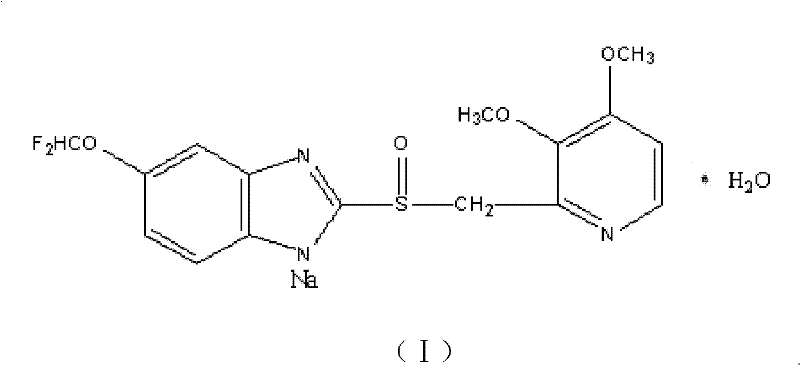
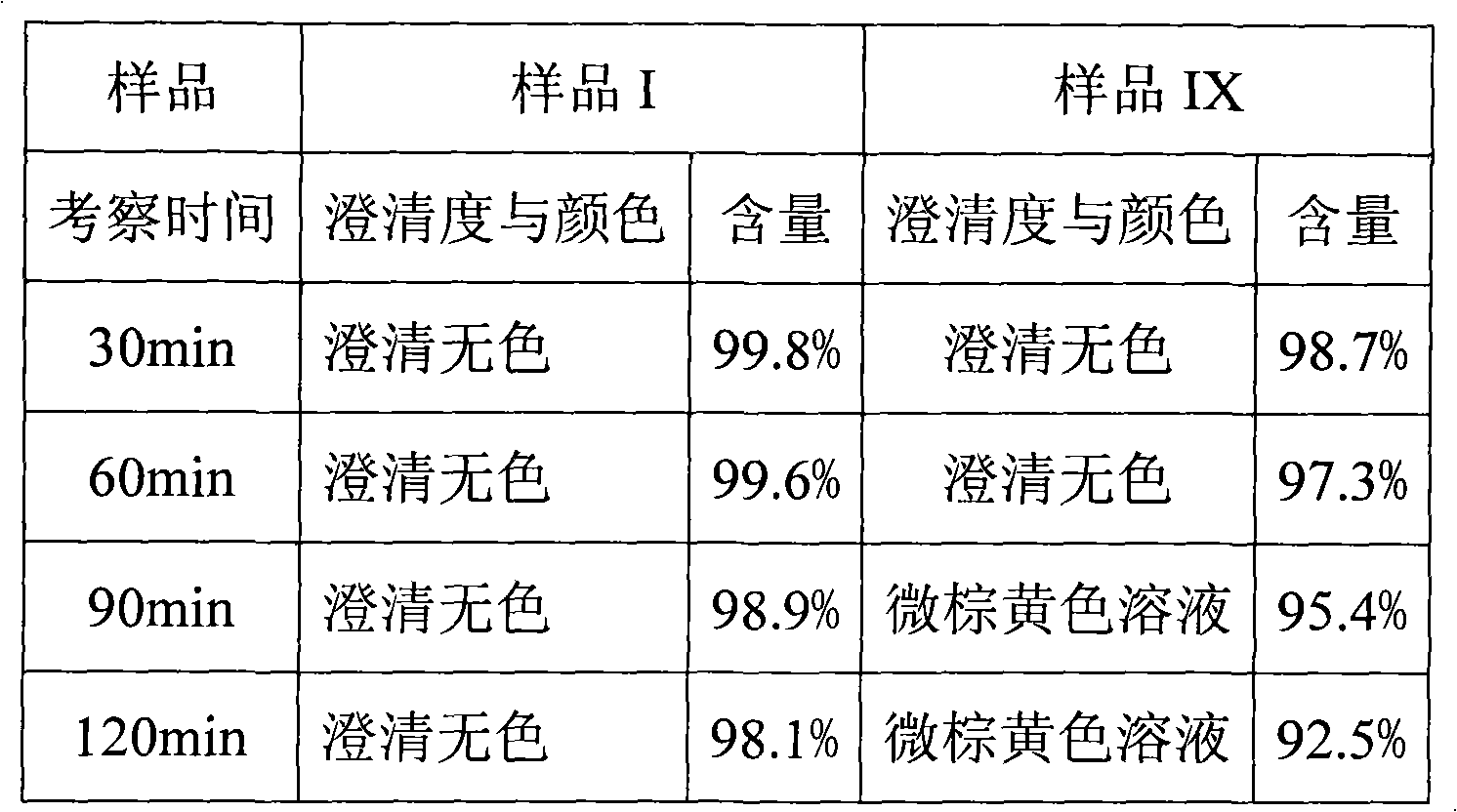
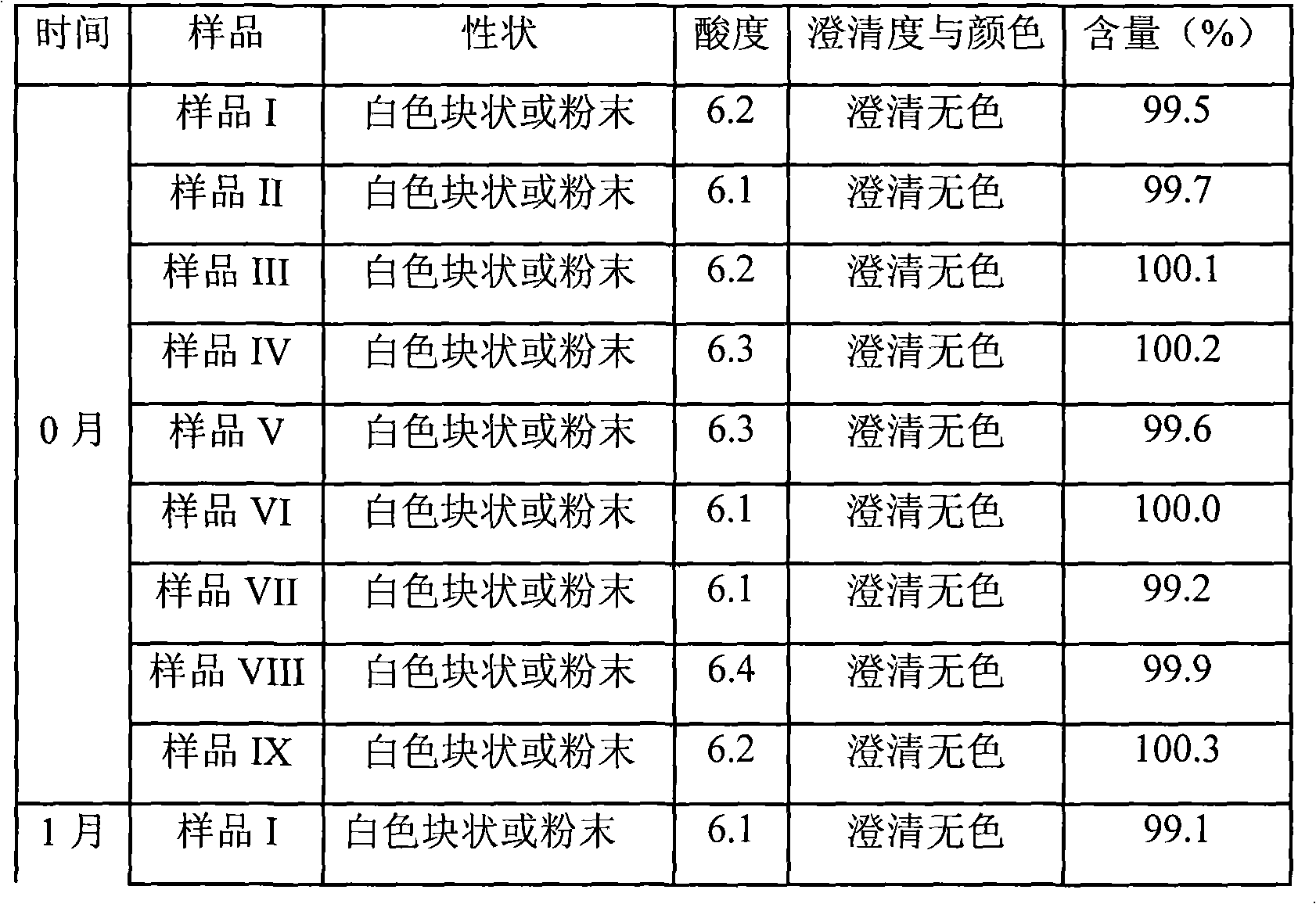
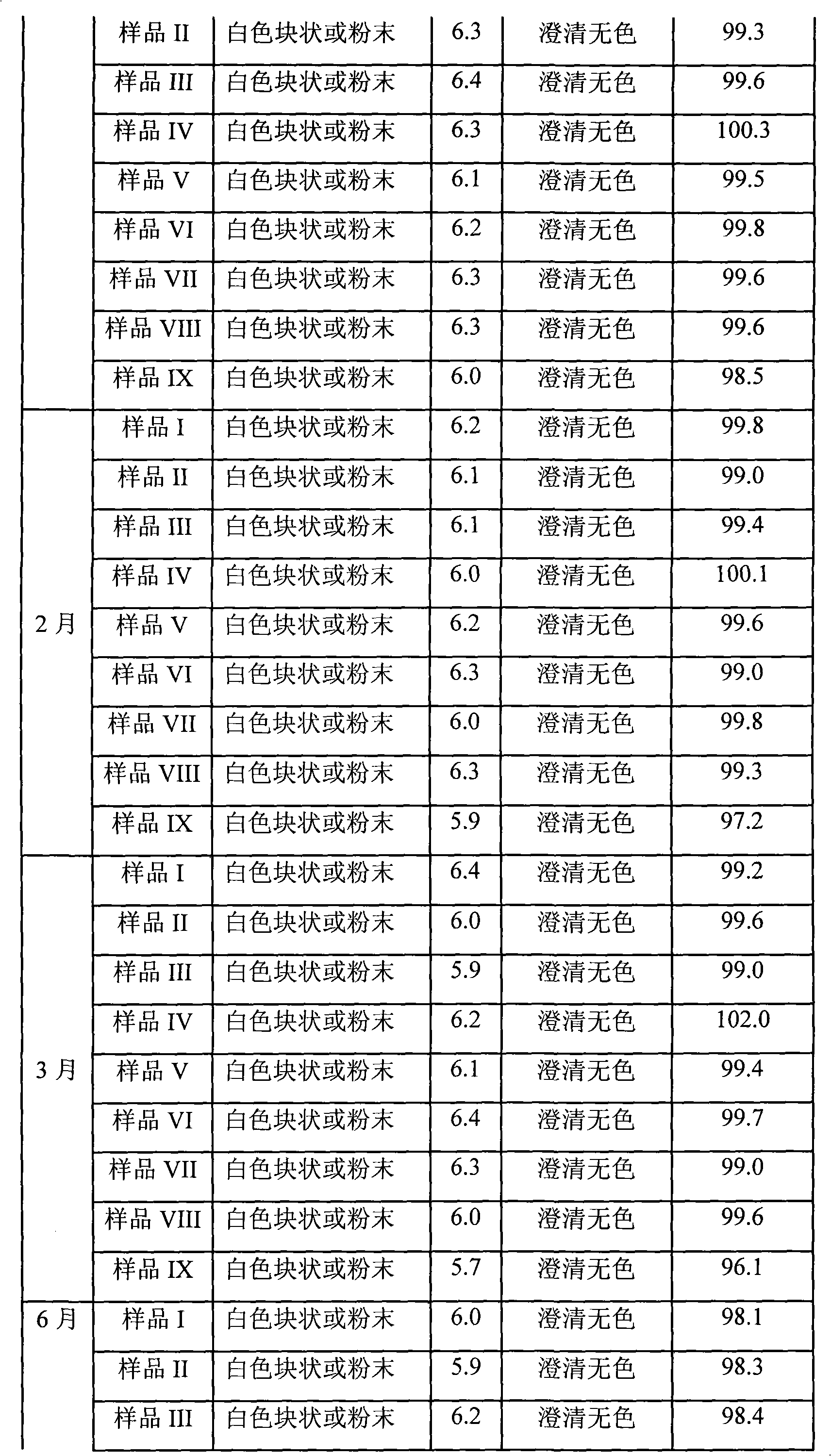
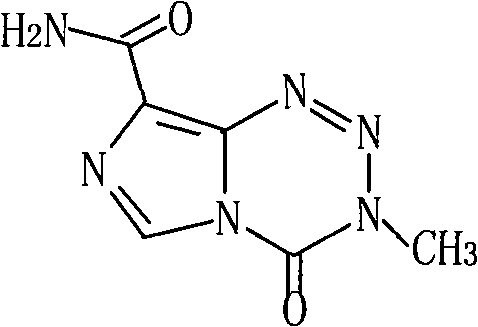
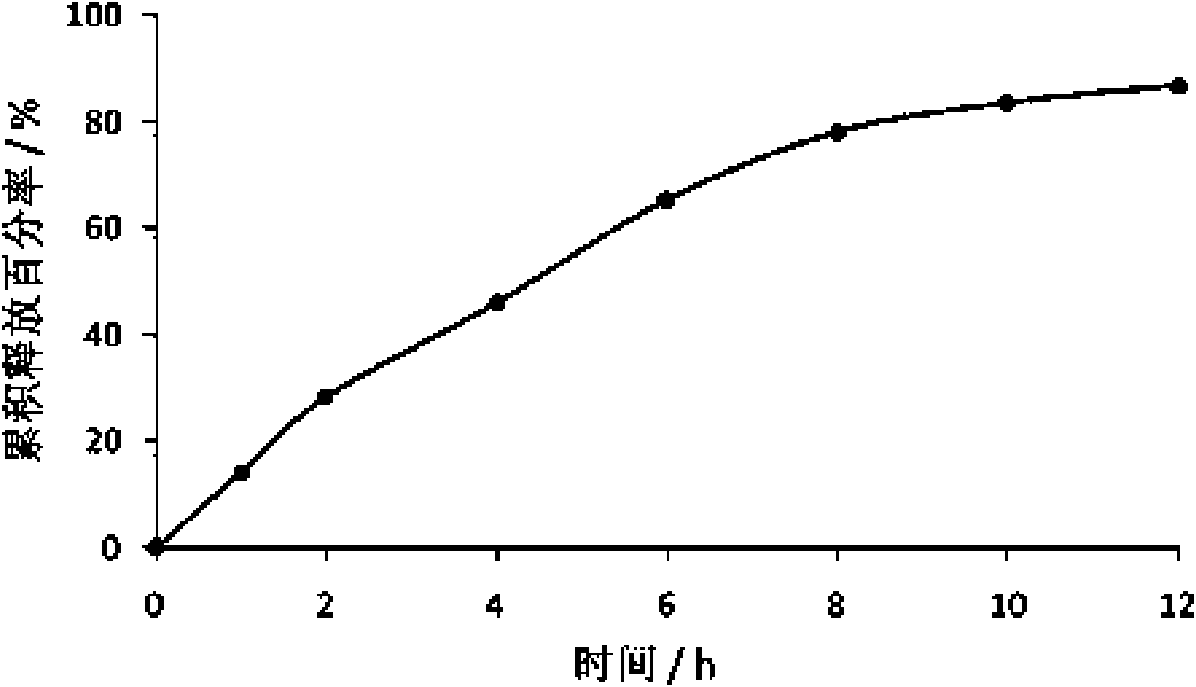
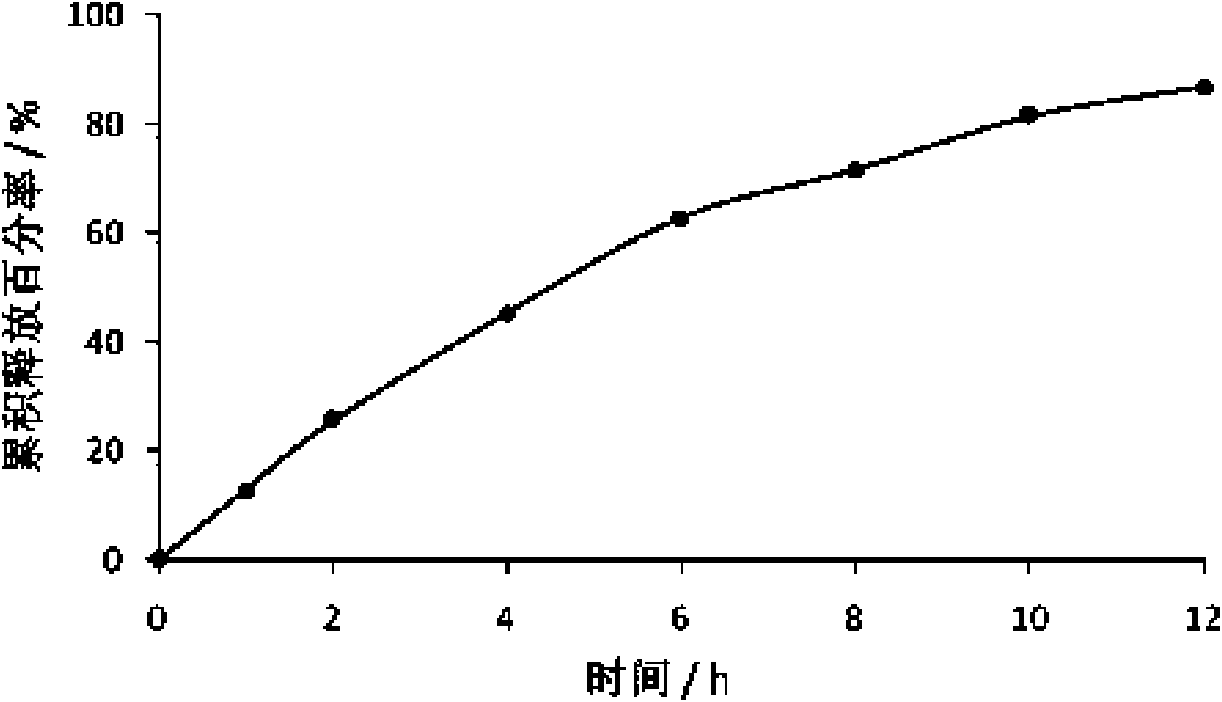
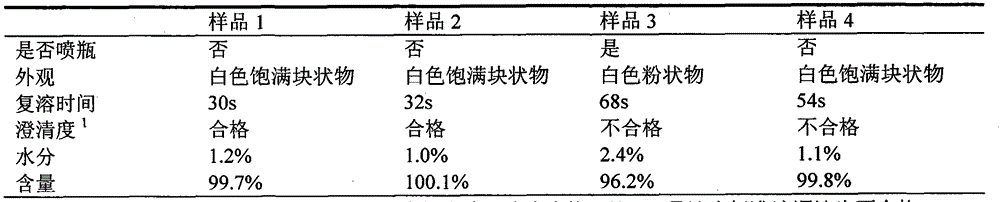
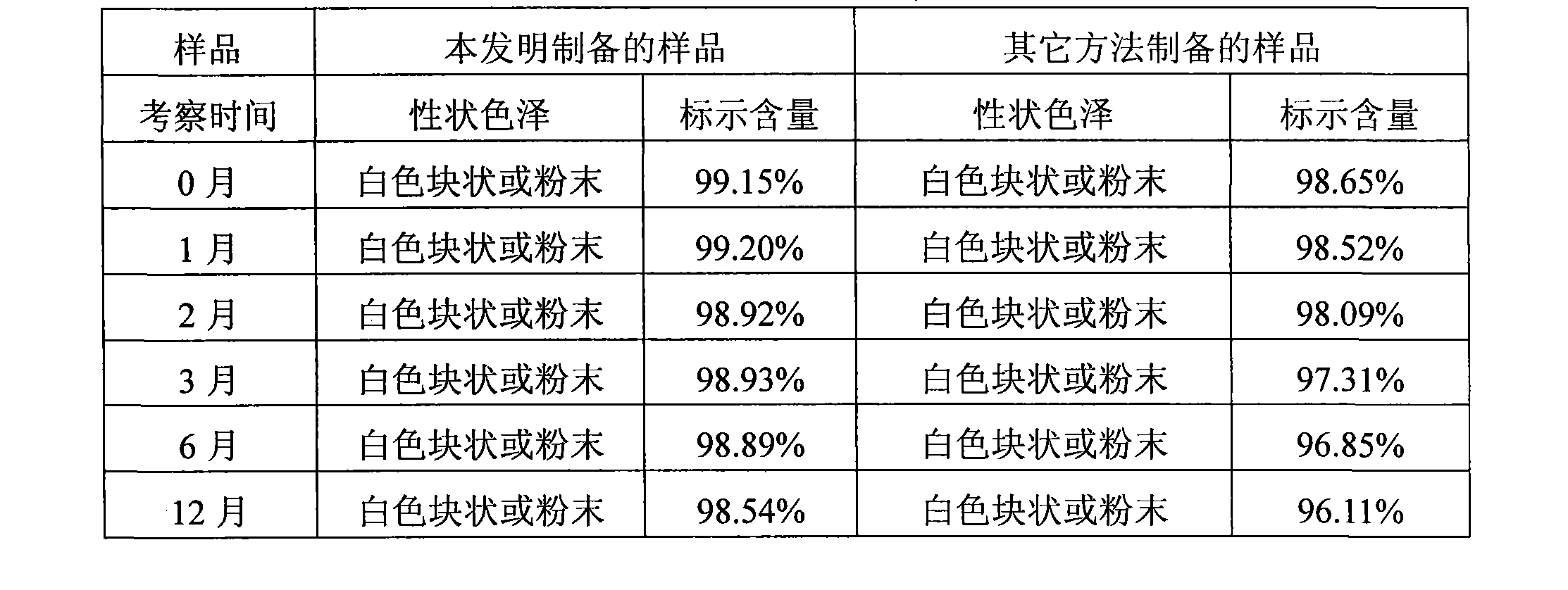
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
411results about How to "Good resolubility" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
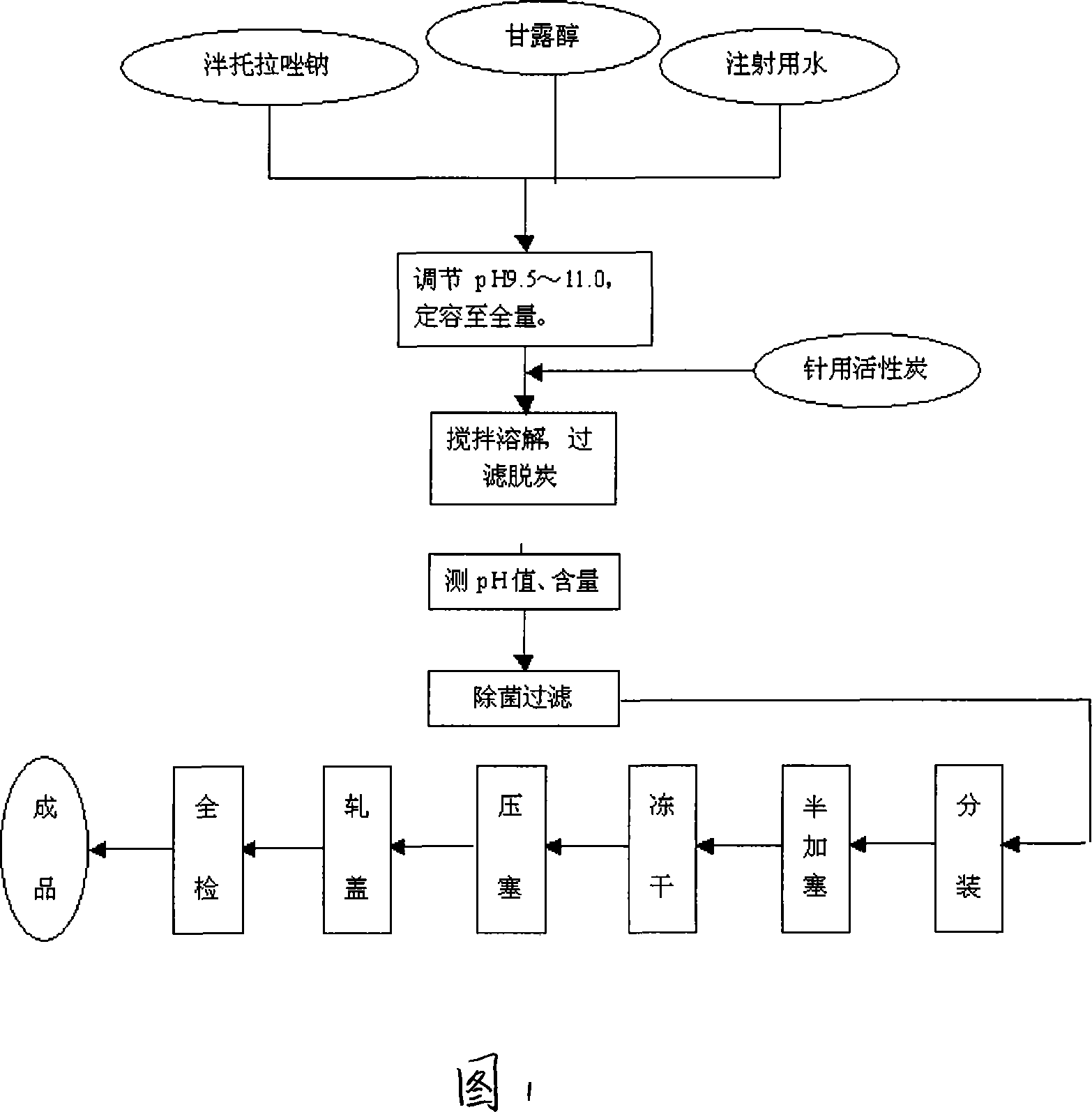
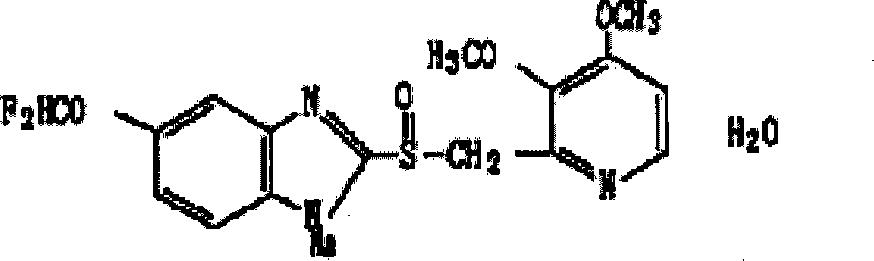
Pantoprazole sodium freeze-dried powder injection and preparing method thereof
ActiveCN101229138ASimple recipeLittle side effectsPowder deliveryOrganic active ingredientsSolubilityMANNITOL/SORBITOL
The invention aims at providing a pantoprazole sodium freeze-dried powder injection and comprises pantoprazole sodium and mannitol with the weight ratio of 1: 2 to 