Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
3227 results about "Sterile water" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Sterile water is water that has no microorganisms living within it. As it cannot transfer pathogens, it is used mainly in the medical setting. Sterile water, though sometimes distilled, is not the same thing as distilled water.
Sterile surgical tray
Owner:DOHENY EYE INST
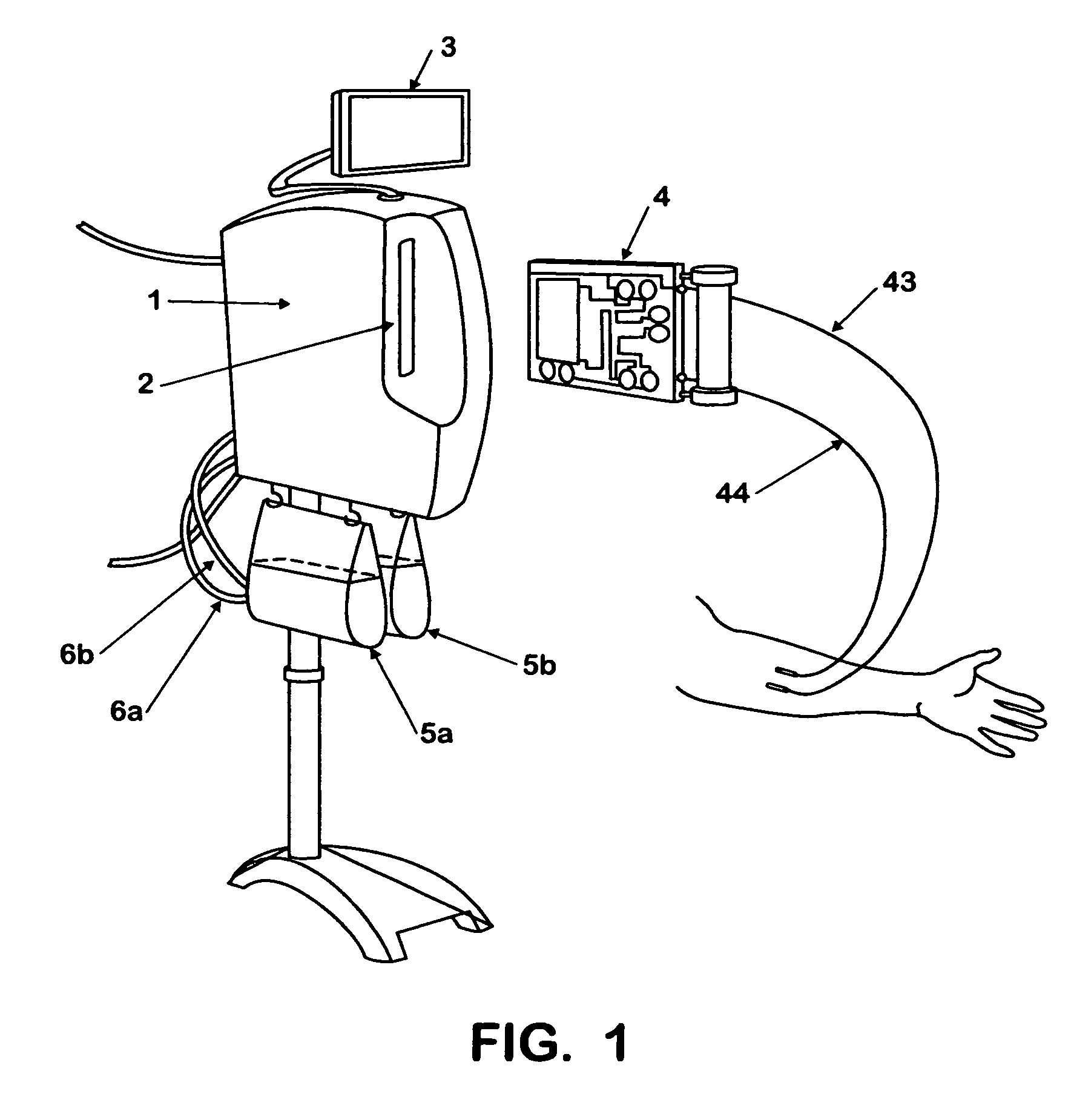
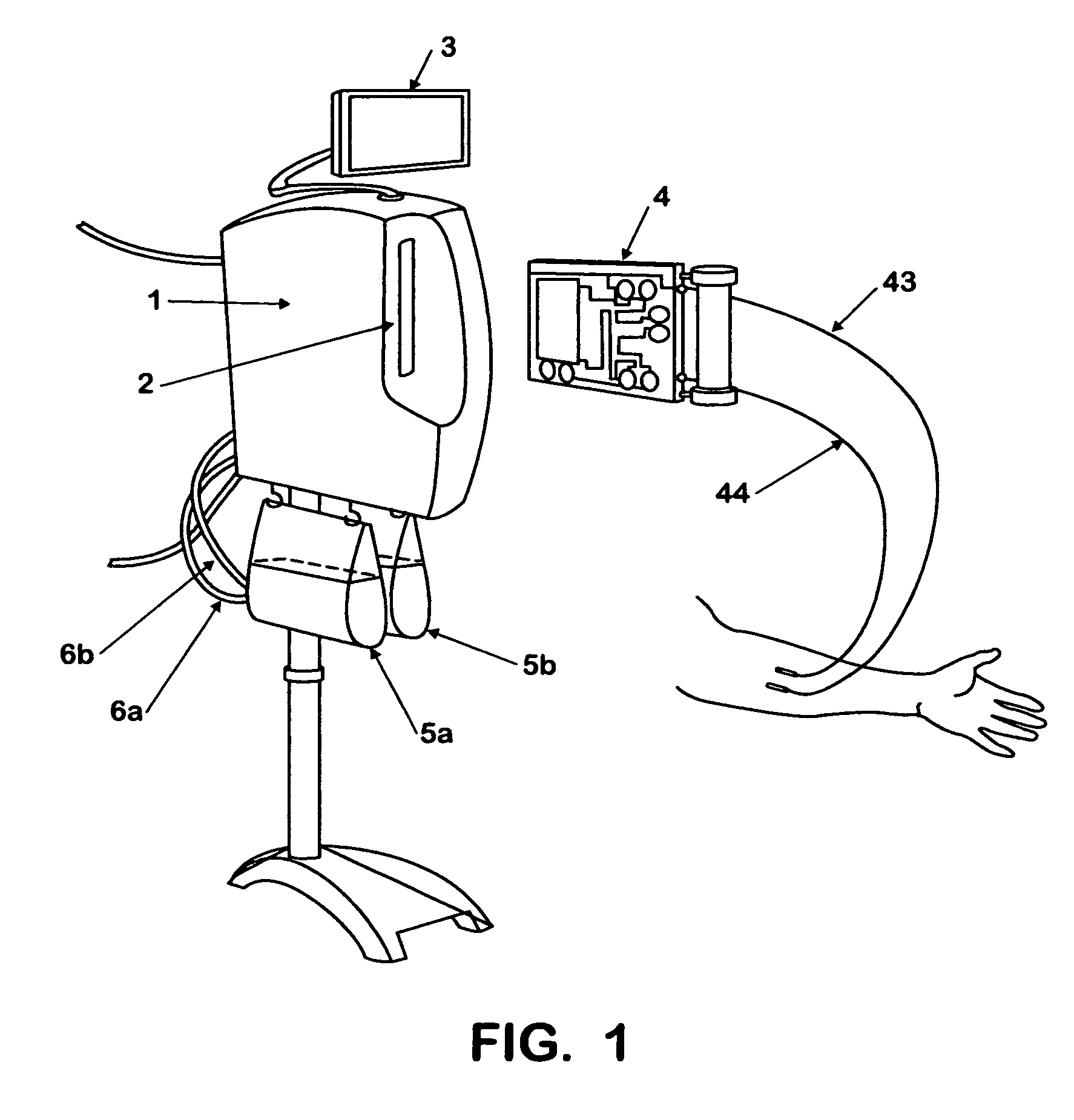
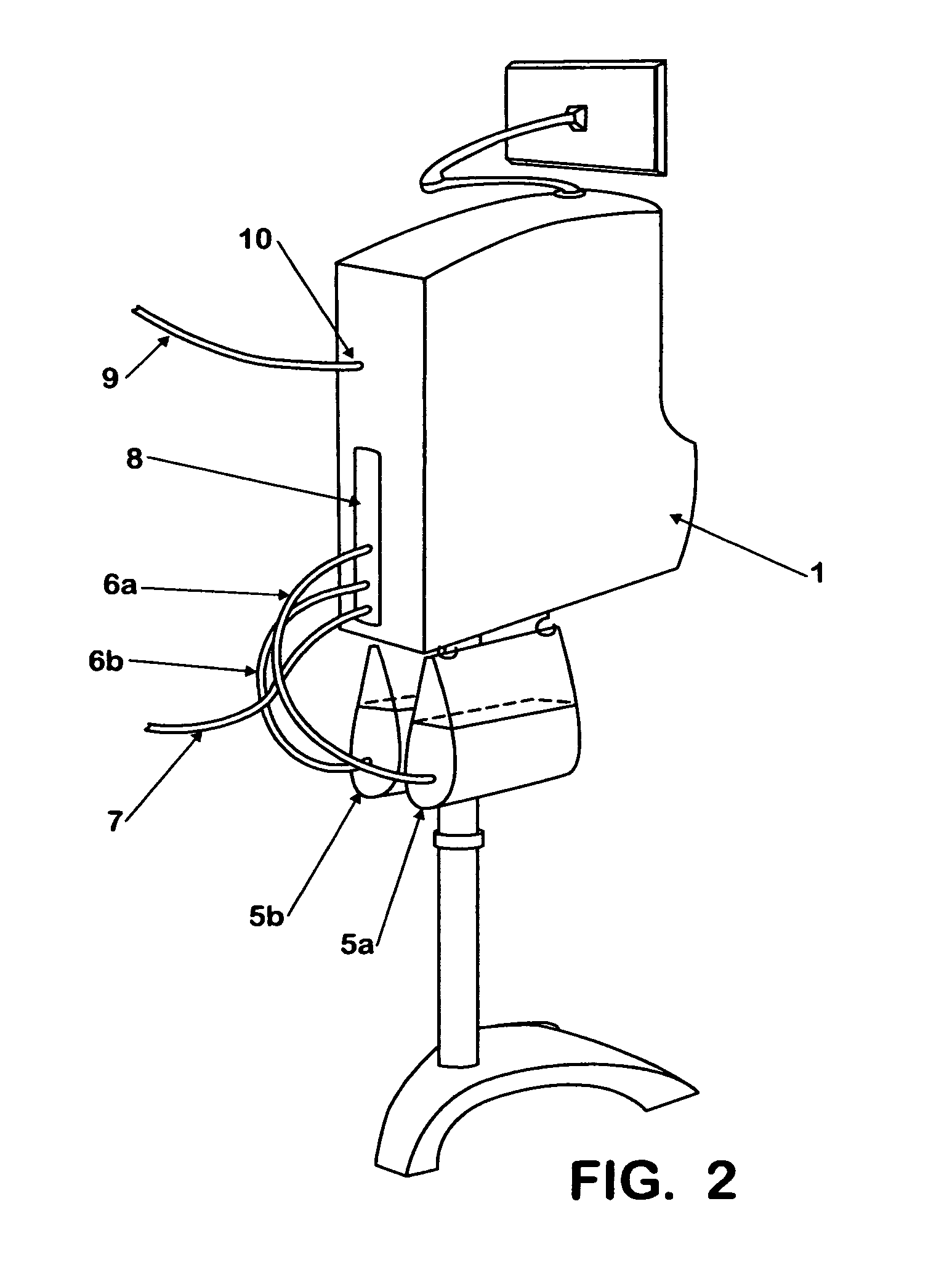
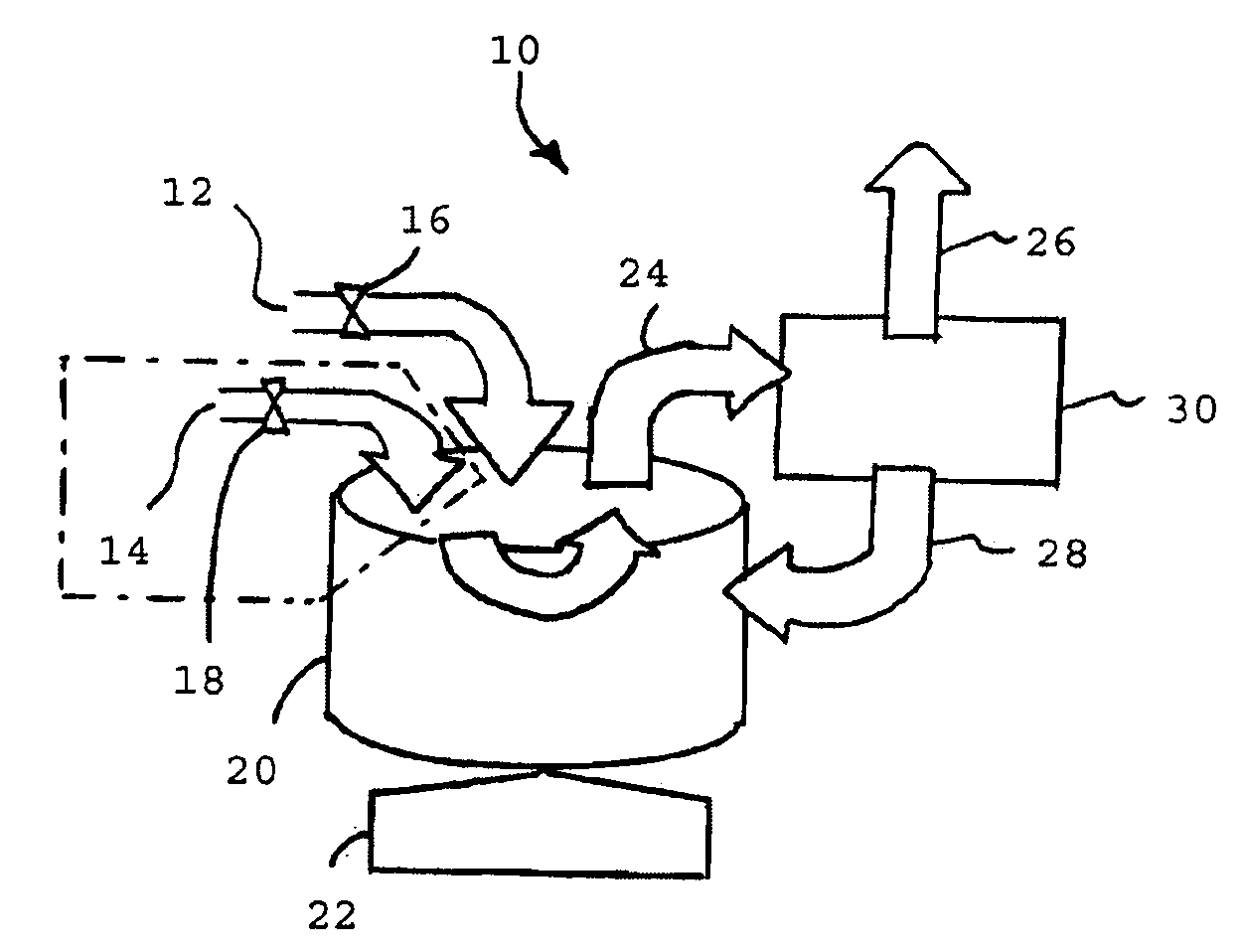
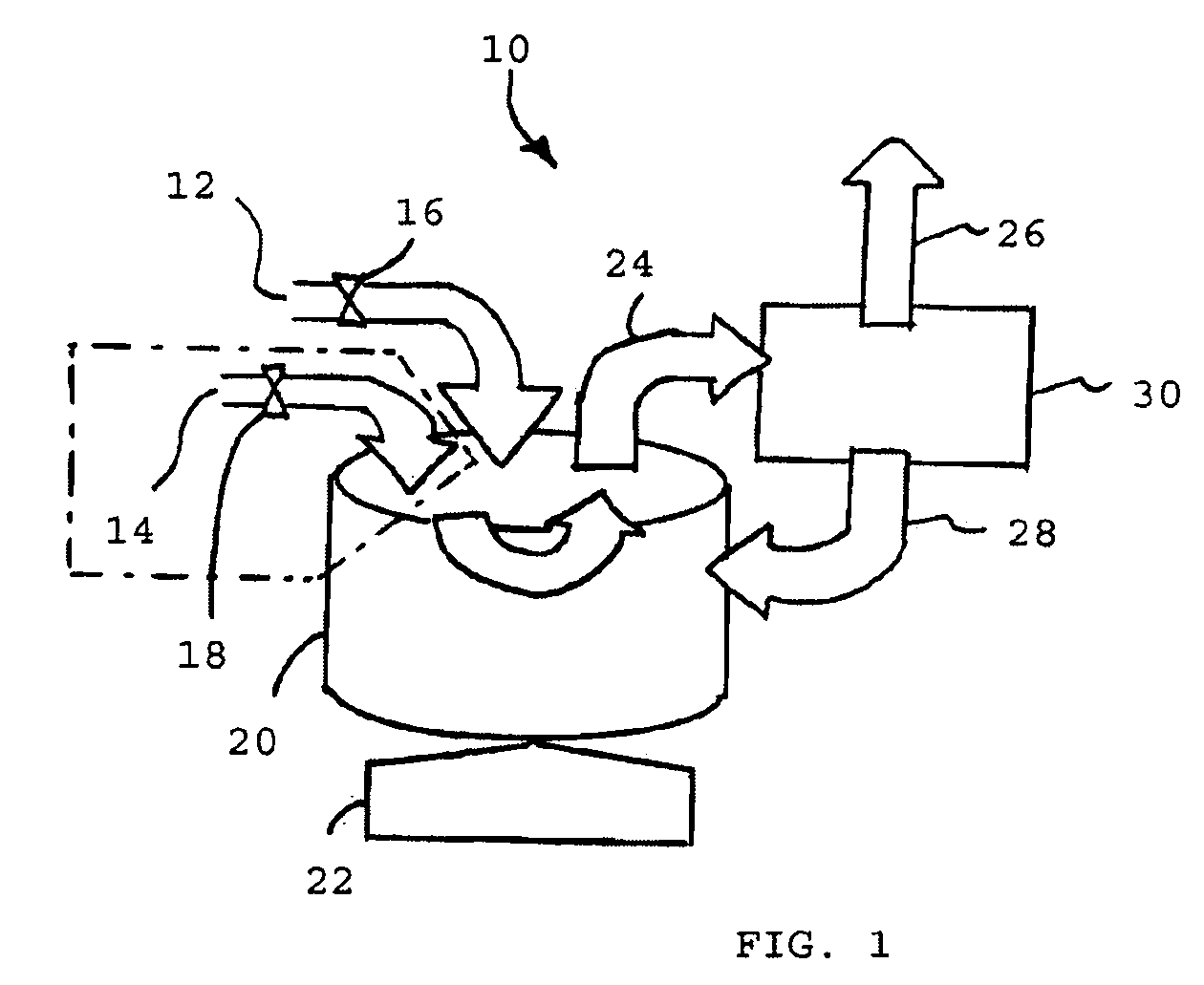
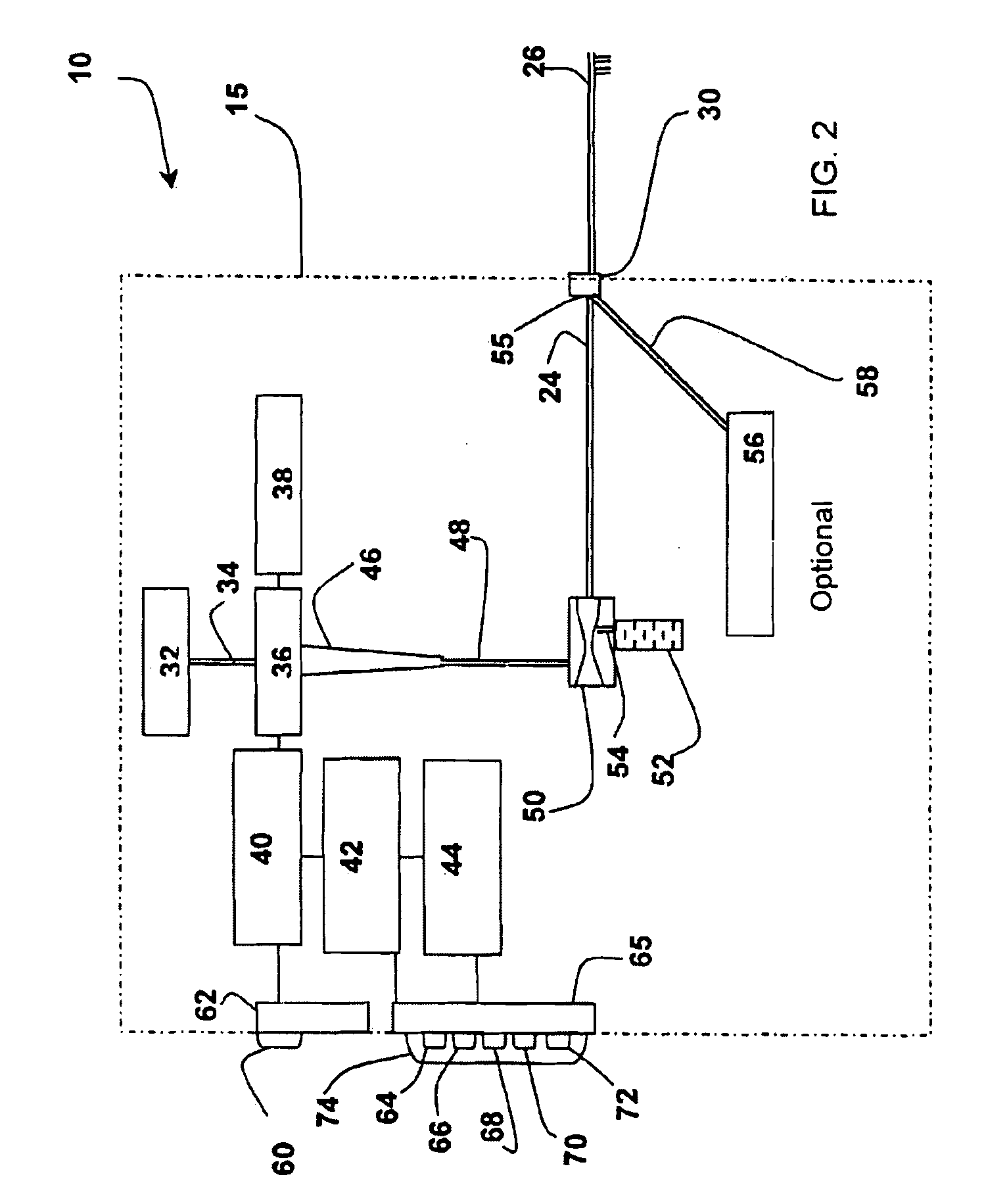
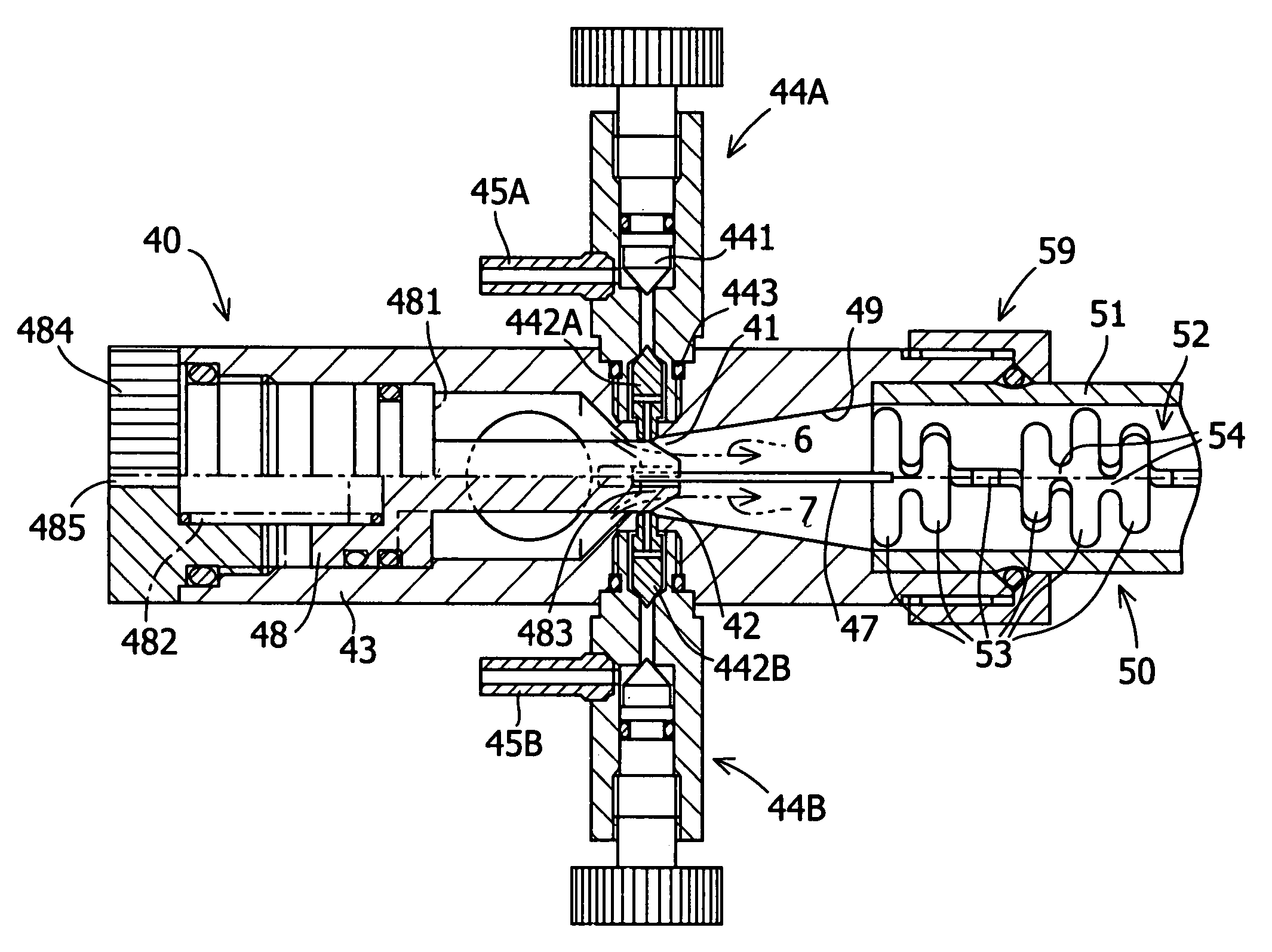
Fluid processing apparatus
ActiveUS8535525B2Need is eliminated and minimisedEliminate needSettling tanks feed/dischargeDialysis systemsSterile waterEngineering
A machine is provided with a slot to releasably receive and retain a cartridge in which dialysis is effected. The machine is configured for supplying to the cartridge, at a controlled temperature and rate, sterile water for use in haemodialysis and is operable to maintain, in a sterile condition, residual water contained therein after completion of a haemodialysis treatment.
