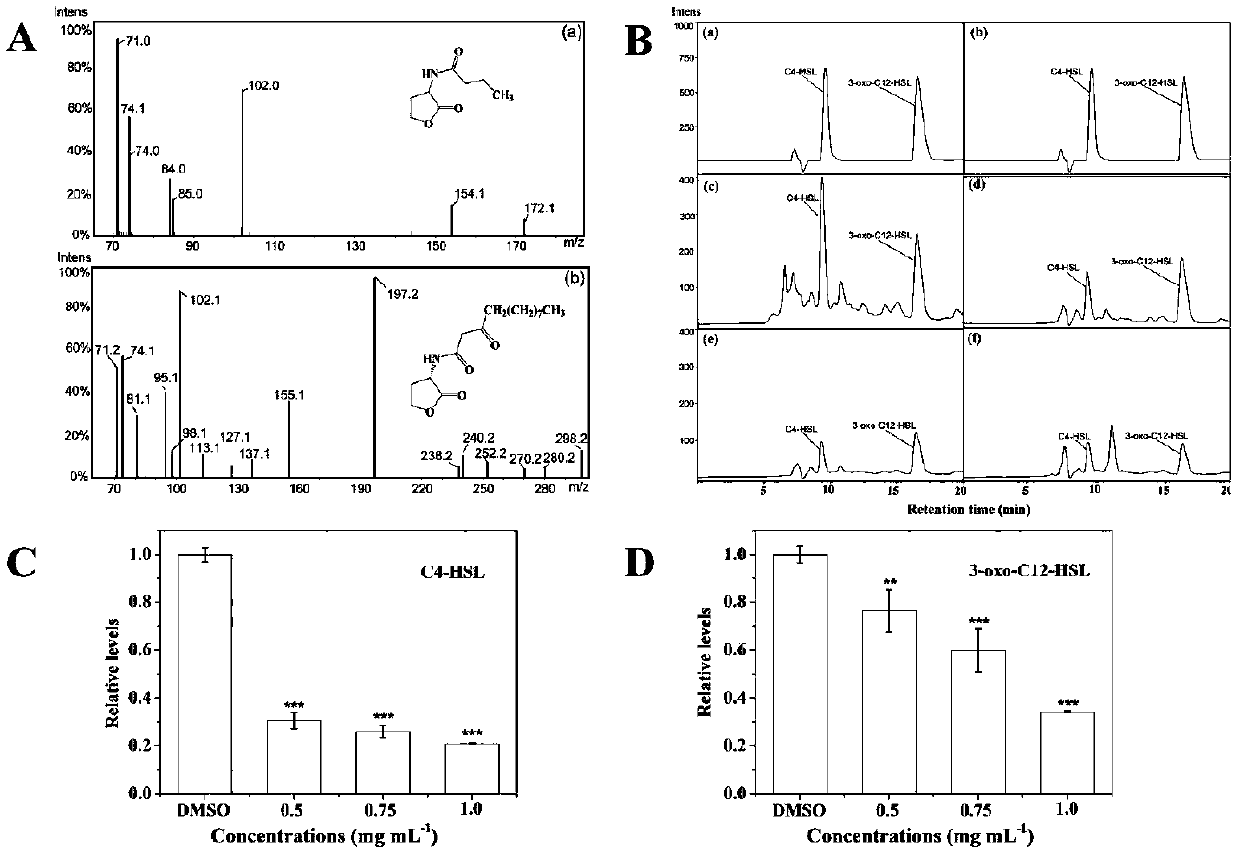
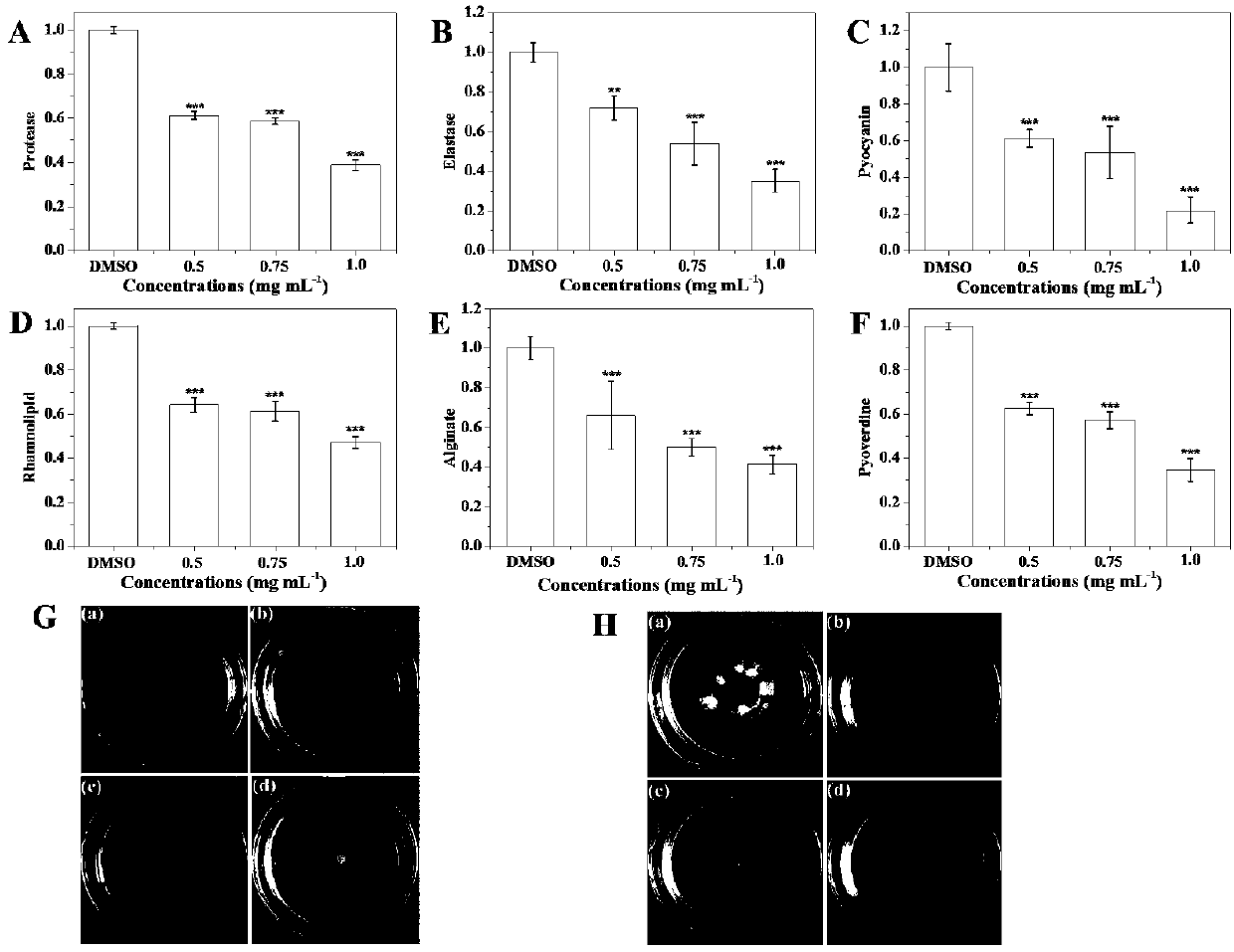
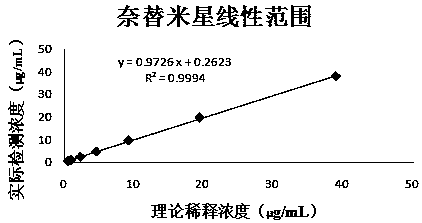
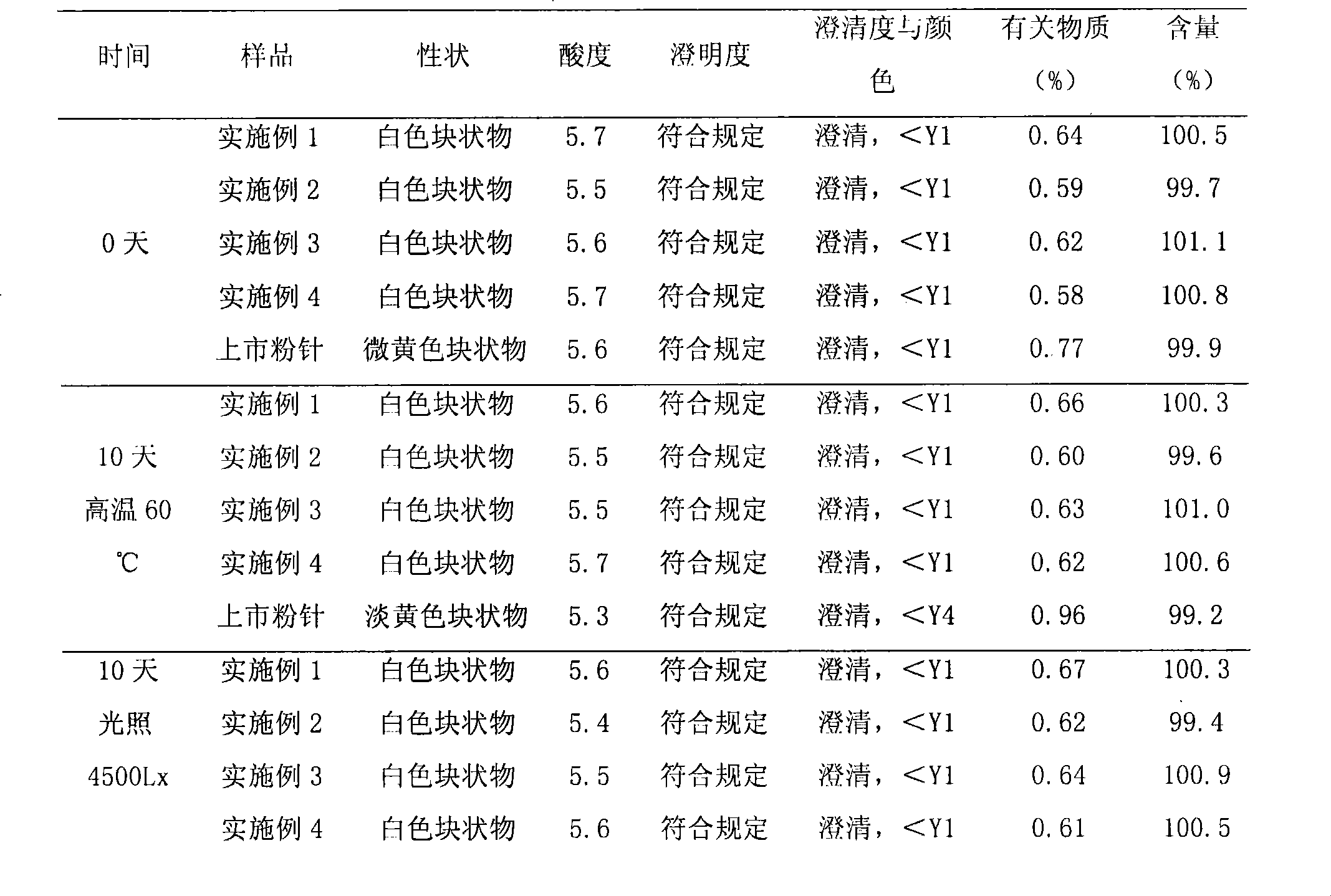
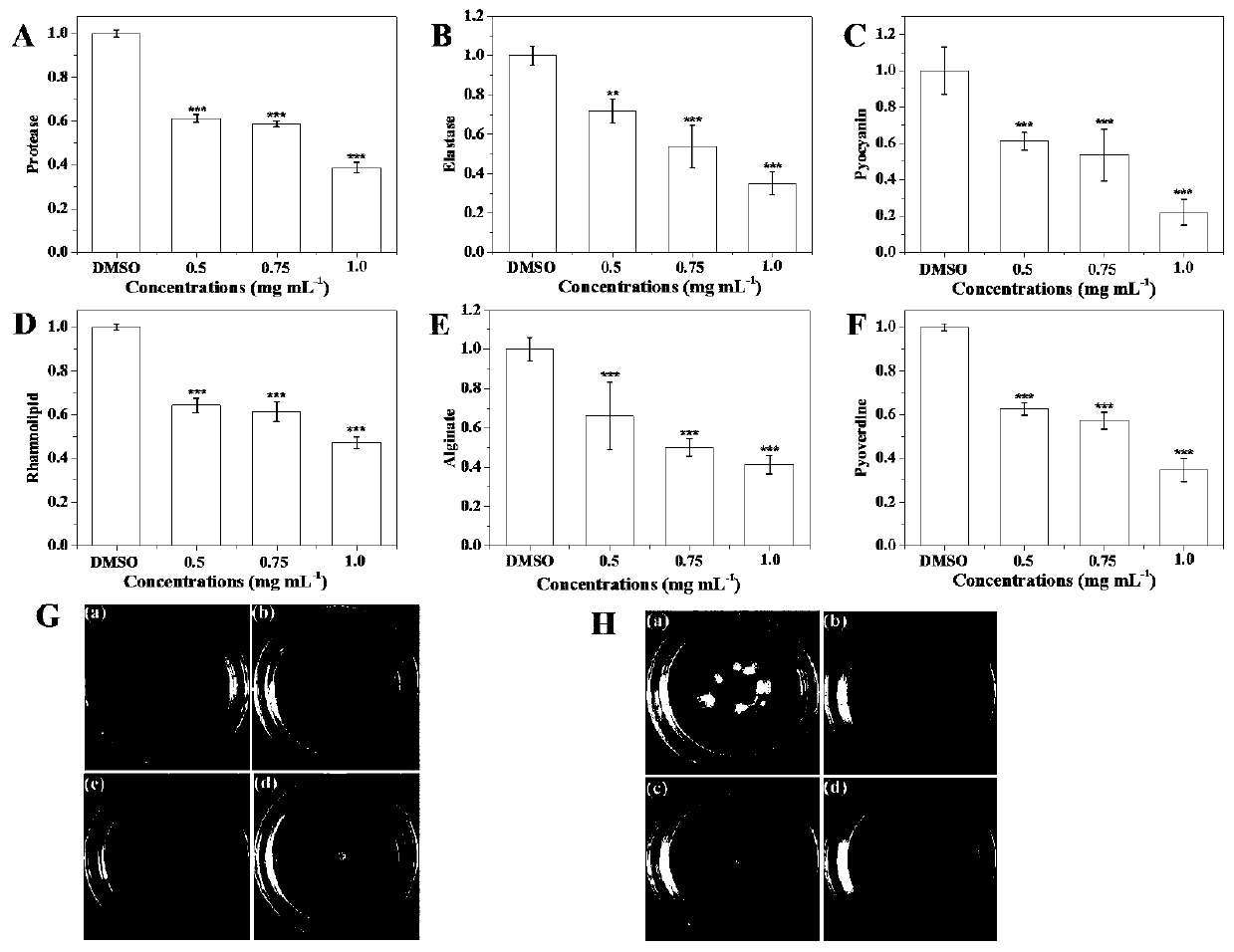
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
34 results about "Netilmicin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Netilmicin is a member of the aminoglycoside family of antibiotics. These antibiotics have the ability to kill a wide variety of bacteria. Netilmicin is not absorbed from the gut and is therefore only given by injection or infusion. It is only used in the treatment of serious infections particularly those resistant to gentamicin.
Application of Netilmicin sulfate in preparation of eye drops
InactiveCN1502335AReduce inflammationNo obvious irritating reactionAntibacterial agentsOrganic active ingredientsIrritationSolvent
The present invention belongs to a preparation process of eye drops with obvious curative effect for curing keratitis due to pseudomonas and infective ophthalmopathies due to gram-negative bacteria, pseudomonas aeruginosa and other bacteria. Said eye drops preparation is of netilamicin sulfate which is used as main raw material and adding chemical inhibitor, antioxidation synergist, preservative, pH regulating agent, isoosmotic regulating agent, stabilizing agent and solvent as auxiliary material.
