Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
33 results about "Tropisetron" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
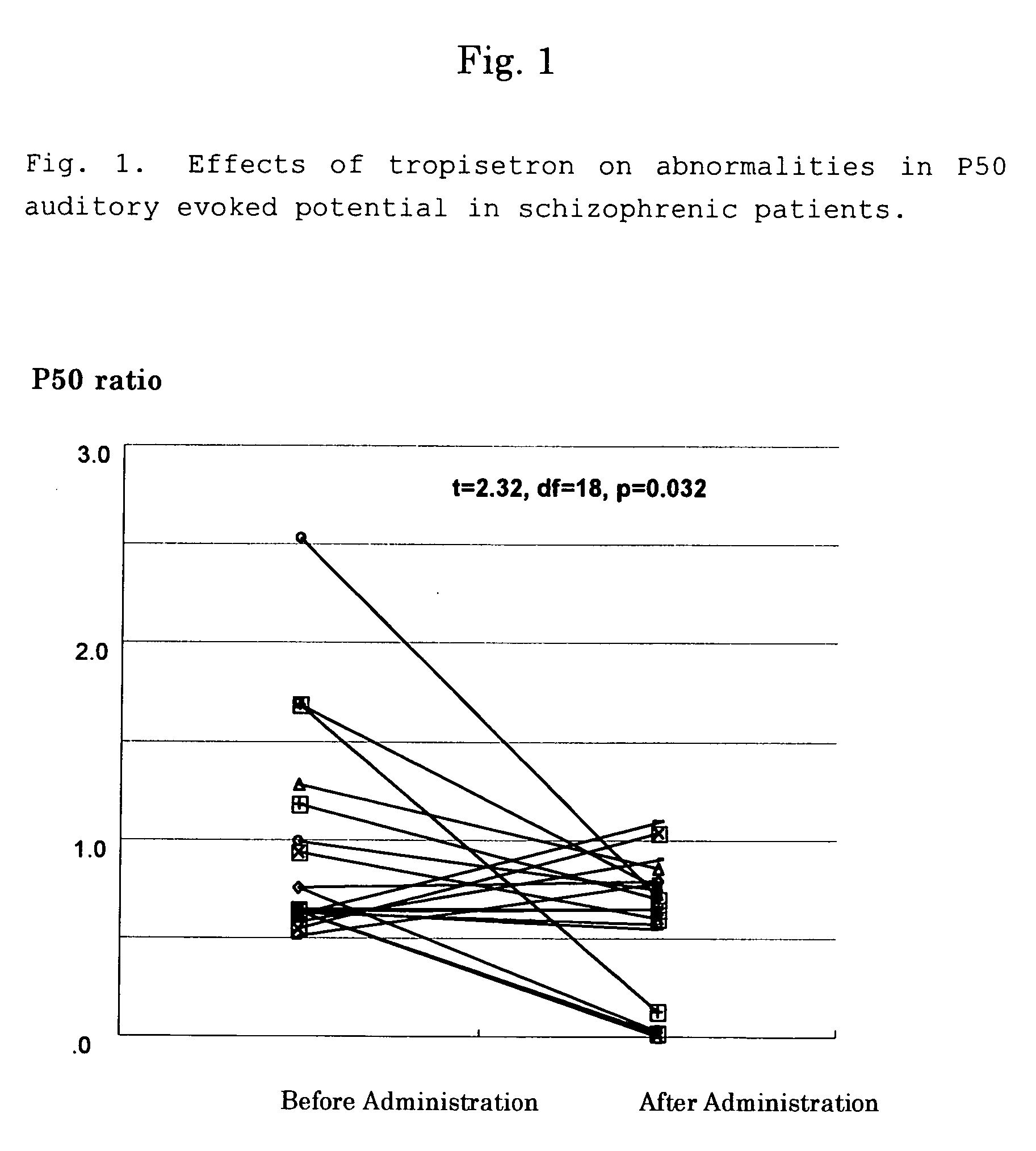
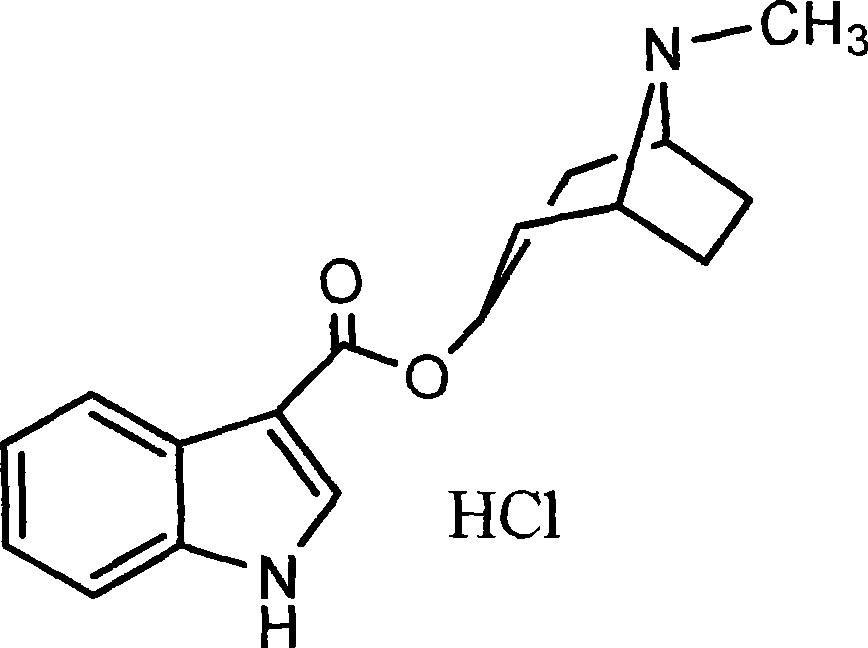
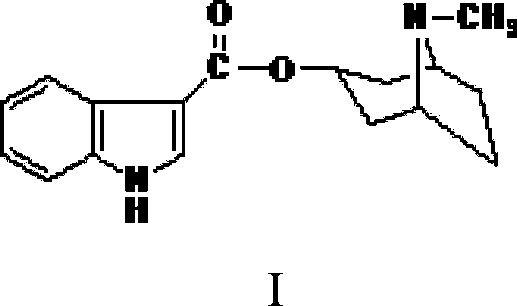
Tropisetron is a serotonin 5-HT₃ receptor antagonist used mainly as an antiemetic to treat nausea and vomiting following chemotherapy, although it has been used experimentally as an analgesic in cases of fibromyalgia.