5. The invention is simple in formula and little in side effect; products prepared by the method are plump in appearance, good in complex solubility and excellent in quality with the adoption of an advanced freezing and drying process.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +1
Pantoprazole sodium compound and pharmaceutical composition thereof
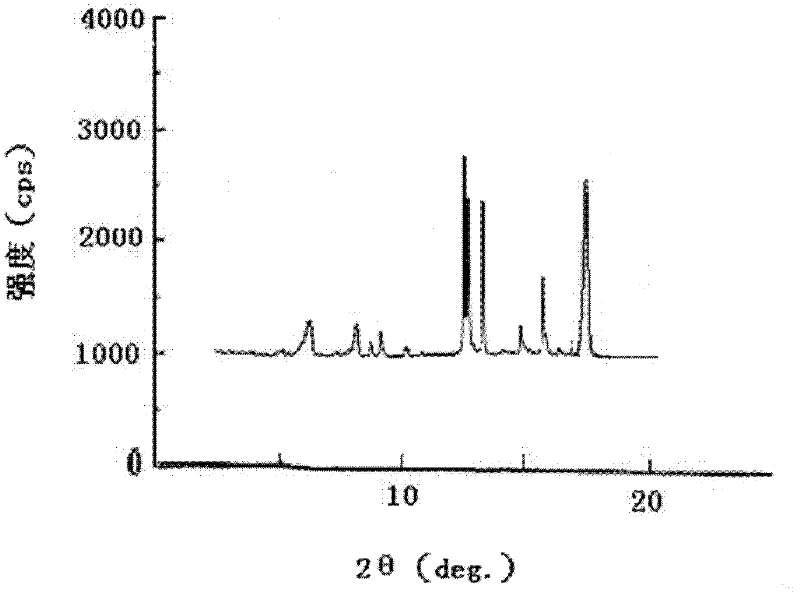
ActiveCN102351844AStable moisture contentNo changeOrganic active ingredientsPowder deliveryFreeze-dryingX-ray
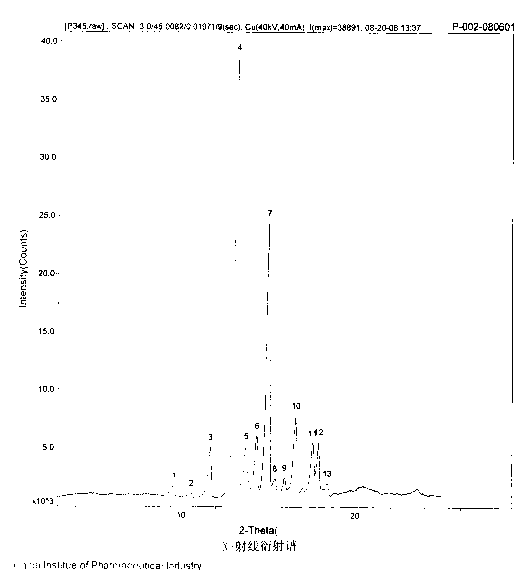
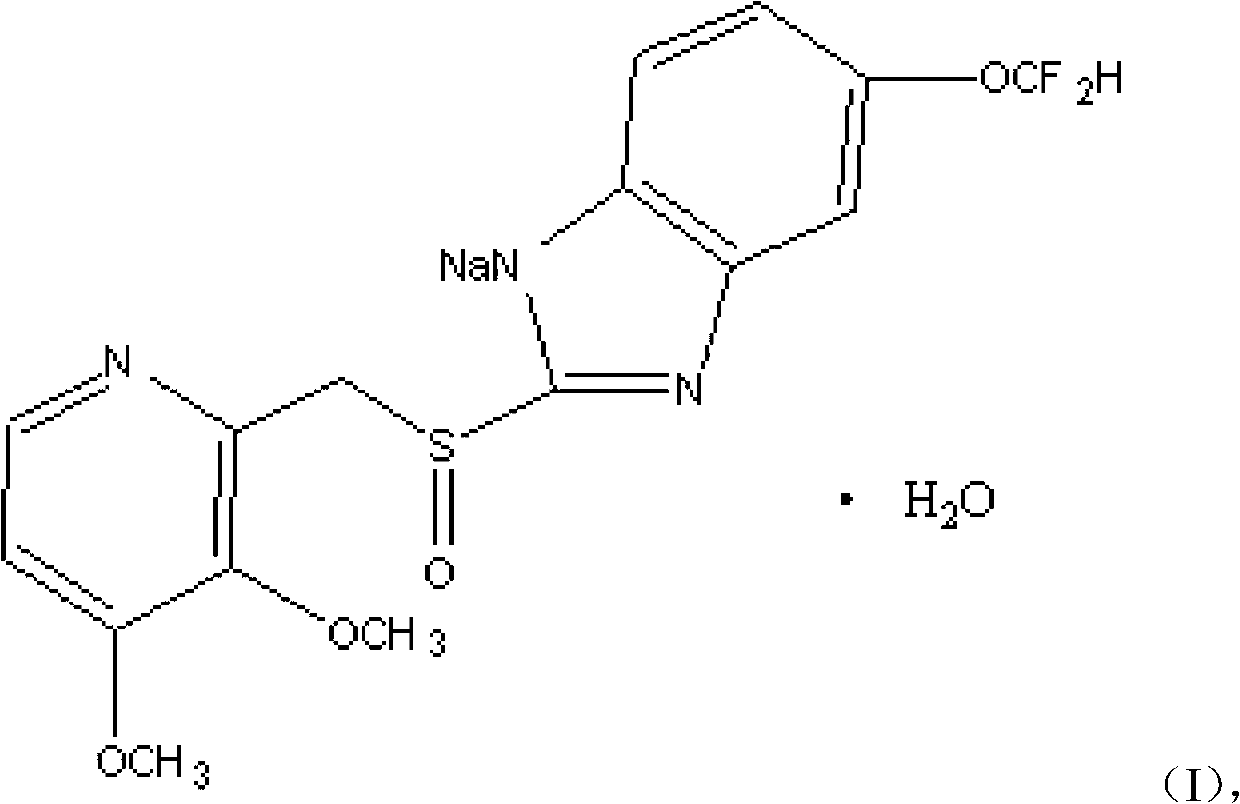
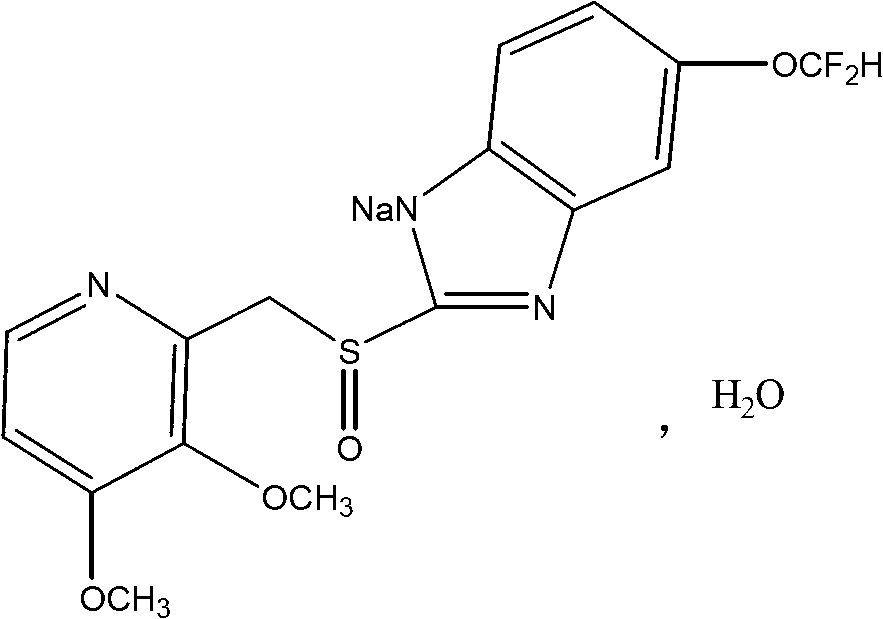
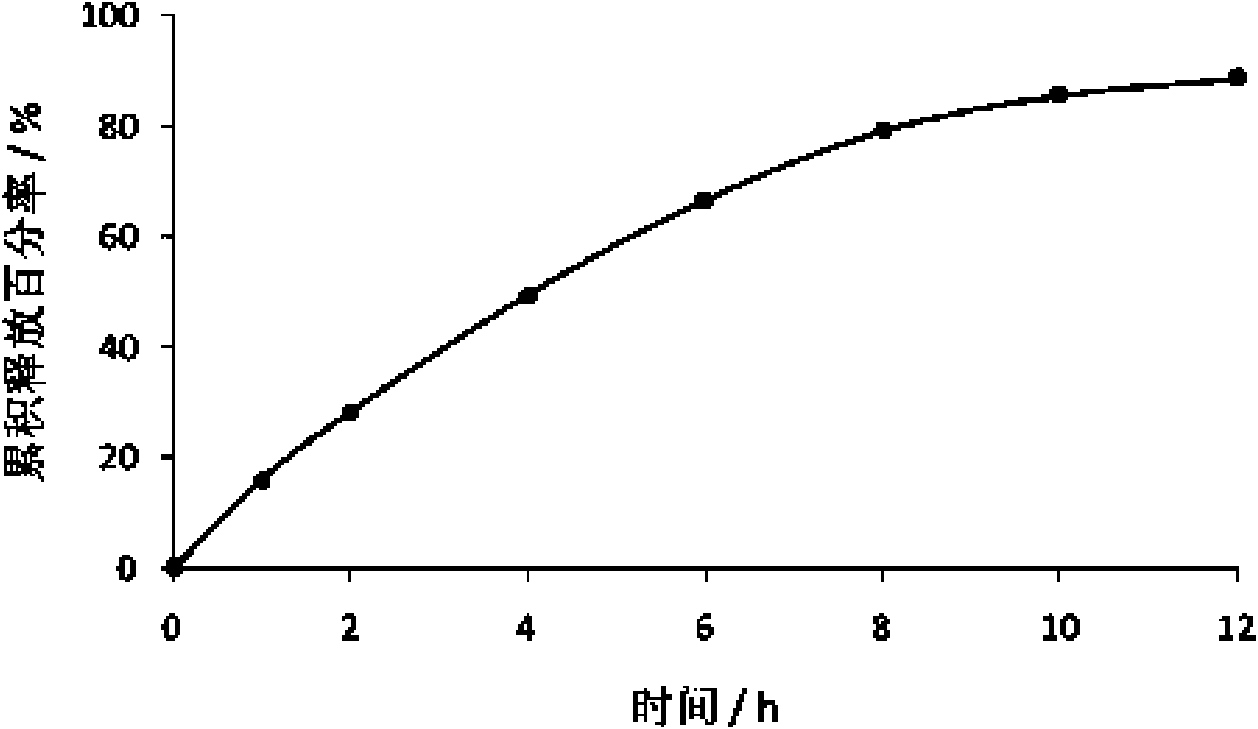
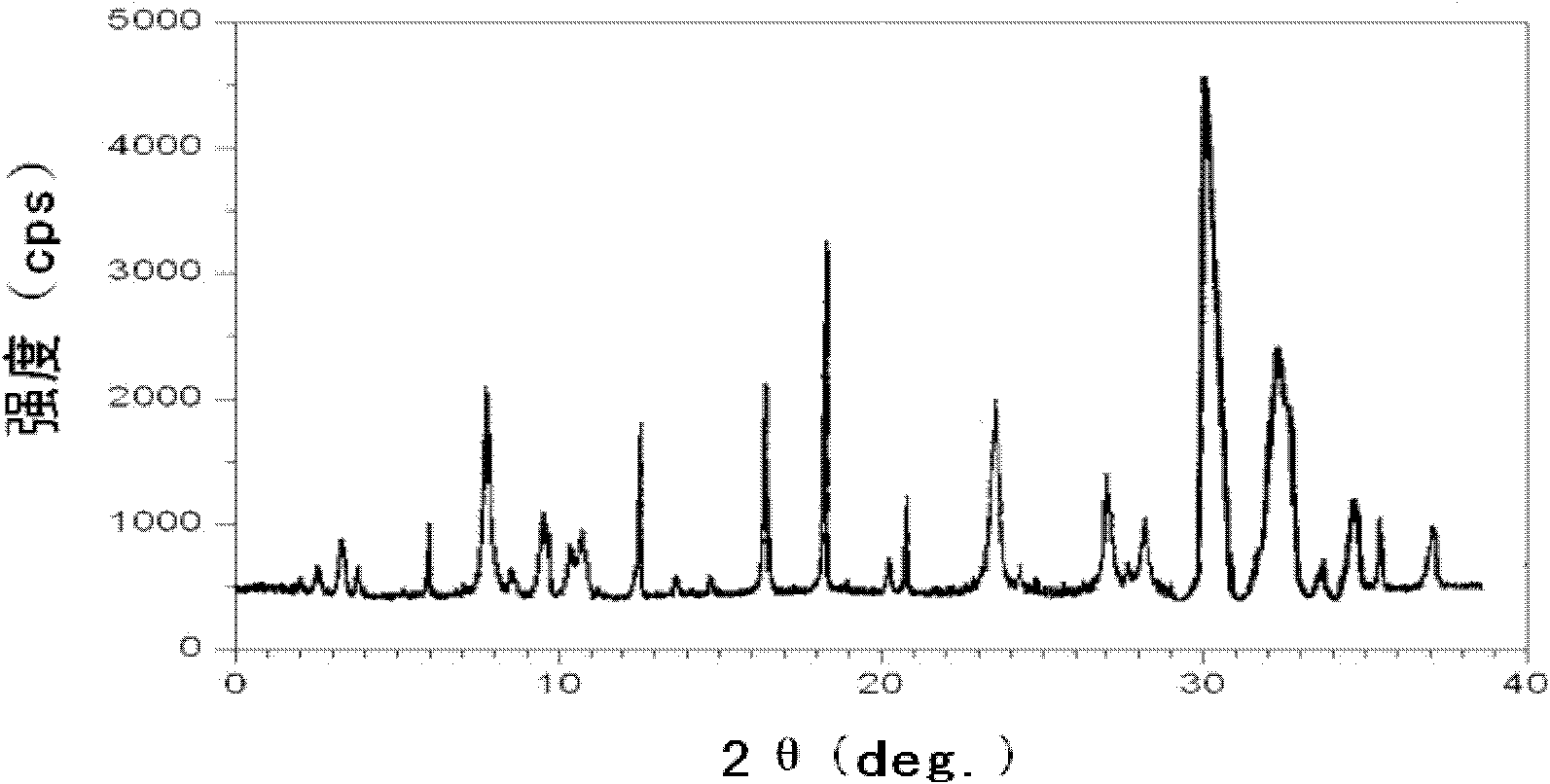
The invention discloses a pantoprazole sodium compound, which is crystal. In X-ray powder diffraction pattern obtained through Cu-Kalpha ray measurement, the characteristic peaks of the pantoprazole sodium compound are shown in positions where 2theta