Owner:QUANTA DIALYSIS TECH LTD
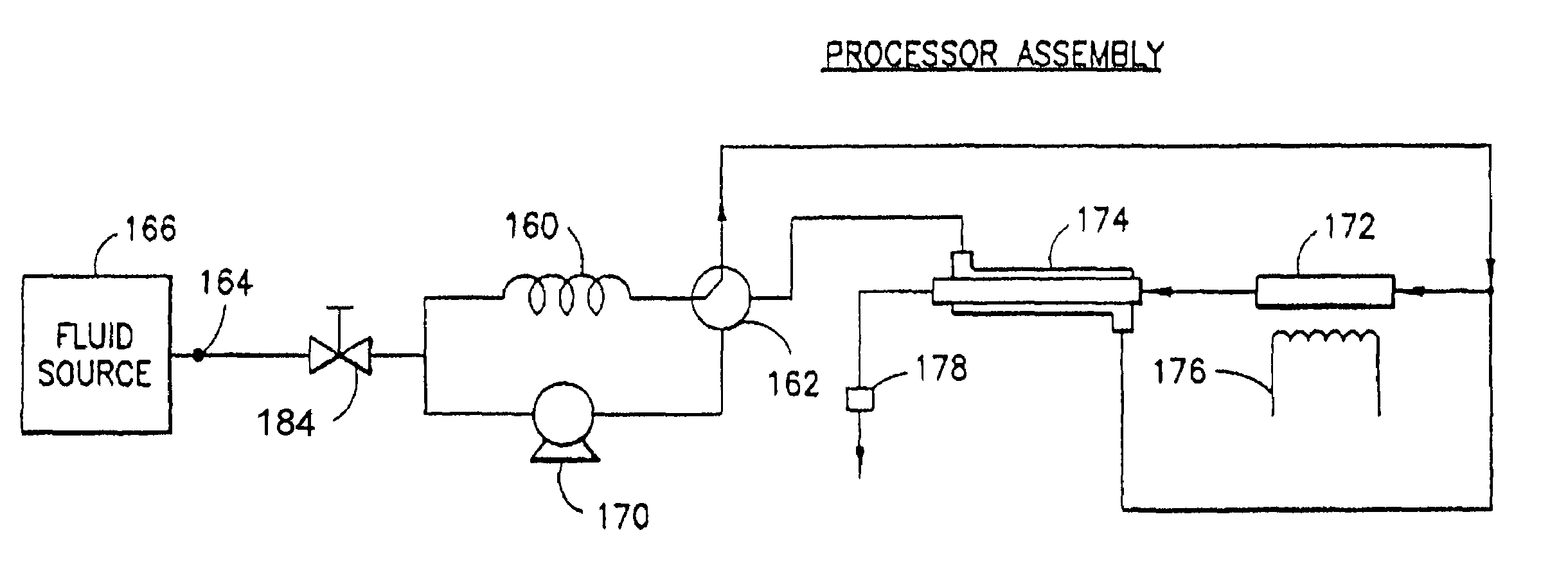
Apparatus and method for continuous depyrogenation and production of sterile water for injection
InactiveUS7122149B2Simple yet dependableReduce contact timeDialysis systemsTreatment involving filtrationDepyrogenationIon exchange
A fluid processor, suitable for the production of sterile water for injection, having a processor assembly and a process control system comprising a pump, a flow splitter, flow restrictors and a pressure relief valve. In a preferred embodiment, the processor assembly comprises a heat exchanger, a reactor and a heater arranged in a nested configuration. The preferred embodiment of the present invention also include a treatment assembly having a combination of filter, reverse osmosis and ion exchange devices and further incorporates an assembly and method allowing for the in situ sanitization of the fluid processor during cold start and shutdown to prevent bacteria growth during storage of the fluid processor. The fluid processor may include an electronic control system comprising a touch screen operator interface, a programmable logic controller and sensors for measuring temperature, pressure, flow rate, conductivity and endotoxin level.