Owner:TIANJIN PHARMA GROUP XINZHENG
Separation and purification method of high-purity netilmicin
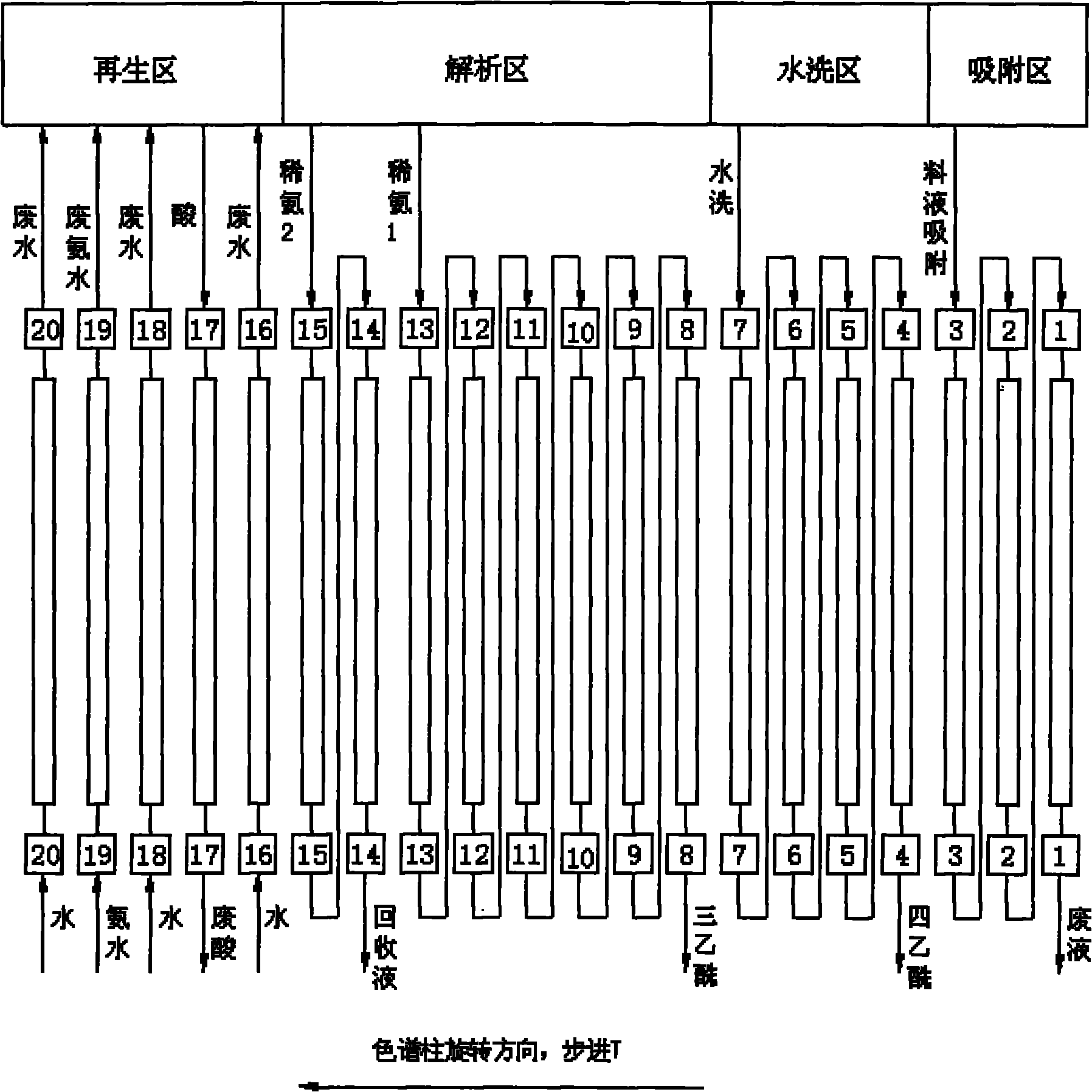
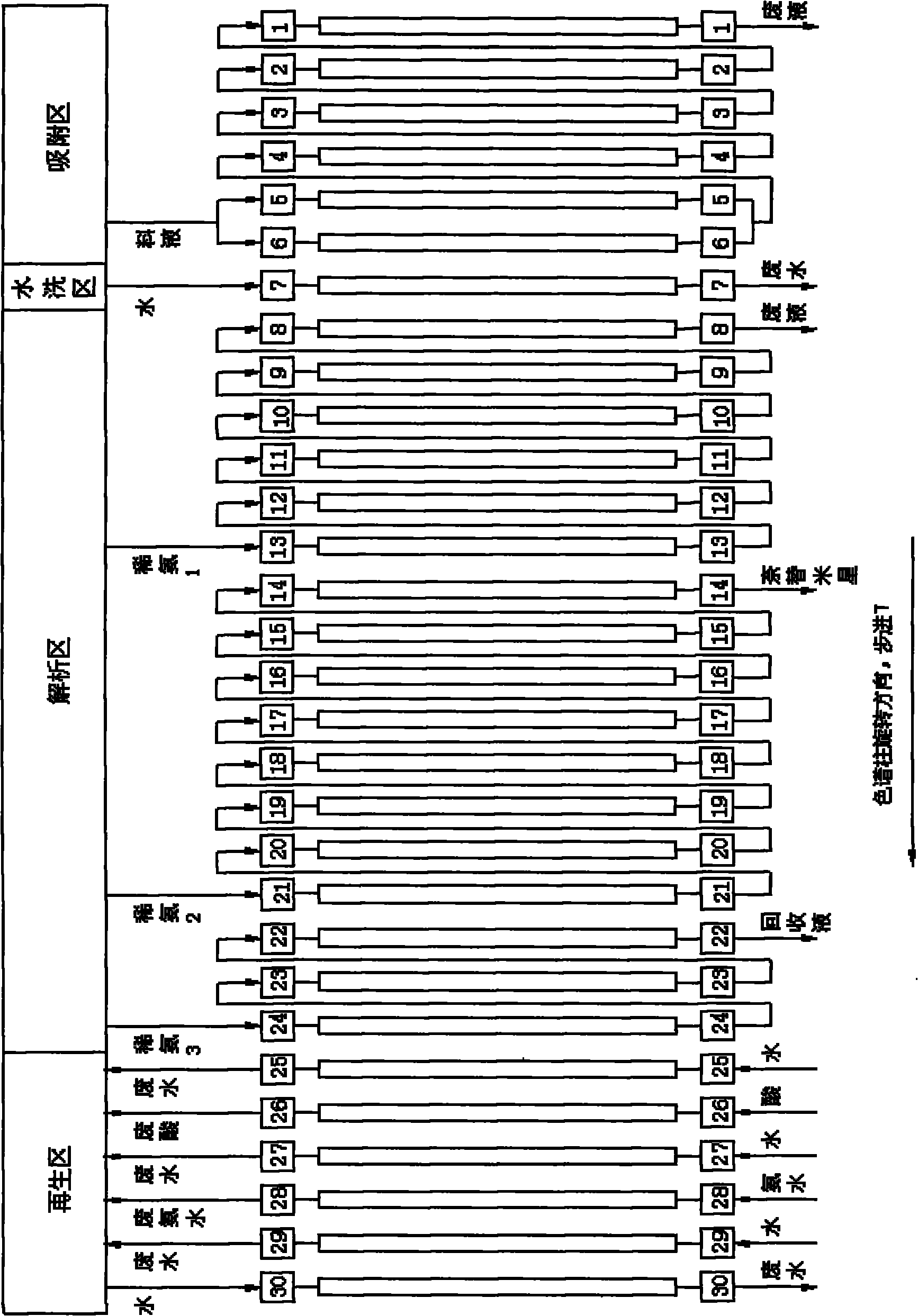
ActiveCN101805382AIncrease profitReduce consumptionSugar derivativesUltrafiltrationChromatographic separationSisomicin
The invention relates to a separation and purification method of high-purity netilmicin, belonging to the technical fields of medical continuous chromatographic separation and binding film separation and purification. In the invention, the continuous chromatographic separation technology is used for separating and purifying triacetyl sisomicin reaction liquid to obtain high-purity triacetyl sisomicin, and then, the industrial chromatographic separation and film separation combined technology is used for purifying and separating netilmicin hydrolyzate to obtain high-purity netilmicin. By adopting a continuous chromatographic separation system combined with film ultrafiltration and nanofiltration technology, the feed liquid of the triacetyl sisomicin and the netilmicin which are used as intermediates can be effectively separated and purified through the continuous chromatographic separation system; in the film separation production process, the reasonable temperature condition is controlled to reduce the generation of degradation products; and meanwhile, the production cost can be reduced, the production cycle can be shortened, the product quality can be improved, and the discharge amount of waste water can be lowered. The netilmicin purity of the product, which is measured by the high performance liquid chromatography (HPLC) method, is greater than or equal to 98%.
