Detection kit for metabolic markers of ondansetron and tropisetron, detection method of detection kit and application of detection kit and detection method
A technology for detection kits and metabolic markers, which is used in the determination/inspection of microorganisms, biochemical equipment and methods, DNA/RNA fragments, etc., which can solve the problems of long detection cycle, easy contamination of amplified products, and complicated operation steps. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1, the preparation of kit
[0047] The rapid reaction kit of the present invention designs specific amplification primers and sequencing primers for CYP2D6 (C100T), CYP2D6 (G1846A) and CYP2D6*5 for amplification and pyrosequencing detection. Designing primers based on rapid amplification technology is one of the keys of the present invention. Gene polymorphism sequence is subject to the public sequence in Genebank.
[0048] (1) The primer sequences of this embodiment are as follows:
[0049]
[0050]
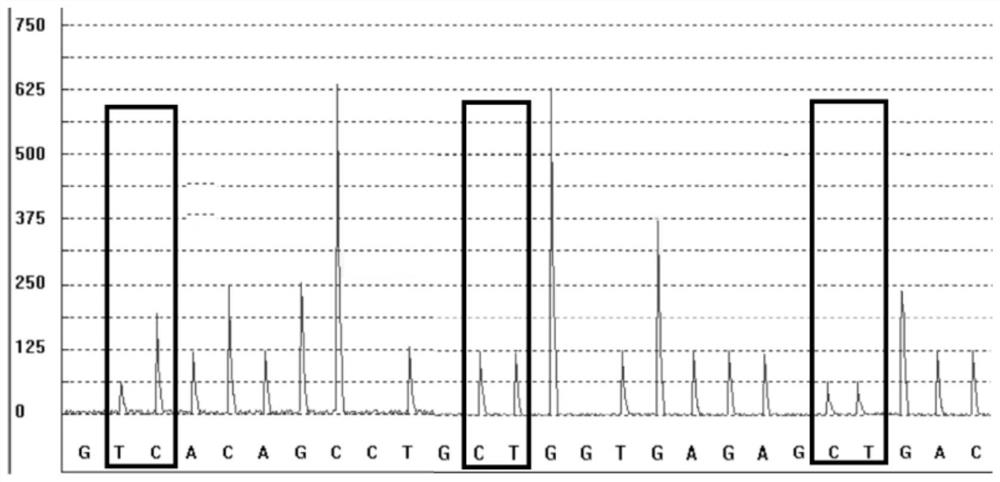
[0051] The copy number detection fragment of CYP2D6*5 is located in exon 9 of the CYP2D6 gene. Through the same sequence as CYP2D6 and CYP2D8 but different from CYP2D7, the related fragments of CYP2D6 and CYP2D8 genes are amplified at the same time, and then the PCR amplification product is used for Pyrosequencing. Since there are only two copies of CYP2D8, the peak height ratios of different bases at the same position in the measured sequence of the C...
Embodiment 2
[0059] Embodiment 2, pyrophosphate detection
[0060] The instruments adopted in the present invention are as follows: amplification instrument, pyrosequencer (Wuhan First Biotechnology Co., Ltd.).
[0061] (1) Reagent preparation (reagent preparation room)
[0062] Take out the reagents in advance, vortex the PCR reaction solution for 15 seconds, and centrifuge at low speed for later use. Determine the number of reactions N, N = number of samples to be tested (n) + number of quality control products (1) + blank control. It is recommended to conduct positive control and blank control analysis for each PCR experiment at the same time. Then the reaction solution was dispensed into PCR reaction tubes at 16 μL / tube.
[0063] (2) Sample testing (sample preparation room)
[0064] Add EDTA anticoagulated whole blood, positive control and blank control into the PCR reaction tube according to the sample volume of 4 μL, close the tube cap tightly, centrifuge at low speed for 15 seco...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com