Owner:APPLIED RES ASSOCS INC
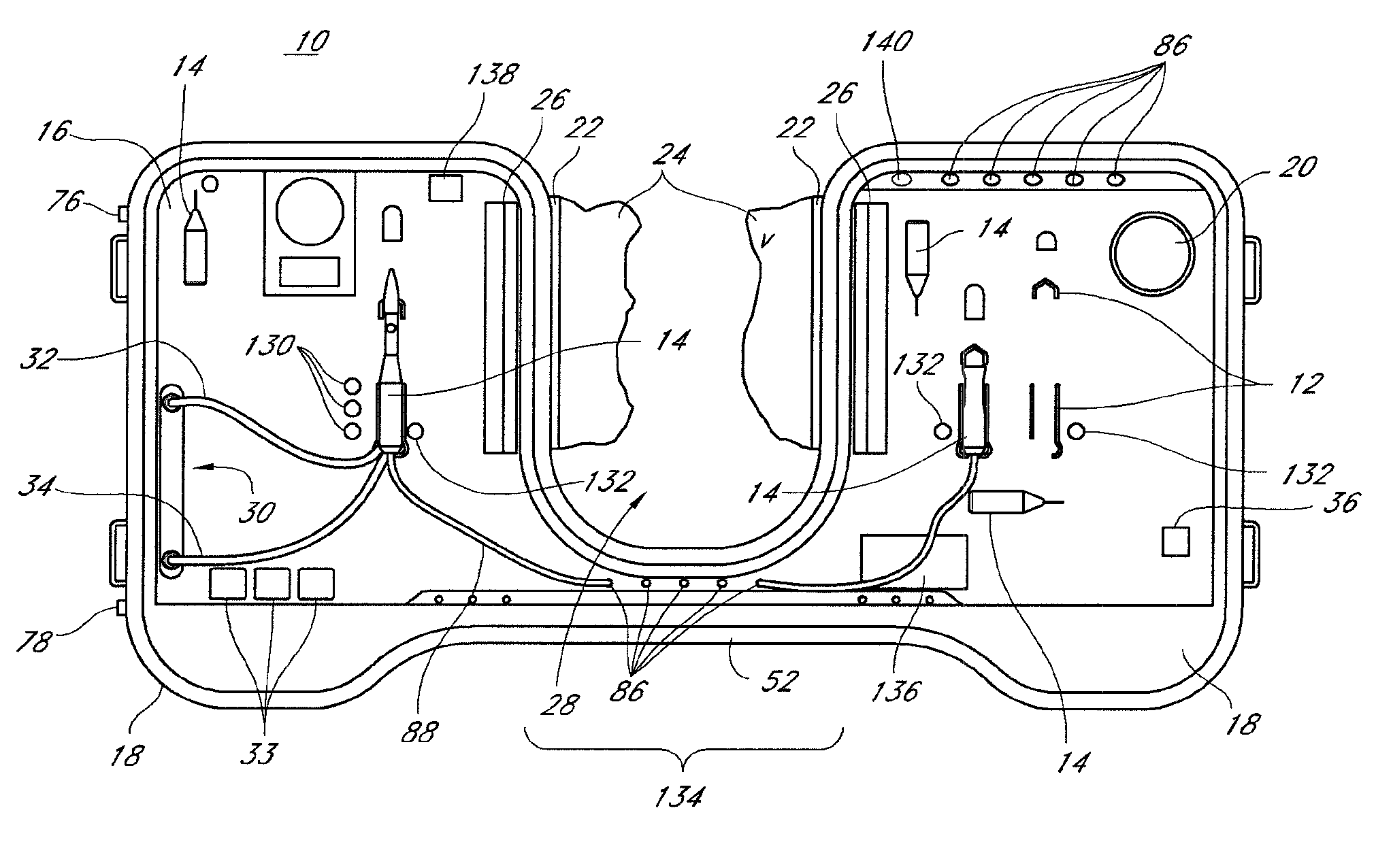
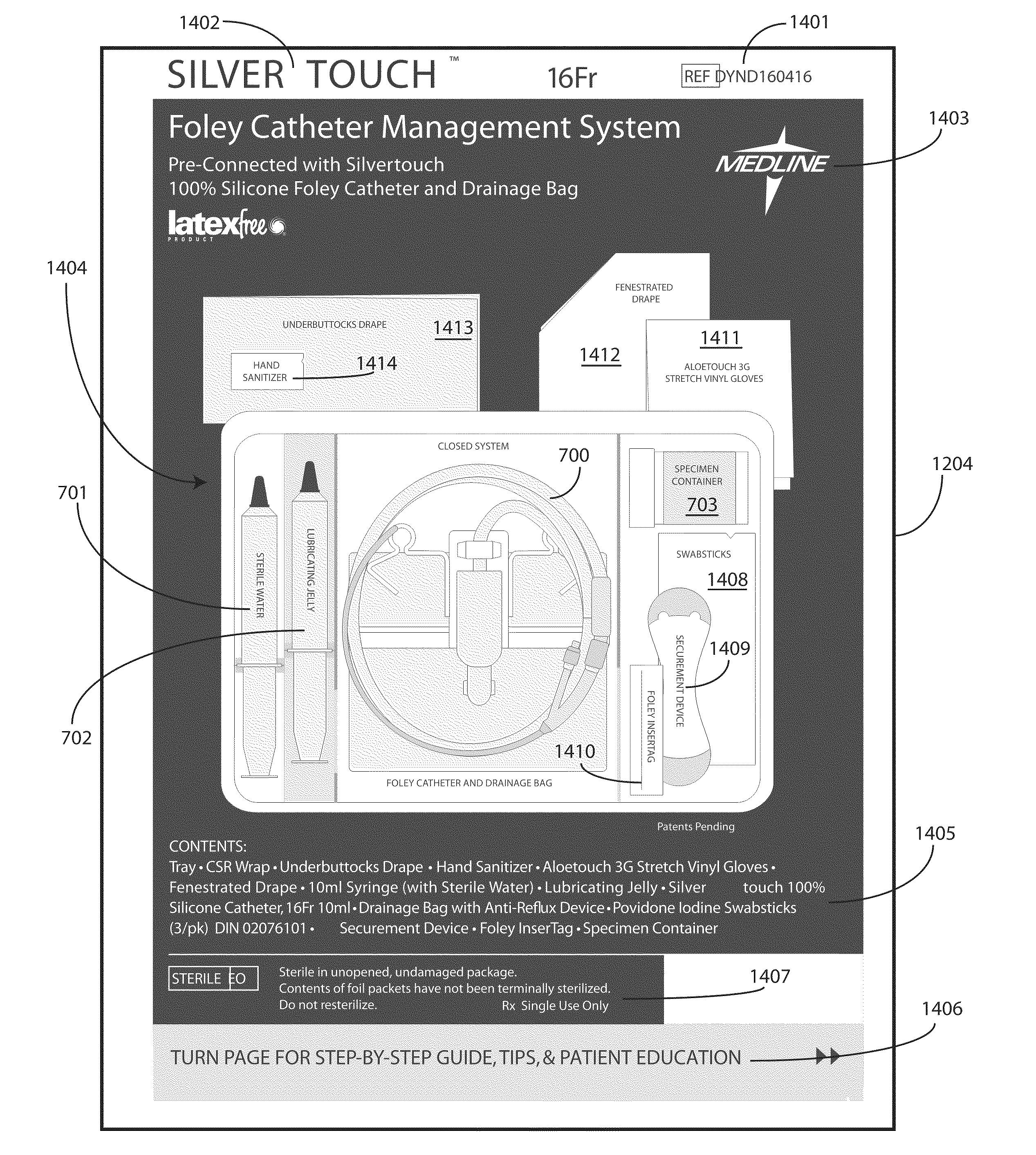
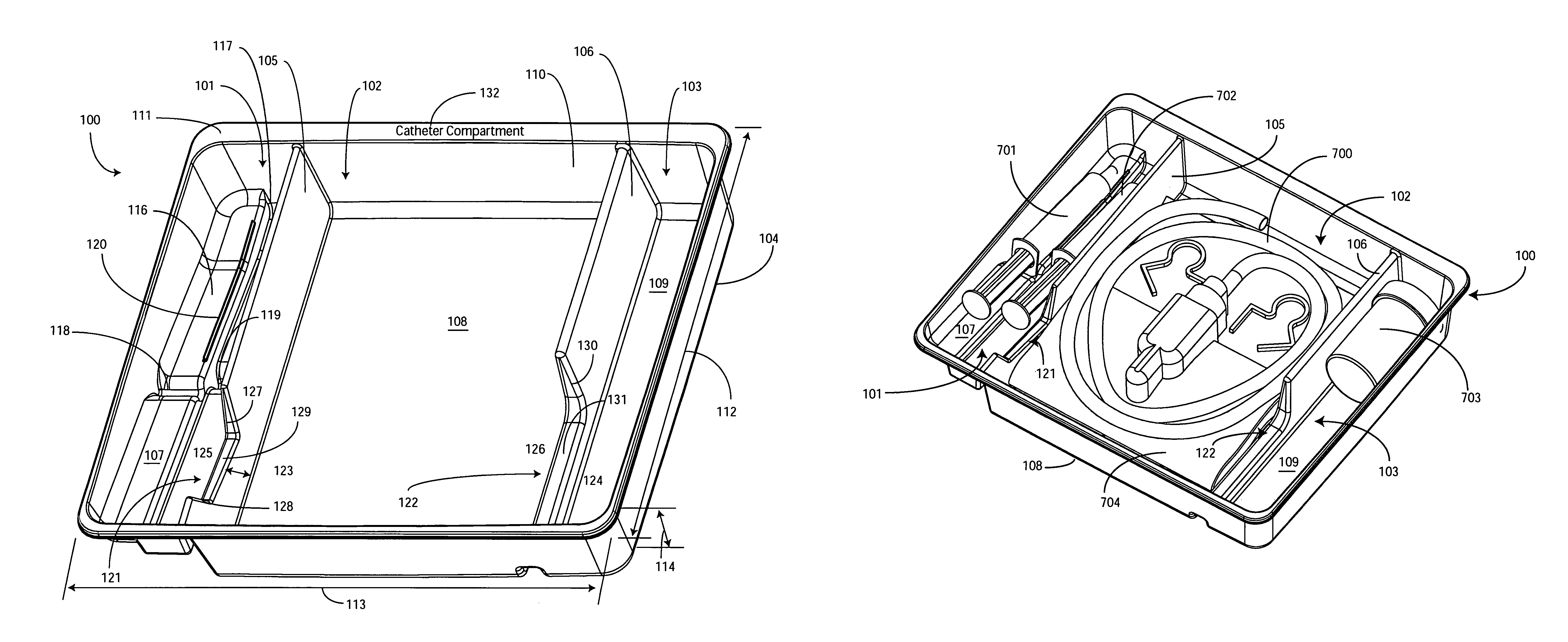
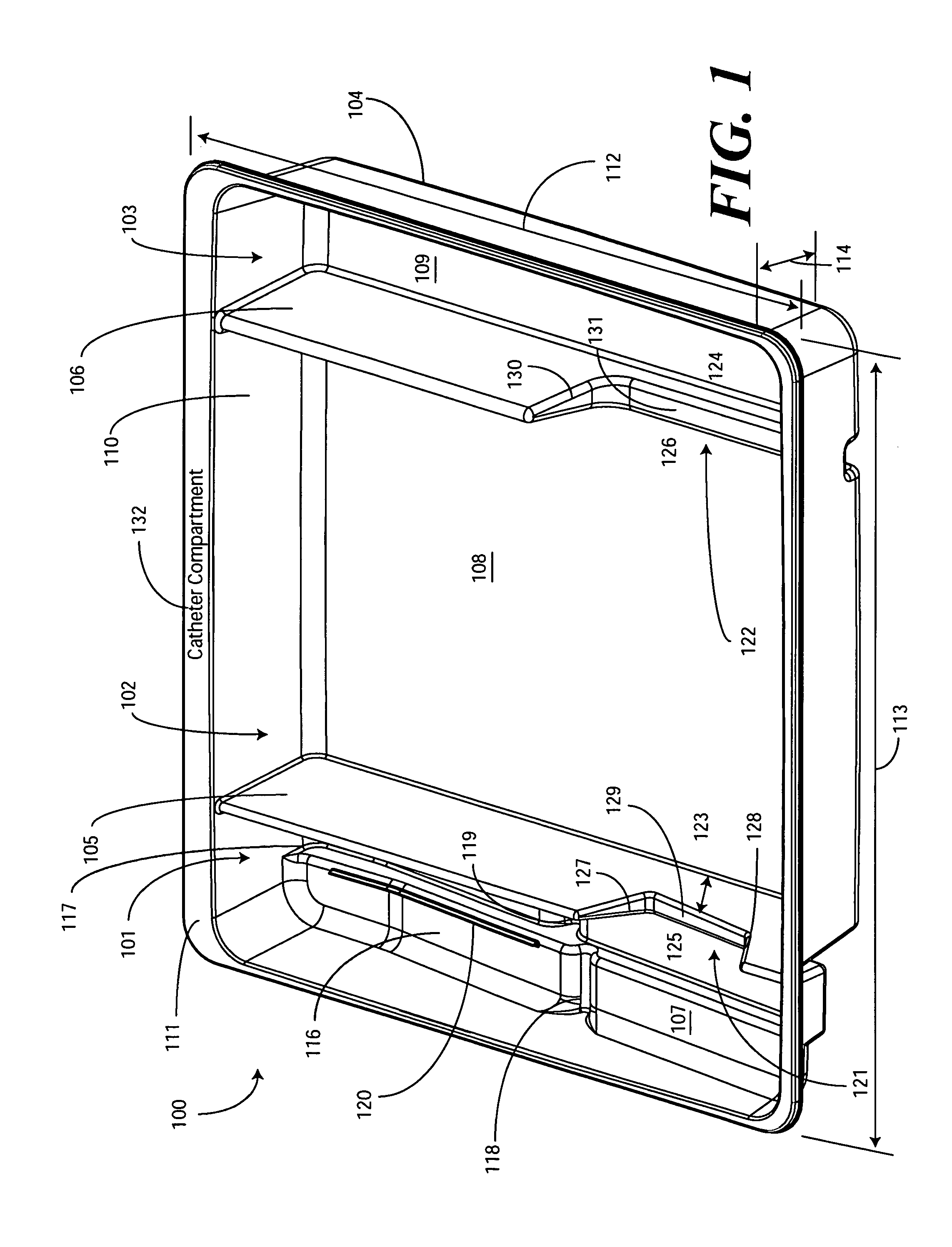
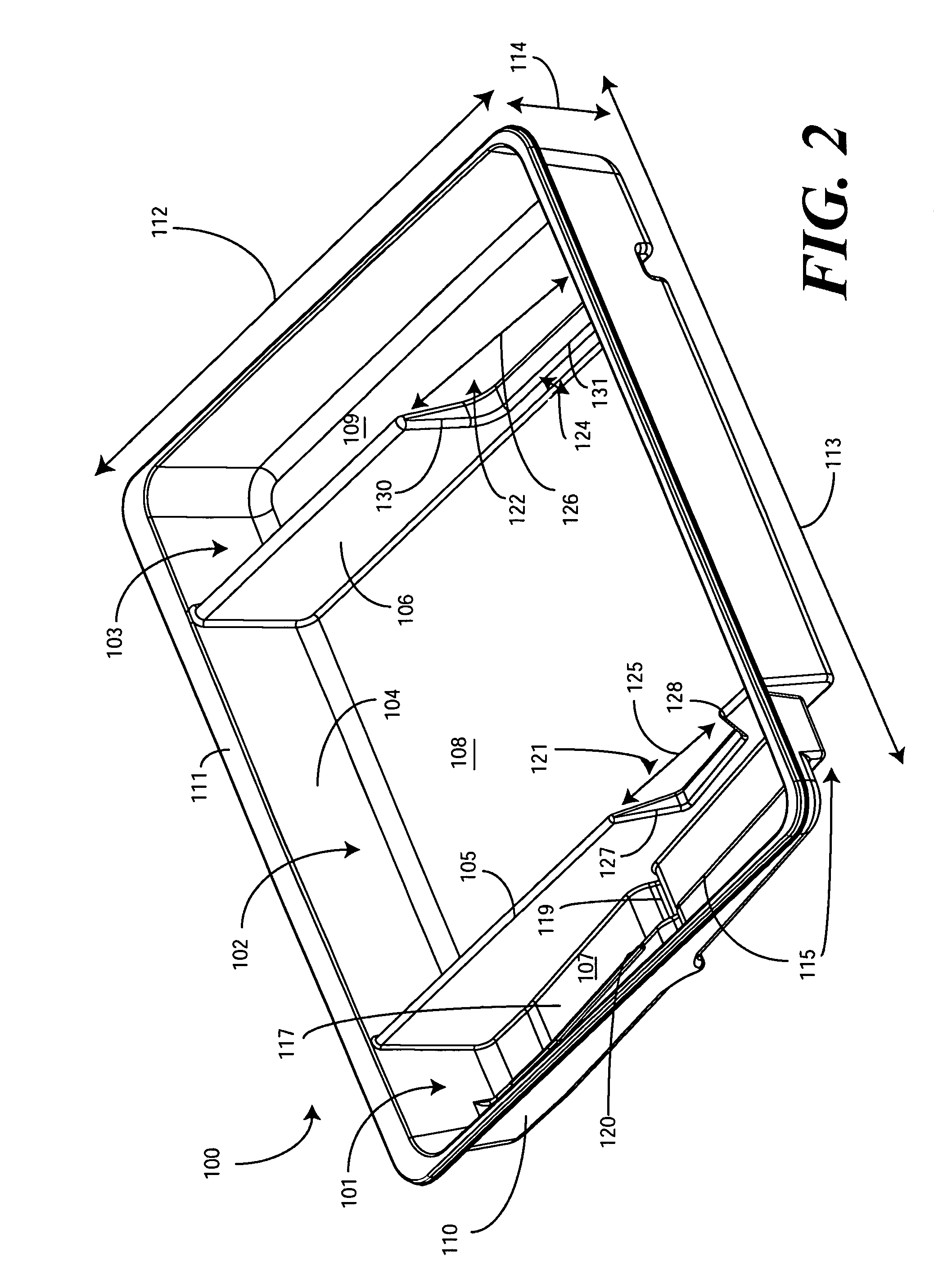
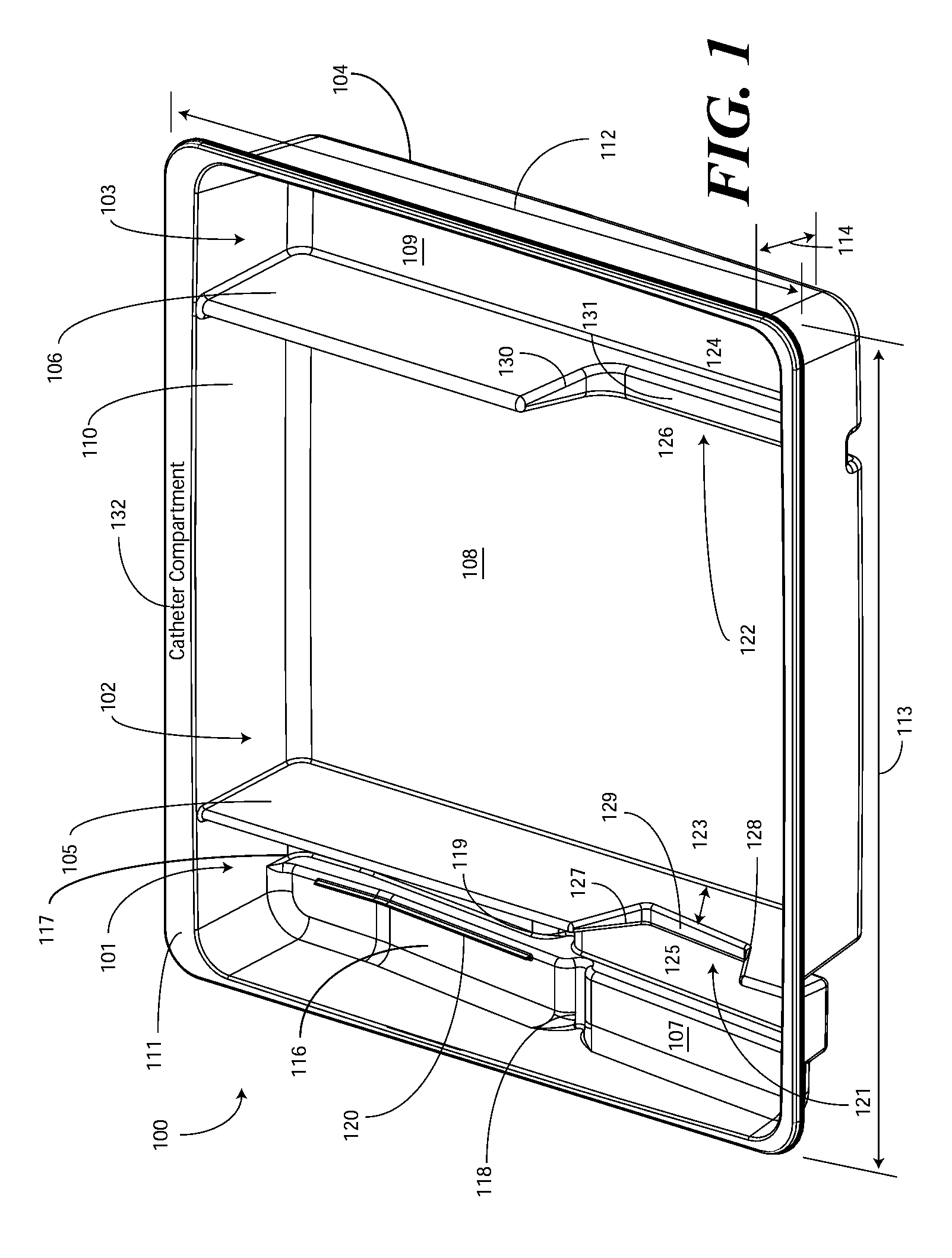
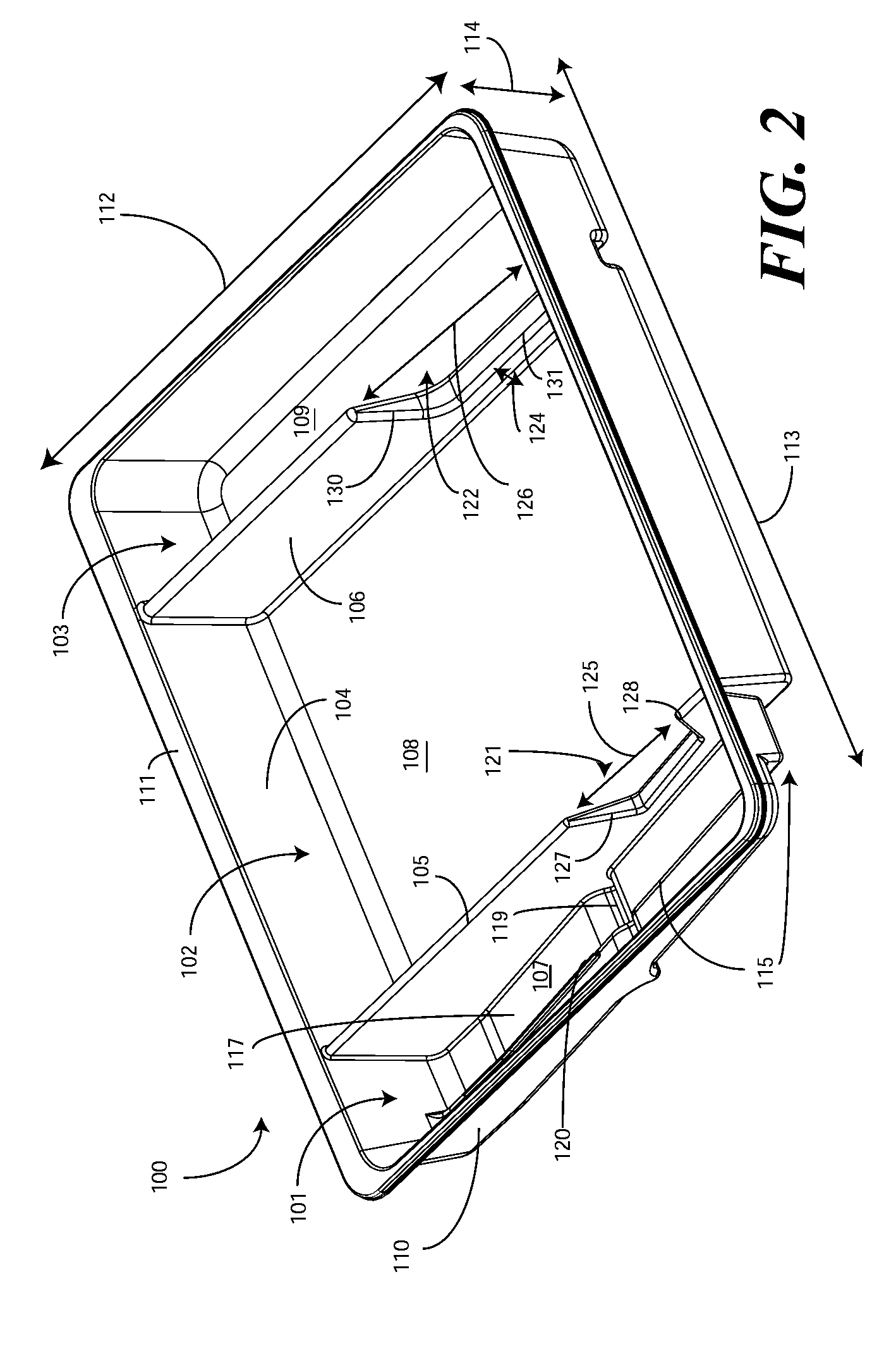
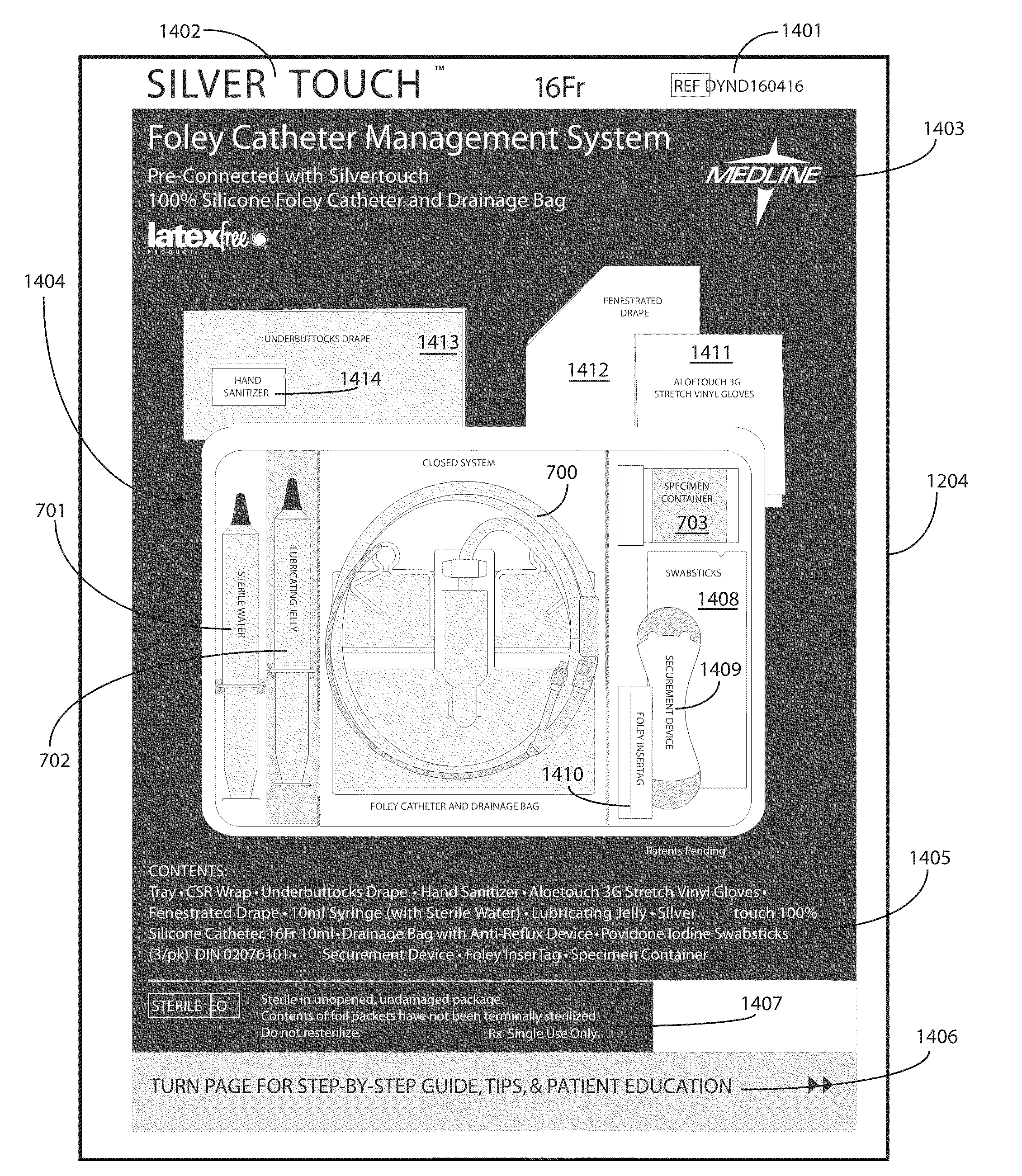
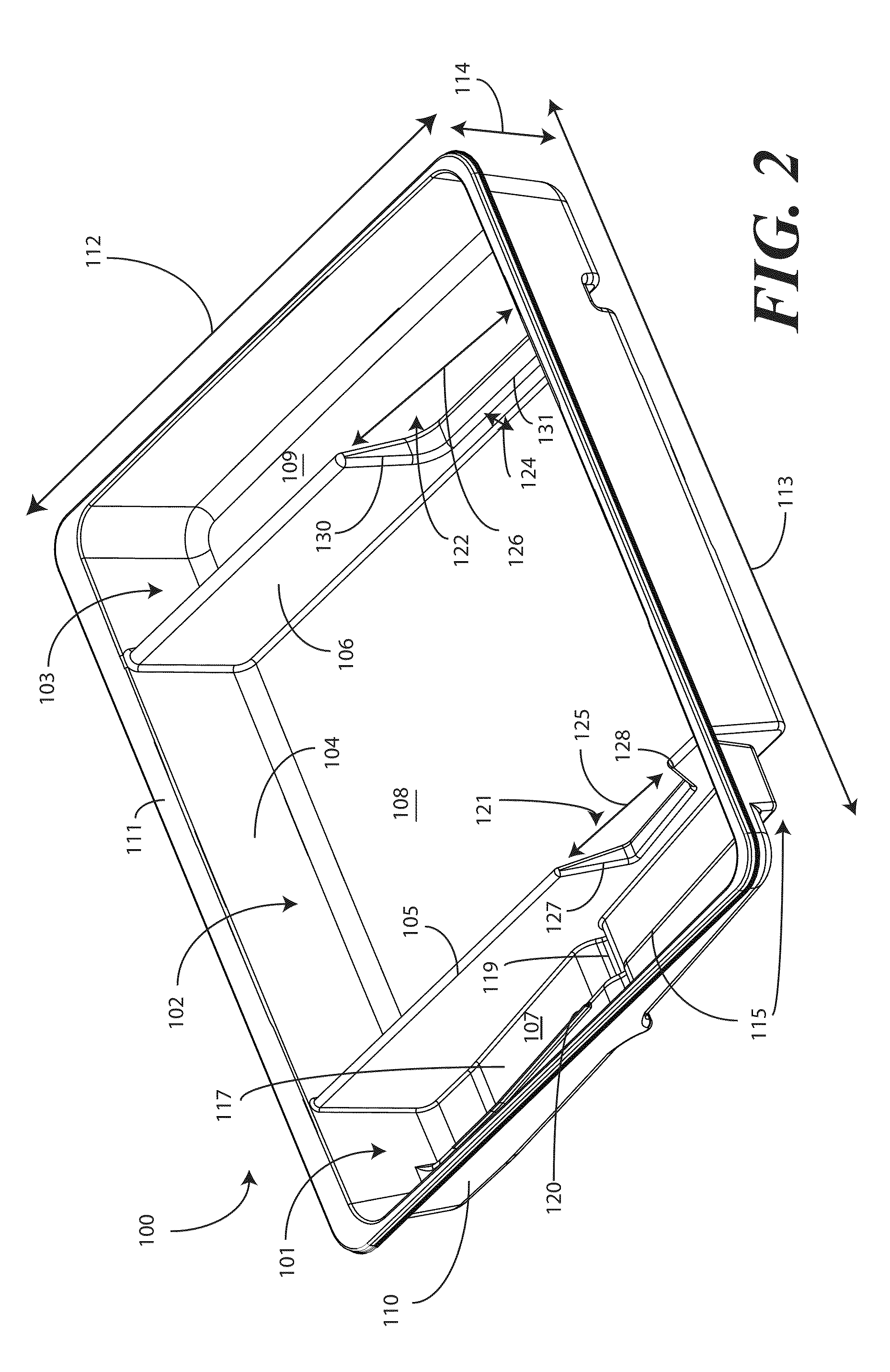
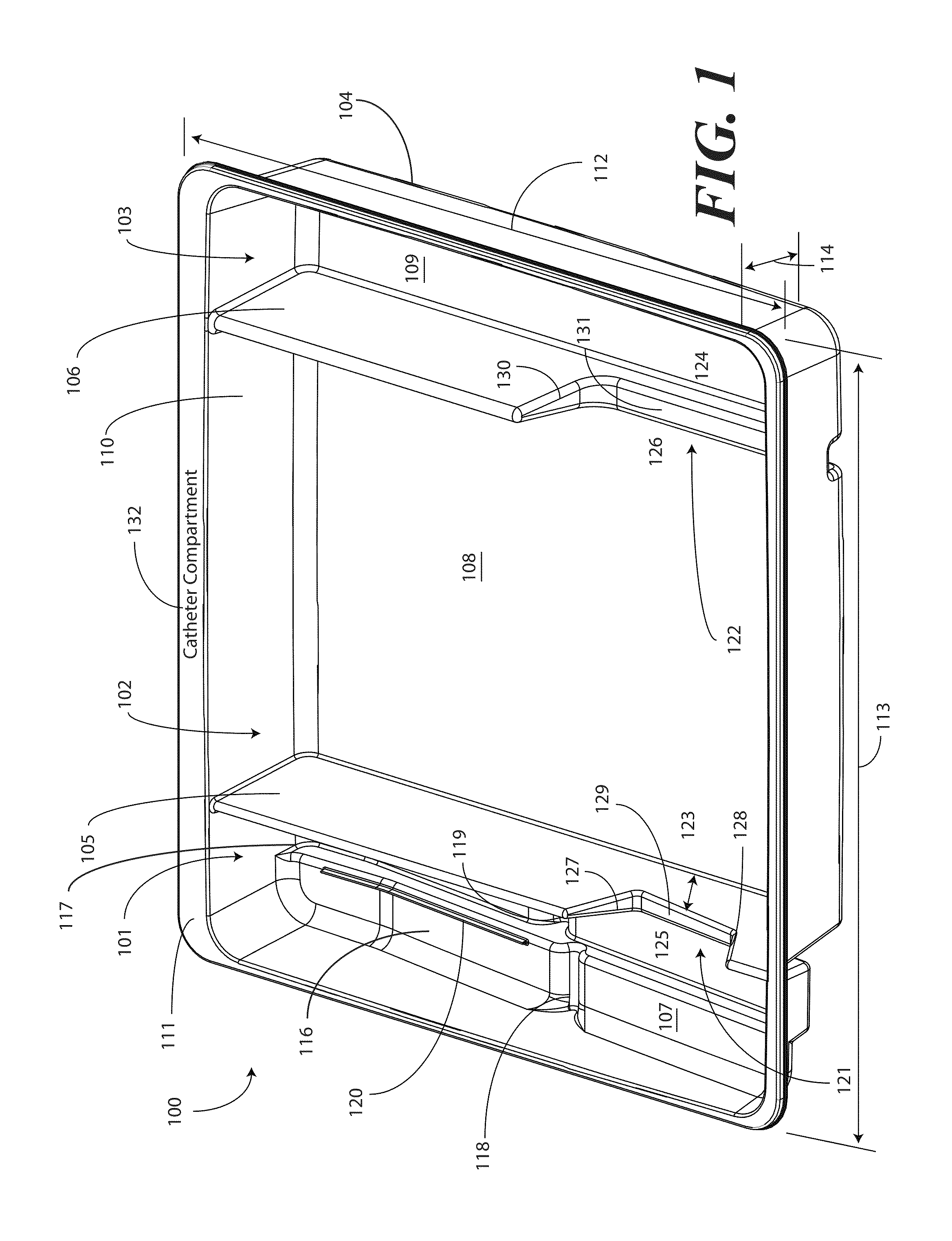
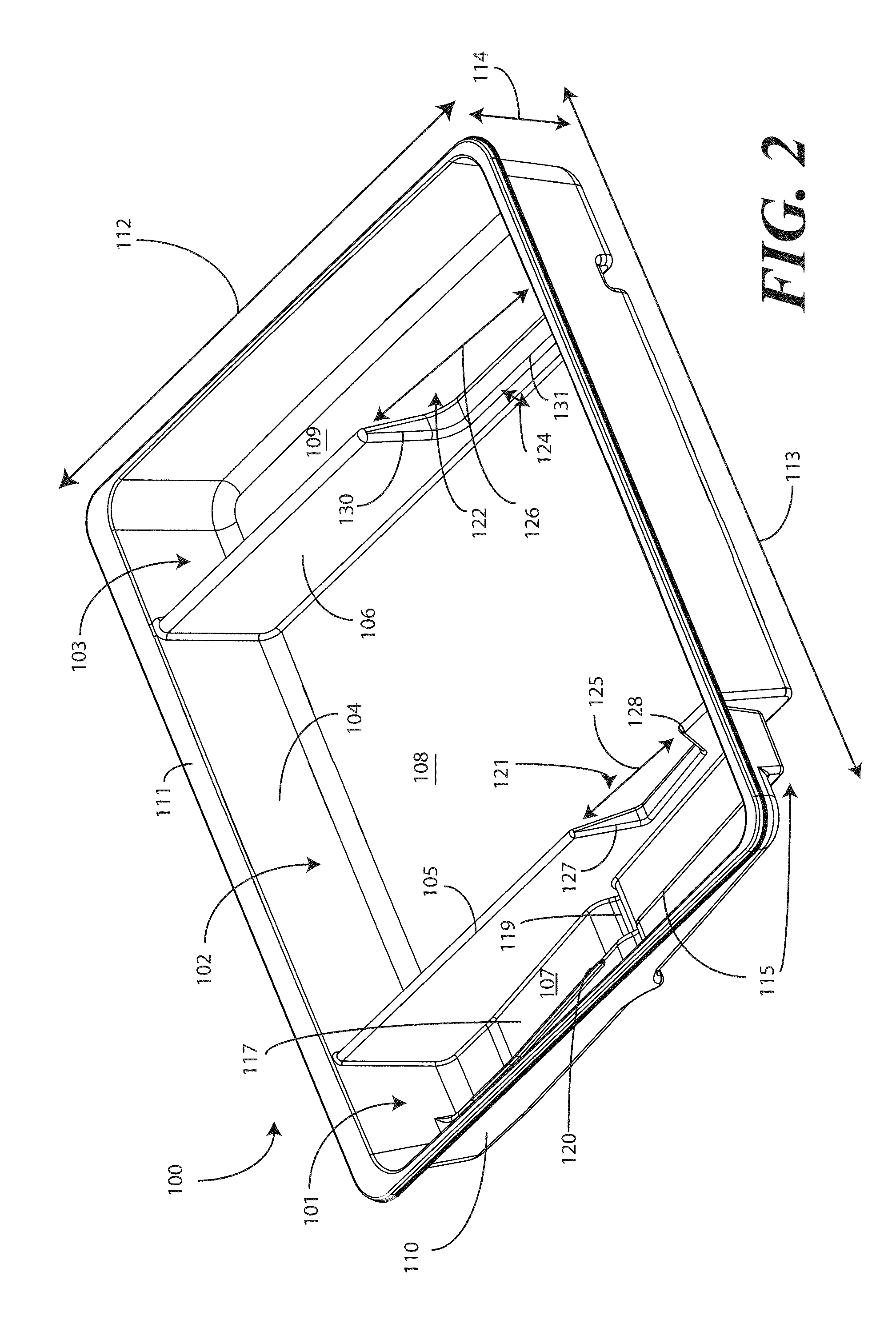
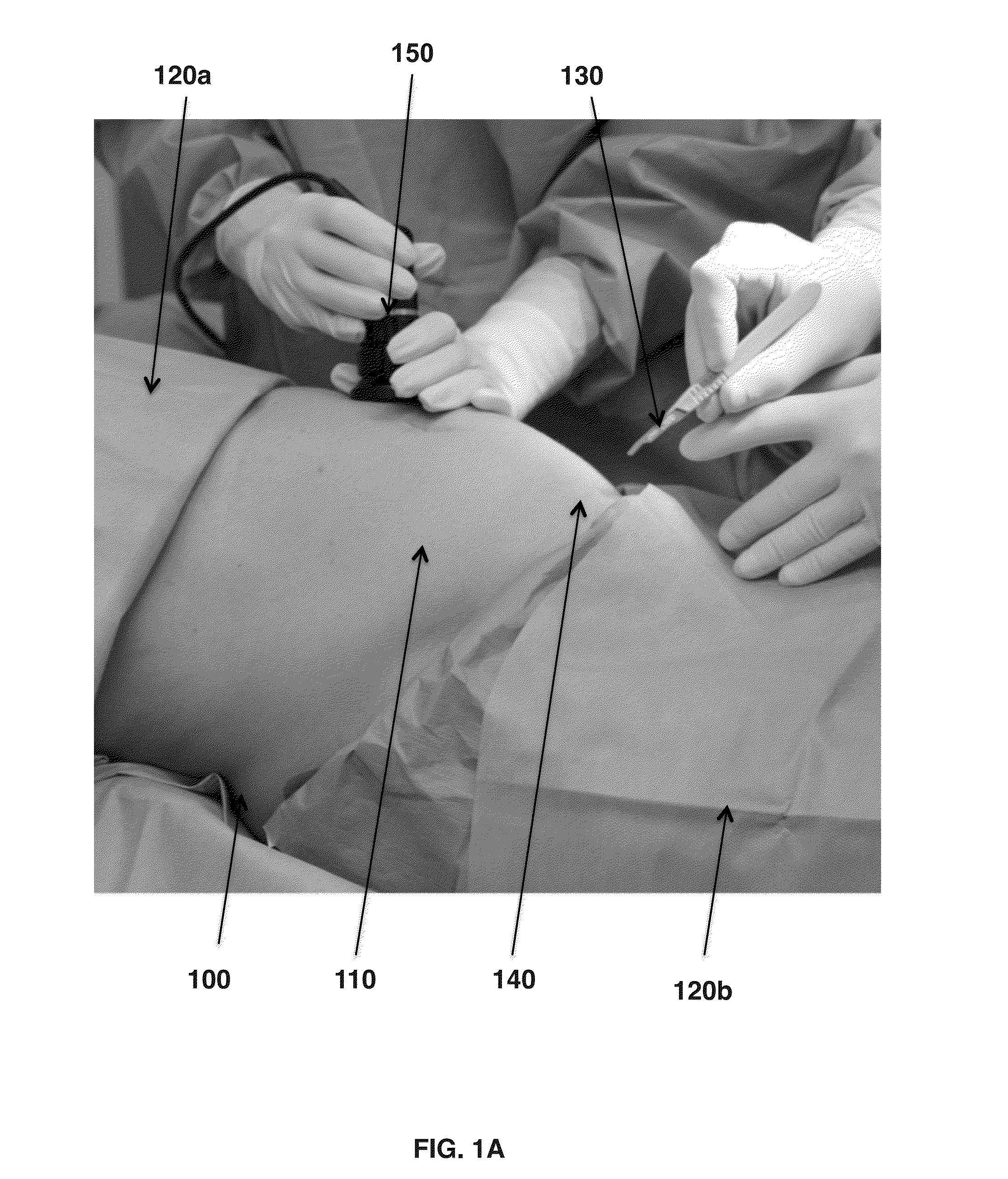
Catheter Tray, Packaging System, and Associated Methods
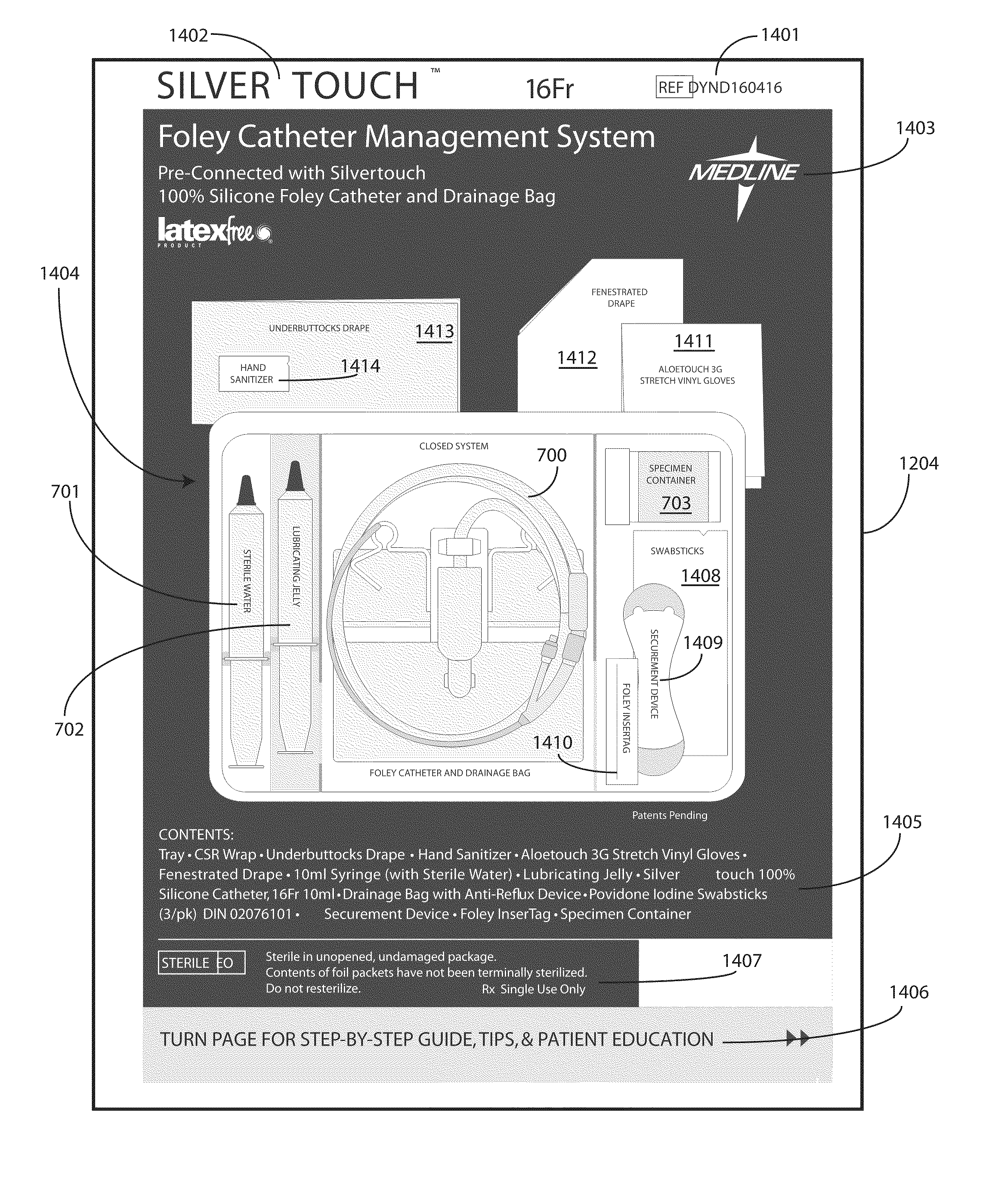
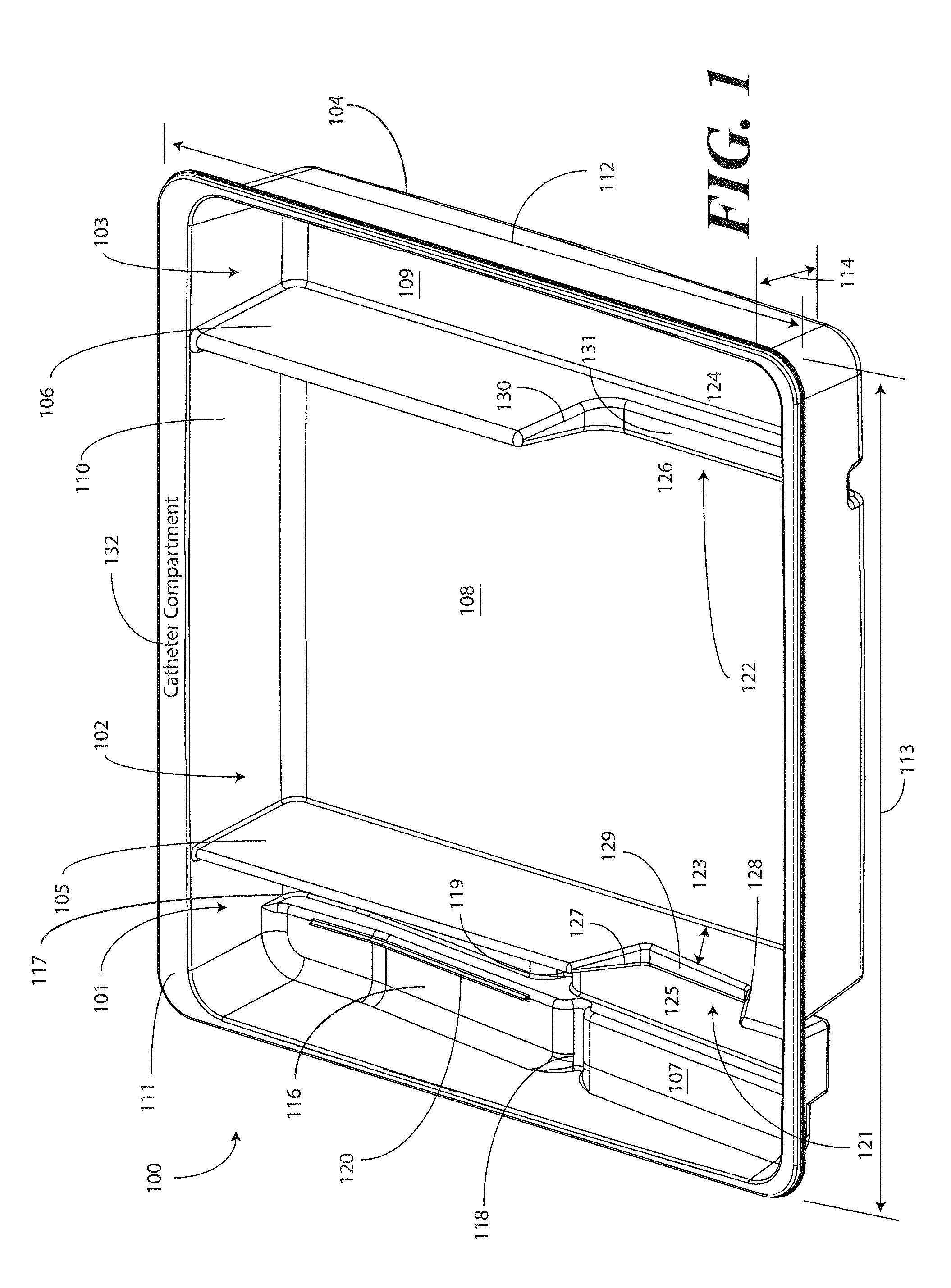
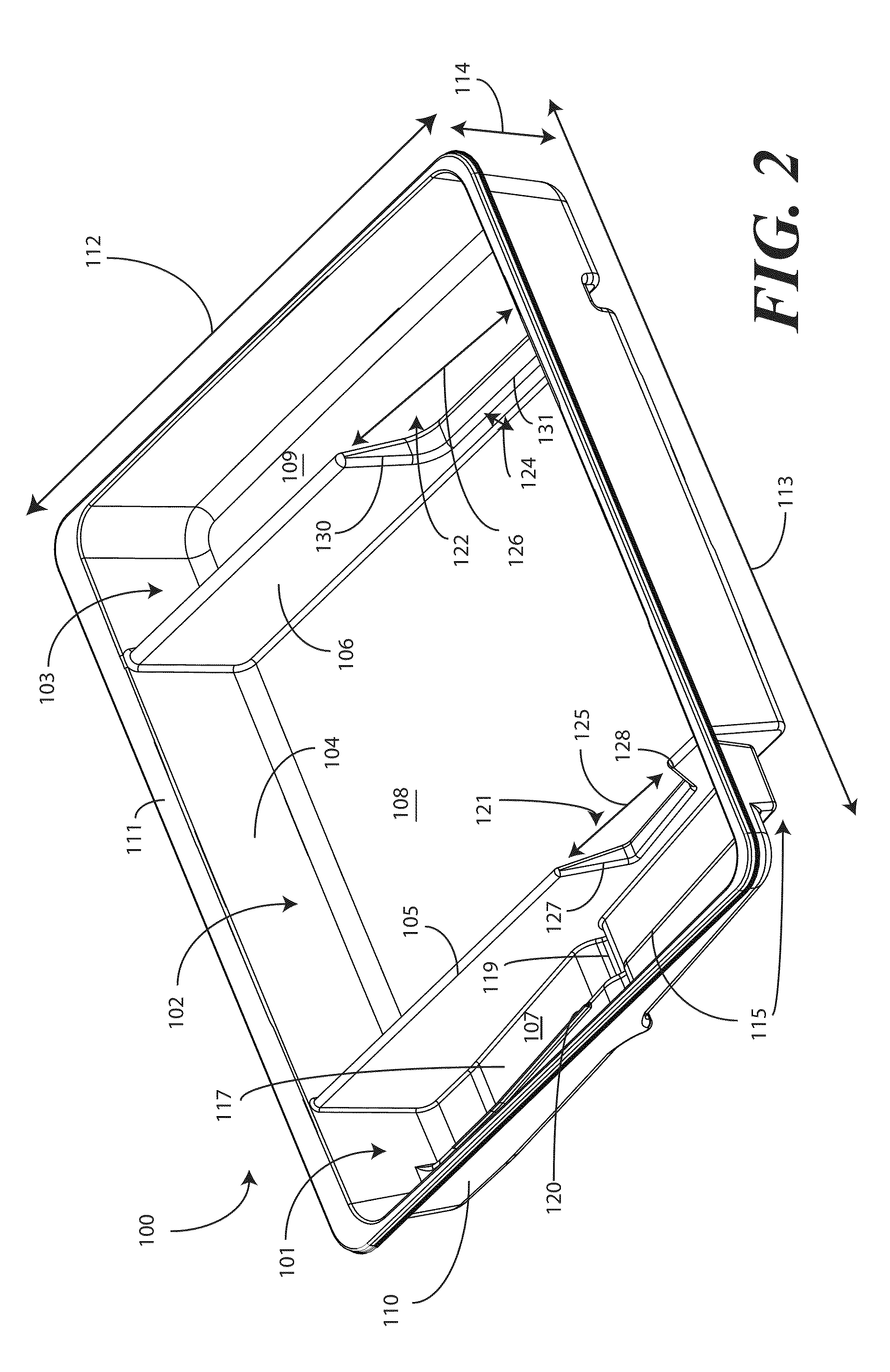
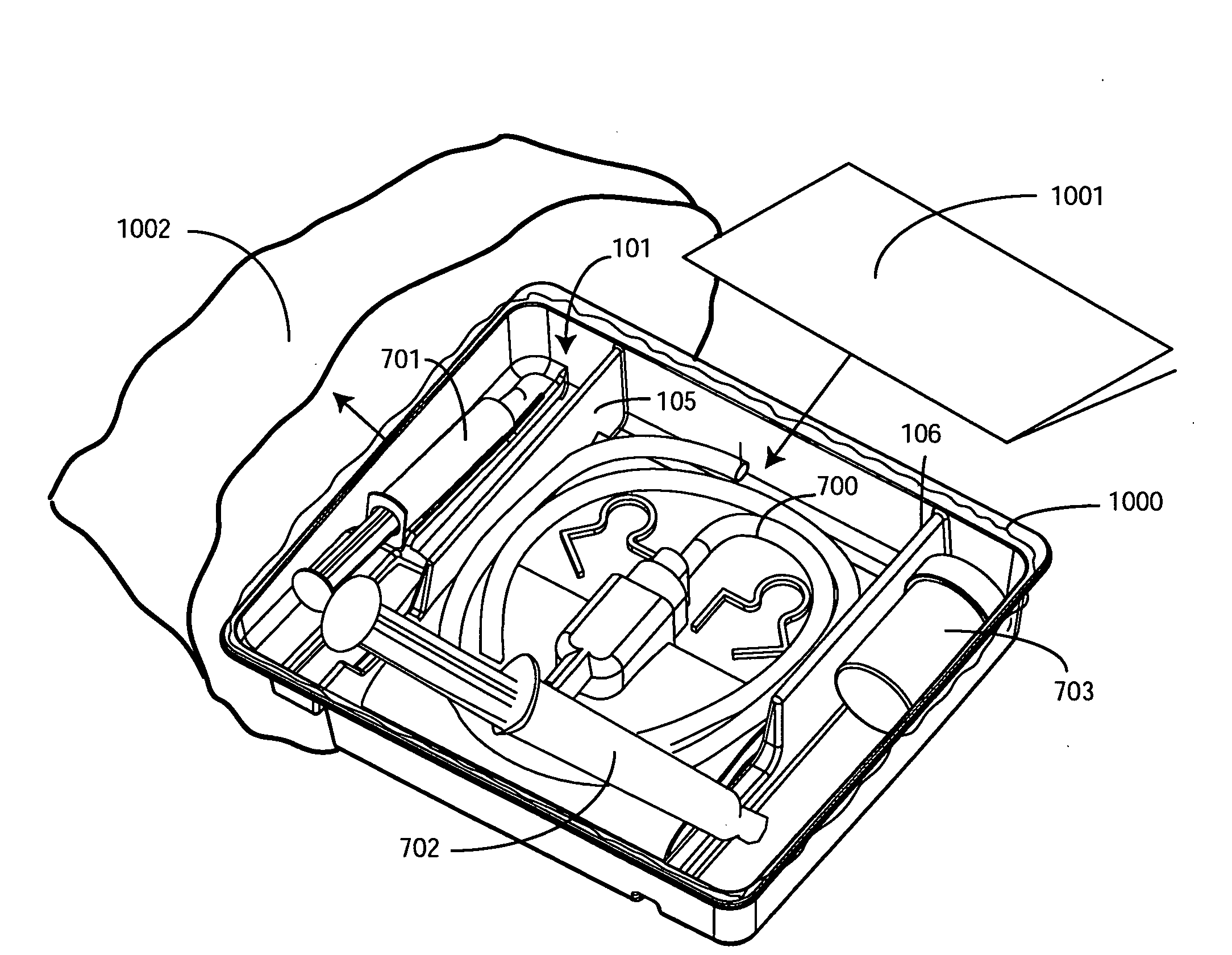
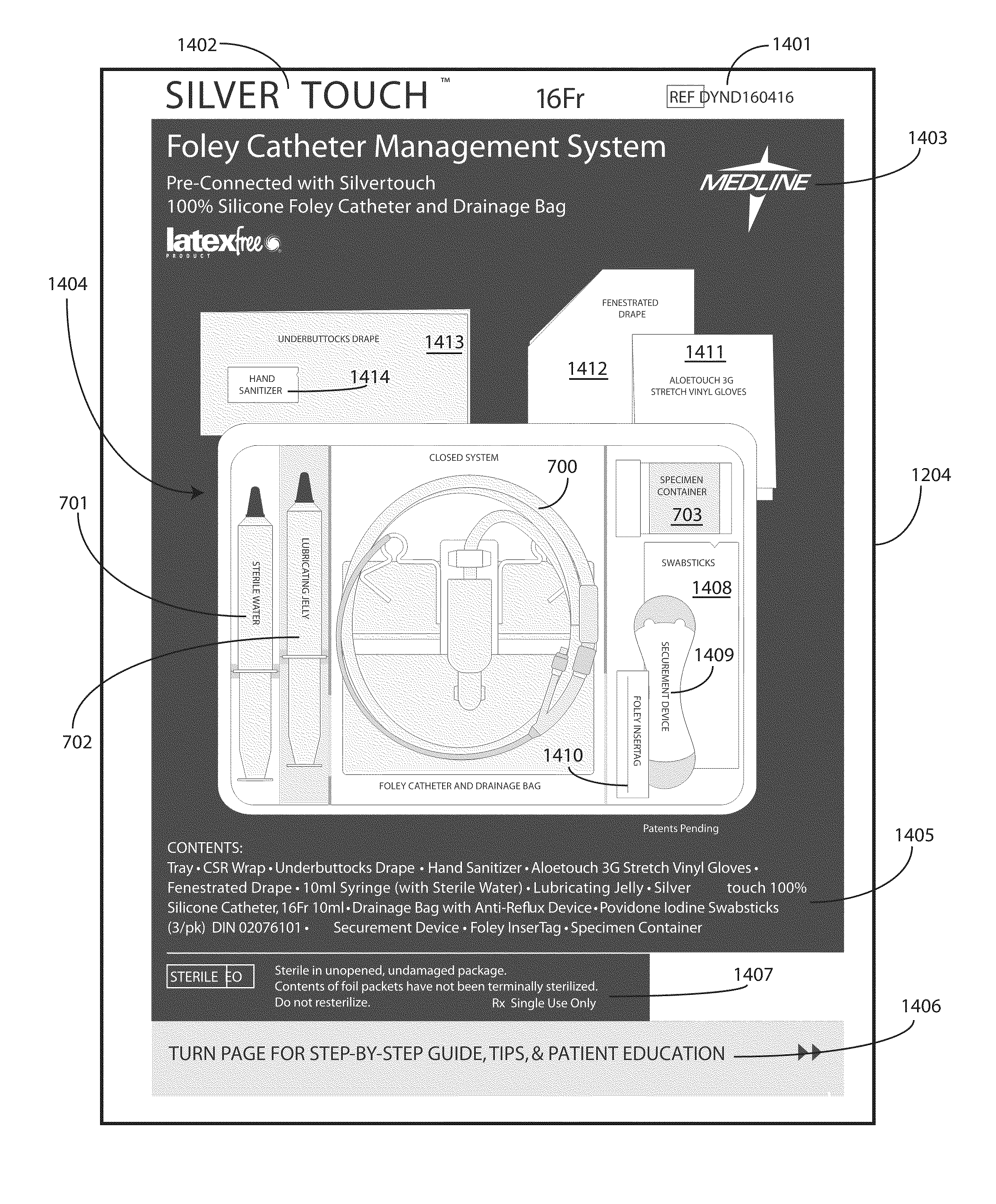
A tray (100) for accommodating a coiled medical device, such as a catheter assembly (700), includes a first compartment (101), a second compartment (102), and a third compartment (103). The catheter assembly (700) and devices associated with a catheterization procedure, such as syringes (701,702) containing sterile water and