is 12.5, 12.6, 13.2, 16.2 and 17.3. The pantoprazole sodium compound can be used together with multiple freeze-drying supporting agents, the prepared freeze-dried powder injection has the advantages of good redissolution, good transparency after redissolution, low impurity content and the like; and moreover, the use amount of the freeze-drying supporting agent is lower, thus saving the pharmaceutical cost and improving the stability of a drug preparation. The invention also discloses a pharmaceutical composition. The pharmaceutical composition comprises a pharmaceutical active ingredient and pharmaceutical auxiliary materials, wherein the pharmaceutical active ingredient is the pantoprazole sodium compound. The stability of the pharmaceutical composition is obviously superior to that of commercial products, and especially, the stability duration of the pharmaceutical composition after being matched with common infusion fluid is prolonged, thus facilitating the clinical application.
Owner:江西新先锋医药有限公司
Omeprazole freeze-dried powder injection and preparation method thereof
InactiveCN101283986AStable color and lusterLittle side effectsOrganic active ingredientsPowder deliveryOmeprazole SodiumSide effect
The invention provides an omeprazole sodium lyophilized powder for injection (pH of 10.8-11.2), comprising omeprazole sodium as the main active component, as well as excipient, metal ion complexing agent, stabilizer, antioxidant and pH regulator. The omeprazole sodium lyophilized powder for injection has higher active component content than that of the conventional omeprazole sodium injection, solves solution clarity problem, and effectively prevents loss of bone calcium due to complexation with calcium ion. The omeprazole sodium lyophilized powder for injection also has the advantages of stable color and properties, low side effects, good stability, good redissolution, and convenient application.