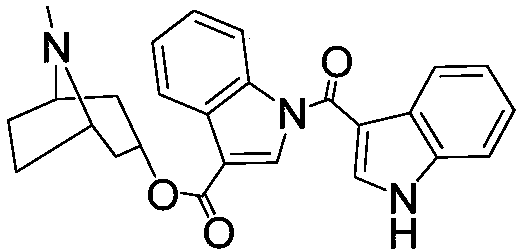
Methods of treating mild cognitive impairment (MCI) and related disorders
ActiveUS20120071468A1Progression from an asymptomatic state to a symptomatic state is prevented or delayedBiocideNervous disorderHonokiolMedicine
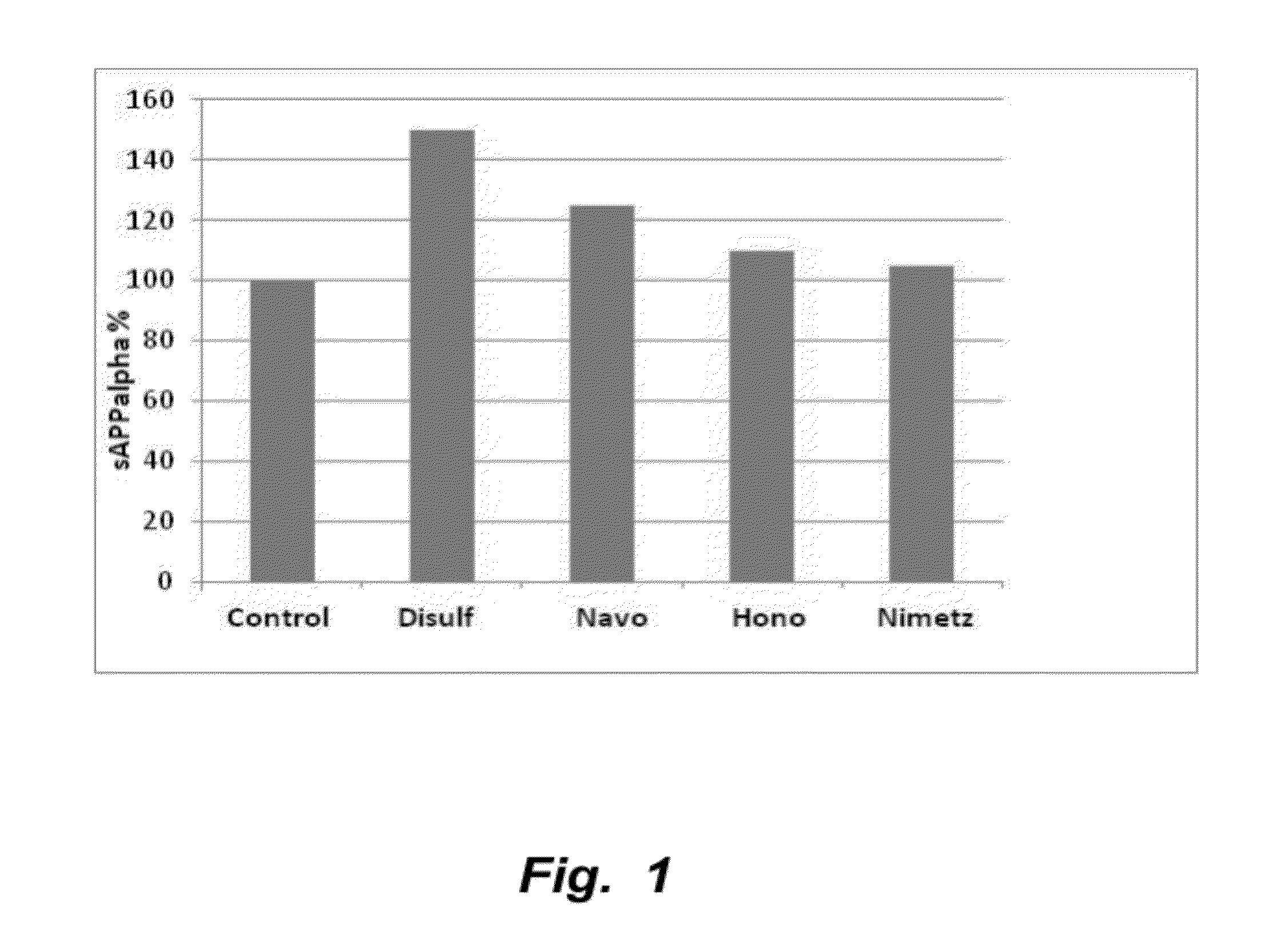
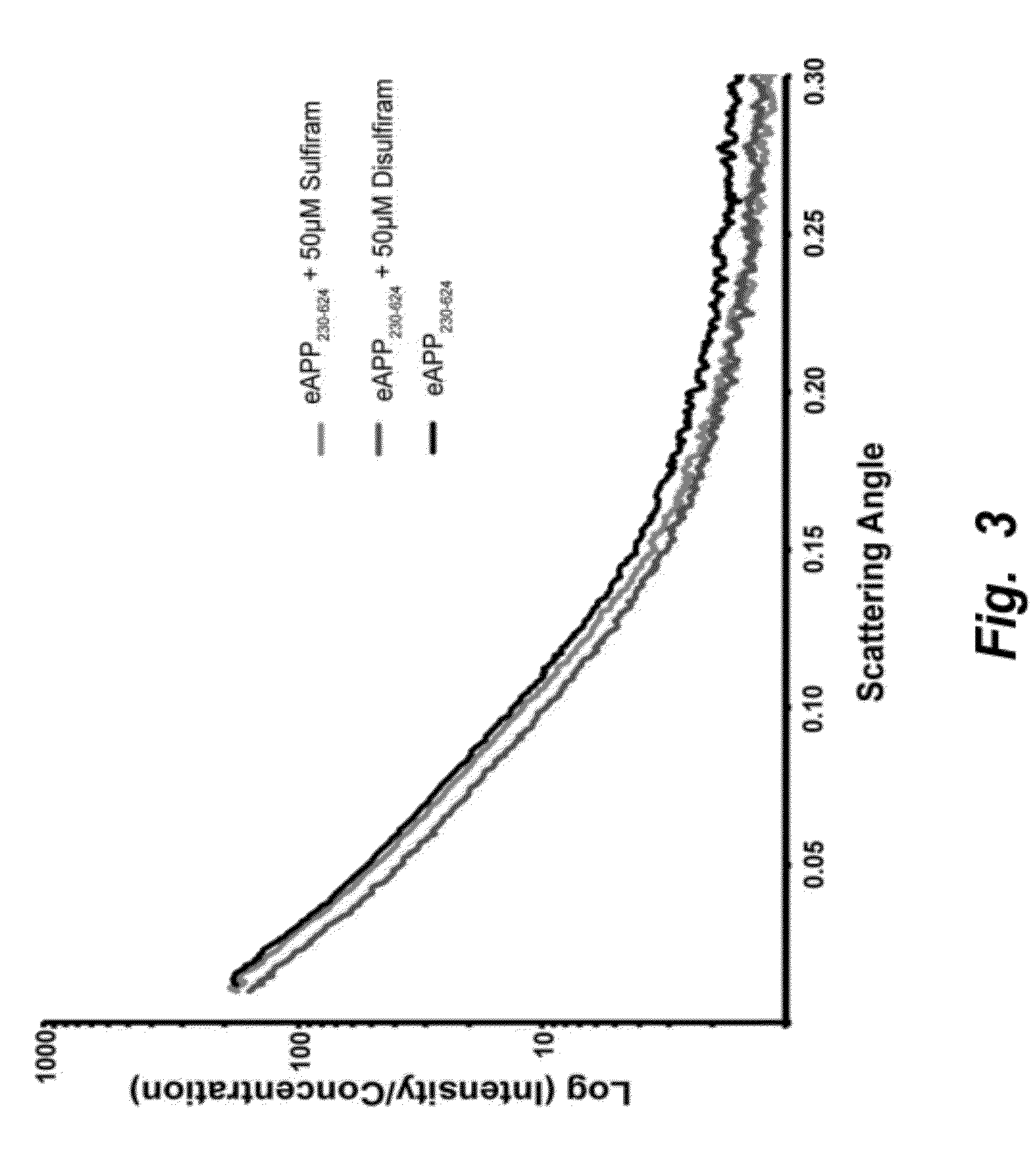
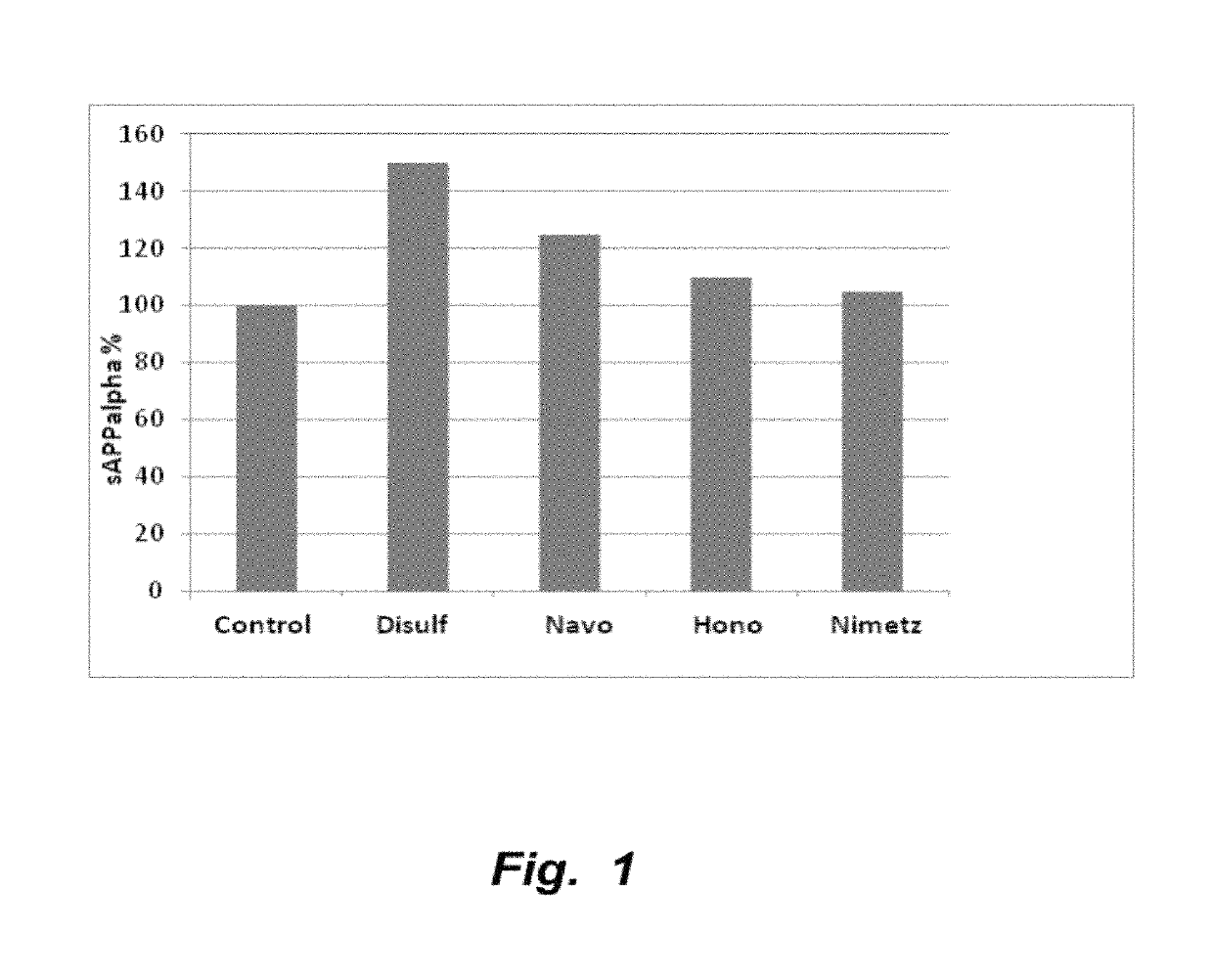
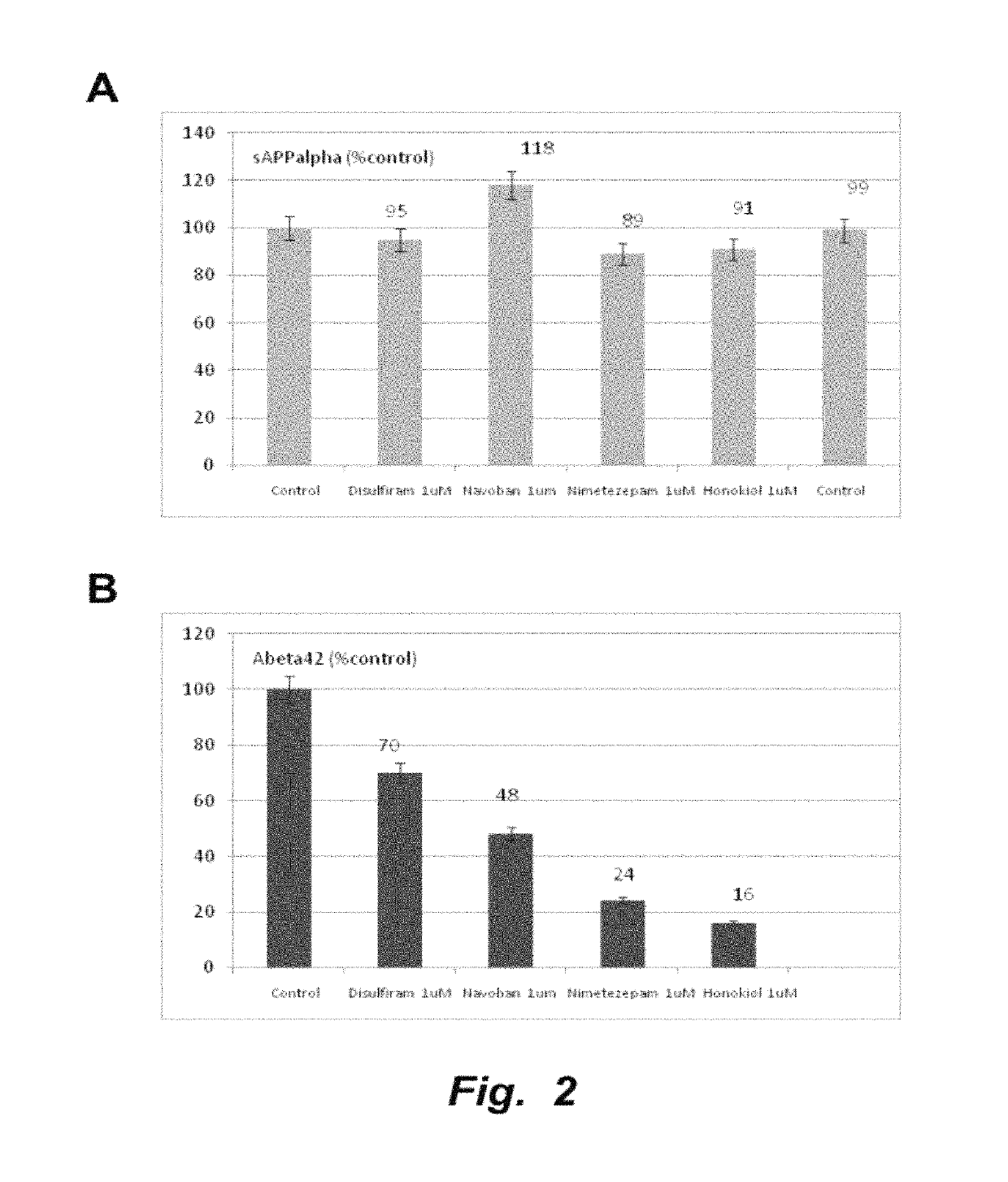
The invention provides compositions and methods for the treatment of mild cognitive impairment (MCI), and for inhibiting, reducing, delaying and / or preventing the progression of MCI to Alzheimer's disease. The methods entail administering an effective amount of one or more compounds selected from the group consisting of tropisetron, disulfuram, honokiol and nimetazepam. The methods also are useful for prophylactic and therapeutic treatment of amyloidogenic diseases, including Alzheimer's disease.