lubricating jelly and a specimen container (703) can be disposed within the tray. Printed instructions (1001) can be included with the tray (100). When a CSR wrap (1000) is disposed about the tray (100), the printed instructions can be placed atop the CSR wrap (1000) but beneath an outer sterile wrap (1002). The printed instructions (1001) can include a patient portion (1202) that is detachably coupled to a health care services portion (1201) such that it can be taken home with the patient after the procedure.
Owner:MEDLINE IND LP
Fluid processing apparatus
ActiveUS20130037465A1Need is eliminated and minimisedEliminate needDialysis systemsMedical devicesSterile waterEngineering
A machine is provided with a slot to releasably receive and retain a cartridge in which dialysis is effected. The machine is configured for supplying to the cartridge, at a controlled temperature and rate, sterile water for use in haemodialysis and is operable to maintain, in a sterile condition, residual water contained therein after completion of a haemodialysis treatment.
Owner:QUANTA DIALYSIS TECH LTD
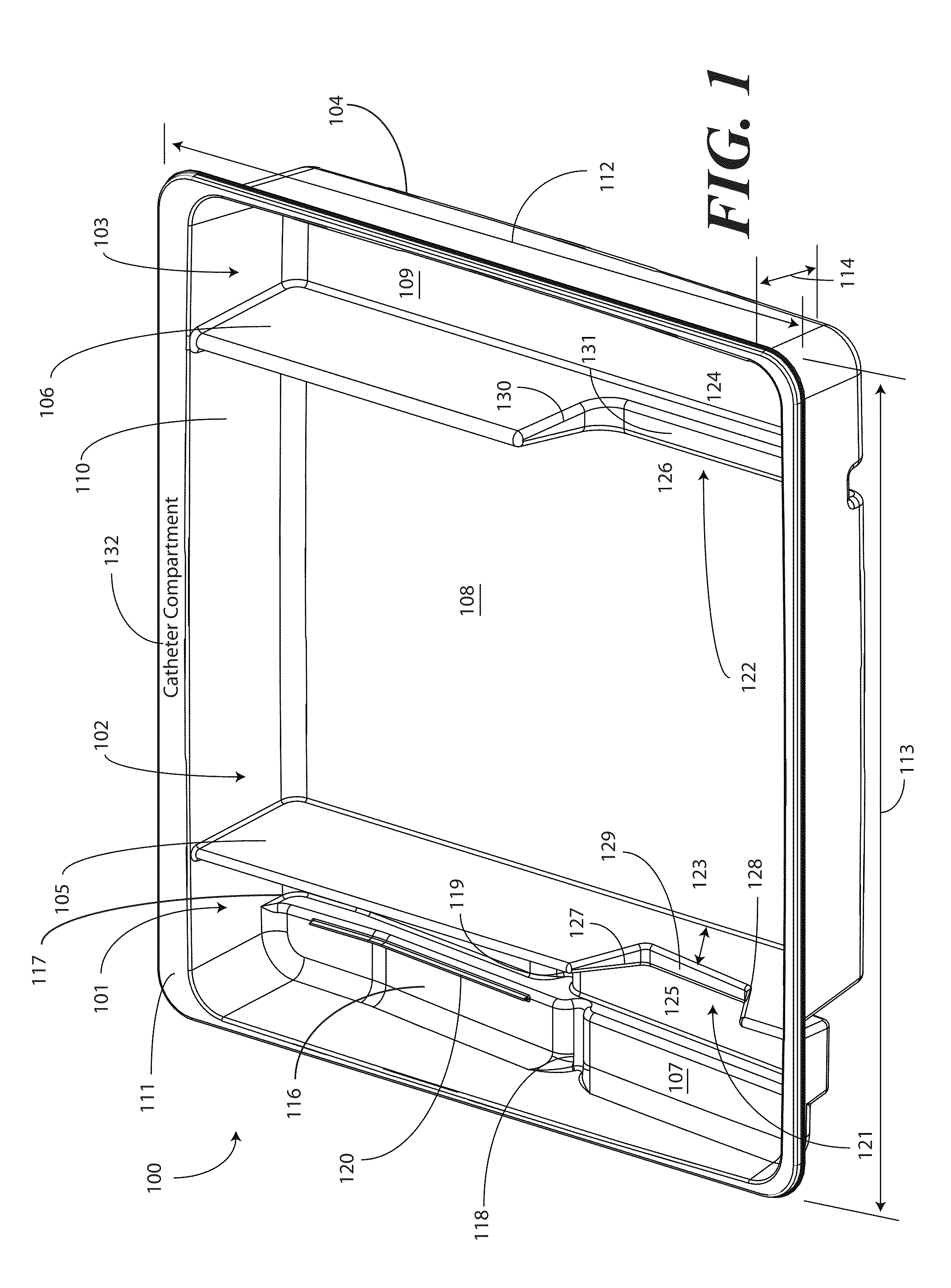
Catheter tray, packaging system, and associated methods