Owner:海南瑞基药物研究有限公司
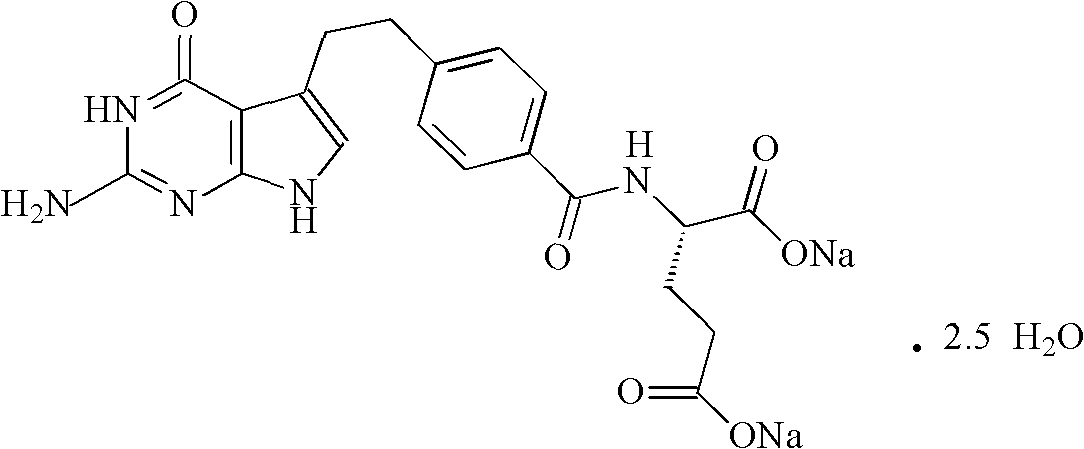
Pemetrexed disodium freeze-dried powder injection and preparation method thereof
ActiveCN102106833AReduce adverse effectsSimple prescriptionOrganic active ingredientsPowder deliveryActivated carbonFreeze-drying
The invention belongs to the technical field of medication, and in particular relates to a pemetrexed disodium freeze-dried powder injection and a preparation method thereof. The pemetrexed disodium freeze-dried powder injection consists of pemetrexed disodium and mannitol, wherein the mass ratio of the mannitol to the pemetrexed disodium is (0.6-2.0):1. The preparation method comprises the following steps: adding injecting water into a liquid preparation tank; adding the pemetrexed disodium weighted according to the formula; stirring until the pemetrexed disodium completely dissolved; adding the mannitol; regulating the pH by utilizing a hydrochloric acid solution or a sodium hydroxide solution; adding activated carbon for decoloration; filtering to remove the carbon; finely filtering with a filter membrane; subpackaging; and freezing and drying. The pemetrexed disodium freeze-dried powder injection has excellent moldability; the appearance of the solution before freezing is clear; the frozen and dry product has good re-dissolubility; and the re-dissolved product has the advantages of good clarity, low impurity content, low moisture content, good stability and controllable quality.
Owner:HAINAN JINRUI PHARMA
Bortezomib freeze-dried powder injection and preparation method thereof
ActiveCN102784114AAvoid growingSmall total impuritiesPowder deliveryDipeptide ingredientsDrugChemistry
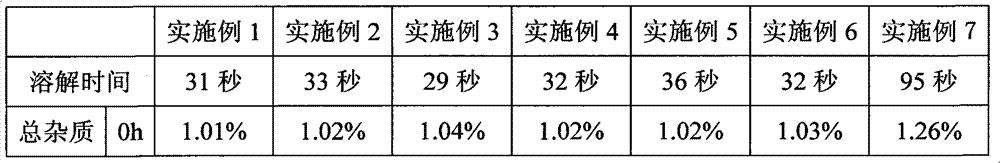
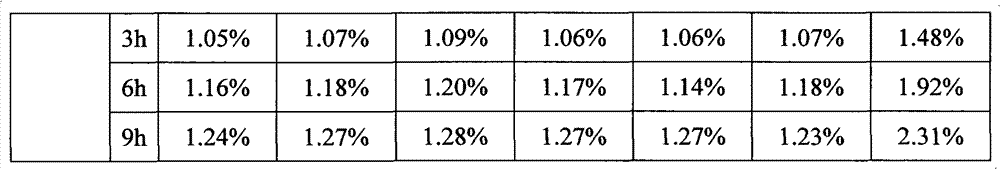
The invention belongs to the technical field of pharmaceuticals, and specifically relates to a bortezomib freeze-dried powder injection and a preparation method thereof. Directed at poor clarity of a redissolved bortezomib preparation, and fast increase of impurities in the bortezomib preparation with standing time, the present invention provides a bortezomib freeze-dried powder injection which includes bortezomib, an excipient, an antioxidant, and a pH adjusting agent. The resulting preparation is good in redissolution with a dissolution time of 29-36 seconds, while the total impurity content of the preparation is not higher than 1.27% after placing for 9 hours at room temperature. The preparation is good in stability, and helps to increase the safety of drug use.
Owner:SHANDONG NEWTIME PHARMA
Aarin preparation for injection and preparing process thereof
InactiveCN1698590AFull shapeGood formabilityPowder deliveryComponent separationMANNITOL/SORBITOLAsarone
Owner:许群
Lornoxicam freeze-dried injection and preparation method thereof
ActiveCN101327193AHigh clarityGood formabilityPowder deliveryOrganic active ingredientsMedicineFreeze-drying
The invention relates to lomoxicam freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection comprises omoxicam, mannite, tromethamine, EDTA and pH regulator. The dosage of the mannite is 3.5 to 8.5 g of mannite per gram of lomoxicam, and the dosage of the EDTA is 0.015 to 0.025g of EDTA per gram of lomoxicam. The invention adopts EDTA to replace EDTA-disodium salt and selects the dosages of the mannite and EDTA so that the clarity of the reconstituted obtained freeze-dried powder is greatly improved, and the formability is good; in addition, the invention adopts two pH regulators, in the process, one pH regulator is used to regulate the solution to a pH range, then the other pH regulator is used to regulate the solution to another pH range, and the freeze drying technology is controlled strictly. The prepared freeze-dried powder injection has great improvement of stability and favorable resolubity.
Owner:HAINAN JINRUI PHARMA CO LTD
Temozolomide freeze-dried preparation
ActiveCN101869551ASolve the speed problemSolve poor resolubilityOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLFreeze-drying
The invention discloses a temozolomide freeze-dried preparation. Every 100ml of the preparation comprises 1 to 2,000 mg of temozolomide, 1 to 2,000 mg of solubilizer, 801 to 2,000 mg of polysorbate, 5 to 5,000 mg of mannitol, 1 to 2,000 mg of buffering agent, 0.5 to 1,000 mg of hydrochloric acid and the balance of water. The preparation of the invention overcomes the shortcomings of slow dissolving speed, poor redissolution, fussy operation and unqualified solution clarity of the freeze-dried preparation, has the technical effects that: the preparation of the invention has high redissolution; solid is completely dissolved to be clear and colorless by adding water into the freeze-dried preparation and shaking slightly; purity is over 99.5 percent and single impurity content is below 0.3 percent by detecting the purity and the content by using HPLC; through stability experimental investigation, relative substances and content of the preparation are not changed remarkably; and quality is stable.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Self-crosslinking polyacrylate rubber latex, preparation method thereof and water-based paper printing ink
InactiveCN101870793APrevent sticking backFast dryingPaper coatingCoatingsWater basedHydrophilic monomer
The invention discloses self-crosslinking polyacrylate rubber latex, a preparation method thereof and water-based paper printing ink. The self-crosslinking polyacrylate rubber latex is prepared by the following steps: mixing an acrylic ester monomer, a hydrophilic monomer and ADH in a head tank A; mixing water, an emulsifier, the acrylic ester monomer and DAAM in a head tank B; adding water, the emulsifier and polymerizable self-emulsifying functional monomer, stirring the mixture, heating the mixture to 75 DEG C, and dripping a mixture in the head tank A and an initiator to prepare a protective colloid first; dripping the mixture in the head tank B and the initiator at 85 DEG C; and performing a constant-temperature reaction. The water-based paper printing ink is prepared by the following steps: dissolving a phenylethylene-acrylic acid polymer and a pH value regulator in water at 60 DEG C with stirring; adding a pigment and necessary assistant and mixing the mixture to prepare color paste; and uniformly mixing the color paste with the self-crosslinking polyacrylate rubber latex, a surface tension regulator and water. The water-based paper printing ink can be diluted by one or a mixture of two of water and ethanol, can be dried quickly and has high printing adaptability.
Owner:SUN YAT SEN UNIV +1
High-covering-power styrene-acrylate emulsion, synthesis method thereof and use thereof in aqueous printing ink
The invention relates to the field of printing ink and discloses high-covering-power styrene-acrylate emulsion, a synthesis method thereof and use thereof in aqueous printing ink. The preparation method comprises: mixing deionized water, an initiator, a lacquer and a composite emulsifier to obtain solution A; regulating the pH value; introducing nitrogen to remove oxygen; mixing a monomer with a molecular weight regulator to obtain solution B; dripping solution B into solution A to perform a chemical reaction; regulating the pH value of shell emulsion and heating to prepare shell emulsion; adding a composite emulsifier and an initiator into the synthesized shell emulsion; dripping a vinyl monomer to synthesize core-shell emulsion; and cooling, regulating the pH value and filtering to obtain the styrene-acrylate emulsion. The invention also provides aqueous printing ink using the high-covering-power styrene-acrylate emulsion. The preparation process is very simple, the reaction processis simple and easy to control, the operability is high, and the method is suitable for large-scale production. The aqueous printing ink disclosed by the invention is high in resolubility, is insusceptible to crusting and blocking printing plate and has high printing adaptability.
Owner:广东天龙油墨有限公司
Production technology for preparing freeze-drying genetic engineering bacterium competence cell and protective agent formula
InactiveCN101264062AReduce moisture contentNot prone to oxidationPowder deliveryGenetic material ingredientsBiotechnology researchFreeze-drying
The invention discloses a manufacturing technique for preparing frozen-out gene engineering bacteria competent cells. The invention also relates to a prescription of protecting agent, which is wide in application by taking as the host bacteria for transferring outer DNA in researching, developing, producing and checking of biotechnology. The protecting agent is one of the most fundamental matching consumption preparations. The invention utilizes vacuum freeze drying technology to deal with the poor gene engineering bacteria competent cells, and can save the cells steadily at a wide temperature range of 20 DEG C below zero to 4 DEG C for long time while keeping high transformation efficiency. The invention enables the cells to store and long-distance transport conveniently. The invention relates to a manufacturing technique for preparing frozen-out competent cells, quality inspection regulation and the frozen-out protecting agent, comprising culture conditions of gene engineering bacteria, technological processes of freezing and drying and the composition and matching of the protecting agent. The protecting agent is formed by water and one or arbitrary combination of the following materials: gelatin, degreasing milk, dextran, trehalose, sucrose, sorbitol or mannitol.
Owner:袁红杰 +2
Self-crosslinked polyacrylate latex and preparation method thereof, and water-based paper ink
InactiveCN102304262APrevent sticking backFast dryingPaper coatingCoatingsHydrophilic monomerPolymer science
The invention discloses self-crosslinked polyacrylate latex and a preparation method thereof, and water-based paper ink. The preparation method for the self-crosslinked polyacrylate latex comprises the following steps of: mixing acrylate monomers, hydrophilic monomers and alcohol dehydrogenase (ADH) in a head tank A; mixing water, an emulsifier, acrylate monomers and diacetone acrylamide (DAAM) in a head tank B; adding water, an emulsifier and polymerization self-emulsification functional monomers into a normal-pressure reaction tank, stirring, heating to 75DEG C, dripping a mixture in the head tank A and an initiator, and preparing protective colloid; dripping a mixture in the head tank B and an initiator at 85DEG C; and reacting at constant temperature. The water-based paper ink is prepared by the following steps of: stirring and dissolving styrene-acrylate copolymer, a pH regulator and water at 60DEG C; adding pigments and necessary aids, and mixing to obtain color paste; and uniformly mixing the color paste, the self-crosslinked polyacrylate latex, a surface tension regulator and water. The water-based paper ink can be diluted by one of water and ethanol or a mixture of water and ethanol, and has high drying speed and good printing adaptability.
