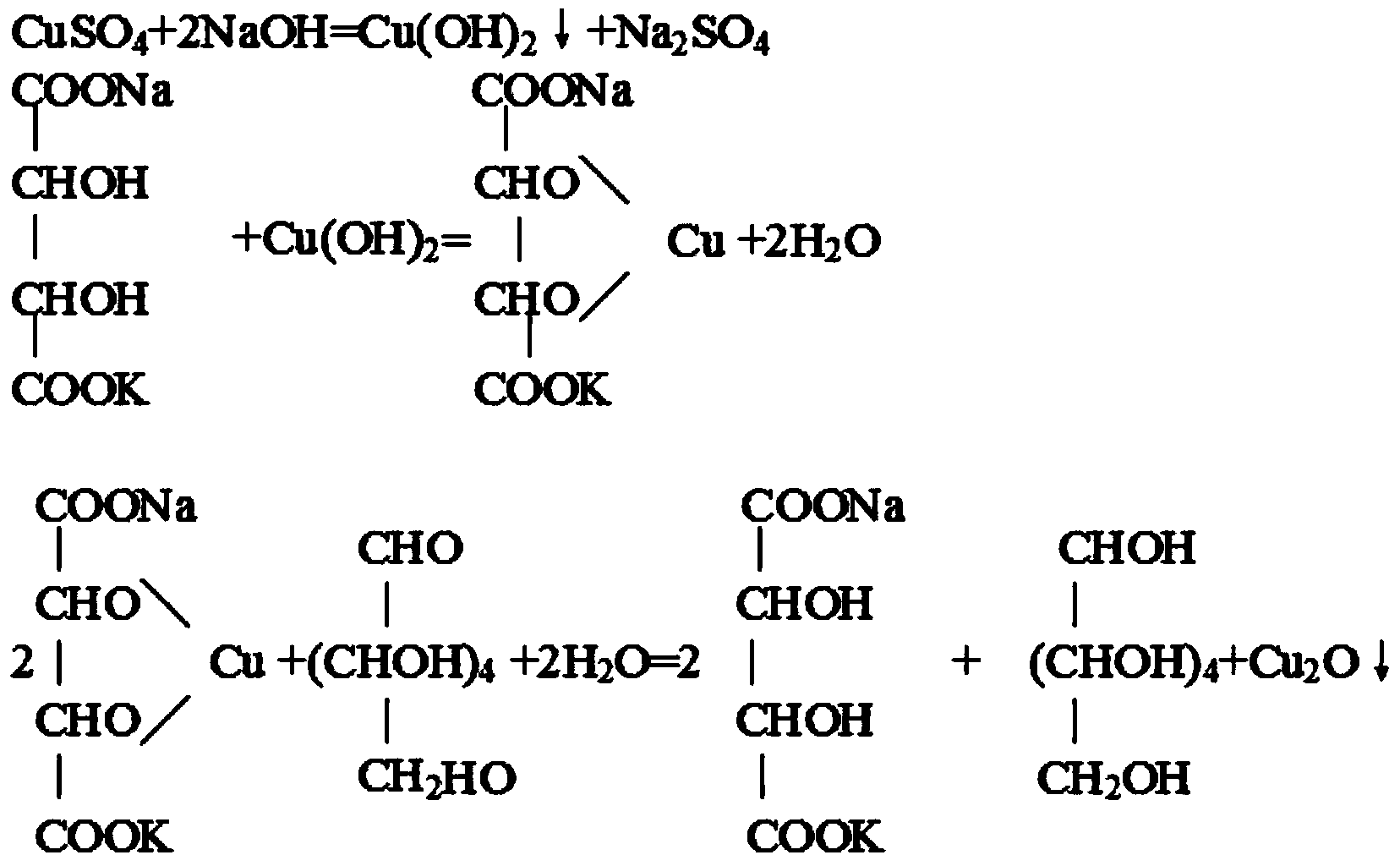
ActiveCN101428020AEasy to separateAccurate determination of contentOrganic active ingredientsPowder deliveryOxymatrineMANNITOL/SORBITOL
The invention provides a kurarinone freeze-dry powder preparation and a preparation method thereof. The preparation method comprises the following steps: filtering the solution of three components - kurarinone, mannitol, disodium edetate or calcio-sodium edetate in an appropriate proportion, freezing and drying, and then obtaining the freeze-dry powder preparation. Compared with the prior product, the preparation has the advantages that the stability is obviously improved, the expiry date can be as long as 24 months, and the characters of the product are not changed obviously; compared with the prior art, the preparation method is more simplified, and the production cost is saved. The invention also provides a method for detecting the content of oxymatrine in the product of kurarinone freeze-dry powder preparation. By optimizing the mobile phase and the detection condition, a highly-effective liquid-phase chromatographic detection method with better differentiation and stronger specificity is established.
Owner:HARBIN ZHENBAO PHARMA
Pepper numb-taste component content detection based on method of quantitative analysis of multi-components by single marker
InactiveCN106770788AEfficient separationAccurate determination of contentComponent separationAmount of substanceStandard curve
The invention discloses a pepper numb-taste component content detection based on a method of quantitative analysis of multi-components by a single marker. The pepper numb-taste component content detection based on the method of quantitative analysis of multi-components by the single marker comprises the steps: (1) establishing a hydroxy-beta-sanshol standard curve; (2) detecting the content of hydroxy-beta-sanshol in a sample to be detected; and (3) using a relative correction factor to calculate the contents of hydroxy-alpha-sanshol and hydroxy-gamma-sanshol in the sample to be detected. According to the pepper numb-taste component content detection based on the method of quantitative analysis of multi-components by the single marker, the method of quantitative analysis of multi-components by the single marker and an external standard method are mutually confirmed and have no obvious difference; and the results of the method of quantitative analysis of multi-components by the single marker and the external standard method are both reliable. The pepper numb-taste component content detection based on the method of quantitative analysis of multi-components by the single marker, disclosed by the invention, has the advantages that the contents of the hydroxy-alpha-sanshol and the hydroxy-gamma-sanshol are calculated by using the relative correction factor and determination of chromatographic peak in case of lacking comparison products of the hydroxy-alpha-sanshol and the hydroxy-gamma-sanshol, the quality controls of main numb-taste components of the hydroxy-alpha-sanshol and the hydroxy-gamma-sanshol in pepper are realized, and a new method is provided for pepper quality control.
Owner:CHENGDU UNIV OF TRADITIONAL CHINESE MEDICINE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com