ActiveUS8631935B2Facilitates more effective and simpler deployment of the deviceSurgical furnitureDispensing apparatusSterile waterSpecimen containers
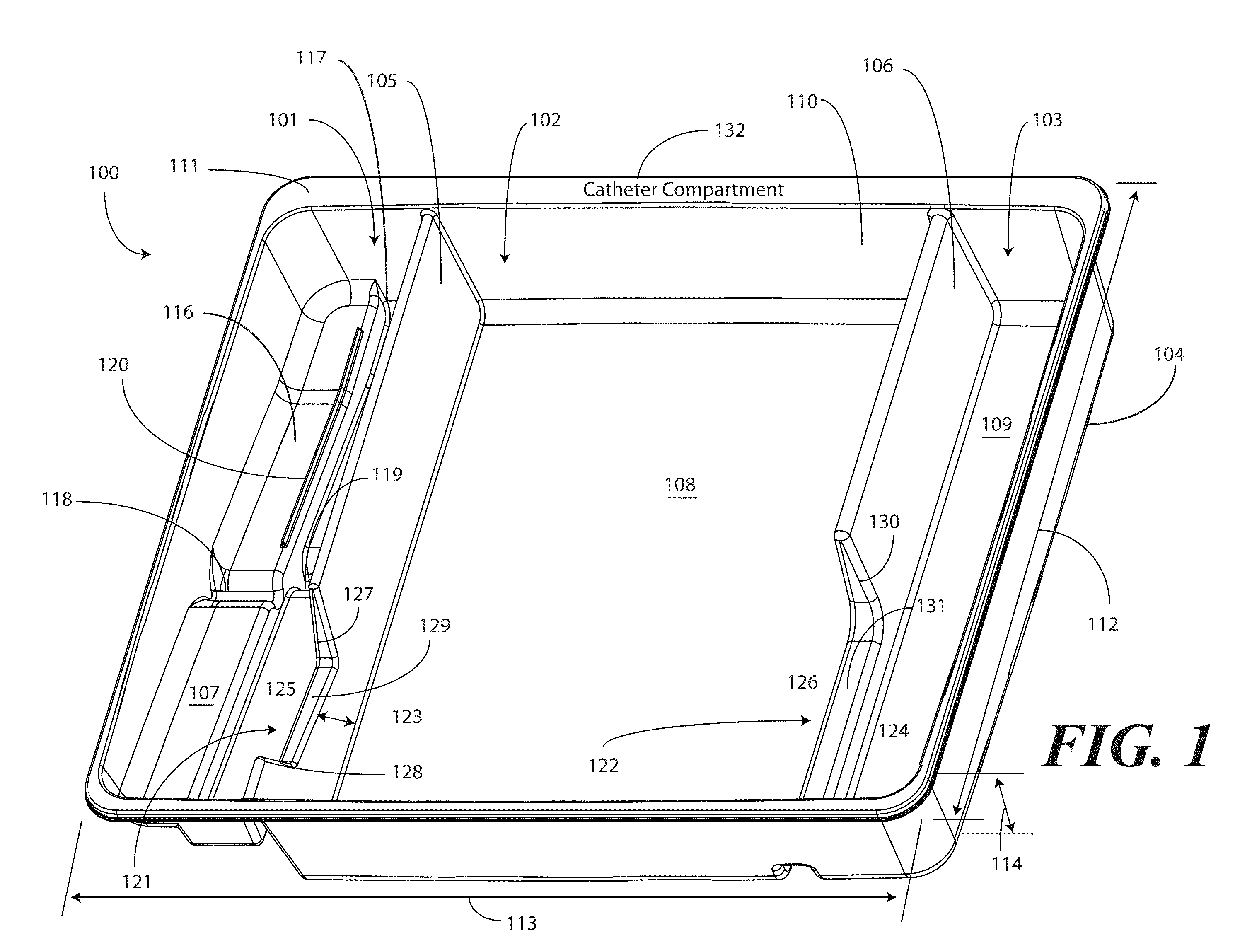
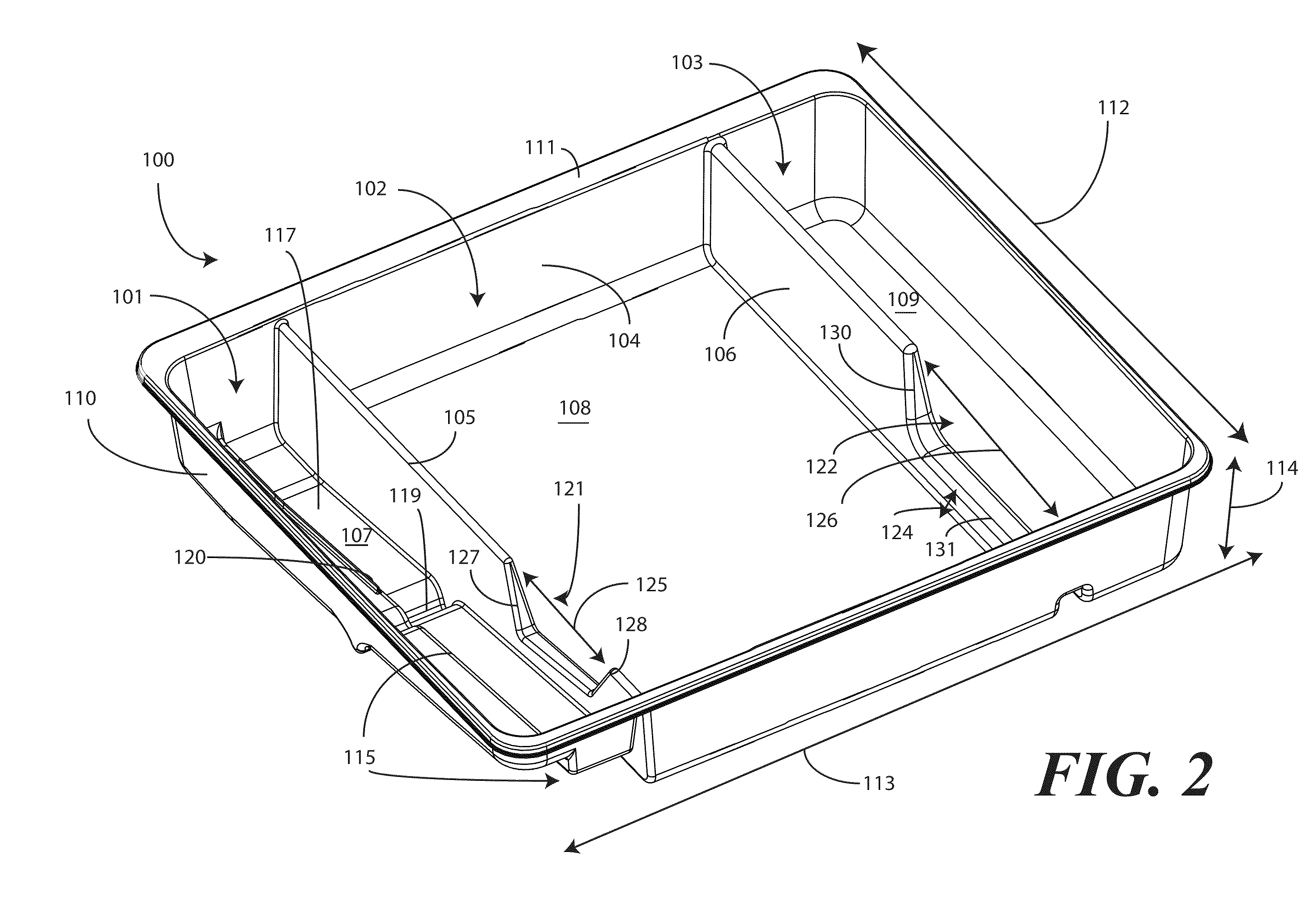
A tray (100) for accommodating a coiled medical device, such as a catheter assembly (700), includes a first compartment (101), a second compartment (102), and a third compartment (103). The catheter assembly (700) and devices associated with a catheterization procedure, such as syringes (701,702) containing sterile water and lubricating jelly and a specimen container (703) can be disposed within the tray. A first barrier (105) and second barrier (106) separate the compartments. The barriers can have openings (121,122) therein to accommodate large syringes or to enable the first compartment (101) to be used as a lubricant applicator for the catheter. The first compartment (101) can include a stair-stepped contour (115) such that the syringes are held at different depths to facilitate ease of use. The various devices can be disposed within the tray (100) in accordance with their order of use in the catheterization procedure.