Owner:WUXI FORTUNE PHARMA
Netilmicin sulphate nano micelle preparation for intravenous injection and preparation method thereof
InactiveCN101461783AImprove stabilityTo achieve the purpose of long-term circulationAntibacterial agentsOrganic active ingredientsPolyethylene glycolNetilmicin sulphate
The invention provides a netilmicin sulfate nano-micelle preparation capable of being intravenously injected and a preparation method thereof. The netilmicin sulfate nano-micelle preparation is prepared from netilmicin sulfate, polyethylene glycol-phospholipid and an additive by the method comprising the following steps: the netilmicin sulfate and the polyethylene glycol-phospholipid are prepared into a polymer lipid membrane, and water or a buffer solution which is dissolved with the additive is added into the polymer lipid membrane for hydration to be prepared into the netilmicin sulfate nano-micelle preparation capable of being intravenously injected. The netilmicin sulfate nano-micelle preparation achieves the aim of long-acting circulation in blood, greatly improves the bioavailability, and also greatly increases the stability.
Owner:HAINAN YONGTIAN PHARMA INST
Chemical medicine composition for treating helicobacter pylori infection
PendingCN111184867AGood synergyGood curative effectAntibacterial agentsOrganic active ingredientsHelicobacter InfectionsIsepamicin
The invention discloses a chemical medicine composition for treating helicobacter pylori (Hp) infection of adults through oral administration. The composition at least comprises fosfomycin or pharmaceutical salts such as a sodium salt, a calcium salt, fosfomycin trometamol and aminoglycoside medicines of the fosfomycin of a common dosage of an adult, and the aminoglycoside medicines are selected from tobramycin, netilmicin, etimicin, isepamicin, amikacin and common salts such as sulfate, of the medicines.
Owner:龚跃明
Method for preparing colon targeted pill of tilmicosin used for poultry and livestock
InactiveCN1969867AGood treatment effectAntibacterial agentsOrganic active ingredientsBiotechnologyAcrylic resin
The invention relates to Tilmicosin colon-targeting pills which comprise Netilmicin as the main ingredient, and blank pellets are employed. The medicinal layer comprises 8% retarding layer of hexadecyl alcohol, 2% retarding layer of HPMC, 15% pH responsive film of acrylic resin S100. The prepared colon-targeting pills are of retarding-and-pH responsive combined type which can achieve the positioned medicinal release on the animal's colons, the curative effects for localized infection and general infection can be improved substantially.
Owner:TIANJIN RINGPU BIO TECH
Method for simultaneously separation determination of content of three aminoglycoside antibiotics
The invention discloses a method for simultaneously separation determination of the content of three aminoglycoside antibiotics (AGs) (tobramycin, etimicin and netilmicin); the method successfully separates a mixture of the three antibiotics, and also is applied in determination of the content of the AGs in tobramycin eye drops, an etimicin sulfate injection and a netilmicin sulfate injection, and an HPLC-RRS combined technology is used for determination of the AGs. In an experiment, used chromatographic columns comprise Agilent Zorbox SB-C18 column (250 mm*4.6 mm i.d., 5 [mu]m) and a protective column (Security Guard C18, 4*2 mm, Phenomenex). A mobile phase is methanol:water (containing 0.2% TFA)=5:95 (v / v), the HPLC flow rate is 0.50 mL*min<-1>, [lambda]ex=[lambda]em=362 nm, the column temperature is 30 DEG C, and the sample injection volume is 20 [mu]L. The probe is a 2.0*10<-5> mol*L<-1> PSB solution, the pH is 9.5, the single-pump flow rate is 0.2 mL*min<-1>, the reaction pipe length is 250 cm, and the internal diameter is 0.5 mm. A brand-new HPLC detection mode established with RRS as a detection method is established, and is successfully applied to determination of the content of the AGs in different preparations. The application range of the RRS technology is widened, and the brand-new detection mode is provided for HPLC.
Owner:CHONGQING MEDICAL UNIVERSITY
Antibiotic coating of porous body, its prepn. and application
The invention describes an antibiotic coating for porous bodies and its use. Into the porous system of non-metallic porous bodies and of metallic porous bodies, a coating made of at least one antibiotic salt that is hardly soluble in water or in an aqueous environment from the group of the netilmicin laurate, the netilmicin myristate, the netilmicin dodecyl sulfate, the sisomicin laurate, the sisomicin myristate, the sisomicin dodecyl sulfate, the gentamicin laurate, the gentamicin myristate, the clindamycin laurate, the amikacin laurate, the amikacin myristate, the amikacin dodecyl sulfate, the kanamycin laurate, the kanamycin myristate, the kanamycin dodecyl sulfate, the tobramycin laurate, the tobramycin myristate, the tobramycin dodecyl sulfate, the ciprofloxacin myristate, the vancomycin dodecyl sulfate, the vancomycin laurate, the vancomycin myristate, the vancomycin teicoplanin and the clindamycin teicoplanin is introduced. The antibiotically coated, porous bodies are used as implants.
Owner:HERAEUS MEDICAL
Reagent of multi-residue simultaneous rapid fluorescence detection of aminoglycoside antibiotics and application thereof
ActiveCN110308289AEasy to storeSolve the problem of information transmissionBiological testingFluorescence/phosphorescenceKanamycinAntibiotic Y
The invention relates to a preparation and using method of a reagent of multi-residue simultaneous rapid fluorescence detection of aminoglycoside antibiotics. Three kinds of quantum dots with the samematerial core and different emission wavelengths are coupled with an aptamer having the high specific recognition ability for kanamycin, netilmicin and tobramycin; the three kinds of coupling mattersand graphene oxide to form a fluorescence resonance energy transfer system; and a reagent capable of realizing simultaneous rapid fluorescence detection of kanamycin, netilmicin and tobramycin residues is prepared. With the provided reagent, rapid, accurate and sensitive detection of kanamycin, netilmicin and tobramycin residues in milk samples can be realized without depending on the separationanalysis technology; and the powerful technical guarantee is provided for the g food safety supervision enhancement.
Owner:JIANGSU UNIV
Anti-infection pharmacy application of hordenine or hordenine combined with antibiotics
ActiveCN107929742AAvoid infectionAvoid damageAntibacterial agentsOrganic active ingredientsSignalling moleculesAntibiotic Y
The invention discloses new application of a natural product hordenine and in particular relates to application of a compound hordenine in preparation of a medicine used for preventing and treating bacterial infection. The new application is mainly reflected in that hordenine can obviously inhibit synthesis of a quorum sensing-regulated signal molecule, hordenine can obviously inhibit expression of a quorum sensing-regulated virulence factor and hordenine can obviously inhibit expression of a quorum sensing-related gene. Besides, drug combination of hordenine and antibiotic netilmicin can obviously inhibit generation of a biofilm, a formed biofilm can be obviously destroyed, and the hordenine is hopeful to becoming a novel anti-infective agent for preventing and treating bacterial infection.