Owner:THE BUCK INST FOR RES ON AGING
Process of preparing troipisetron
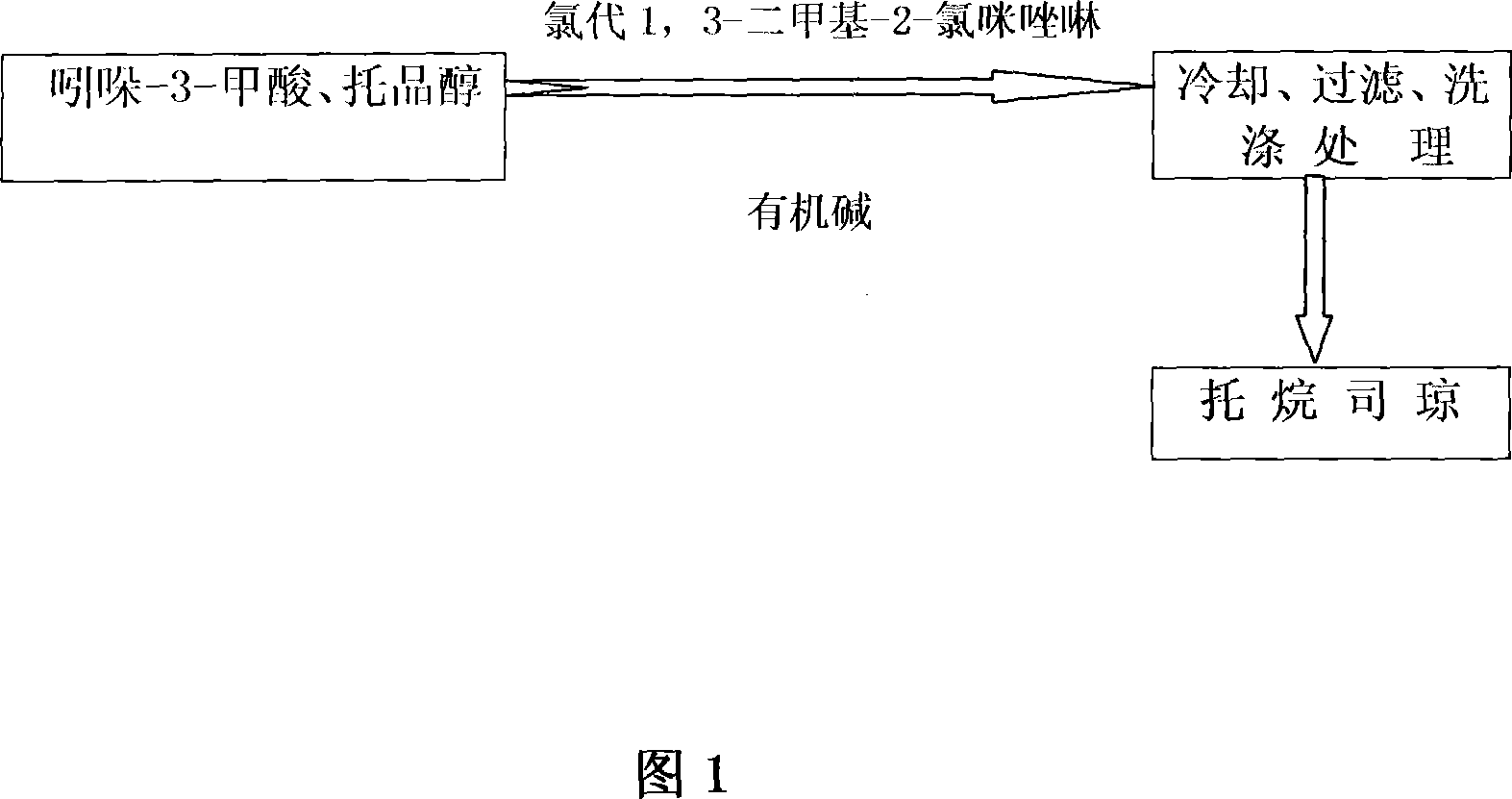
This invention relates to a synthesizing technology for preparing tropisetron with chloro 1, 3-dimethyl-2-chlorin imidazoline as the condensation agent, in which, indole-3-formic acid is reacted with tropaic alcohol for 12-24h under room temperature then to be cooled, filtered and cleaned to get the objective product tropisetron, and the mol ration of the indole-3-formic acid, tropaic alcohol and chloro 1, 3-dimethyl-2-chlorin imidazoline for condensation reaction, and the option is 1:1-1.5:1-1.5.
Owner:北京成宇化工有限公司
Citric acid tropisetron raw material medicine and preparation technology of raw material medicine and injection liquid
ActiveCN101838266AGood curative effectImprove securityOrganic chemistryDigestive systemTropaneFormate
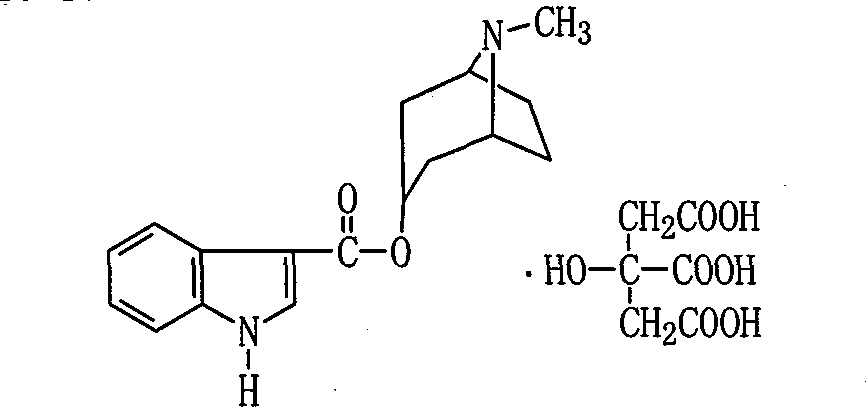
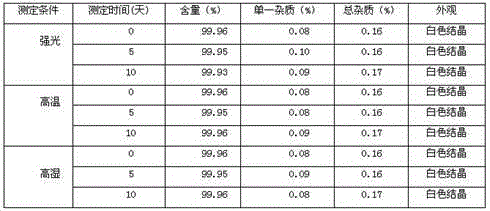
The invention relates to a citric acid tropisetron raw material medicine and a preparation technology of the citric acid tropisetron raw material medicine and an injection liquid, and belongs to the field of chemical pharmacy; in the process of tropisetron salifying, the citric acid is used as acid radical; the chemical name of the citric acid tropane tropisetron is [(1alpha H, 5alpha H)-8-methyl-azepine bicyclo-(3, 2, 1) octyl group-3alpha-]-1H- benzpyrole-3- formic ether citrate; and the molecular formula is C23H28N209. In the process of tropisetron salifying, the tropisetron is dissolved in ethanol; the mixture is added with ethanol solution of the citric acid, evenly stirred and stands till the needed solid is separated out; the crude citric acid tropisetron is filtered, heated and dissolved in distilled water, is added with the activated carbon for backflowing and decoloring, cools and stands after being filtered so as to separate out white cystal; the mixture is filtered; the filter cake is dried and then distilled water is used for the secondary recrystallization to obtain the refined citric acid tropane tropisetron. The clinical research shows that compared with the imported muriatic acid tropisetron injection solution, the citric acid tropisetron injection solution has similar curative effect and safety.