Owner:MEDLINE IND LP
Catheter tray, packaging system, instruction insert, and associated methods
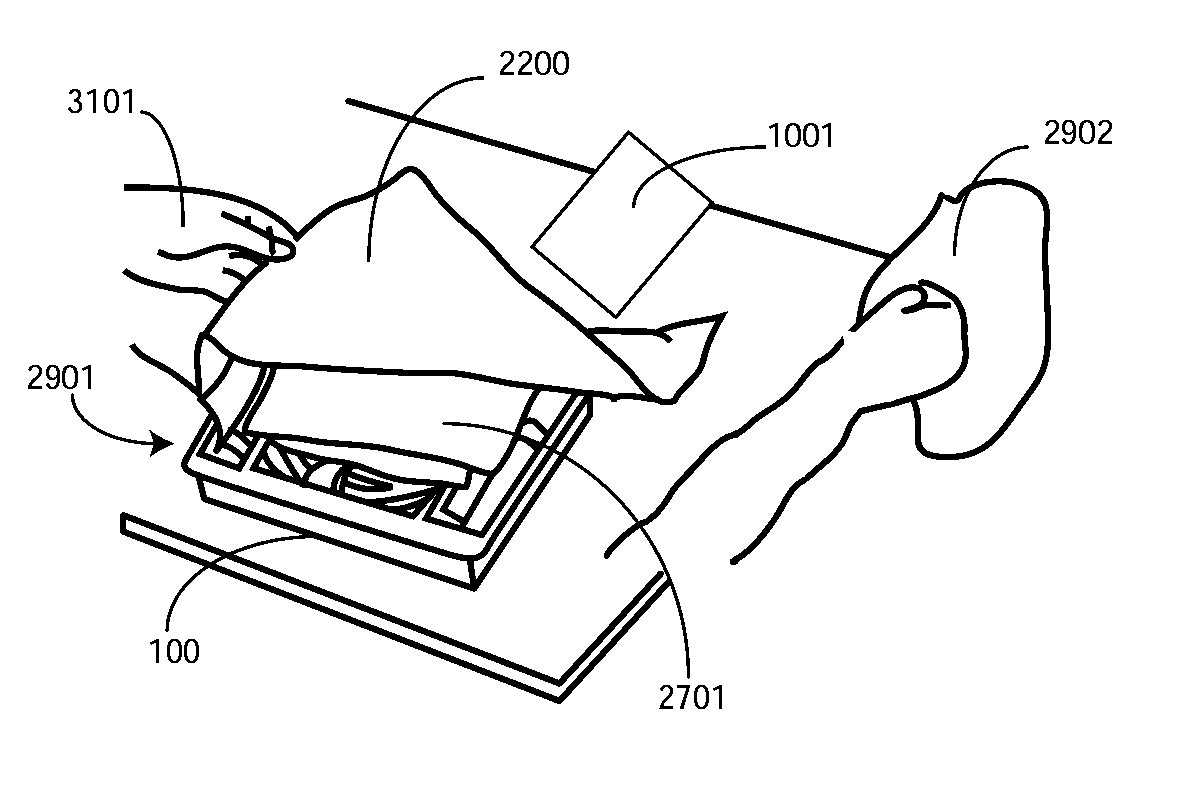
A tray (100) for accommodating a coiled medical device, such as a catheter assembly (700), includes a first compartment (101), a second compartment (102), and a third compartment (103). The catheter assembly (700) and devices associated with a catheterization procedure, such as syringes (701,702) containing sterile water and lubricating jelly and a specimen container (703) can be disposed within the tray. Printed instructions (1001) can be included with the tray (100). One or more layers of wrap material (2200) can be folded about the tray (100) to enclose the tray (100) and other items, such as an additional layer of wrap material (2701), packaged liquid hand sanitizer (2401), and packaged gloves (2402). When a health care services provider (3101) unfolds the wrap material, the same can be used to create a sterile field beneath a patient (3201).
Owner:MEDLINE IND LP
Soft tissue processing
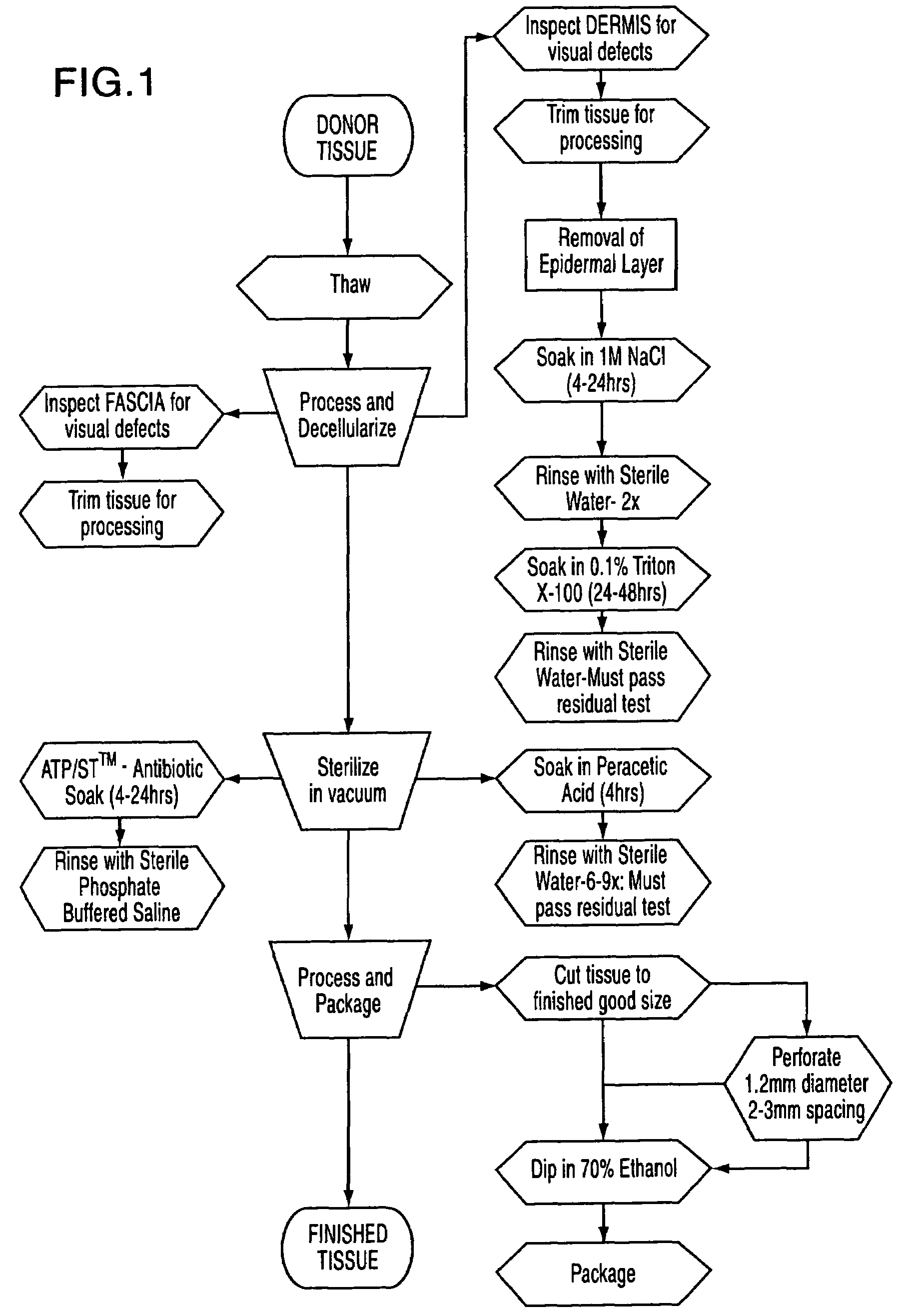
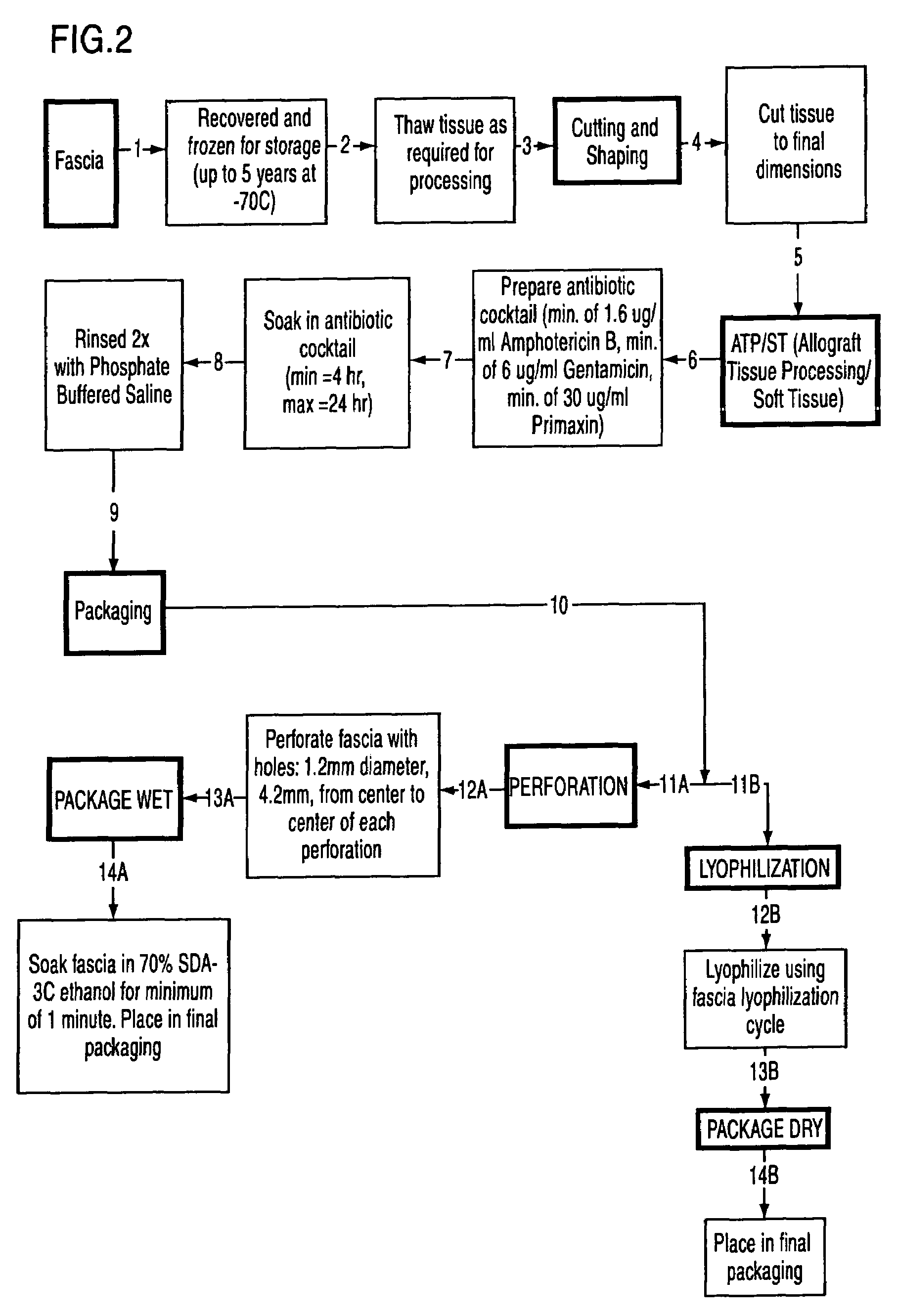
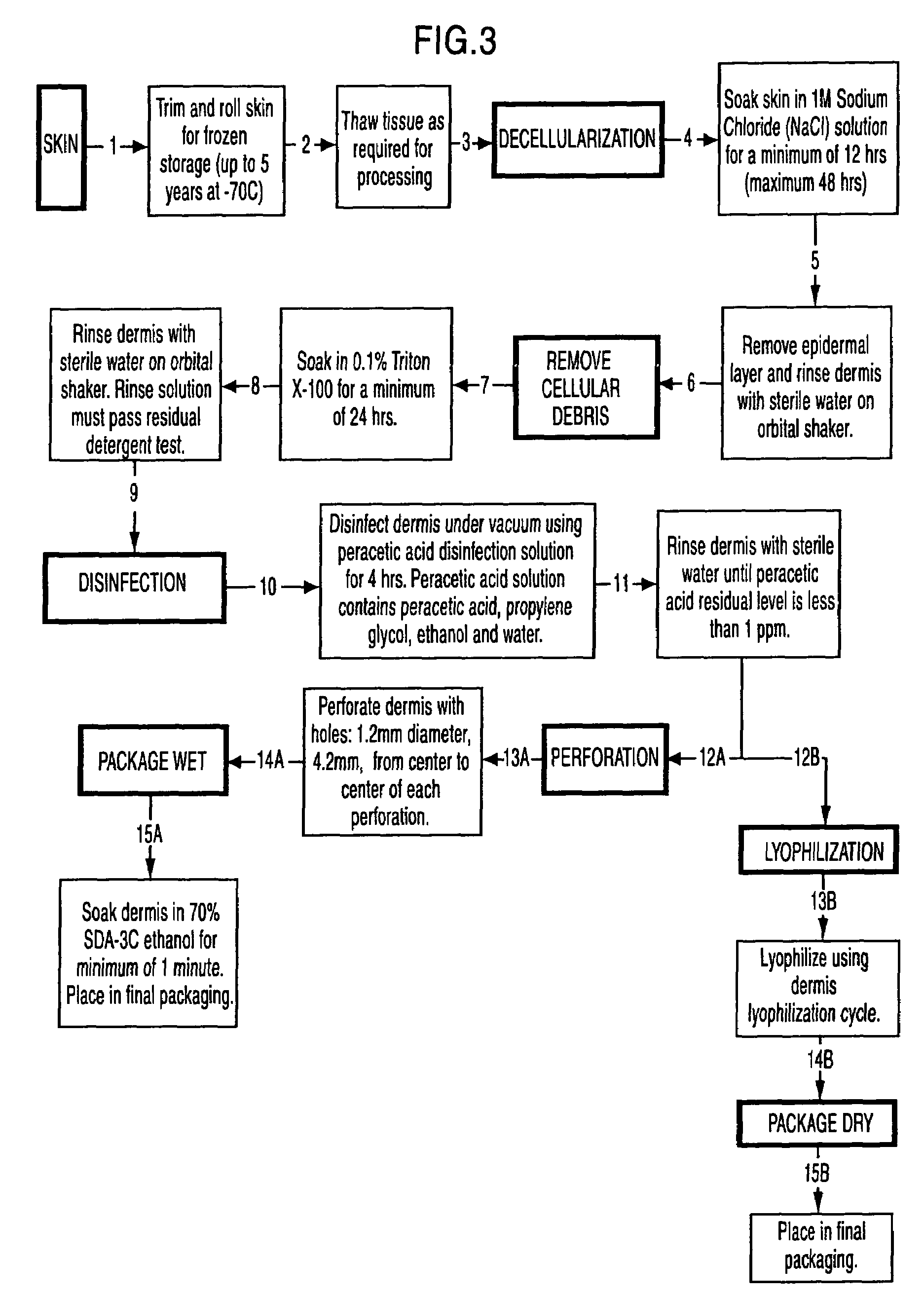
The present invention is a process for preparing soft tissue such as tendons, ligaments, cartilage, fascia, dermis, human valves and human veins for implant in a human and removes cellular components and forms an decellular matrix having as major components collagens and elastins while sterilizing the tissue. The process comprises the following steps:(1) isolating from a suitable donor a desired soft tissue sample of the biological material;(2) processing and decellularizing the soft tissue including inspection for visual defects, trimming and soaking the tissue in a detergent depending on whether the tissue is fascia or dermis and rinsing same with sterile water;(3) sterilizing the soft tissue in a vacuum and soaking the tissue in an antibiotic composition or peracetic acid depending on whether the soft tissue is fascia or dermis and rinsing same;(4) processing the tissue by cutting the tissue to size and perforating the tissue; and(5) dipping the tissue in 70% ethanol and packaging the tissue.
Owner:MUSCULOSKELETAL TRANSPLANT FOUND INC
Catheter tray, packaging system, instruction insert, and associated methods
A tray (100) for accommodating a coiled medical device, such as a catheter assembly (700), includes a first compartment (101), a second compartment (102), and a third compartment (103). The catheter assembly (700) and devices associated with a catheterization procedure, such as syringes (701,702) containing sterile water and lubricating jelly and a specimen container (703) can be disposed within the tray. Printed instructions (1001) can be included with the tray (100). One or more layers of wrap material (2200) can be folded about the tray (100) to enclose the tray (100) and other items, such as an additional layer of wrap material (2701), packaged liquid hand sanitizer (2401), and packaged gloves (2402). When a health care services provider (3101) unfolds the wrap material, the same can be used to create a sterile field beneath a patient (3201).
Owner:MEDLINE IND LP
Catheter Tray, Packaging System, and Associated Methods
ActiveUS20100307942A1Facilitates more effective and simpler deployment of the deviceSurgical furnitureDispensing apparatusSterile waterSpecimen containers
A tray (100) for accommodating a coiled medical device, such as a catheter assembly (700), includes a first compartment (101), a second compartment (102), and a third compartment (103). The catheter assembly (700) and devices associated with a catheterization procedure, such as syringes (701,702) containing sterile water and lubricating jelly and a specimen container (703) can be disposed within the tray. A first barrier (105) and second barrier (106) separate the compartments. The barriers can have openings (121,122) therein to accommodate large syringes or to enable the first compartment (101) to be used as a lubricant applicator for the catheter. The first compartment (101) can include a stair-stepped contour (115) such that the syringes are held at different depths to facilitate ease of use. The various devices can be disposed within the tray (100) in accordance with their order of use in the catheterization procedure.
Owner:MEDLINE IND LP
Pulmonary Rehabilitation Providing Respiratory Assistance by Application of Positive Airway Pressure
InactiveUS20090007911A1Improve mobilityRespiratorsOther heat production devicesPositive airway pressureSterile water
A system for pulmonary rehabilitation by the application of positive airway pressure, characterized in that the system comprises a source of pressurized air, a source of pressurized oxygen, control arrangement for enabling a control of the sources of pressurized air and pressurized oxygen, a source of sterile water, a source of heat, and an output arrangement for providing pressurized and heated air and oxygen to a patient. The system can be rendered portable by use of a mobile casing. Means can be provided for enabling an automatic following of a patient by the casing.
Owner:CLEARY DOREEN +1
Catheter Tray, Packaging System, Instruction Insert, and Associated Methods
A tray (100) for accommodating a coiled medical device, such as a catheter assembly (700), includes a first compartment (101), a second compartment (102), and a third compartment (103). The catheter assembly (700) and devices associated with a catheterization procedure, such as syringes (701,702) containing sterile water and lubricating jelly and a specimen container (703) can be disposed within the tray. Printed instructions (1001) can be included with the tray (100). One or more layers of wrap material (2200) can be folded about the tray (100) to enclose the tray (100) and other items, such as an additional layer of wrap material (2701), packaged liquid hand sanitizer (2401), and packaged gloves (2402). When a health care services provider (3101) unfolds the wrap material, the same can be used to create a sterile field beneath a patient (3201).
Owner:MEDLINE IND LP
Biaxially oriented hollow thermoplastic bodies and improved method for sterilization
InactiveUS7141190B2Less energy transferExcellent heat transfer mediumDomestic articlesHollow articlesShell moldingSterile water
A process of molding thermoplastic preforms into bottles and similar containers wherein pressurized liquids, such as water, are used in the stretching and shaping process instead of pneumatic gases, such as heated air. The result is greater control over the crystallization of the thermoplastic material and economies of scale. Addition of peroxides, or similar materials are used for sterilization can be added to the liquid thereby conditioning the container for immediate filling with sterile product and eliminating the need for an additional sterilization step following completion of the molding step. As an added step, dry, sterile air can be used to vent and dry the container just prior to introduction of product. Also use of liquid, rather than heated air, provides a washing or cleansing of actealdehydes or ethanol, which may be present in the extruded preform, from the finished container.
Owner:HEKAL IHAB M
Biological preservative for fruits and vegetable and its prepn
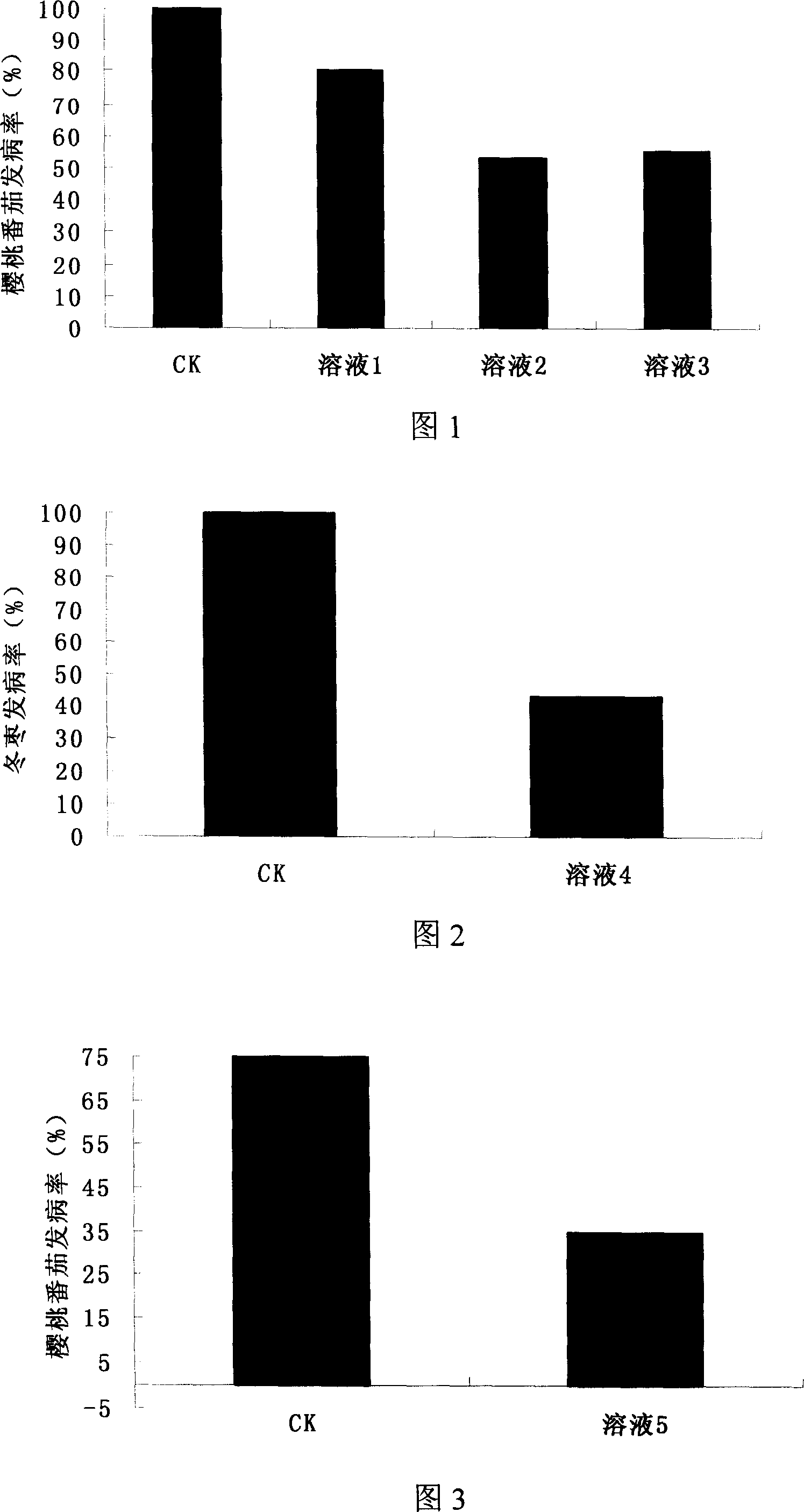
InactiveCN101019572AImprove securityReduce rotFungiFruit and vegetables preservationYeastPreservative
The present invention discloses one kind of biological bactericide for fruits and vegetable and its preparation process. The biological bactericide consists of 10<8>-10<11> CFU / ml concentration Tallman yeast cell suspension 100-1000 ml, and sodium ethyl hydroxyl benzoate 0.6-1.2 g or sodium butyl hydroxyl benzoate 0.6-1.2 g. The preparation process includes the following steps: 1. inoculating the saccharommycete to NYDB culture medium inside a flask, culturing at 26-28 deg.c and 150-200 rpm for 18-30 hr, centrifuging the fermented liquid at 2000-3000 rpm for 5-10 min to collect thallus, twice washing with abacterial water, counting with a blood counting chamber and regulating the concentration of the suspension to 10<8>-10<11> CFU / ml; and 2. mixing the suspension, sodium hydroxyl benzoate and water in the ratio of 100 to 1 to 105-100 to 1 to 106 via stirring.