Owner:HAINAN UNIVERSITY
Netilmicin sulfate injection and preparing method
The invention relates to a netilmicin sulfate injection and a preparing method. Particularly, the netilmicin sulfate injection is prepared from netilmicin sulfate, sodium sulfite or sodium hydrogen sulfite, cysteine, edetate disodium or sodium calcium edentate and water for injection. The invention further relates to the preparing method for the netilmicin sulfate injection. The prepared netilmicin sulfate injection has one or more excellent characteristics described in the description.
Owner:成都天台山制药股份有限公司
Netilmicin liposome and technique of preparing the same
InactiveCN101176718AImprove stabilityLarge particle sizeAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention discloses a Netilmicin liposome and the preparation technique. The Netilmicin liposome is composed by the following materials in weight ratio: one share of Netilmicin, two to seven shares of cholesterol and four to eighteen shares of lecithin. The preparation technique includes the infusing and ultrasonic method and the multiple emulsion and ultrasonic method, with the following steps: firstly, dissolve a matched proportion of Netilmicin medicine into a neutral-pH buffer solution of phosphate as an aqueous phase; secondly, dissolve a matched proportion of the cholesterol and the lecithin into a proper proportion of Ethyl ether as an organic phase; thirdly, drop the organic phase into the aqueous phase uniformly and stir simultaneously; and finally with the water ultrasonic extraction method, an uniform emulsion, the Netilmicin liposome with a particle diameter of one hundred to two hundreds nanometers, is formed with renal toxicity decreased greatly. The invention has the advantages that due to simplified operation of processing, the liposome with less particle diameter and better stability can be formed; the particle diameter is notably reduced while the uniformity is improved; additionally, the envelope rate rises to fifty percents or above.
Owner:谢开智
Netilmicin immunoassay reagent and preparation and detection method thereof
The invention discloses a netilmicin immunoassay reagent and preparation and detection method thereof. The netilmicin immunoassay reagent includes enzyme-labeled netilmicin and an indicator reagent for detection of a netilmicin antibody-enzyme-labeled netilmicin complex; the enzyme-labeled netilmicin is conjugated by netilmicin and glucose dehydrogenase. The netilmicin immunoassay reagent can accurately and rapidly determine the content of the netilmicin in human blood and other samples. Compared with the existing detection reagents on the market, the netilmicin immunoassay reagent has the advantages of convenience, rapidity, high sensitivity, strong specificity, accurate quantification and so on, and is conducive to clinical popularization and use.
Owner:太原瑞盛生物科技有限公司
Netilmicin sulphate nano micelle preparation for intravenous injection and preparation method thereof
InactiveCN101461783BImprove stabilityTo achieve the purpose of long-term circulationAntibacterial agentsOrganic active ingredientsNetilmicin sulphatePolyethylene glycol
The invention provides a netilmicin sulfate nano-micelle preparation capable of being intravenously injected and a preparation method thereof. The netilmicin sulfate nano-micelle preparation is prepared from netilmicin sulfate, polyethylene glycol-phospholipid and an additive by the method comprising the following steps: the netilmicin sulfate and the polyethylene glycol-phospholipid are preparedinto a polymer lipid membrane, and water or a buffer solution which is dissolved with the additive is added into the polymer lipid membrane for hydration to be prepared into the netilmicin sulfate nano-micelle preparation capable of being intravenously injected. The netilmicin sulfate nano-micelle preparation achieves the aim of long-acting circulation in blood, greatly improves the bioavailability, and also greatly increases the stability.
Owner:HAINAN YONGTIAN PHARMA INST
Western medicine composition for treating leucoderma
InactiveCN106511973ANo discomfortReduce economic pressurePeptide/protein ingredientsHydroxy compound active ingredientsTreatment effectSide effect
The invention discloses a western medicine composition for treating leucoderma. The composition is prepared from, by weight, 0.1-1 part of vitamin C, 0.1-2 parts of vitamin p, 0.1-1 part of vitamin E, 2-5 parts of placentin, 2-8 parts of hair, 10-25 parts of thymosin injection, 0.2-5 parts of retinoic acid, 1-3 parts of netilmicin, 2-5 parts of acetylspiramycin, 2-10 parts of pazufloxacin mesilate, 1-5 parts of asparaginic acid, 0.5-1 part of glycine, 2-5 parts of compound kaliziran tincture and 1-5 parts of vindesine. The medicine for treating leucoderma has the advantages that the medicine is good in treatment effect, takes effect quickly and is short in treatment period and small in toxic and side effects, leucoderma is not likely to relapse after drug withdrawal, and the medicine is convenient to take. Formation of melanin around a leukoplakia portion is benefited, and the effective rate is as high as 80%. A preparation method is simple, cost is low, and economic burden of patients is effectively reduced.
Owner:郑州莉迪亚医药科技有限公司
Fructose injection of antibiotic medicine
InactiveCN108721625AEasy to useSuitable for useAntibacterial agentsPharmaceutical delivery mechanismFluconazoleNorfloxacin
The invention relates to fructose injection of an antibiotic medicine. The fructose injection of the antibiotic medicine consists of antibiotics, fructose and water and also comprises proper additives, wherein the antibiotics comprise gatifloxacin, levofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, caderofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin as well as medicinal acid addition salt, esterification compounds, derivatives and the like; the fructose injection is prepared from the antibiotics; the advantages that the injection is convenient to use and takes effect rapidly are achieved; and compared with the glucose injection, the fructose injection is easier to absorb and utilize, more suitable for antisepsis and anti-inflammation, energy supply and body liquid supplementation of patientssuffering from diabetes, heart diseases and liver diseases, and enlarges the use range.
Owner:WEIHAI HAOTONG MEDICAL SCI & TECH
Fructose injection of antibiotic drug
InactiveCN106668862AEasy to useSuitable for useAntibacterial agentsPharmaceutical delivery mechanismFluconazoleNorfloxacin
The invention provides a fructose injection of an antibiotic drug. The injection is composed of antibiotics, fructose and water. The injection also can contain proper additives. The antibiotic comprise gatifloxacin, levofloxacin, ofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, caderofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin and medicinal acid additive salts, esterified compounds, derivatives and the like. The antibiotics are prepared into the fructose injection. Besides the advantages of convenience in use and quickness to take effect of the injection, compared with a gluconic infection, the injection provided by the invention is more easily absorbed and utilized, is more suitable for preventing bacteria and diminishing inflammation, supplying energy and supplementing body fluids for patients with diabetes, heart disease and liver disease, so that the application range of the injection is expanded.
Owner:威海恒基伟业信息科技发展有限公司
Separation and purification method of high-purity netilmicin
ActiveCN101805382BReduce layoutLess investmentSugar derivativesUltrafiltrationChromatographic separationSisomicin
The invention relates to a separation and purification method of high-purity netilmicin, belonging to the technical fields of medical continuous chromatographic separation and binding film separation and purification. In the invention, the continuous chromatographic separation technology is used for separating and purifying triacetyl sisomicin reaction liquid to obtain high-purity triacetyl sisomicin, and then, the industrial chromatographic separation and film separation combined technology is used for purifying and separating netilmicin hydrolyzate to obtain high-purity netilmicin. By adopting a continuous chromatographic separation system combined with film ultrafiltration and nanofiltration technology, the feed liquid of the triacetyl sisomicin and the netilmicin which are used as intermediates can be effectively separated and purified through the continuous chromatographic separation system; in the film separation production process, the reasonable temperature condition is controlled to reduce the generation of degradation products; and meanwhile, the production cost can be reduced, the production cycle can be shortened, the product quality can be improved, and the discharge amount of waste water can be lowered. The netilmicin purity of the product, which is measured by the high performance liquid chromatography (HPLC) method, is greater than or equal to 98%.