Owner:SUN YAT SEN UNIV +1
Pantoprazole compound, preparation methods and pharmaceutical preparations thereof
ActiveCN102796078AGood resolubilityHigh clarityOrganic active ingredientsPowder deliverySolubilityDrug compound
The invention belongs to the technical field of pharmaceutical compounds, and relates to a pantoprazole sodium compound entity, especially a pantoprazole sodium crystal form, preparation methods and pharmaceutical preparations thereof. The pantoprazole sodium compound is crystal, and measured by X-diffraction powder diffraction, and the diffraction pattern has the following diffraction angles (2Theta) in turn: 9.5 degrees, 10.4 degrees, 11.6 degrees, 13.1 degrees, 13.8 degrees, 14.2 degrees, 15.0 degrees, 15.3 degrees, 15.9 degrees, 16.5 degrees, 17.5 degrees, 18.0 degrees and 18.2 degrees. The pantoprazole sodium compound entity may be associated with a variety of lyophilization supporting agents and the prepared lyophilized powder for injection has good solubility, good clarity and low content of related substances, etc. simultaneously the use level of the used lyophilization supporting agent is relatively less, the cost of the products is reduced, and the stability and quality of the products are improved.
Owner:杭州澳亚生物技术股份有限公司
Yinhuang powder injection for intravenous injection and its prepn process
The present invention discloses a Yinhuang powder injection for intravenous injection and its preparation process. The formula includes honeysuckle extractive in 0.1-0.3 weight portions and skullcap root extractive in 0.1-0.6 weight portions. The preparation process includes the steps of dissolution of honeysuckle extractive and skullcap root extractive in water solution of sodium hydroxide, heating to slightly boiling for 15-20 min, filtering, disinfection, refrigerating for one week, pressurized filtering, freeze drying or spray drying for the filtrate liquid to obtain the said powder.
Owner:李大鹏
Tacrolimus sustained-release preparation and preparation method thereof
InactiveCN101664394AOvercome the problems of poor water solubility and narrow therapeutic windowImprove securityOrganic active ingredientsGranular deliverySolubilityCyclodextrin
The invention provides a tacrolimus sustained-release preparation and a preparation method thereof. The invention adopts a solid dispersion technology, or cyclodextrin inclusion technology or a solubilizing method for micronizing drugs and then adding one or more types of surfactants and the like, and obviously improves solubility thereof so as to improve the bioavailability thereof, then adds oneor more types of skeleton materials and other auxiliary materials so as to prepare a skeleton type sustained-release preparation, or adopts a sustained-release material to carry out coating so as toprepare a diaphragm-controlled type or osmotic pump type sustained-release preparation. The tacrolimus sustained-release preparation has better solubility and dissolution rate, high bioavailability and sustained-controlled-release effect, thus maintaining stable blood and drug concentration, reducing the occurrence rate of adverse reaction, and improving clinical drug safety; in addition, the invention has easy obtaining for materials, simple and feasible preparation technique, high yield and low cost, can realize large-scale industrialized production and has obviously economical benefit.
Owner:宋洪涛
Preparation method of human coagulation factor VIII
ActiveCN104231073ASuitable for a wide range of peopleHigh potencyFactor VIIPeptide/protein ingredientsAlcohol sugarsDiabetic patient
The invention discloses a preparation method of a human coagulation factor VIII. The human coagulation factor VIII prepared by the method does not contain human serum albumin or other animal-derived protein, does not contain sugar or sugar alcohol, does not have the risk for transmitting other viruses or pathogene, and is wide in applicable crowd scope, and can be used by diabetic patients; the human coagulation factor VIII prepared by the method is fast to redissolve and good in redissolving effect, and still keeps high titer and high specific activity which are respectively larger than 80 percent and 40 IU / mg; in addition, the preparation method is simple, the cost is low, the human coagulation factor VIII is safe and effective, and has a good industrial application prospect.
Owner:广东双林生物制药有限公司
Freeze-drying process for preparation of bortezomib for injection
InactiveCN106310217AGood resolubilityClarity qualifiedPowder deliveryDipeptide ingredientsSolubilityFreeze-drying
The invention provides a freeze-drying process for preparation of bortezomib for injection, and according to the process, after subpackage of a bortezomib liquor, freeze-drying is performed, main features of a pre freezing stage comprise two times of annealing, in a sublimation drying stage, drying is kept for 30-40h at -30 DEG C. The bortezomib for injection prepared by the process is not spurted from a bottle, has full and complete appearance, good solubility, qualified clear degree and high safety, and is suitable for industrial production.
Owner:BEIJING JIMEITANG MEDICINE RES CO LTD
Novel water-based ink binder self-crosslinking emulsion for flexographic printing and preparation method thereof
The invention discloses a novel water-based ink binder self-crosslinking emulsion for flexographic printing and a preparation method of the novel water-based ink binder self-crosslinking emulsion. Theself-crosslinking emulsion is a copolymer emulsion prepared from the following components through emulsion copolymerization, by weight, 30-70 parts of deionized water, 5-35 parts of water-based resin, 2-40 parts of alkyl acrylate monomer with the total carbon number being 4-12, 0-25 parts of methacrylate monomer with the total carbon number being 5-10, 0-25 parts of a styrene monomer, 0.2-5 partsof a hydroxyl (meth)acrylate monomer, 0.5-5 parts of a crosslinking monomer, 0.01-0.5 part of a metal ion crosslinking agent, 0.01-0.1 part of an initiator and 0-10 parts of a pH regulator. The water-based ink binder self-crosslinking emulsion can be subjected to a self-crosslinking reaction at room temperature, and has excellent resolubility, water resistance and printing transfer performance.
Owner:BEIJING UNIV OF CHEM TECH +1
Tropisetron preparation for injection and preparation method thereof
InactiveCN101444508AFlat surfaceNot crackedDigestive systemMacromolecular non-active ingredientsMANNITOL/SORBITOLActive component
The invention provides a tropisetron preparation for injection. The main active components of the tropisetron preparation are tropisetron, beta-cyclodextrin or hydroxypropyl-beta-cyclodextrin and mannitol. The method comprises the following steps: dissolving tropisetron hydrochloride and the beta-cyclodextrin or the hydroxypropyl-beta-cyclodextrin, then adding the mannitol for dissolving, adjusting the pH value with citric acid-disodium hydrogen phosphate buffer solution to obtain a liquid medicine, quickly prefreezing the liquid medicine after filling, and then lyophilizing to obtain the tropisetron preparation. The tropisetron preparation for injection has flat surface, is fine and smooth and uniform, and is free from crack, breakage and sticking to bottles. The tropisetron preparation is a white loose block which is well formed and very easily dissolved, has good redissolution performance, clean and transparent solution, stable product quality, and practicability.
Owner:海南瑞基药物研究有限公司
Asarone injection and preparation method thereof
ActiveCN101647774AHigh solubilizing powerReduce capacityPowder deliveryNervous disorderAsaronePolyethylene glycol
The invention provides an alpha-asarone injection. In the preparation of the alpha-asarone injection, alpha-asarone (with the chemical name of 2,4,5-trimethoxy-1-propenylbenzene) is used as an activecomponent and carbowax-12-hydroxy stearate is adopted as a solubilizing agent to prepare an injection, thereby effectively solving the problem that the asarone is not dissolved in water, and greatly enhancing the medicament carrying quantity of the injection. The injection prepared by the preparation method has the advantages of good stability and simple preparation technology.
Owner:SICHUAN UNIV
Preparation and application of polymer composition loaded with sirolimus compound or its derivative
InactiveCN103284948AHigh drug loadingHigh encapsulation efficiencyOrganic active ingredientsPowder deliveryPercent Diameter StenosisFreeze dry
The invention relates to a polymer composition loaded with a sirolimus compound or its derivative. The composition can have diversified forms. A necessary link includes preparing the sirolimus compound or its derivative and a polymer carrier into a micelle. Then according to needs, the micelle can be further prepared into a freeze-dried composition by a freeze-drying technology, or the micelle and other polymer and carrier can be prepared into a solid or semi-solid preparation. The polymer composition can be used for treating tumors, reducing rejection reactions after organ and tissue transplantation, promoting cell regeneration and repair, preventing excessive scar tissue growth after injury and preventing vascular restenosis and blood coagulation embolism, treating or inhibiting autoimmune diseases, and treating or inhibiting inflammation, etc.
Owner:单颖
Stable phytosterol nanometer emulsion compounded by soy protein-stevioside, and preparation method and application thereof
ActiveCN108618146AGood resolubilityUniform particlesMilk preparationFood ingredientsProtein solutionOil phase
The invention discloses stable phytosterol nanometer emulsion compounded by soy protein-stevioside, and a preparation method and application thereof. The method comprises the steps of mixing a soy isolate protein solution and a stevioside solution to prepare a composite stable system; dissolving phytosterol into sunflower seed oil being 90 to 99 DEG C to obtain an oil phase; adding thw oil phase dissolved with the phytosterol into a water phase of soy protein-stevioside; controlling the mass ratio of the oil phase to the water phase to be 10:90-50:50; after the homogenizing, performing ultrasonic treatment or high-pressure micro jet flow treatment to obtain the stable phytosterol nanometer emulsion compounded by soy protein-stevioside. A powder product with high redissolving performance loaded with phytosterol can be prepared through spray drying treatment on the nanometer emulsion. The process conditions are simple and mild; the natural effect and safety are realized; the fast continuous production can be performed; products applied to food, medicine and cosmetics can be prepared through operating and controlling the process conditions; the industrialized and scaled application values are realized.