Owner:JIANGXI DONGFU PHARMA CO LTD
Nasal spray agent
The present invention is nasal spray of setron medicine includes the medicine component selected from Ondansetron, tropisetron and granisetron, osmotic pressure regulator and water.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Remedy For Psychoneurotic Diseases
InactiveUS20080103166A1Effective preventionEffective treatmentBiocideNervous disorderDiseaseMental nerve
A medicinal preparation for preventing and / or treating psychoneurotic diseases such as integration dysfunction and Alzheimer's disease which contains tropisetron or its pharmaceutically acceptable salt as the active ingredient.
Owner:CHIBA UNIVERSITY
Mouth spray for preventing and treating nausea and emesis after tumor chemotherapy and radiotheraphy and preparation method thereof
InactiveCN101385712AEasy to increase or stop doseReduce efficacyAerosol deliveryPharmaceutical non-active ingredientsDiseaseAdditive ingredient
The invention relates to an oral spray for controlling nausea and vomit of chemotherapy and radiation therapy, the formula of the oral spray is composed of ingredients with the following parts by weight: 5-50 parts of drug absorption enhancer, 2-20 parts of drug active ingredient and 30-90 parts of buffer, the drug absorption enhancer can be any one or the combination of more of the following ingredients: azone, propylene glycol, polysorbate (Tween), ethylene glycol deoxycholic acid sodium salt, brij, sodium decanoate, lauric acid, stearic acid, sodium lauryl sulphate, stearyl alcohol sodium sulfate, dioctyl succinate sodium sulfonate, oleic acid, GK2, menthol and borneol; the drug active ingredient can be any one combination of the following ingredients: palonosetron hydrochloride, granisetron, ondansetron, azasetron and tropisetron; and the ingredients of the buffer are sodium citrate buffer solution and phosphate buffer solution. The oral spray provides a formulation which is safer, painless and convenient for patients with advanced tumor, elderly and weak patients, children patients and the patients who suffer from the metal illness and do not obey the oral administration or the injection drug administration.
Owner:陆飚 +1
Snuff
The present invention is snuff of setron medicine includes the medicine component selected from Ondansetron, tropisetron and granisetron; and medicine carrier selected from glucose, mannitol, lactose, sorbitol, microcrystalline cellulose and beta-cyclodextrin.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Transdermal composition of antivomiting agent and preparation containing the same
InactiveCN1364083AHydrocarbon active ingredientsHydroxy compound active ingredientsAlcoholOndansetron
A transdermal composition of the present invention comprises (a) a matrix containing (i) 20 to 80 % by weight of an alcohol, (ii) 1 to 50 % by weight of a skin penetration enhancer selected from the group consisting of a fatty acid and a derivative thereof, a fatty alcohol and a derivative thereof, an amide, a terpene, a surfactant and a mixture thereof, and (iii) 15 to 80 % by weight of water; and (b) 1 to 15 % by weight, based on the weight of the matrix, of an antivomiting agent selected from the group consisting of tropisetron, ondansetron, granisetron and pharmaceutically acceptable salts thereof, which is capable of delivering the antivomiting agent efficiently over a period of a day or more without skin irritation.
Owner:SAMYANG BIOPHARMLS CORP
Method for testing tropine alcohol of residual intermediate product in synthesizing Troipisetron
A method for detecting residual intermediate of tropaic alcohol in tropisetron synthesis includes preparing test solution and control solution , dropping them separately on the same silica gel G thin layer plate , using acetone ¿C ammonia water as developing agent to carry out thin layer chromatography determination, developing chromatographic cylinder, using developer of bismuth potassium iodide solution with assistance of sodium nitride solution to develop color and checking colour developed result.
Owner:QINGDAO PURUISEN MEDICINE SCI & TECH CO LTD
Detection method for alpha-tropine as impurity in hydrochloric acid tropisetron
ActiveCN102565226AImprove controllabilityDirect analysis methodComponent separationReference samplePeak area
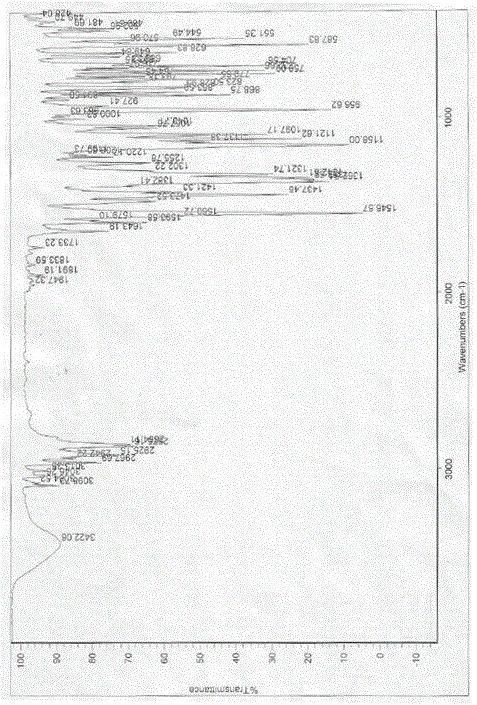
The invention relates to a detection method for alpha-tropine as impurity in hydrochloric acid tropisetron, which is characterized by adopting the gas chromatography for detection, and particularly includes the following steps: (1) preparing sample hydrochloric acid tropisetron solution with concentration of 10mg / m1 to 30mg / ml; (2) preparing reference sample alpha-tropine solution with the concentration of 0.02mg / m1 to 0.06mg / m1; (3) performing headspace sample injection and chromatogram acquisition; and (4) calculating the result through the appearance method based on the peak area. The invention has the characteristics that the detection method is convenient, quantitative and sensitive, and can be widely applied to the control and improvement of the medicine quality.
Owner:SHANDONG QIDU PHARMA
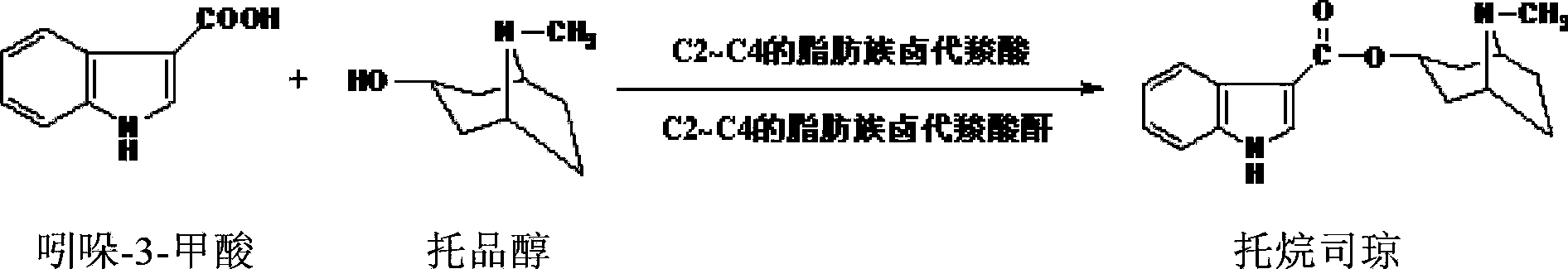
Preparation method of tropisetron
ActiveCN102887893ASimple and fast operationMild reaction conditionsOrganic chemistryCarboxylic acidSolvent
The invention provides a preparation method of tropisetron, which comprises the following steps: in an inert solvent, activating indolyl-3-formic acid into mixed anhydride by using C2-C4 aliphatic halogenated carboxylic acids as a catalyst and C2-C4 aliphatic halogenated carboxylic acid anhydride as an activating reagent, and dropwisely adding a tropine alcoholic solution dissolved in the inert solvent to carry out condensation reaction, thereby obtaining the tropisetron. The method provided by the invention is simple to operate, has the advantages of mild reaction conditions, high safety coefficient, fewer side reactions, high yield and high purity, lowers the production cost and operating risk, and is especially suitable for large-scale industrial production.