Owner:WUXI FORTUNE PHARMA
Preparation method of netilmicin sulfate sterile powder injection for injection
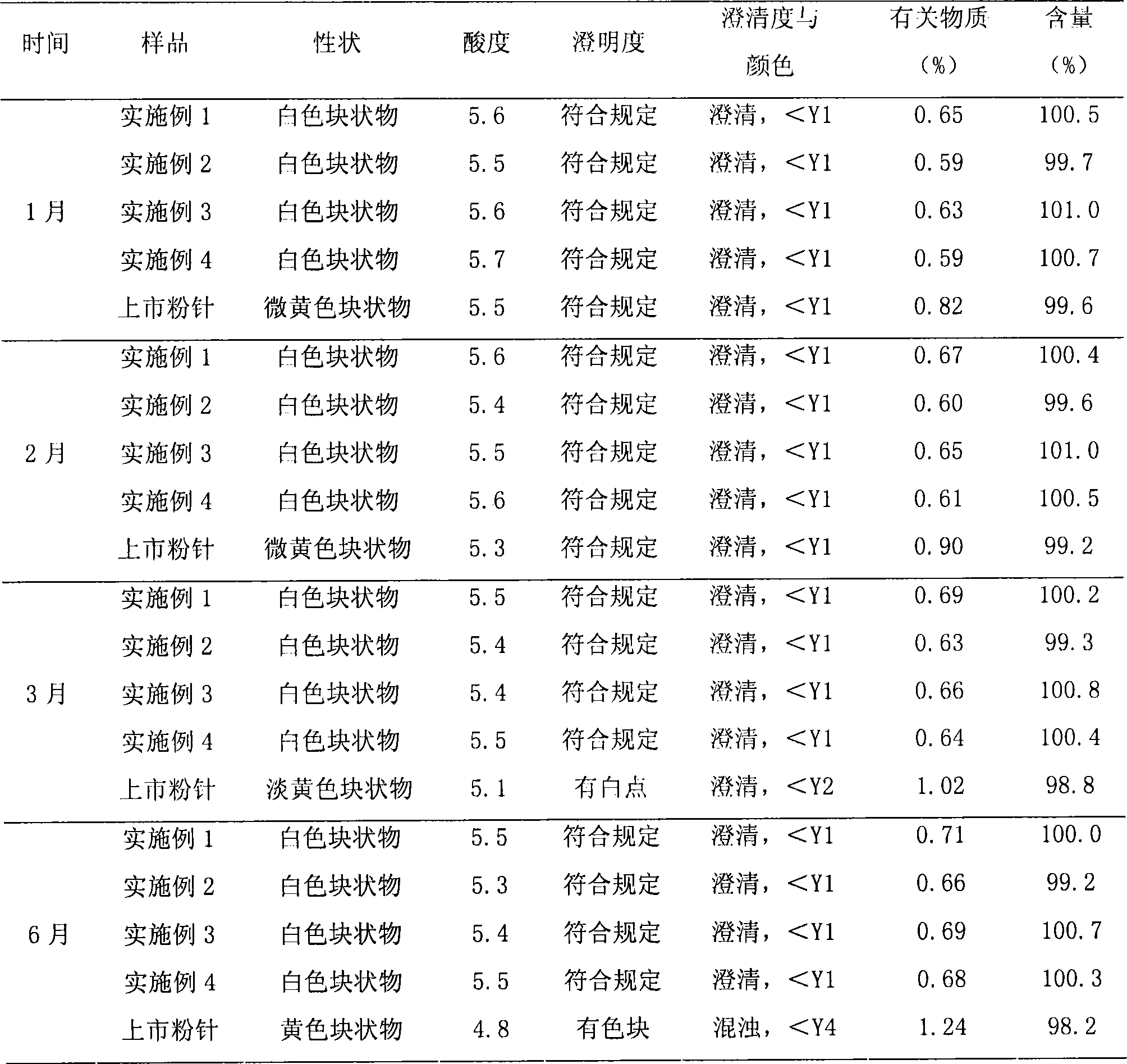
InactiveCN113171348AImprove stabilityHigh content of active ingredientsAntibacterial agentsPowder deliveryAntioxidantFreeze-drying
The invention discloses a preparation method of netilmicin sulfate sterile powder injection for injection, which comprises the following steps: taking water for injection, adding an antioxidant and an excipient, and conducting dissolving to obtain a first solution; cooling the first solution to 5-15 DEG C, and adding netilmicin sulfate for dissolving to obtain a second solution; adjusting the pH value of the second solution to 5.0-5.5 by using a pH value regulator to obtain a third solution; decoloring, degerming and filtering the third solution to obtain a fourth solution; and freezing and drying the fourth solution to obtain the netilmicin sulfate sterile powder injection. The netilmicin sulfate freeze-dried powder injection prepared by the invention has the beneficial effects that the stability is good, the content of active ingredients of the product is high, the resolubility is excellent, and the long-term storage is facilitated.
Owner:HAINAN GENERAL & KANGLI PHARMA
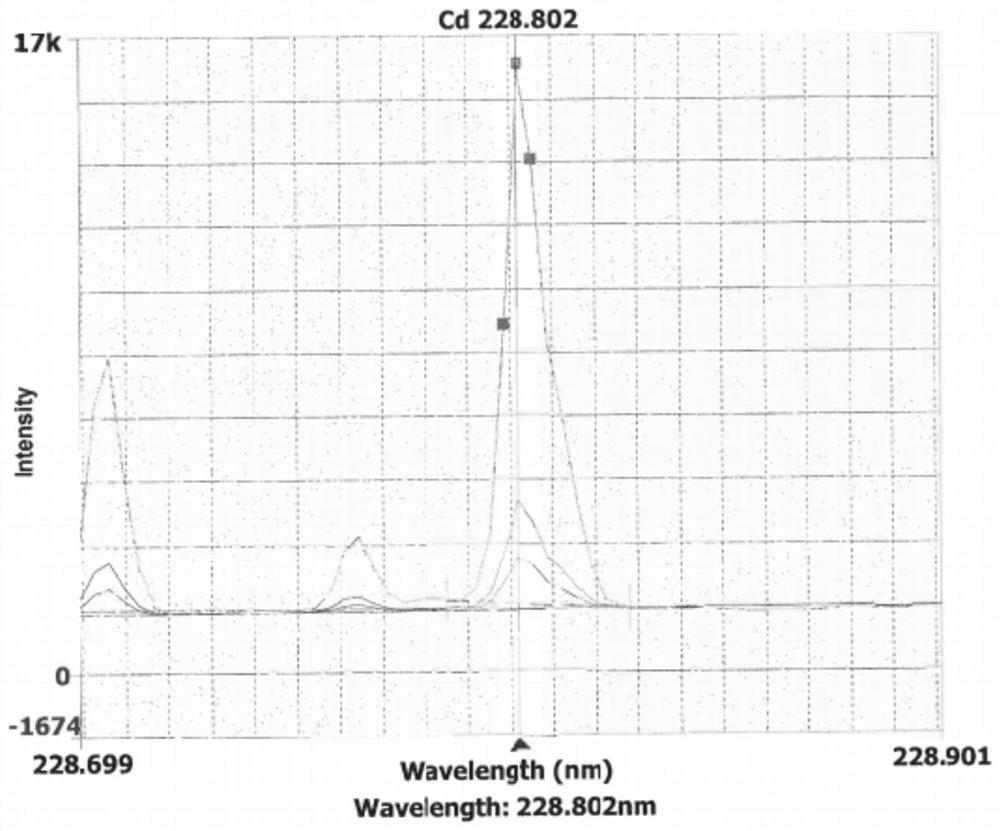
Method for detecting residual amount of mercury in netilmicin sulfate
InactiveCN112697775AEfficient determinationEnsure safetyPreparing sample for investigationAnalysis by thermal excitationNetilmicin sulphateNetilmicin Sulfate
The invention provides a method for detecting the residual amount of mercury in netilmicin sulfate, which comprises the following five steps: (1) preparing a linear stock solution and a linear solution from a mercury element; (2) preparing a test solution; (3) preparing a standard test solution; (4) measuring the linear solution in ICP, recording a spectrogram, and carrying out linear regression by taking the sample injection concentration as an abscissa and the peak area as an ordinate to obtain a regression equation; and (5) sequentially determining the test solution and the standard test solution according to the steps (2) and (3), and determining the repeatability intermediate precision and the recovery rate of the standard test solution. According to the method disclosed by the invention, the efficient determination of the residual quantity of Cd, Pb, As, Co, V and Ni in netilmicin sulfate is realized through five steps, the operation steps are simple, the sensitivity is good, and the safety, effectiveness and quality controllability of medicines are ensured.