Owner:ZHEJIANG UNIV
Apparatus for producing sterilized water
InactiveUS7416326B2Easy constructionRestrains fluctuation of pressure affecting feedingFlow mixersMixing methodsChlorine dioxideSterile water
Owner:FAMILY LIFE
Rose paste and preparation technique thereof
InactiveCN101455327APromote dissolutionFacilitated releaseFood preparationPlant ingredientsBiotechnologySterile water
The present invention provides a rose sauce and the preparation process thereof. The rose sauce is produced by novel edible rose through the following steps: cleaning; beating; using 3% citric acid solution to adjust the pH value of slurry to 3.7 to 5.5; based on mass ratio, mixing a slurry obtained by 10 parts of fresh rose through the above-mentioned steps with 8 to 12 parts of white sugar, 4 to 8 parts of grape syrup, 1 part of honey, 0.01 parts of potassium sorbate, putting into a fermentation cylinder, heating to 65 DEG C-85 DEG C for sterilizing 10 to 30 minutes, cooling to 20 DEG C-50 DEG C; according to a proportionality that each g of fresh rose using 0.1-0.2u of cellulase and 0.2-1.0u of tannase , entirely dispersing and dissolving the two enzymes required by 10 parts of rose in 0.5 parts of sterile water; pouring the sterile water in the fermentation cylinder and mixing uniformly, hydrolyzing the mixture in the pot, wherein, the hydrolysis temperature is 20-50 DEG C, the hydrolysis time is 24-240 hours; and then heating to 90 DEG C, heat preservation 5 minutes to kill the enzymes activity, and then obtaining the products after cooling down to room temperature.
Owner:KUNMING UNIV OF SCI & TECH
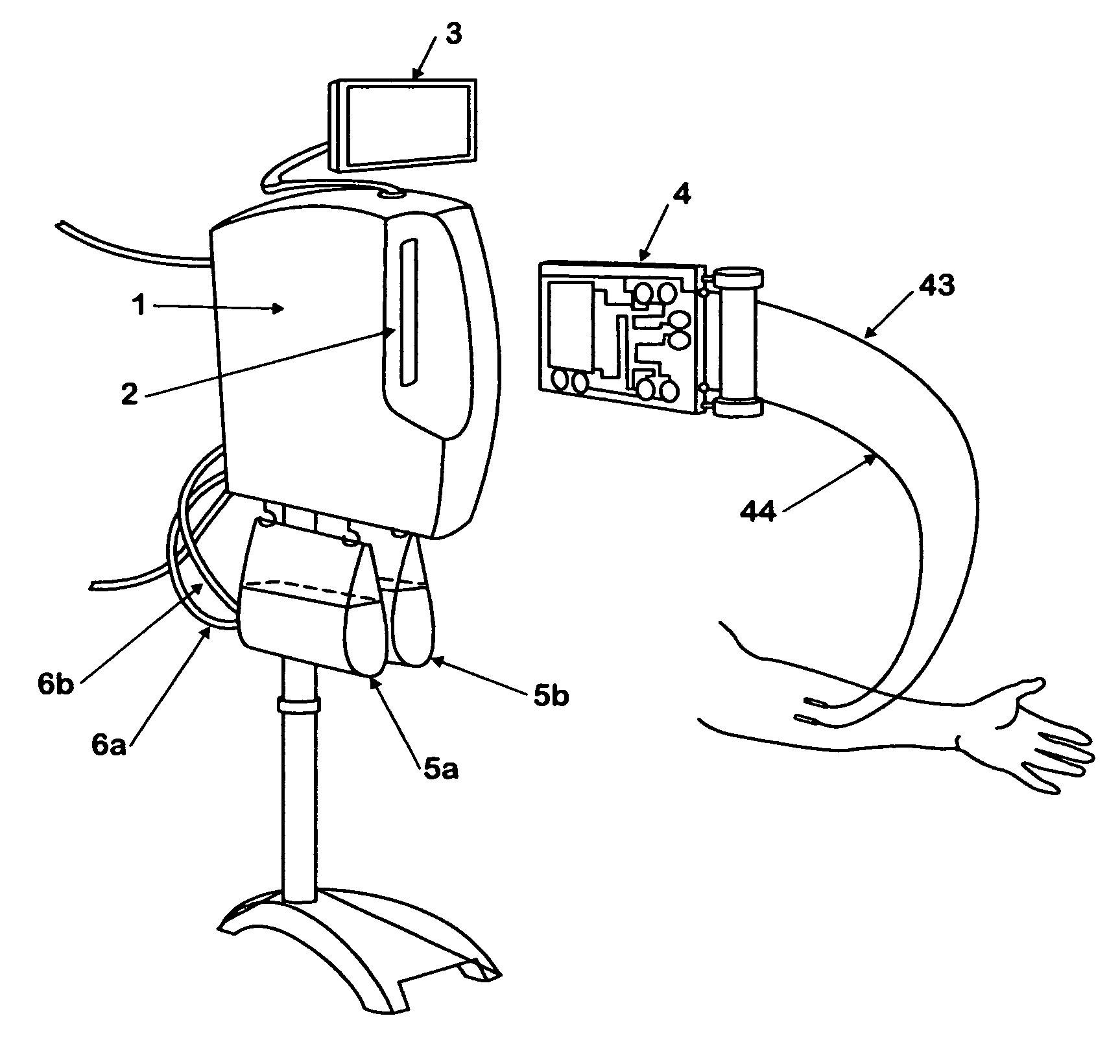
Injection device
InactiveUS20030236500A1Easy to operateReliably trappedFiltering accessoriesTube connectorsSkin surfaceSterile water
An injection device for injecting an injection solution prepared from a powdered medication in sterile water immediately before use which is administered by a syringe. A winged cannula has a hollow needle and a needle holder with wings. A female Luer Lock connector is joined to the winged cannula by a flexible connecting tube and has an insertion region for the connecting tube and a connection region for placing the syringe, filled with the injection solution, against a skin surface, and also has a continuous bore that is continuous from the insertion region to the connection region of the Luer Lock connector. A porous filter element permeable to the injection solution is provided in the continuous bore of the Luer Lock connector.
Owner:SCHEU ROLF RAINER
Composite fresh-keeping method for large yellow croaker
ActiveCN103976005ANo effect on sensory qualityImprove food qualityMeat/fish preservation by coatingMeat/fish preservation using chemicalsAcetic acidSterile water
The invention relates to a fresh-keeping method for aquatic products, specifically to a composite fresh-keeping method for large yellow croaker. The fresh-keeping method mainly comprises a step of modified atmosphere packaging of cultured large yellow croaker with tea polyphenol, nisin and chitosan as biological fresh-keeping agents after sterilization with ozone water. The method comprises the following concrete steps: freezing fresh and alive cultured large yellow croaker to death with crushed ice; treating the frozen cultured large yellow croaker with ozone water for 1 to 8 min; soaking the treated large yellow croaker in a solution for 20 to 40 min, wherein the solution is prepared by fully dissolving tea polyphenol and nisin in sterile water; taking the soaked large yellow croaker in a chitosan (dissolved with 1% acetic acid) solution for 30 s; and carrying out draining and film forming and then successively carrying out modified atmosphere packaging and refrigeration at a temperature of 0 to 4 DEG C. The composite fresh-keeping method has a scientific conception, poses no influence to sensory quality of the cultured large yellow croaker, is safe and harmless to human health, can substantially prolong the shelf life of the cultured large yellow croaker under refrigeration conditions, improves eating quality of large yellow croaker and broadens the marketing range of large yellow croaker.
Owner:ZHEJIANG GONGSHANG UNIVERSITY
Catheter Tray, Packaging System, Instruction Insert, and Associated Methods
A tray (100) for accommodating a coiled medical device, such as a catheter assembly (700), includes a first compartment (101), a second compartment (102), and a third compartment (103). The catheter assembly (700) and devices associated with a catheterization procedure, such as syringes (701,702) containing sterile water and lubricating jelly and a specimen container (703) can be disposed within the tray. Printed instructions (1001) can be included with the tray (100). One or more layers of wrap material (2200) can be folded about the tray (100) to enclose the tray (100) and other items, such as an additional layer of wrap material (2701), packaged liquid hand sanitizer (2401), and packaged gloves (2402). When a health care services provider (3101) unfolds the wrap material, the same can be used to create a sterile field beneath a patient (3201).
Owner:MEDLINE IND LP
Preparation method of agaric ferment
ActiveCN103549385AWide range of usesSimple processMulti-step food processesFood ingredient as taste affecting agentAgaricSterile water
The invention discloses a preparation method of agaric ferment. The preparation method adopts the following components in parts by weight and a technical process; the components are mainly as follows: 2-10 parts of dry agaric, 40-100 parts of sugars and 200-500 parts of sterile water. The technical process is as follows: (1), soaking and re-watering the dry agaric; (2), draining off after flushing; (3), cutting the agaric into pieces; (4), spacing and layering the agaric pieces and the sugars to be placed into a fermentation barrel, wherein adding amount of the sugars is 20-60 parts; and adding 50-200 parts of the sterile water; (5), naturally fermenting under the normal temperature, stirring once every day, and releasing gases for 3-5 times during the period; (6), naturally fermenting for over 45 days to obtain primary fermentation agaric ferment liquor; (7), adding 20-40 parts of sugars and 50-150 parts of the sterile water for secondary natural fermentation for over 30 days after grinding the agaric pieces into a thick liquid; and (8), combining the twice fermentation liquor, homogenizing and standing for over 10 days to obtain the agaric ferment.
Owner:天津市星河系科技有限公司
Aerosolisation system
The invention provides a combination of a micro pump 27 or a micro valve with a vibrating mesh nebuliser 2. This is powered by a controller 3. The controller 3 may have modifications to provide the electrical drive mechanism for the pump 27 in addition to fulfilling the aerosol / nebuliser drive requirements. In one case the system is used for humidifying gas in a ventilator circuit. A humidifying agent (sterile water or sterile saline) is aerosolised and then delivered to a ventilator circuit 100 coupled to the respiratory system of a patient.
Owner:STAMFORD DEVICES LTD
Method for directly seedling dendrobe seedlings by using plastic greenhouse
ActiveCN103004445AImprove seedling rateLow costSeed and root treatmentClimate change adaptationDiseaseSymbiotic bacteria
A method for directly seedling dendrobe seedlings by using a plastic greenhouse comprises the following steps: constructing a greenhouse; making a seedling bed; preparing a base material and performing sterilization, which can be realized by mixing pine barks and sheep manure, filtering the pine barks and the sheep manure through a screen after completely fermenting the pine barks and the sheep manure, soaking the pine barks and the sheep manure by using carbendazim, spreading the pine barks and the sheep manure on the seedling bed, placing the pine barks and the sheep manure on the seedling bed for 5-10 days, and controlling the water content; inoculating the symbiotic bacteria, which can be realized by taking a two years old base material of the dendrobe and uniformly scattering the two years old substrate on the seedling bed on which a new substrate is laid; pre-treating seeds, which can be realized by completely moisturizing the seeds by using sterile water containing naphthyl acetic acid; sterilizing the seeds in the greenhouse, which can be realized by fumigating every cubic meter of seeds by using 4 g mushroom protector, fumigating the seeds for one time before sowing, and fumigating the seeds every half a month in the future; scattering, which can be realized by after the seeds are scattered and before the seedlings are transplanted, controlling temperature in the greenhouse, water content of the seedling bed and air humidity, treating the seeds by weak lights within the first month of the scattering, and gradually enhancing illumination after budding; managing water, fertilizers and diseases of the seedlings; and transplanting the seedlings. The method for directly seedling the dendrobe seedlings by using the plastic greenhouse, provided by the invention has the advantages that as the dendrobe are transplanted after directly becoming to the seedlings, the seedling breeding cost is effectively reduced, the seedling rate of the seeds is more than 90 percent, and the transplanting survival rate of the seedlings is 100 percent.
Owner:戴亚峰
Fresh-keeping method for fresh wet noodle
The invention relates to a method for keeping raw wet noodle fresh, which includes the steps that: (1) before dough making, ultraviolet lamp is switched on to irradiate the dough for 30min to 1.5h; (2)during the dough making, purified water or sterile water with an amount controlled between 25 to 32 percent is added to the dough and at least one of the three antistaling agents, salt, sodium dehydroacetate and calcium propionate in proportions respectively of 2 to 7 percent, 0.02 to 0.05 percent and 0.1 to 0.3 percent, is added to the water; additionally, 0.1 to 0.3 percent of phosphate, 0.1 to 0.3 percent of sucrose ester, 0 to 0.3 percent of guar gum and 0 to 0.3 percent of edible alkali as well as 5 to 10 percent of wheat gluten powder are added; (3) sterilization treatment is applied to cooking equipment, strip press and the like and noodle strip after pressing is thermally insulated and cooked; (4) asepsis treatment is applied to wet noodle strip and the moisture of the product is controlled between 18 to 28 percent; (5) sterilization is applied to the product before packaging. The method of the invention does not affect the flavor and color of products and the raw wet noodle is characterized by bright appearance, cream white color and strong tenacity and has a shelf life of two months at normal temperature and as long as six months at the temperature below 10 DEG C (cold storage).
Owner:CENTRAL SOUTH UNIVERSITY OF FORESTRY AND TECHNOLOGY
Method for Obtaining Sterile Human Amniotic Fluid and Uses Thereof
ActiveUS20150025366A1Improve stabilityFacilitating and minimizing costSurgeryMedical devicesWound healingMedicine
Provided herein is a method for sterilely filtering amniotic fluid from selected caesarean sections of an individual. The amniotic fluid is first centrifuged at 5,000 to 10,000 rpm for 30 to 60 minutes and filtered through filters with about 5 to about 10 μm pore size. Next, the fluid is sequentially filtered through a series of membrane filters with the pore sizes 1 μm and 0.45 or / and 0.2 μm. The filtrate is then aseptically transferred to and sealed in syringes or vials. The fluid is subsequently lyophilized to obtain the lyophilisate of amniotic fluid. Amniotic fluid is reconstituted by adding sterile water to the lyophilisate, and the reconstituted fluid is used for wound healing, cosmetic, orthopedic or ophthalmic applications, particularly for the treatment of dry eyes.
Owner:MAM HLDG OF WEST FLORIDA L L C
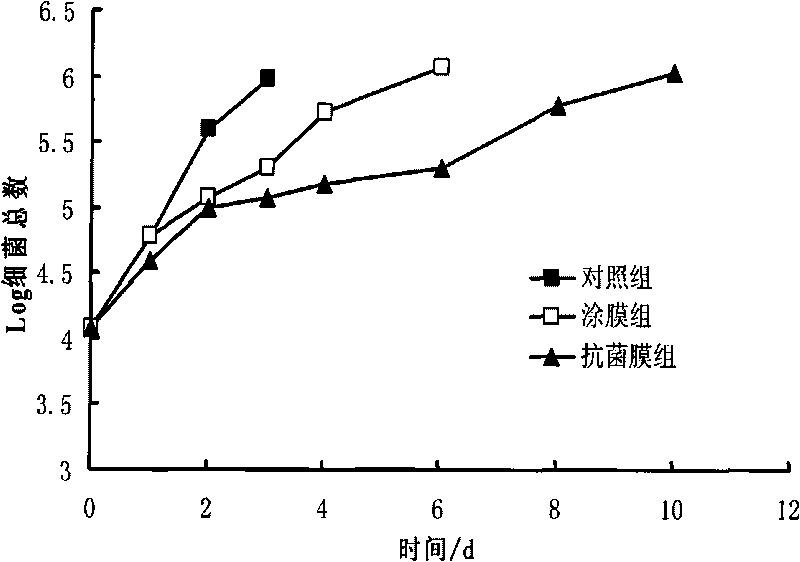
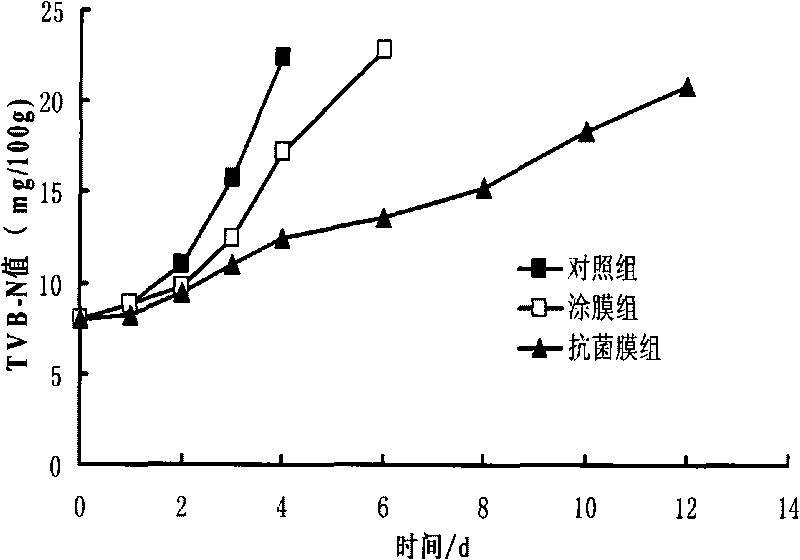
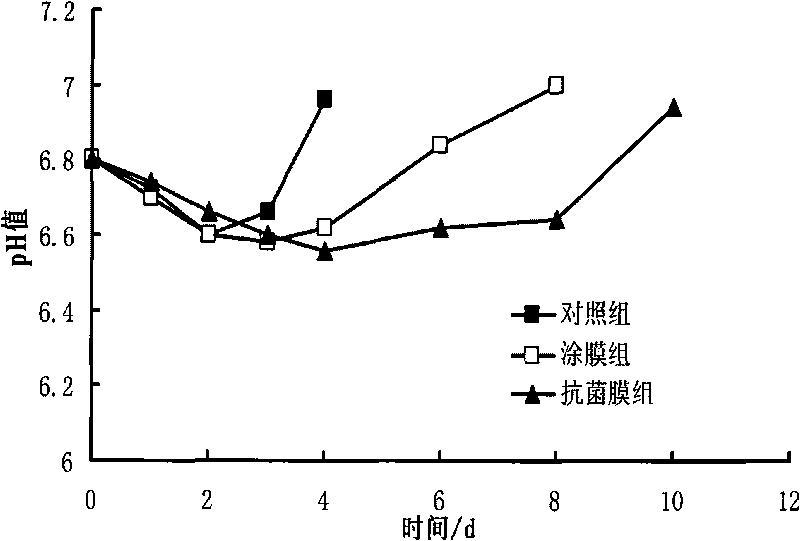
Method for preparing novel ocean lysozyme antibiotic preservative coated film
InactiveCN101703093AMeat/fish preservation by coatingMeat/fish preservation using chemicalsPreservativeGlycerol
The invention relates to a method for preparing a novel ocean lysozyme antibiotic preservative coated film. The method is characterized by comprising the following steps of: preparing solution of sodium alginate by dissolving sodium alginate and glycerol in sterile water; adding a compound preservative formed by cean lysozyme, potassium sorbate and tea polyphenol in the solution of sodium alginate to prepare solution of antibiotic film; and colloidizing the solution of antibiotic film in 4 to 6 weight percent CaC12 solution to obtain the film. The method has the advantages of simple process, low cost, high performance-to-price ratio, good preservative effect, safety and wide application to freshness keeping of various aquatic products.
