Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
35 results about "CYP2D6" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Cytochrome P450 2D6 (CYP2D6) is an enzyme that in humans is encoded by the CYP2D6 gene. CYP2D6 is primarily expressed in the liver. It is also highly expressed in areas of the central nervous system, including the substantia nigra.
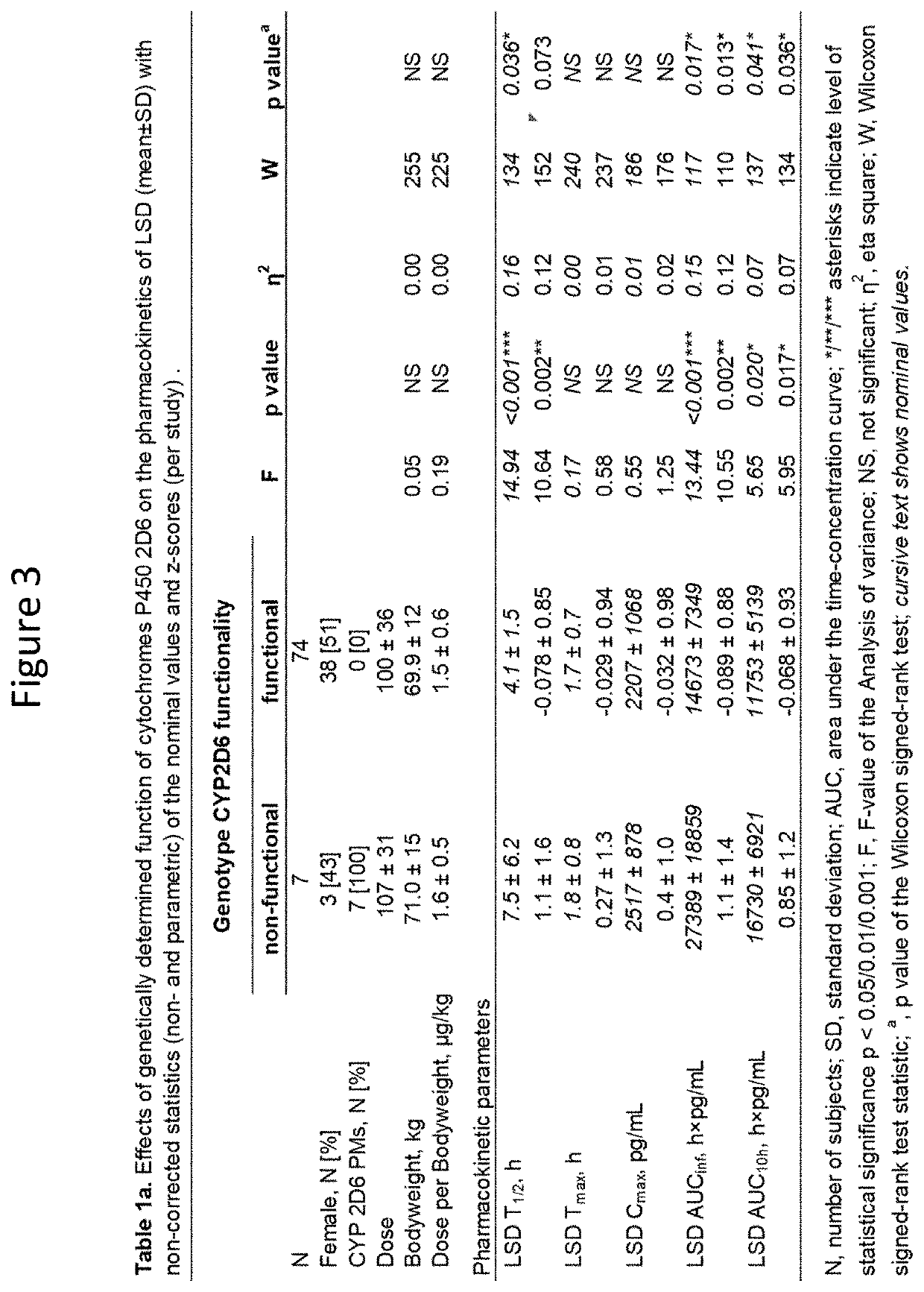
Preparation method, detection method and application of probe drug composition for determination of metabolic activity of cytochrome P450
InactiveCN102650620AHigh sensitivityStrong specificityComponent separationIn-vivo testing preparationsDrugs solutionMicroparticle
The invention relates to a preparation method, a detection method and application of a probe drug composition for determination of metabolic activity of cytochrome P450. The composition mainly comprises a preparation made with a specific probe with major isoforms of CYP450, i.e. CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4, as an active component. Cocktail probe drug solution is prepared, the probe drug composition is injected into an animal or liver microsomes for in vitro co-incubation, and the concentration of each probe drug is determined to assess the metabolic activity of the CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4. In the early stage of research and development of new drugs, the effects of the drugs on the activity of each isoform of the cytochrome P450 are screened in a high-throughput way, and the interactions of the drugs can be predicted. In the stage of clinical research, the testing can be performed with the probe drug composition in an in-vivo probe method, and the effects of the drugs on the in-vivo metabolic activity of different isoforms of the human liver CYP450 can be examined.