Owner:QILU PHARMA HAINAN +1
Hydrochloric-acid tropisetron freeze-dried powder injection for injection and preparation method thereof
ActiveCN105125505APrevent oxidationImprove stabilityPowder deliveryDigestive systemFreeze-dryingBULK ACTIVE INGREDIENT
The invention belongs to the technical field of pharmaceutical preparations, and particularly relates to a hydrochloric-acid tropisetron freeze-dried powder injection for injection and a preparation method thereof. The hydrochloric-acid tropisetron freeze-dried powder injection comprises a medicinal active ingredient, excipients and pH (potential of hydrogen) stabilizers, wherein the medicinal active ingredient is hydrochloric-acid tropisetron, the excipients include mannitol and sodium chloride, and the pH stabilizers include citric acid and sodium citrate. According to the hydrochloric-acid tropisetron freeze-dried powder injection, the mannitol and the sodium chloride serve as the excipients, the pH value of a midbody solution is controlled to be stabilized within 4.6-5.2 by citric acid and sodium citrate buffer solutions, oxidation of a main drug is inhibited, and stability of the main drug is improved; in six months of an acceleration test, the injection is stable in content, changes of related substances are small, and the storage period of the injection is prolonged. The preparation method is simple in process and suitable for industrial production.
Owner:REYOUNG PHARMA
Remedy for psychoneurotic diseases
InactiveUS8470846B2Effective in prevention and treatmentAvoid problemsBiocideNervous disorderDiseaseBULK ACTIVE INGREDIENT
A medicinal preparation for preventing and / or treating psychoneurotic diseases such as integration dysfunction and Alzheimer's disease which contains tropisetron or its pharmaceutically acceptable salt as the active ingredient.
Owner:CHIBA UNIVERSITY
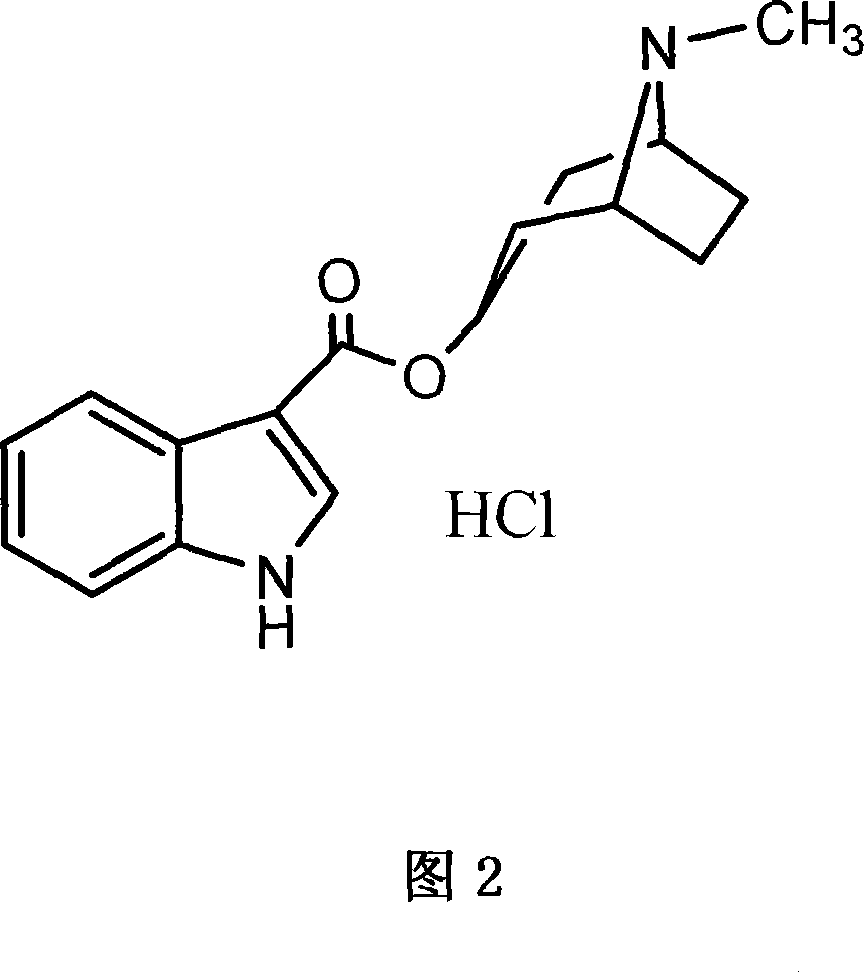
Tropiseiron hydrochloride compound
The invention belongs to the technical field of medicines, and particularly relates to a tropiseiron hydrochloride compound and a preparation method thereof. The invention also relates to application of an injection containing the tropiseiron hydrochloride compound in preparation of drugs for treating nausea and vomit.
Owner:TIANJIN HANKANG PHARMA BIOTECH
Method for testing tropine alcohol of residual intermediate product in synthesizing Troipisetron
A method for detecting residual intermediate of tropaic alcohol in tropisetron synthesis includes preparing test solution and control solution , dropping them separately on the same silica gel G thin layer plate , using acetone - ammonia water as developing agent to carry out thin layer chromatography determination, developing chromatographic cylinder, using developer of bismuth potassium iodide solution with assistance of sodium nitride solution to develop color and checking colour developed result.
Owner:QINGDAO PURUISEN MEDICINE SCI & TECH CO LTD
Trans-dermal composition of anti-vomiting agent and preparation containing the same
InactiveCN1209108CHydrocarbon active ingredientsHydroxy compound active ingredientsFatty acidFatty alcohol
A transdermal composition of the present invention comprises (a) a matrix containing (i) 20 to 80 % by weight of an alcohol, (ii) 1 to 50 % by weight of a skin penetration enhancer selected from the group consisting of a fatty acid and a derivative thereof, a fatty alcohol and a derivative thereof, an amide, a terpene, a surfactant and a mixture thereof, and (iii) 15 to 80 % by weight of water; and (b) 1 to 15 % by weight, based on the weight of the matrix, of an antivomiting agent selected from the group consisting of tropisetron, ondansetron, granisetron and pharmaceutically acceptable salts thereof, which is capable of delivering the antivomiting agent efficiently over a period of a day or more without skin irritation.
Owner:SAMYANG BIOPHARMLS CORP
Compound preparation used for treating melancholia and senile dementia
ActiveCN101850119AEffective therapeutic effectNervous disorderHeterocyclic compound active ingredientsHeadachesAprepitant
The invention discloses a compound preparation used for treating depression and senile dementia. The compound preparation comprises the following components in part by weight: 1 part of rolipram and 0.25 to 200 parts of antiemetic medicament, wherein the antiemetic medicament is granisetron, ondansetron, tropisetron, aprepitant or metoclopramide. The compound preparation is prepared from the rolipram and the granisetron, the ondansetron, the tropisetron, the aprepitant or the metoclopramide. The compound preparation has an effective curative effect of treating the melancholia and the senile dementia, and simultaneously solves the technical problems that in the prior art, the rolipram causes adverse reactions such as vomition, sicchasia, headache and the like.