Owner:青岛华美加生物科技有限公司
Complex of organic medicines and beta-cyclodextrin derivatives and its preparing process
InactiveUS20050215520A1Fast dissolutionImprove stabilityBiocideNervous disorderSimple Organic CompoundsOrganic solvent
The present invention relates to a process of preparing a water-soluble complex of water-insoluble or sparingly-soluble organic medicines and beta-cyclodextrin derivatives: a. dissolving successively or simultaneously the organic medicines and the beta-cyclodextrin derivatives in organic solvent(s) at certain mole ratio or mass ratio in the presence of suitable amount of water; b. removing the organic solvent from the solution of the step a to obtain aqueous solution of the organic medicine and beta-cyclodextrin derivative; and c. removing water from the aqueous solution to obtain the complex of the organic medicine and beta-cyclodextrin derivative. The invention also provides a sterile water-soluble complex of water-insoluble or sparingly-soluble organic medicines and beta-cyclodextrin derivatives. A fully-water-soluble complex can be prepared from any water-insoluble or sparingly-soluble organic medicines or other organic compounds according to the present invention. Thus, appreciably convenient means are provided for industrial uses of such organic medicine preparations and of other organic compounds.
Owner:ADVANCED LIUS PHARMATECH LLC
Preparation method of virus-free seedlings of sweet potato
ActiveCN104067821AEarly germinationEasy to stripPlant tissue cultureHorticulture methodsBudSomatic cell
The invention provides a preparation method of virus-free seedlings of sweet potato. The method comprises the following steps: placing sweet potato blocks that are exposed under the sun and matured banana together for 3 to 5 days; planting the sweet potatoes in sandy soil to accelerate the germination in a culture box; setting relatively high temperature; picking 1-2cm top end of the seedling based on the length; cutting off the visual leaves; disinfecting the surface; rinsing with sterile water; picking off stem tip with 1 to 2 leaf primordium; inoculating to the corresponding culture medium to respectively induce differentiation of adventitious buds and generation of somatic embryogenesis; performing virus detection to a regeneration plant; performing subculture for the virus-free seedling; hardening-seedling; directly planting into a plastic greenhouse; performing flood irrigation with more water; preventing direct exposing under sun at noon in the first two weeks after transplanting; applying urea three weeks after transplanting; watering to obtain virus-free seedlings. According to the preparation method of the virus-free seedlings of sweet potato, the virus-free test-tube plantlets are acclimated and then directly planted into the plastic greenhouse, thus the survival rate is obviously raised, and the operation processes are decreased; the process of acclimating in an acclimating room is saved, and as a result, labor, materials and property are saved.
Owner:QINGDAO AGRI UNIV
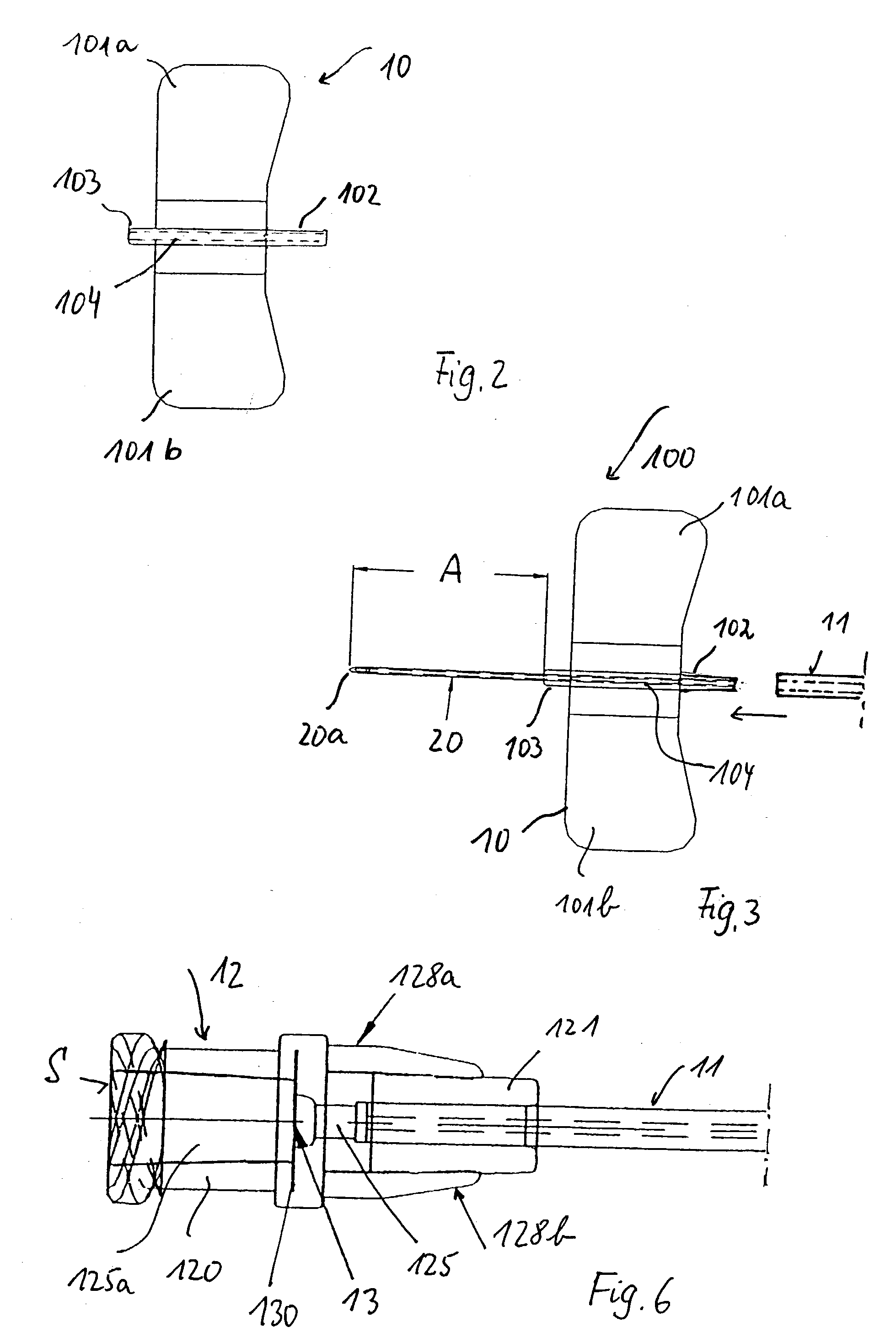
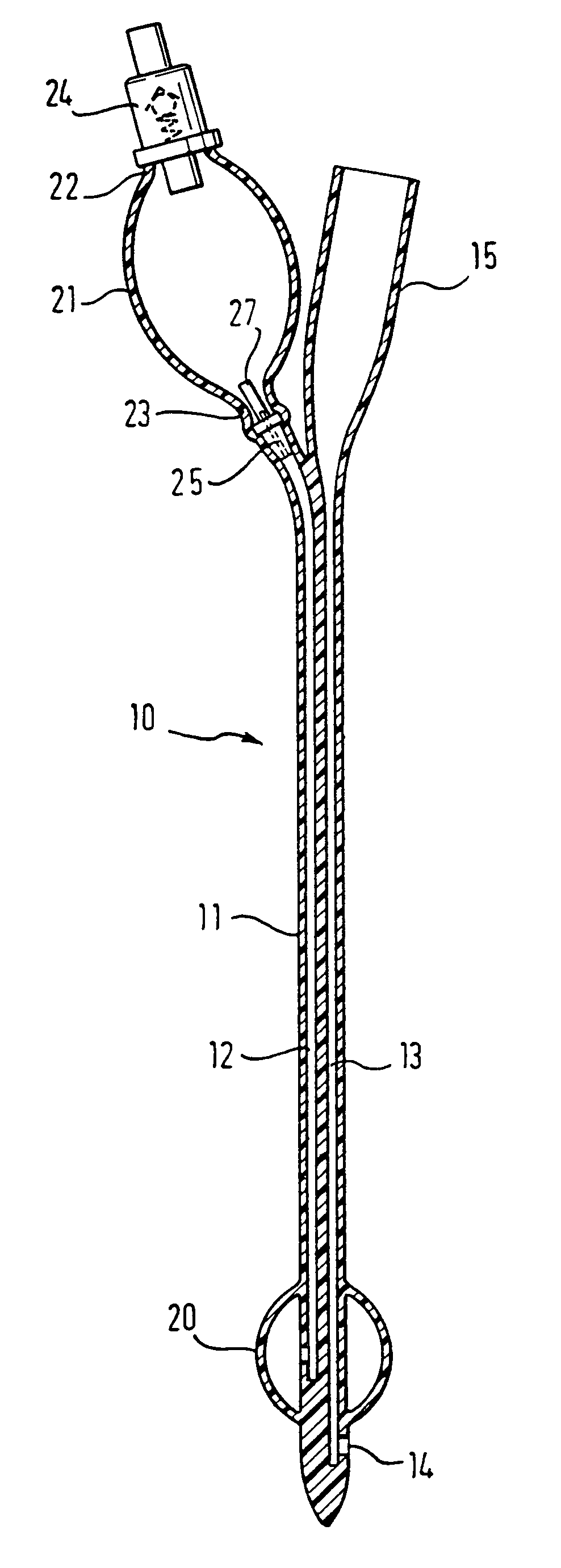
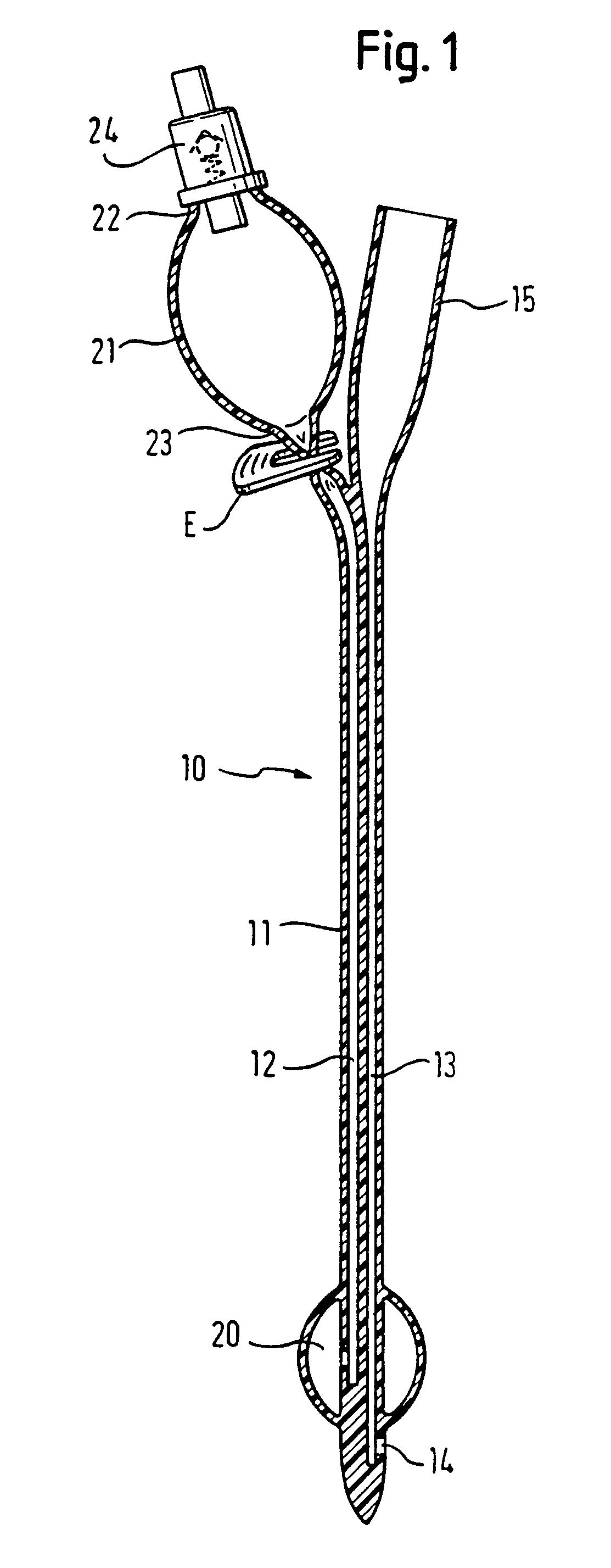
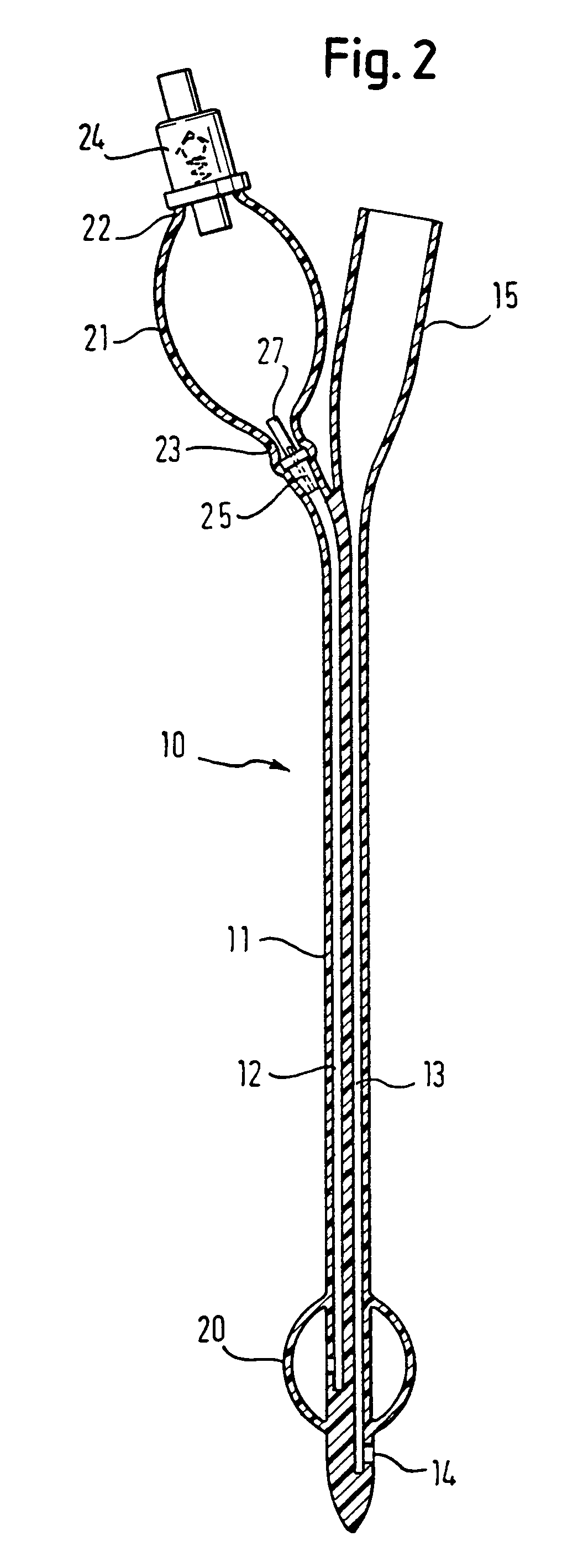
Medical device with elastomeric bulb
In a pre-filled Foley catheter for urine drainage, the conventional clip for releasing sterile water from a bulb (21) at the proximal end of the catheter, to fill the anchor bulb (20) at the distal end of the device, is replaced by a plug (25) which has an annular portion connected to a thin stem (27) by a circle of weakness. Snapping the stem (27) from the annulus provides a tactile signal through the opaque latex lumen that the catheter has been actuated. There is no separate clip to be disposed of. The interface between the latex and the plug remains undisturbed. Further improvement in the shelf-life and convenience of use of the catheter is delivered by the use of a sleeve, which can be of shrink-wrap material, around the bulb (21) and optionally also around the drain coupling (15) of the catheter. Apparatus for placing the plug and the sleeve is also described.
Owner:CR BARD INC
Broad-spectrum bioactive composite foliar fertilizer and its preparation method
InactiveCN102464502AGuaranteed stabilityImprove the durability of fertilizer effectOrganic fertilisersSodium acetateDisease
The invention relates to a broad-spectrum bioactive composite foliar fertilizer and its preparation method. The preparation method of the broad-spectrum bioactive composite foliar fertilizer comprises: (by weight of solid substances) mixing 14.2% of potassium dihydrogen phosphate, 14.2% of urea, 17.7% of brown sugar, 30% of composite amino acid powder, 7.1% of salt, 9.1% of fulvic acid, 0.4% of sodium acetate, 0.2% of magnesium sulfate, 7.6% of trace elements, and molasses with a concentration of 2.5ml / L, a composite liquid strain of equivalent lactic acid bacteria, yeasts, actinomycetes, aspergilli and phosphorus bacteria and with a concentration of 10ml / L, as well as sterile water at a temperature of 35DEG C, and conducting microaerobic fermentation for 192-288h, then adding photosynthetic bacteria with a concentration of 1ml / L for stirring, leaving the mixture to 48-96h of illumination, then adjusting the PH value, thus obtaining a bioactive composite foliar fertilizer. The bioactive composite foliar fertilizer of the invention can promote crop photosynthesis, activate the activity of each enzyme, enhance disease prevention and antibacterial properties of plants, and improve the stress resistance of plants. Characterized by no pollution, no public hazard, lasting fertilizer effect, the fertilizer of the invention can strengthen seedlings, resist diseases, enhance output, improve agricultural product quality, and bring products to market about 7-15 days earlier, thus improving agricultural economic benefits.
Owner:江苏邦德生物科技发展有限公司
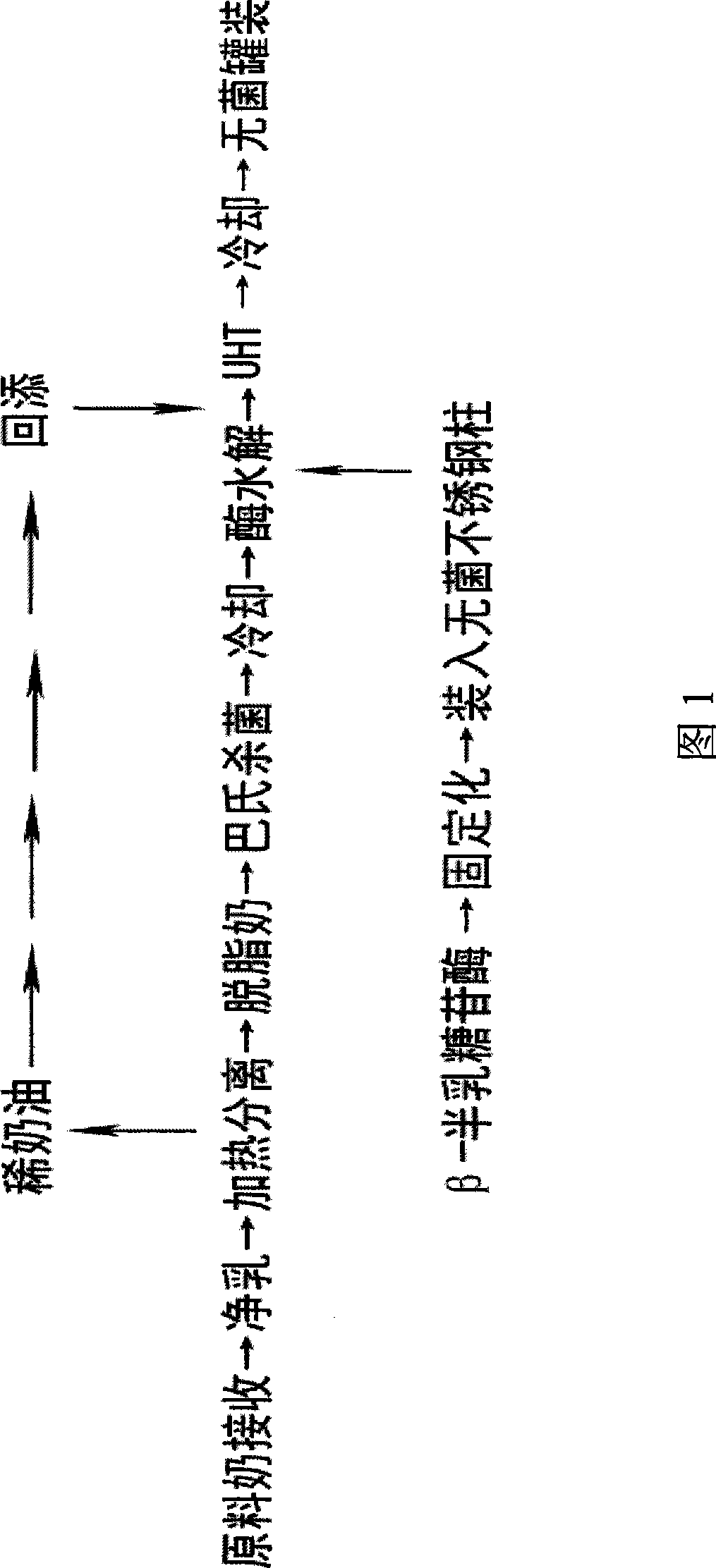
Production method of milk rich in galacto-oligosaccharides
The invention relates to a production method used for milk rich in galacto-oligosaccharide, comprising the steps as follows: heating milk, separating fat to get skim milk, pasteurizing, cooling, hydrolyzing by immobilizing Beta-galactosidase, UHT sterilizing, cooling and packaging. The carrier of the immobilized Beta-galactosidase is granular carrier with hydrazide group; immobilization method of the Beta-galactosidase comprises the steps as follows: the grains with the hydrazide group are added in glutaraldehyde and statically arranged for 0.5-1 hours; glutaraldehyde which is residual on the activation carrier is washed away by aseptic water; subsequently, Beta-galactosidase is added and statically arranged for 2-4 hours; the residual Beta-galactosidase is washed away by the aseptic water, thus gaining the immobilized Beta-galactosidase. The method greatly reduces the content of the lactose in the milk, meanwhile, the method generates galacto-oligosaccharide of high content; therefore, the method not only solves the problem of lactose intolerance but also adds galacto-oligosaccharide bifidus factor in the milk.
Owner:INNER MONGOLIA MENGNIU DAIRY IND (GRP) CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com