Owner:SOUTH CHINA UNIV OF TECH
Water-based gravure yellow ink for soft PVC decoration film, and preparation method thereof
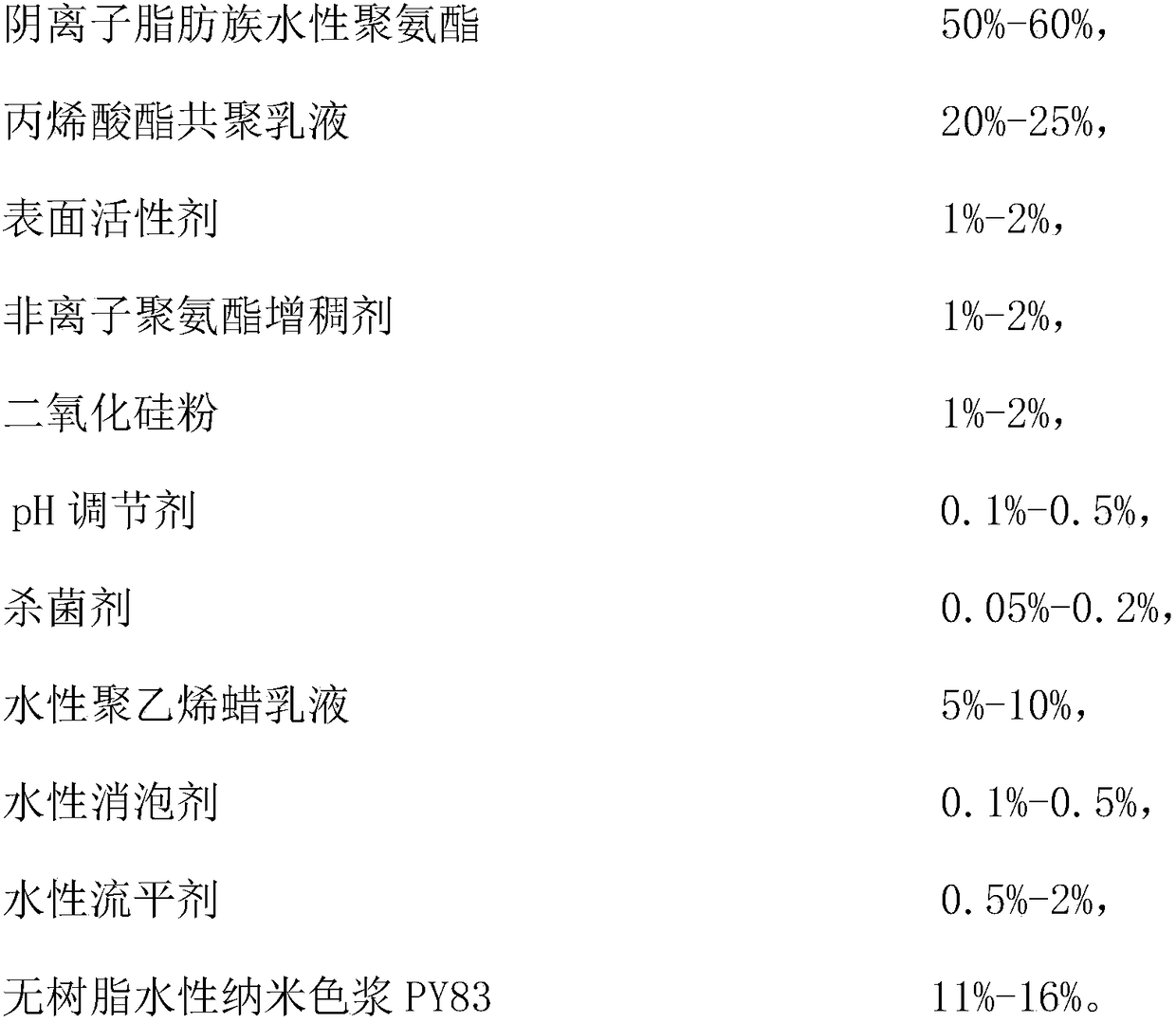
The invention relates to a water-based gravure yellow ink for a soft PVC decoration film. The water-based gravure yellow ink comprises, by mass, 50-60% of anionic aliphatic waterborne polyurethane, 20-25% of an acrylate copolymer emulsion, 1-2% of a surfactant, 1-2% of a nonionic polyurethane thickener, 1-2% of silica powder, 0.1-0.5% of a pH adjuster, 0.05-0.2% of a bactericide, 5-10% of an aqueous polyethylene wax emulsion, 0.1-0.5% of an aqueous defoamer, 0.5-2% of an aqueous leveling agent and 11-16% of a resin-free aqueous nanometer color paste PY83. The invention also provides a preparation method of the aqueous gravure yellow ink. The ink has the advantages of good resolubility, no clogging, good adhesion to a substrate, no after-tack, few bubbles in the use process, convenience inpackaging and construction, high bonding strength, and excellent resistance to yellowing and weathering; and the preparation method has the advantages of simplicity, easiness in control, no grinding process, and improvement of the production efficiency.
Owner:中山创美涂料有限公司
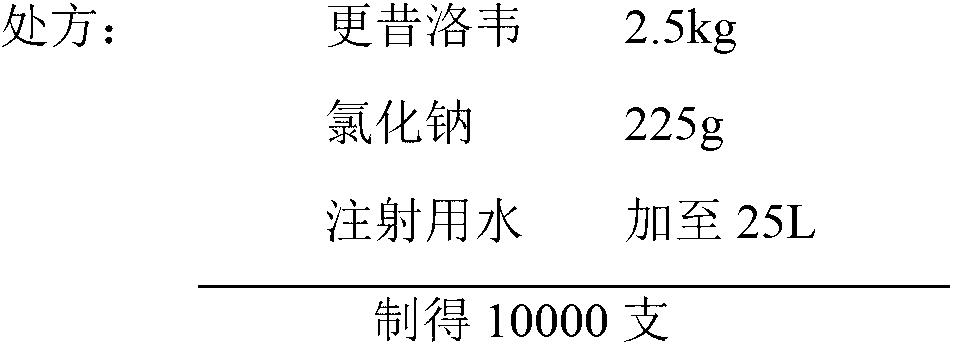
Ganciclovir for injection and preparation method thereof
ActiveCN103054819AAdvantages and Notable ImprovementsImprove yieldPowder deliveryInorganic non-active ingredientsMedicineFreeze-drying
The invention discloses ganciclovir for injection and a preparation method thereof. The preparation is formed by regulating the pH of a solution containing ganciclovir and sodium chloride to 10.0-11.5 by adopting a pH regulator, and freezing and drying repetitively. The freeze-dried powder injection of the ganciclovir for injection has the advantages of high yield, good re-dissolubility, more stable quality and the like; change of related substances during the period of accelerated test is not obvious; and the ganciclovir for injection is safer for clinical application.
Owner:BEIJING WANPENGLANGGE PHARMA TECH
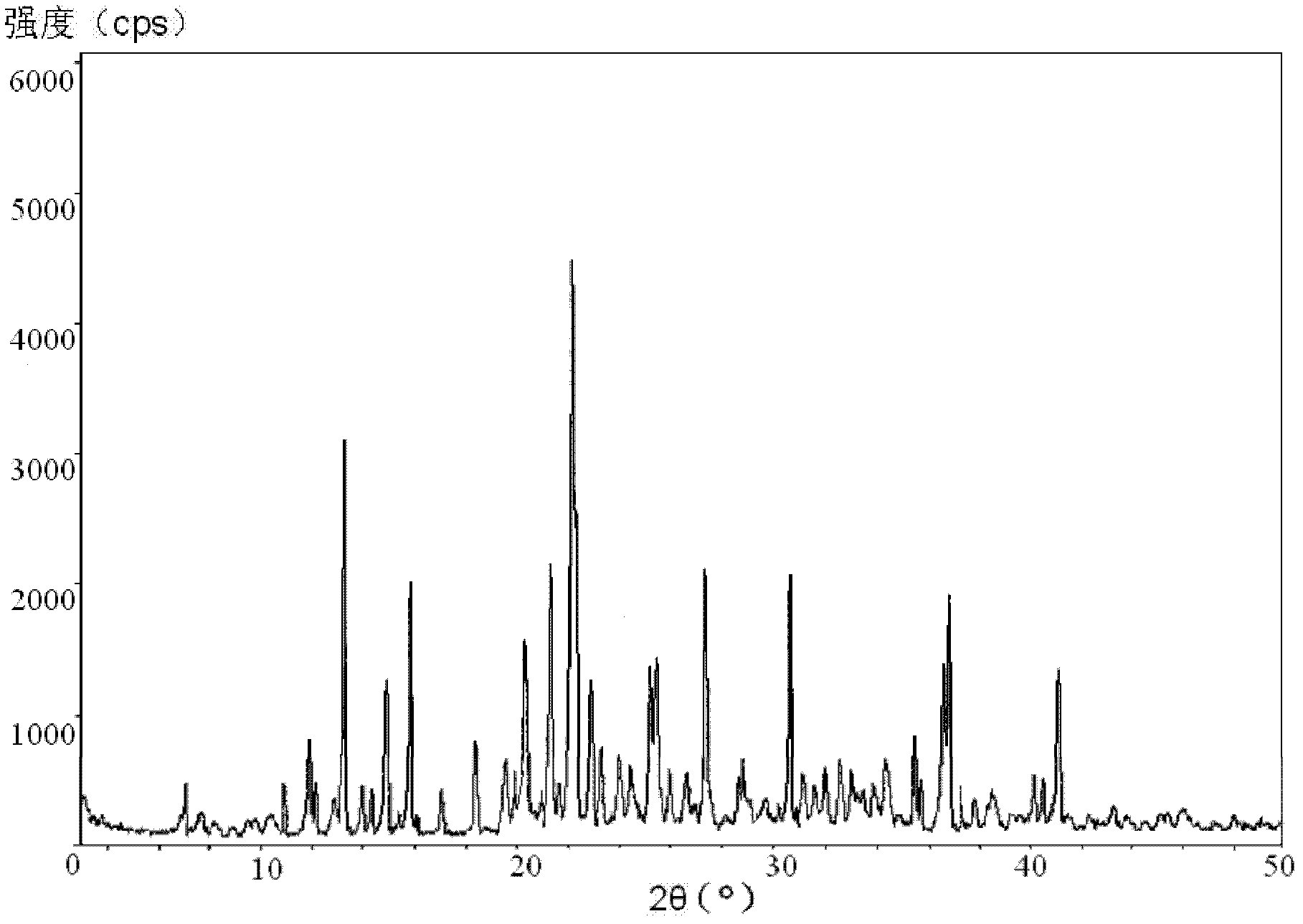
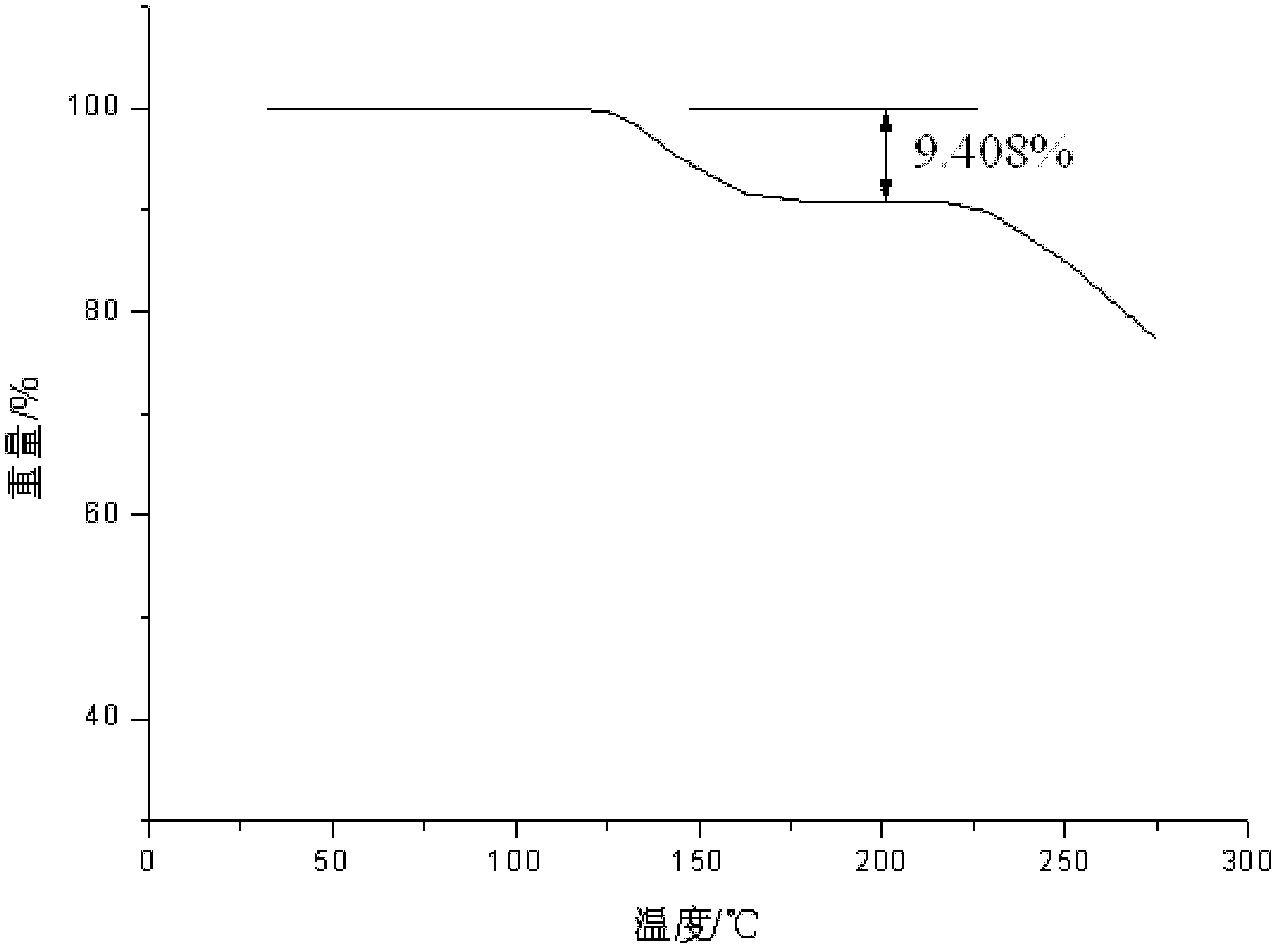
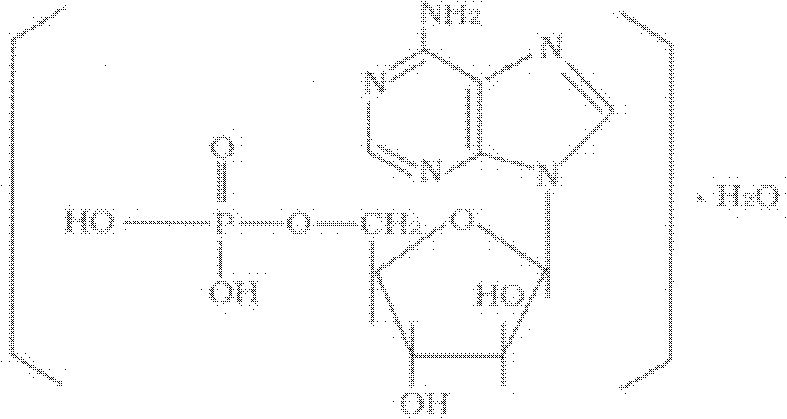
Vidarabine monophosphate freeze-dried powder injection and preparation method thereof
ActiveCN102379853AGood formabilityHigh clarityPowder deliveryOrganic active ingredientsFreeze-dryingPhosphoric acid
The invention relates to a vidarabine monophosphate freeze-dried powder injection and a preparation method thereof, and the vidarabine monophosphate freeze-dried powder injection is prepared by freeze-drying vidarabine monophosphate, sodium hydroxide solution and water for injection, wherein the sodium hydroxide solution is used as a pH regulator, and the sodium hydroxide solution is used until the pH value of liquid medicine is 7.0-7.5. The vidarabine monophosphate freeze-dried powder injection provided by the invention is few in types of auxiliary materials, low in using amount, good in forming property of a freeze-dried product, good in re-dissolving property, clear in appearance of the solution before freezing, good in clarity after being freeze-dried, low in content of impurities, good in stability and controllable in quality, and can reduce the potential safety hazard of the vidarabine monophosphate freeze-dried powder injection, and improve the efficacy of the vidarabine monophosphate freeze-dried powder injection.