Owner:兰晟生物医药(苏州)有限公司
Synthesis method of N-hydroxy tropisetron
ActiveCN111362935AHigh purityHigh yieldOrganic chemistryBulk chemical productionDrugs synthesisPharmaceutical drug
The invention discloses a synthetic method of tropisetron oxide, namely N-hydroxy tropisetron, belonging to the field of drug synthesis. According to the invention, 2-nitrophenyl acetate is used as araw material, and six reactions are successively conducted to synthesize the tropisetron oxide, namely N-hydroxy tropisetron; optimal preparation steps and reaction conditions are screened out througha large number of experiments; and the purity of the prepared target product can reach 99% or above, and the prepared target product does not contain tropisetron serving as a crude drug, can be usedfor research on pharmacokinetics, crude drug impurity identification and the like, and has important application value in impurity identification of tropisetron, research on metabolic mechanism of tropisetron and research design of novel drugs of tropisetron.
Owner:TLC NANJING PHARMA RANDD CO LTD
Tropisetron composition for injection
InactiveCN103356618AGood treatment effectImprove immunityPowder deliveryDigestive systemTreatment effectRegimen
The invention provides a tropisetron composition for injection and relates to the technical fields of medicines and medicine preparation. The tropisetron composition is characterized in that the main medicines are tropisetron and melatonin, wherein the melatonin comprises a quick release part and a slow release part included by cyclodextrin. According to the tropisetron composition provided by the invention, the treating effect of the tropisetron is improved; problems of instability and rapidness in distribution and clearing caused when MT (melatonin) is orally absorbed are avoided, and a first-pass effect of the MT is reduced; the use level of the tropisetron is lowered; a medicine administration design in which a quick release manner and a slow release manner are combined is accordant with physiological secretion characteristics of the MT, the problem of the MT that the half-life period is short is solved, and the biological availability of a product is improved; the treatment of the tropisetron to CINV (Chemotherapy Induced Nausea and Vomiting) is enhanced, after the tropisetron and the melatonin are combined, not only the CINV can be treated, the treatment effect of the tropisetron to the CINV can be improved, the treatment course can be shortened, but also the use level of the tropisetron is lowered, the side effect of the tropisetron is reduced, and the immune capability of a human body can be improved; by maintaining the melatonin with a certain concentration in the blood of the human body, a stress reaction of the body can be effectively reduced, and the treatment of the CINV is benefited.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Snuff
The present invention is snuff of setron medicine includes the medicine component selected from Ondansetron, tropisetron and granisetron; and medicine carrier selected from glucose, mannitol, lactose, sorbitol, microcrystalline cellulose and beta-cyclodextrin.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
A kind of pharmaceutical composition of tropisetron
ActiveCN103893109BPromote absorptionPromote efficacyAerosol deliveryDigestive systemAdhesiveMethyl group
Owner:SHENZHEN SOUTH CHINA PHARMA
Compound preparation used for treating melancholia and senile dementia
ActiveCN101850119BEffective therapeutic effectNervous disorderHeterocyclic compound active ingredientsHeadachesAprepitant
The invention discloses a compound preparation used for treating depression and senile dementia. The compound preparation comprises the following components in part by weight: 1 part of rolipram and 0.25 to 200 parts of antiemetic medicament, wherein the antiemetic medicament is granisetron, ondansetron, tropisetron, aprepitant or metoclopramide. The compound preparation is prepared from the rolipram and the granisetron, the ondansetron, the tropisetron, the aprepitant or the metoclopramide. The compound preparation has an effective curative effect of treating the melancholia and the senile dementia, and simultaneously solves the technical problems that in the prior art, the rolipram causes adverse reactions such as vomition, sicchasia, headache and the like.
Owner:兰晟(崇左)医药有限公司
Combination drugs or kit products and applications for treating gastrointestinal reactions after chemotherapy
ActiveCN111450175BImprove therapeutic efficacyReduce adverse reactionsHeavy metal active ingredientsDigestive systemMedicinal herbsClinical efficacy
The invention relates to the field of medicines, in particular, it provides a combined medicine or medicine box product and application for treating gastrointestinal reactions after chemotherapy. Through repeated exploration and a large number of experiments, the inventor found through clinical application that the traditional Chinese medicine composition with woody, sandalwood, agarwood, musk, magnolia officinalis, citrus aurantium, rhubarb, croton cream, jujube and chuanxiong as main medicinal materials and tropane The combined use of setron or its pharmaceutically acceptable derivatives can improve the clinical efficacy of chemotherapy-induced nausea and vomiting, acute vomiting, and delayed vomiting. The combination of the two has a synergistic effect on gastrointestinal reactions, and can Improve the curative effect of chemotherapy-induced gastrointestinal reactions, and reduce the adverse reactions caused by the drug itself.
Owner:津药达仁堂集团股份有限公司乐仁堂制药厂
Application of tropisetron in preparation of daily chemicals or medicines for improving skin
PendingCN111773129AImprove skinGood for UV damage repairCosmetic preparationsToilet preparationsBiological bodyHave Nausea
The invention belongs to the technical field of medicines, and particularly relates to an application of tropisetron in preparation of daily chemicals or medicines for improving skin, and the tropisetron is used for improving skin. Tropisetron is used for preventing and treating nausea and vomiting caused by cancer chemotherapy. The inventor accidentally finds that the tropisetron has an excellenteffect of improving the skin, so that the skin of a human body can better exert the function of a first organism protective film.
Owner:PLASTIC SURGERY HOSPITAL CHINESE ACAD OF MEDICAL SCI
Process of preparing troipisetron
The invention relates to a synthesis process for preparing tropisetron by using 2-chloro-1,3-dimethylimidazoline chloride as a condensing agent. Under the action of imidazoline chloride and an organic base, react indole-3-carboxylic acid and tropinol at room temperature for 12 to 24 hours in an inert solvent, and undergo cooling, filtering, washing and other processing steps to obtain the target Product tropisetron; the molar ratio that the above-mentioned indole-3-carboxylic acid, tropinol and 2-chloro-1,3-dimethylimidazoline chloride carry out condensation reaction is: 1: 1~2: 1~3; Preferably it is 1:1 to 1.5:1 to 1.5. The synthesis process of the invention is safe, pollution-free, easy to operate, high in product yield and good in purity, and can meet the requirements of pharmaceutical production.