Owner:WUXI FORTUNE PHARMA
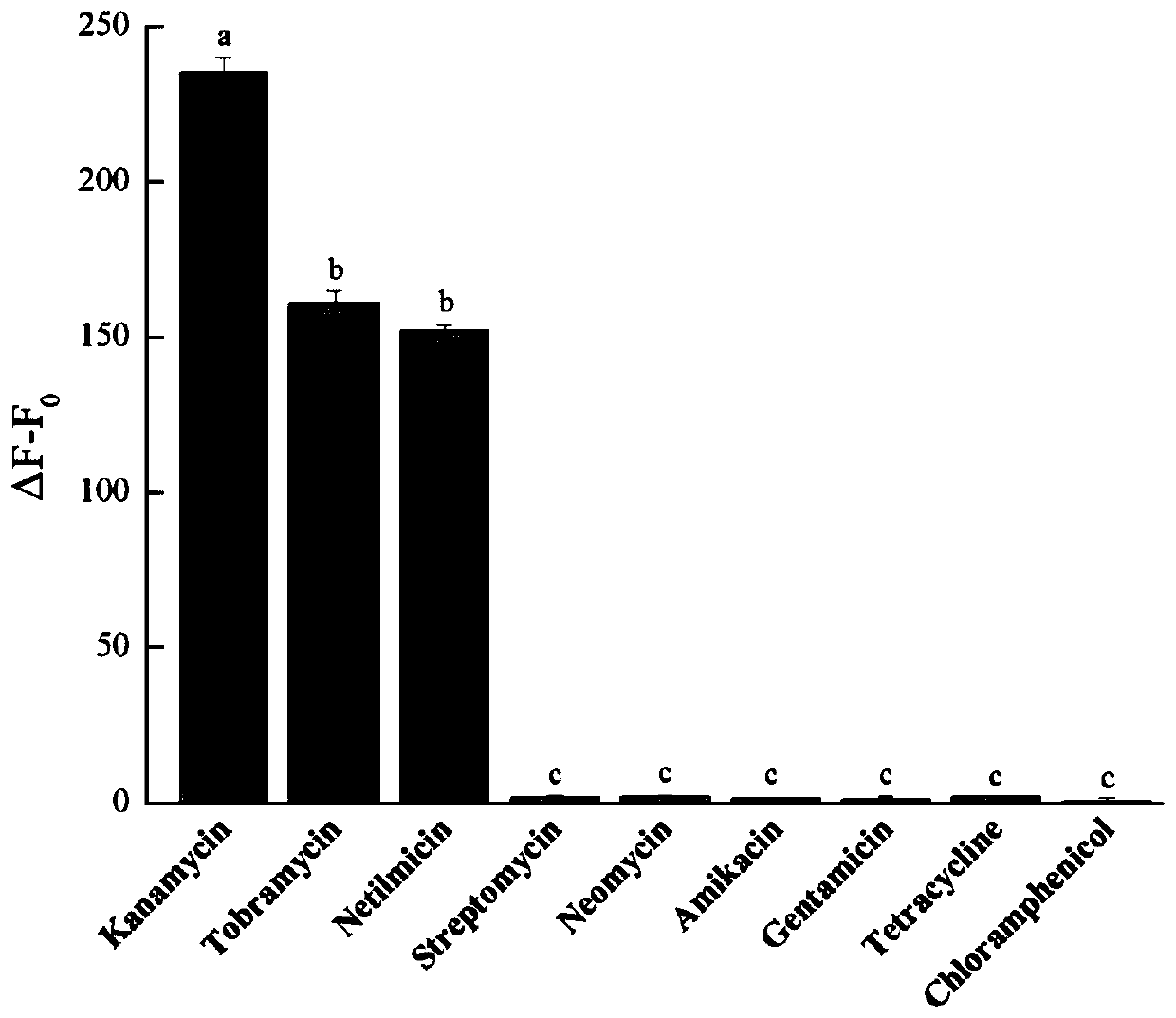
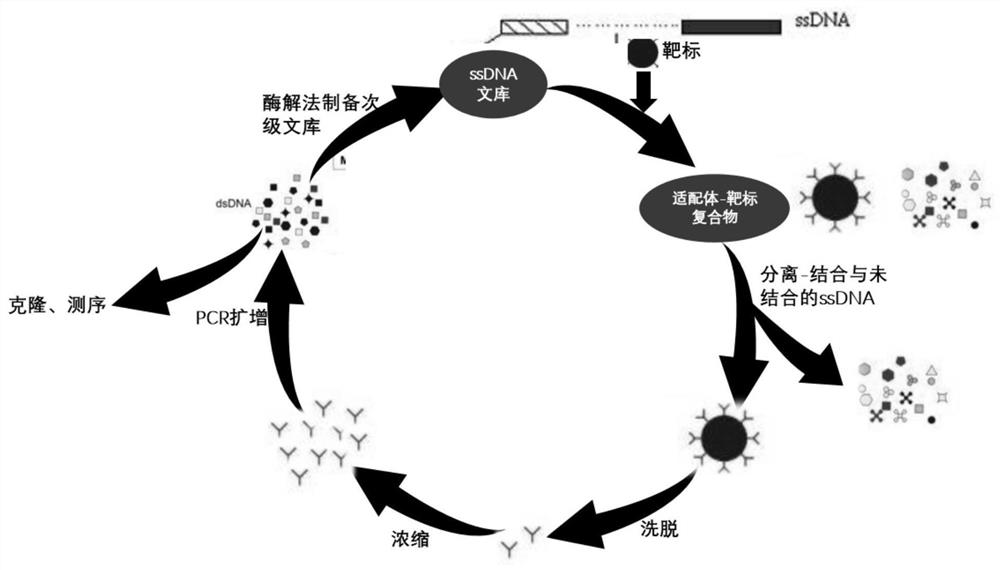
Netilmicin nucleic acid aptamer and its screening and application in the detection of netilmicin
ActiveCN113073100BThe detection method is simpleEasy to operateBiological testingDNA preparationAptamerSimple sample
Owner:浙江首信检测有限公司
Netilmicin sulfate injection and its quality control method
ActiveCN105213301BImprove performanceAntibacterial agentsOrganic active ingredientsMedicineNetilmicin sulphate
The invention relates to netilmicin sulfate injection and a quality control method thereof. Specifically, the netilmicin sulfate injection of the present invention comprises: netilmicin sulfate, sulfite, cysteine, edetate, and water for injection. The present invention also relates to a preparation method of the netilmicin sulfate injection and a method for detecting the quality index of the netilmicin sulfate injection. The netilmicin sulfate injection prepared by the present invention has at least one excellent property as described in the specification, and the method for detecting quality indicators of the present invention has excellent detection performance.
Owner:CHENGDU TIANTAISHAN PHARMA
Anti-infective pharmaceutical use of hordenine or its combined antibiotics
ActiveCN107929742BAvoid infectionAvoid damageAntibacterial agentsOrganic active ingredientsSignalling moleculesAntibiotic drug
Owner:HAINAN UNIV
Aminoglycoside antibiotic multi-residue simultaneous rapid fluorescence detection reagent and application
ActiveCN110308289BEasy to storeSolve the problem of information transmissionBiological testingFluorescence/phosphorescenceGlycosideKanamycin
The invention relates to a method for preparing and using an aminoglycoside antibiotic multi-residue simultaneous rapid fluorescence detection reagent. Three kinds of quantum dots with the same core material and different emission wavelengths were coupled to aptamers with high specific recognition ability for kanamycin, netilmicin and tobramycin respectively. The conjugates and graphene oxide respectively constitute a fluorescence resonance energy transfer system, and reagents for simultaneous and rapid fluorescence detection of multiple residues of kanamycin, netilmicin and tobramycin can be prepared. By adopting the detection reagent of the present invention, rapid, accurate and sensitive detection of multiple residues of kanamycin, netilmicin and tobramycin in milk samples can be realized without relying on separation and analysis techniques, which provides a basis for strengthening food safety supervision Strong technical support.
