Fingerprint spectrum detection method of medicinal preparation
A detection method and fingerprint technology, applied in the field of detection and analysis of pharmaceutical ingredients, can solve the problems that cannot be used to evaluate the internal quality of Chinese patent medicines, and the operation steps are cumbersome
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: The detection method of drug fingerprint of the present invention:
[0057] 1 Instruments and reagents
[0058] ACQUITYH-CLASS type ultra-high performance liquid chromatography system (Waters, USA), including binary ultra-high pressure solvent system, FTN automatic sample injection manager, PDA detector and Empower 3 chromatography workstation; Sartorius CPA225D type 1 / 100,000 electronic analysis Balance (Sartorius Scientific Instrument Co., Ltd., Germany); KQ-300DE CNC ultrasonic cleaner (Kunshan Ultrasonic Instrument Co., Ltd.).
[0059] Actascoside (batch number: 111920-201505, purity: 97.1%) was purchased from China National Institutes for Food and Drug Control, Sangbaritin A (batch number: MUST-17060301, purity: 99.42%), hydroxysafflor yellow A (batch number: MUST-16092910, purity: 99.88% HPLC), amygdalin (lot number: MUST-17042810, purity: 99.28% HPLC), paeoniflorin (lot number: MUST-17031901, purity: 99.30%), rosmarinic acid (lot number: MUST-170405...
Embodiment 2
[0100] Embodiment 2: Assay of pharmaceutical preparation of the present invention:
[0101] 2.1 Content determination
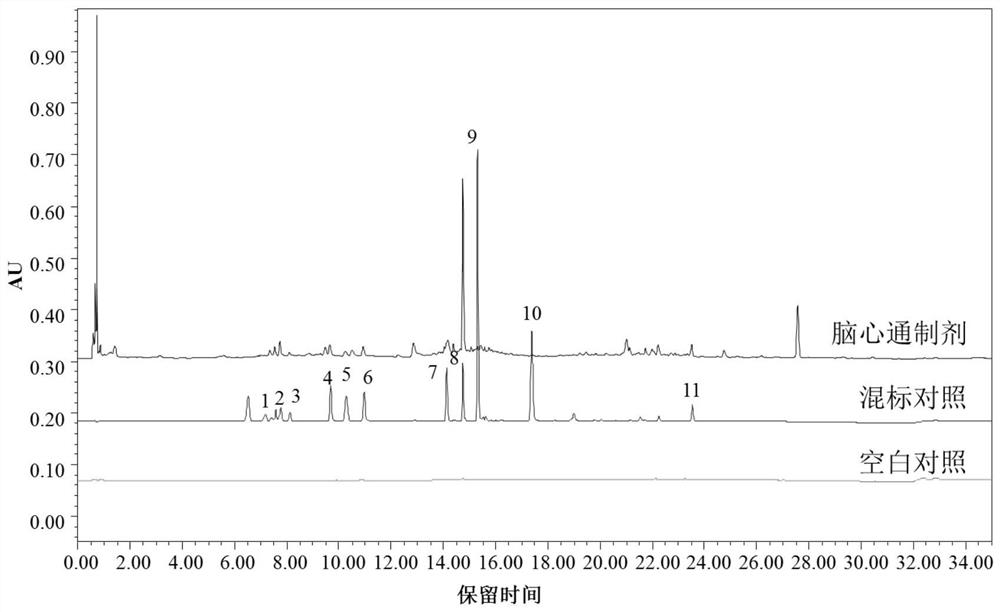
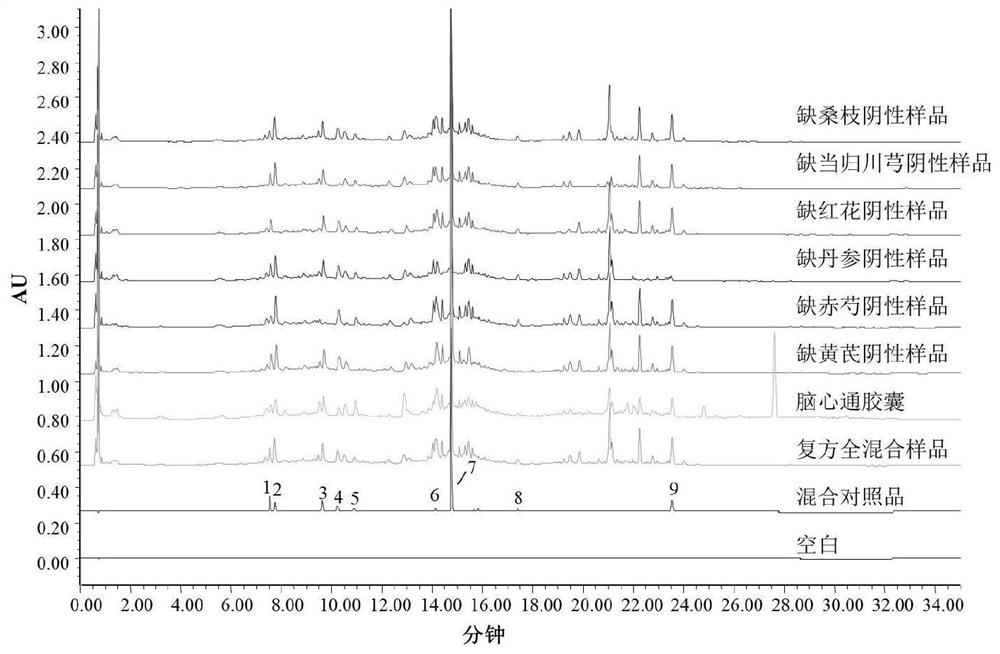
[0102] 2.1.1 Chromatographic conditions
[0103] Using ACQUITY BEH C18 (50mm×2.1mm, 1.7μm) chromatographic column, the mobile phase is 0.2% formic acid-water solution (A)-acetonitrile solution (B), the gradient elution program is 0~2min, 2%B; 2~7min, 2 %~14%B; 7~9min, 14%~15%B; 9~11min, 15%~16%B; 11~14min, 16%~35%B; 14~16min, 35%B; 16~21min , 35%~70%B; 21~23min, 70%B; 23~28min, 70%~100%B; 28~30min, 100%B; 30~32min, 100%~2%B; after running for 5min; The volume flow rate is 0.2ml / min; the column temperature is 35°C; the detection wavelength is 254nm; the injection volume is 5μl.
[0104] 2.1.2 Preparation of mixed reference solution
[0105] Accurately weigh the appropriate amount of mulberry glycoside A, hydroxysafflower yellow A, paeoniflorin, ferulic acid, verbascoside, rosmarinic acid, salvianolic acid B, formononetin and tanshinone IIA reference subst...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com