Owner:北京成宇化工有限公司
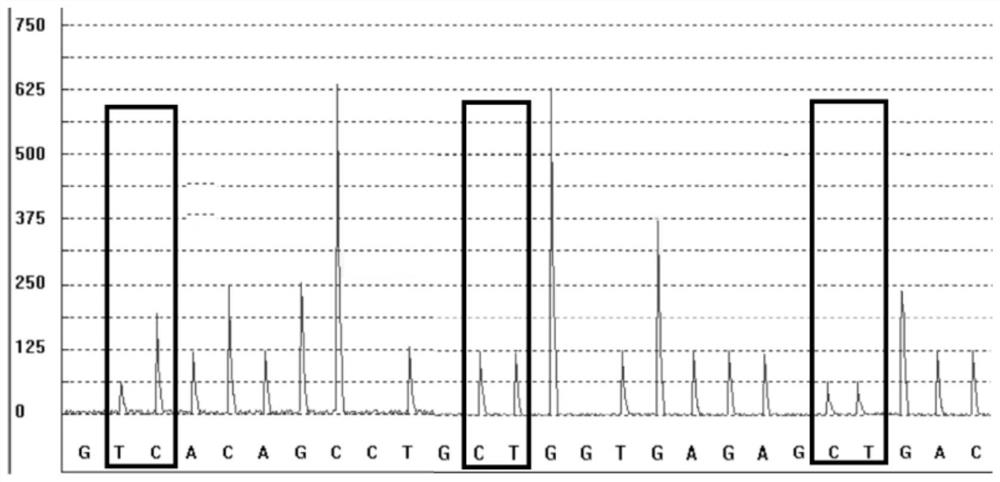
Detection kit for metabolic markers of ondansetron and tropisetron, detection method of detection kit and application of detection kit and detection method
The invention discloses a detection kit for metabolic markers of ondansetron and tropisetron, a detection method of the detection kit and application of the detection kit and the detection method. The detection kit is used for detecting gene polymorphism of the metabolic markers CYP2D6C100T, CYP2D6G1846A and CYP2D6*5 of the ondansetron and the tropisetron. According to the kit, specific amplification primers and sequencing primers are designed according to the polymorphism of the three genes CYP2D6C100T, CYP2D6G1846A and CYP2D6*5. The kit comprises the following components of an amplification reaction solution, the CYP2D6C100T sequencing primer, the CYP2D6G1846A sequencing primer, the CYP2D6*5 sequencing primer and a positive control. According to the invention, blood direct expansion, rapid amplification and optimized pyrosequencing technologies are combined to detect gene polymorphism related to prediction of drug effects and adverse reactions of the ondansetron and the tropisetron, and suggestions from the gene perspective are provided for clinical use of the ondansetron and the tropisetron.
Owner:上海普然生物科技有限公司
Preparation method of tropisetron
ActiveCN102887893BSimple and fast operationMild reaction conditionsOrganic chemistryCarboxylic acidSolvent
The invention provides a preparation method of tropisetron, which comprises the following steps: in an inert solvent, activating indolyl-3-formic acid into mixed anhydride by using C2-C4 aliphatic halogenated carboxylic acids as a catalyst and C2-C4 aliphatic halogenated carboxylic acid anhydride as an activating reagent, and dropwisely adding a tropine alcoholic solution dissolved in the inert solvent to carry out condensation reaction, thereby obtaining the tropisetron. The method provided by the invention is simple to operate, has the advantages of mild reaction conditions, high safety coefficient, fewer side reactions, high yield and high purity, lowers the production cost and operating risk, and is especially suitable for large-scale industrial production.
Owner:QILU PHARMA HAINAN +1
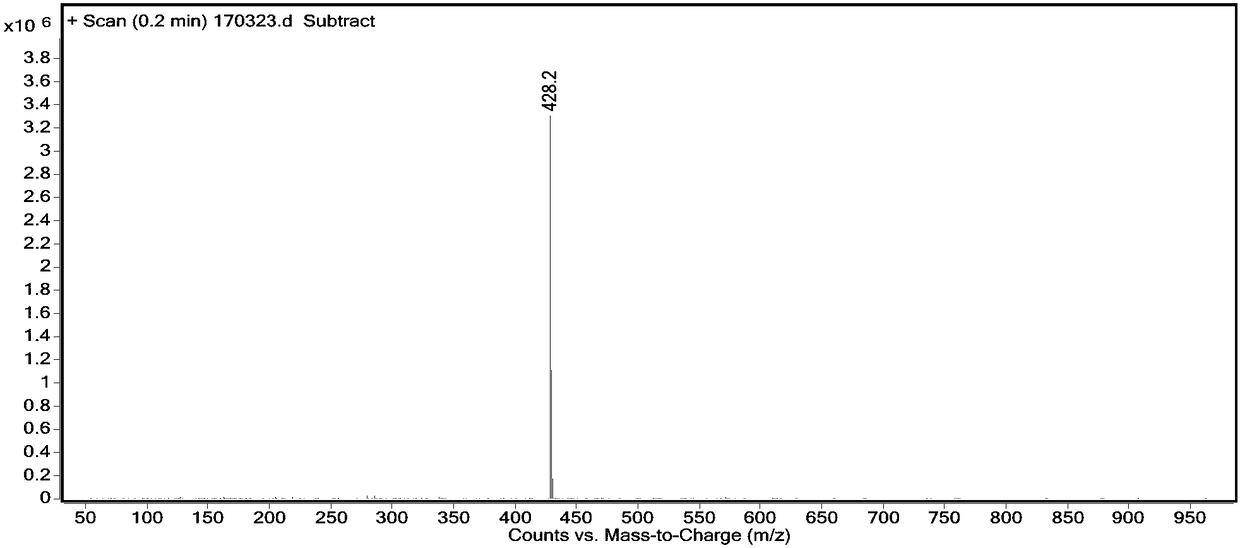
Preparation method of tropisetron bisindole impurities
InactiveCN108148059AHigh purityEasy to operateOrganic chemistry3-indolecarboxylic acidRoom temperature
The invention belongs to the technical field of drug synthesis, and particularly relates to a preparation method of tropisetron bisindole impurities. The preparation method of the tropisetron bisindole impurities comprises the following steps: (1) dissolving 3-indolecarboxylic acid and an acylation reagent in a solvent and reacting, and carrying aftertreatment to obtain 3-indoloformyl chloride; and (2) dissolving tropisetron in tetrahydrofuran, cooling to the temperature of minus 15-20 DEG C, adding alkali, then dropwise adding a tetrahydrofuran solution of the 3-indoloformyl chloride, reacting for 2-10 hours at a room temperature after dropwise adding is finished, and carrying out aftertreatment to obtain tropisetron bisindole impurities. The preparation method is simple to operate, gentle in reaction, high in yield and high in purity of product, is suitable for mass spectrometry on crude drugs, and has quite high commercial value.
Owner:SHANDONG QIDU PHARMA
Methods of treating mild cognitive impairment (MCI) and related disorders
ActiveUS10449177B2Progression from an asymptomatic state to a symptomatic state is prevented or delayedBiocideNervous disorderTherapeutic treatmentDisease cause
The invention provides compositions and methods for the treatment of mild cognitive impairment (MCI), and for inhibiting, reducing, delaying and / or preventing the progression of MCI to Alzheimer's disease. The methods entail administering an effective amount of one or more compounds selected from the group consisting of tropisetron, disulfuram, honokiol and nimetazepam. The methods also are useful for prophylactic and therapeutic treatment of amyloidogenic diseases, including Alzheimer's disease.
Owner:THE BUCK INST FOR RES ON AGING
Tropisetron pharmaceutical composition
ActiveCN103893109APromote absorptionPromote efficacyAerosol deliveryDigestive systemTemperature sensitiveGel state
The invention relates to a tropisetron pharmaceutical composition, which comprises tropisetron salts, carbomer, hydroxypropyl methylcellulose, poloxamer, 1-methyl-4-isopropylcyclo-3-alcohol and other additives. The tropisetron pharmaceutical composition disclosed by the invention is in the liquid state in vitro; after being used for a mouth mucosa, the tropisetron pharmaceutical composition is in the semi-solid gel state and serves as temperature-sensitive gel; the composition gel has a certain intensity and biological adhesive and can stay in a drug application position, so that drugs are continuously released; furthermore, drugs can be promoted to be absorbed through the mouth mucosa; the pharmacological effect is taken.
Owner:SHENZHEN SOUTH CHINA PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com