Owner:JIANGSU UNIV
Sustained-release injection containing netilcosin
InactiveCN101283973AIncrease concentrationReduce concentrationAntibacterial agentsOrganic active ingredientsPeptostreptococcusBacillus acnes
A sustained-release injection containing netilmicin comprises sustained-release microspheres and solvent. The microspheres contain sustained-release adjuvants and aminoglycoside antibiotics, and the solvent is a special solvent containing suspending agent such as sodium carboxymethylcellulose and having viscosity of 100-3000cp (20-30 DEG C); and the sustained-release adjuvants are selected from poly(ethylene-co-vinylacetate) (EVAc), polifeprosan, poly(lactic acid) (PLA), poly(lactic-co-glycolic acid) (PLGA), sebacic acid copolymer, albumin glue and gelatin; the sustained-release microspheres can also be made into sustained-release implant or ointment. The sustained-release implant or injection can be locally placed or injected into foci to locally sustained-release of drug for 5-30 days, so as to obtain and maintain local drug effective concentration while remarkably reduce the systemic toxicity of the drug. The sustained-release injection has distinct and unique therapeutic effect on local infection diseases caused by Staphylococci, Streptococci, Peptostreptococcus, Propionibacterium acnes, Enterobacter, Mycobacterium tuberculosis, Gonococcus or Meningococcus, such as chronic osteomyelitis, severe decubital ulcer, refractory skin ulcer, diabetic foot, femoral head necrosis, abscess, etc.
Owner:JINAN SHUAIHUA PHARMA TECH
Antibiotic coating of porous body, its prepn. and application
The invention describes an antibiotic coating for porous bodies and its use. Into the porous system of non-metallic porous bodies and of metallic porous bodies, a coating made of at least one antibiotic salt that is hardly soluble in water or in an aqueous environment from the group of the netilmicin laurate, the netilmicin myristate, the netilmicin dodecyl sulfate, the sisomicin laurate, the sisomicin myristate, the sisomicin dodecyl sulfate, the gentamicin laurate, the gentamicin myristate, the clindamycin laurate, the amikacin laurate, the amikacin myristate, the amikacin dodecyl sulfate, the kanamycin laurate, the kanamycin myristate, the kanamycin dodecyl sulfate, the tobramycin laurate, the tobramycin myristate, the tobramycin dodecyl sulfate, the ciprofloxacin myristate, the vancomycin dodecyl sulfate, the vancomycin laurate, the vancomycin myristate, the vancomycin teicoplanin and the clindamycin teicoplanin is introduced. The antibiotically coated, porous bodies are used as implants.
Owner:HERAEUS MEDICAL
Netilmicin sulfate aerosol
PendingCN114177140ASolve the problem of high water solubility and non-absorption after oral administrationImprove medication complianceOrganic active ingredientsDispersion deliveryUse medicationNetilmicin sulphate
The invention provides a netilmicin sulfate aerosol. The netilmicin sulfate aerosol is prepared from the following raw materials: 5 to 10g of netilmicin sulfate, 100 to 200ml of solvent, a propellant and 0.05 to 0.5 g of additive. According to the netilmicin sulfate aerosol provided by the invention, the medicine can directly reach the acting part, the netilmicin sulfate aerosol is uniform in distribution, quick in action, convenient to use, free of drinking water, suitable for people of all ages and beneficial to improving the medication compliance of patients, and the netilmicin sulfate aerosol can be pressed once. On the other hand, due to the fact that the medicine is used for local action, the administration dosage is obviously reduced, and the occurrence rate of side effects such as ototoxicity and renal toxicity is reduced.
Owner:ZHUOHE PHARM GRP CO LTD
Fructose injection of antibiotic drug
InactiveCN109953943AEasy to useSuitable for useAntibacterial agentsOrganic active ingredientsFluconazoleSupplement use
A fructose injection of an antibiotic drug comprises antibiotics, fructose and water and can contain proper additives. The antibiotics comprise gatifloxacin, levofloxacin, ofloxacin, ciprofloxacin, pazufloxacin, fleroxacin, sparfloxacin, moxifloxacin, pefloxacin, rufloxacin, lomefloxacin, norfloxacin, cadrofloxacin, azithromycin, telithromycin, ornidazole, secnidazole, tinidazole, metronidazole, clindamycin, lincomycin, fluconazole, etimicin, netilmicin, amikacin as well as pharmaceutical acid added salts, esters and derivatives thereof. The antibiotics are prepared into the fructose injection, beside injection advantages of being convenient to use and taking effect quickly, compared with glucose injections, the injection is easier to absorb and utilize and more suitable for antisepsis andanti-inflammation, energy supply and body fluid supplement use for patients suffering from diabetes, heart disease and hepatopathy, and the use range of the injection is enlarged.
Owner:李芳凯
Eye drops containing netilmicin sulfate and preparation method of eye drops
InactiveCN112545985ASimple preparation processIncrease productivityAntibacterial agentsOrganic active ingredientsNetilmicin sulphatePharmaceutical Aids
The invention discloses eye drops containing netilmicin sulfate. Every 1000ml of the eye drops comprises the following components: 1-10g of netilmicin sulfate, 60-80g of a preservative, 1-10g of an isoosmotic adjusting agent and the balance of purified water. In the eye drops provided by the invention, netilmicin sulfate is used as a main drug, and the preservative, the isoosmotic adjusting agentand the purified water are used as auxiliary materials. The whole preparation process is simple, the production efficiency is high, and the eye drops can be used for treating various eye infections sensitive to netilmicin.
Owner:ZHUOHE PHARM GRP CO LTD
Method for detecting residual quantity of Cd, Pd, As, Co, V and Ni in netilmicin sulfate
InactiveCN112697776AEfficient determinationEnsure safetyPreparing sample for investigationAnalysis by thermal excitationNetilmicin sulphateNetilmicin Sulfate
The invention provides a method for detecting the residual quantity of Cd, Pd, As, Co, V and Ni in netilmicin sulfate, which comprises the following five steps: (1) preparing a linear stock solution and a linear solution; (2) preparing a test solution; (3) preparing a standard test solution; (4) measuring the linear solution in ICP, recording a spectrogram, and carrying out linear regression by taking the sample injection concentration as an abscissa and the peak area as an ordinate to obtain a regression equation; and (5) sequentially determining the test solution and the standard test solution according to the steps (2) and (3), and determining the repeatability intermediate precision and the recovery rate of the standard test solution. According to the method disclosed by the invention, the efficient determination of the residual quantity of Cd, Pb, As, Co, V and Ni in the netilmicin sulfate is realized through five steps, the operation steps are simple, the sensitivity is good, and the safety, effectiveness and quality controllability of medicines are ensured.
Owner:WUXI FORTUNE PHARMA
Aminoglycoside antibiotics for use as vap-1/ssao inhibitors
InactiveUS20070093433A1Substance reductionAnti-inflammatory effectBiocideSenses disorderAntibiotic YStreptomycin
The present invention concerns the use of a compound comprising one or more sugar moieties, which optionally are aminosubstitutes, and possibly other moieties, wherein said compound is a molecule comprising at least two aminosubstituents, said aminosubstituents being primary, secondary or tertiary amino groups, wherein said aminosubstituents are either attached to one single sugar moiety or attached to several sugar moieties or other moieties of the molecule, or to chains connecting two moieties or to chains being substituents to the molecule, for the manufacture of a pharmaceutical preparation useful as an agent capable of influencing an amine oxidase enzyme activity, more particularly inhibiting VAP-1 / SSAO activity. The preferred compounds are aminoglycoside antibiotics, e.g., streptomycin, netilmicin, geneticin, gentamicin, puromycin, tobramycin and amikacin. The preferred conditions to be treated are inflammatory diseases or conditions, diseases related to carbohydrate metabolism, diseases related to aberrations in adipocyte differentiation or function or smooth muscle cell function, and vascular diseases.
Owner:FARON PHARMA OY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com