Preparation method of netilmicin sulfate sterile powder injection for injection
A technology of netilmicin sulfate and sterile powder injection, which is applied in the field of preparation of sterile netilmicin sulfate powder for injection, can solve the problems of difficult storage for a long time, poor stability and the like, and achieves high content of active ingredients in the product , good stability, less impurity content
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
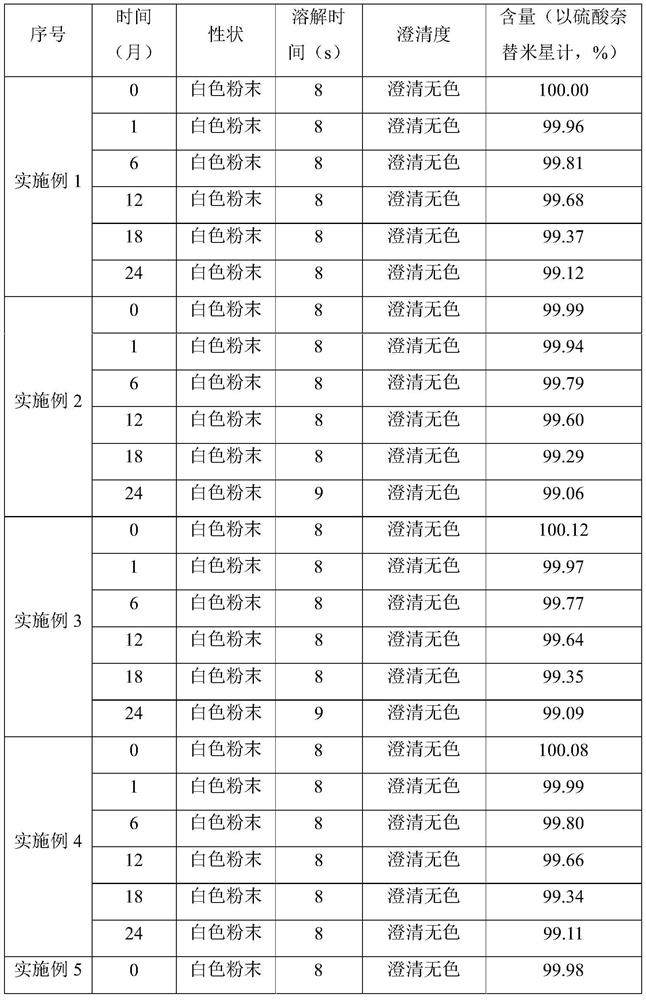
Embodiment 1
[0027] A preparation method of netilmicin sulfate sterile powder for injection is prepared by the following steps:
[0028] Take 500ml of water for injection and add 5g of sodium sulfite and 2.5g of dextran to dissolve to obtain the first solution; use a 0.45μm microporous membrane filter to remove insoluble matter and some residual bacteria and microorganisms, cool the first solution to 5°C, and add 100g of netil sulfate Dissolve Mixing to obtain the second solution; use a small amount of aqueous solution containing 0.1mol / L sodium bicarbonate to adjust the pH value of the second solution to 5.0 to obtain the third solution; maintain the system temperature at 5°C during the whole process, and then add 2g of medicinal Activated carbon, stirred at 5°C for 30 minutes, filtered, sterilized and filtered through a 0.18 μm primary filter, and then sterilized and filtered through a 0.18 μm secondary filter to effectively remove bacteria and microorganisms. Put the sterile drug soluti...
Embodiment 2
[0030] Take 500ml of water for injection and add 10g of cysteine and 5g of amino acid to dissolve to obtain the first solution; use a 0.45μm microporous membrane filter to remove insoluble matter and some residual bacteria and microorganisms, cool the first solution to 10°C, and add 100g of sulfuric acid Dissolve netilmicin to obtain the second solution; use a small amount of aqueous solution containing 0.1mol / L sodium bicarbonate to adjust the pH value of the second solution to 5.1 to obtain the third solution; maintain the system temperature at 10°C during the whole process, and then add 2g Activated carbon for medicinal purposes, stirring and decolorizing at 10°C for 60 minutes, and filtering. Filtration is sterilized and filtered through a 0.18 μm primary filter, and then sterilized and filtered with a 0.18 μm secondary filter to effectively remove bacteria and microorganisms. The sterile liquid obtained is then protected under nitrogen. Put the sterile drug solution into...
Embodiment 3
[0032] Take 500ml of water for injection and add 15g of phosphoric acid and 7.5g of lactose monohydrate to dissolve to obtain the first solution; use 0.45μm microporous membrane filter to remove insoluble matter and some residual bacteria and microorganisms, cool the first solution to 15°C, add 100g Dissolve netilmicin sulfate to obtain the second solution; use a small amount of aqueous solution containing 0.1mol / L sodium bicarbonate to adjust the pH value of the second solution to 5.2 to obtain the third solution; maintain the system temperature at 15°C during the whole process, and then add 2g of medicinal activated carbon, stirred and decolorized at 15°C for 90 minutes, filtered, sterilized and filtered through a 0.18μm primary filter, and then sterilized and filtered through a 0.18μm secondary filter to effectively remove bacteria and microorganisms. Under protection, put the sterile drug solution into a freeze-drying box that has been cooled to -45°C, keep the drug at -35°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com