Owner:HAINAN JINRUI PHARMA CO LTD
Novel omeprazole sodium compound and medicinal composition thereof
ActiveCN102351846AHigh thermodynamic stabilityHigh yieldOrganic active ingredientsPowder deliveryOmeprazole SodiumFreeze-drying
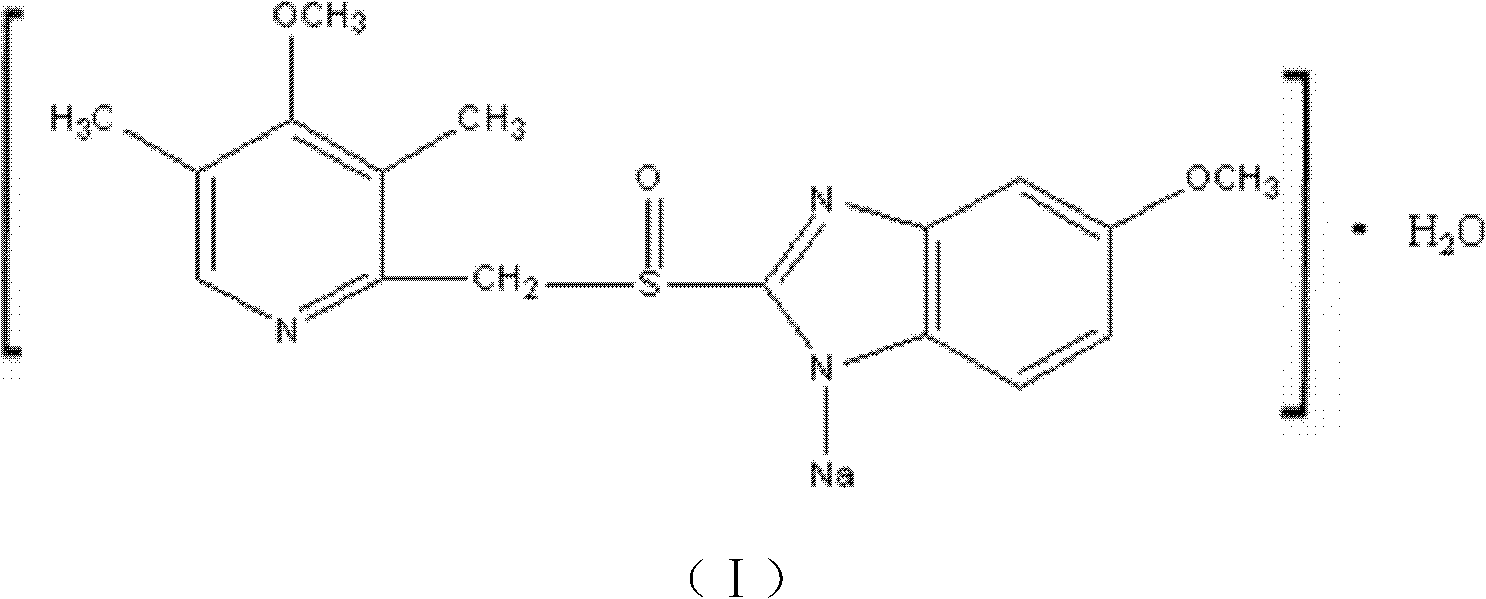
The invention discloses a novel omeprazole sodium compound. Characteristic peaks of the omeprazole sodium compound in an X-ray powder diffraction pattern obtained through Cu-K alpha-ray measurement are displayed when 2 theta is 3.2, 5.9, 7.6, 9.2, 10.5, 10.6, 12.3, 16.2, 18.2, 20.8, 23.4, 27.1, 30.4, 32.3 and 34.6. The omeprazole sodium compound has a new crystal form different from the prior art, has the thermodynamic stability higher than that with known crystal form, and has the advantage of not absorbing moisture basically. The invention also discloses a medicinal composition, which comprises the novel omeprazole sodium compound. Omeprazole sodium freeze-dried powder injection can be directly prepared under the condition of not adding lyoprotectants, and the omeprazole sodium freeze-dried powder injection has the advantages of high stability and high re-dissolubility.
Owner:周晓东
Tirofiban hydrochloride lyophilized powder injection and preparation method thereof
ActiveCN101756915AGood resolubilityExcellent indicatorsPowder deliveryOrganic active ingredientsWestern medicineTirofiban Hydrochloride
The invention relates to a tirofiban hydrochloride lyophilized powder injection and a preparation method thereof, belonging to the field of western medicine preparation. The tirofiban hydrochloride lyophilized powder injection contains 1 part by weight of tirofiban hydrochloride and 0.5-100 parts by weight of dextran, and the pH value of solution before lyophilization is 1.8-2.6. Preferably, the weight ratio between the dextran and the tirofiban hydrochloride is 2:1-20:1. The tirofiban hydrochloride lyophilized powder injection has good composite dissolubility and stability as well as high safety.
Owner:鲁南新时代生物技术有限公司
Ropivacaine mesylate freeze-dried powder injection
ActiveCN102038651AAvoid the risk of safety accidentsGood freeze-dried appearancePowder deliveryPharmaceutical product form changeFreeze-dryingPhysical chemistry
The invention relates to a ropivacaine mesylate freeze-dried powder injection which consists of ropivacaine mesylate and a PH regulator and is prepared by using the following freeze drying method: (1) a section quick-freezing stage: keeping bulked ropivacaine mesylate solution at the temperature of 0 DEG C for 10-30 minutes, and then keeping the bulked ropivacaine mesylate solution at the temperature of minus 35 DEG C-minus 45 DEG C for 1-2 hours; (2) a lyophilization stage: heating to 0 DEG C under the vacuum degree of 10-20 Pa and at the speed of 2-10 DEG C / h, and then keeping the temperature for 1-3 hours; and (3) a desorption drying stage: heating to 30 DEG C under the vacuum degree of 0-10 Pa and at the speed of 5-10 DEG C / h and keeping the temperature for 2-5 hours. The freeze-dried powder injection provided by the invention has the advantages of high yield, good re-dissolubility, more stable quality and the like.
Owner:鲁南新时代生物技术有限公司
Metaduocine freeze-dried powder injection preparation and its preparation method
InactiveCN1476834AGood resolubilityReduce moisturePowder deliveryOrganic active ingredientsAlcoholismsRat liver
The present invention relates to a medicine for curing alcoholism-metadoxine freeze-dried powder injection and its preparation process. The proper quantity of anti-oxidant and chelator can be added in its medicine preparation formula composition so as to raise the stability of said medicinal component. The pharmacodynamic tests show that it has remittence action for rat liver injury due to carbontetrachloride. Said injection is stable, and is favorable for storage and transportation.
Owner:杭州容立医药科技有限公司
Acrylate emulsion of water-based ink for soft PVC (Polyvinyl Chloride) and preparation method thereof
The invention discloses acrylate emulsion of water-based ink for soft PVC (Polyvinyl Chloride) and a preparation method thereof. The acrylate emulsion is prepared from the following raw materials in parts by weight: 4.0 to 4.8 parts of an emulsifier A, 40.0 to 60.0 parts of a mixture A, 2.0 to 6.0 parts of an emulsifier B, 25.5 to 33.0 parts of a mixture B, 25.5 to 31.5 parts of a mixture C, 0.66 to 1.34 parts of an initiator, 4.8 to 18.0 parts of alkali salt and 150.0 to 230.0 parts of water. The acrylate emulsion of the water-based ink is specifically synthesized aiming at application to the soft PVC, and the preparation method of the acrylate emulsion adopts a novel a core-shell emulsion polymerization technology and an interpenetrating polymer network emulsion polymerization technology; an acrylate chain segment with stronger hydrophily is used as a core layer of the acrylate emulsion, and better re-dissolution property and better compatible stability with water-based ink color paste are realized; since the interpenetrating polymer network emulsion polymerization technology is adopted, the strength of an emulsion coating film is greatly increased, the adhesive force of the water-based ink on a soft PVC base material is increased, and the water resistance is good.
Owner:HUBEI ENG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com