Owner:TIANJIN MEDICAL UNIV
Method and composition to evaluate cytochrome P450 2D6 isoenzyme activity using a breath test
InactiveUS20070026480A1Organic active ingredientsIn-vivo radioactive preparationsMetaboliteOral medication
The present invention relates, generally to a method of determining and assessing cytochrome P450 2D6 isoenzyme (CYP2D6)-related metabolic capacity in an individual mammalian subject via a breath assay, by determining the relative amount of 13CO2 exhaled by a the subject upon intravenous or oral administration of a 13C-labeled CYP2D6 substrate compound. The present invention is useful as an in vivo phenotype assay for evaluating CYP2D6-related activity using the metabolite 13CO2 in expired breath and to determine the optimal dosage and timing of administration of CYP2D6 substrate compound.
Owner:OTSUKA AMERICA PHARMA INC
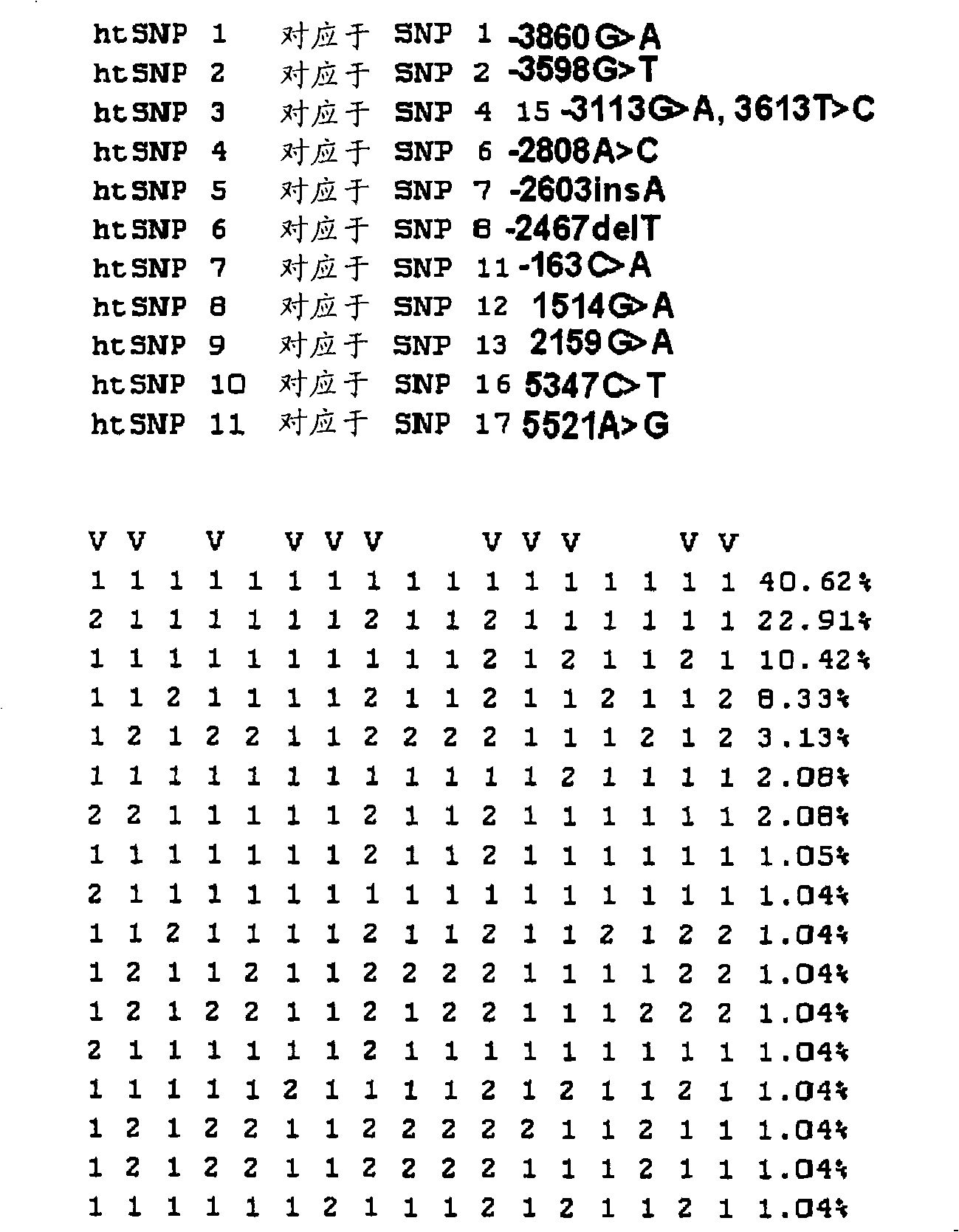
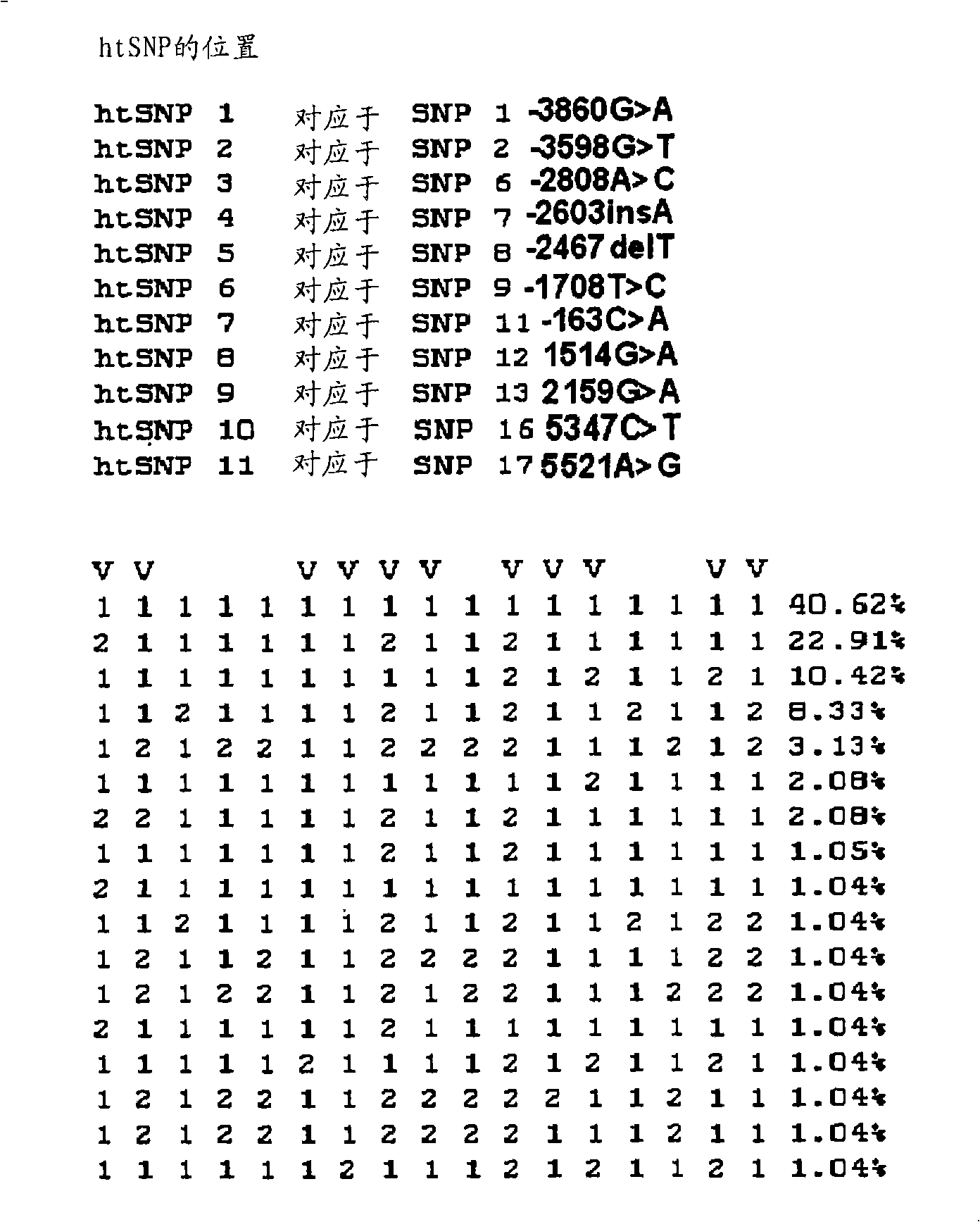
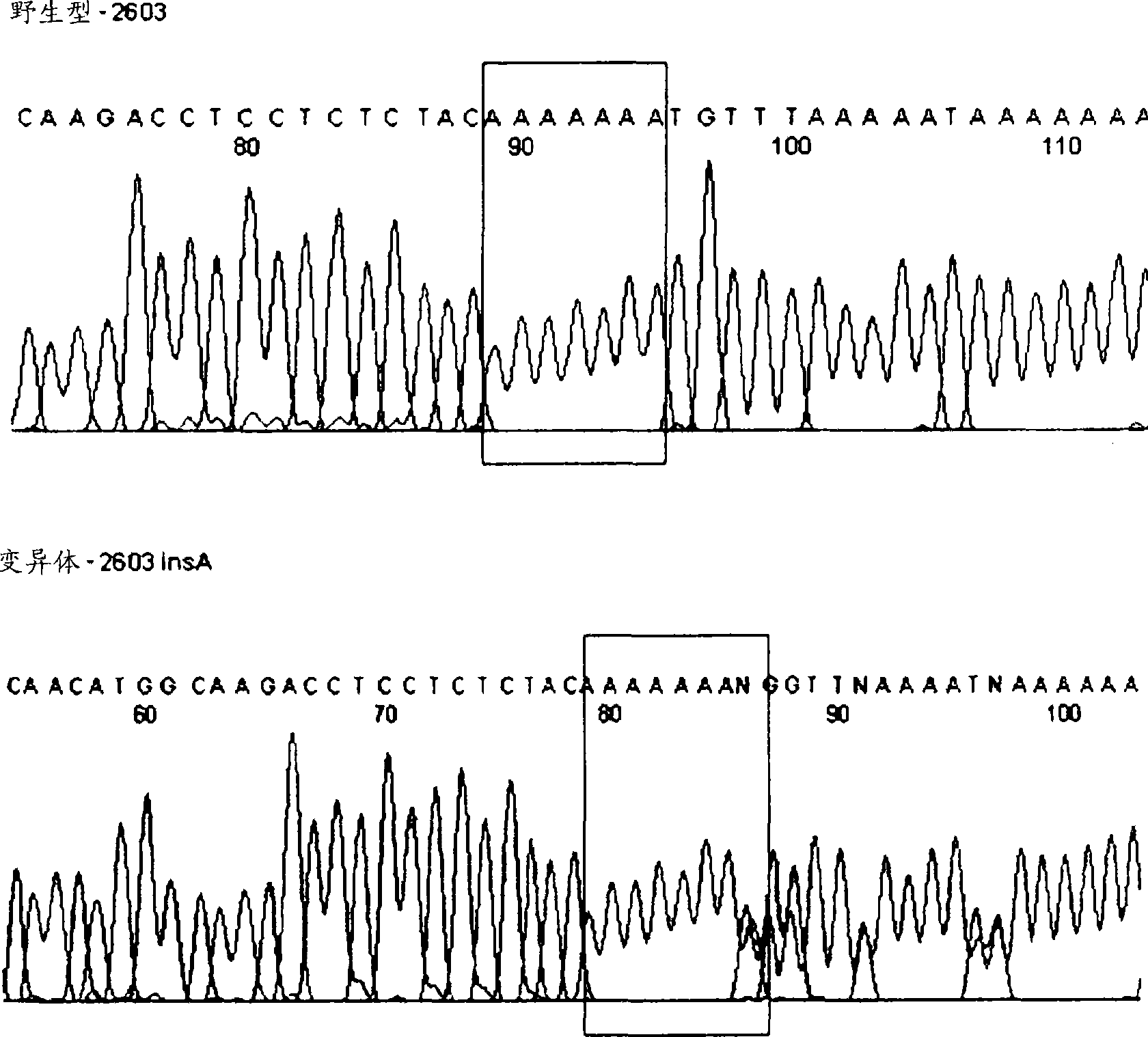
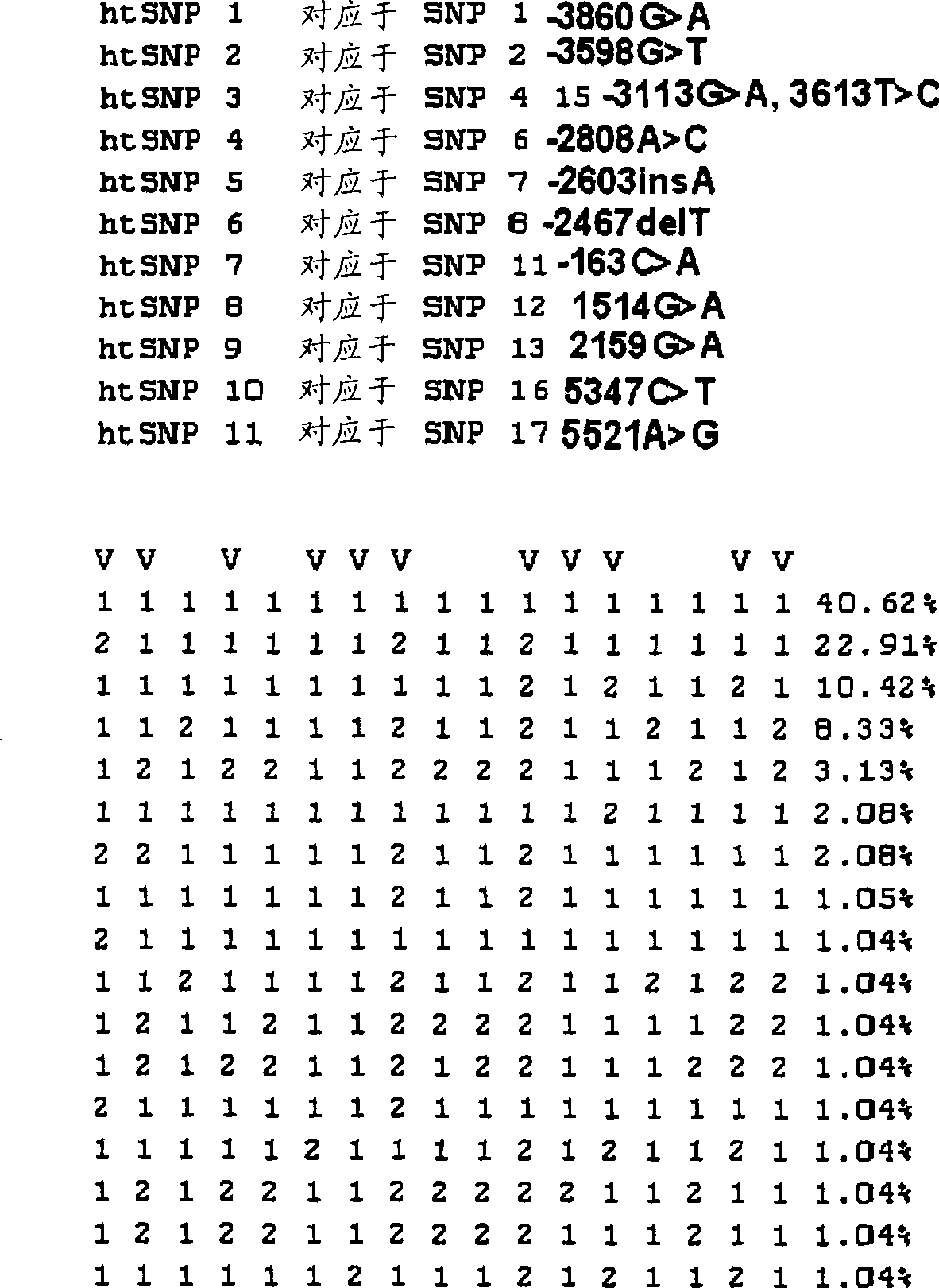
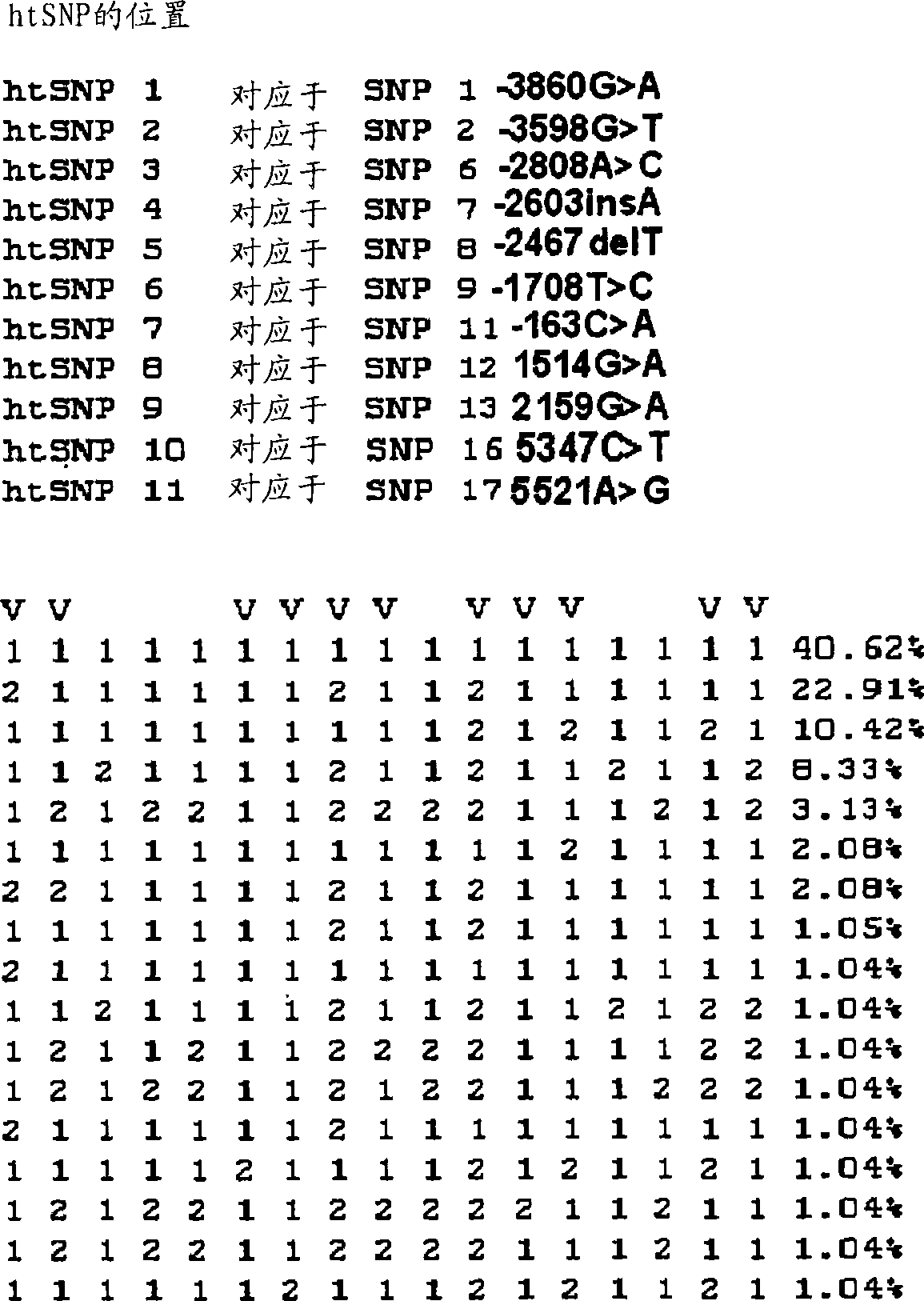
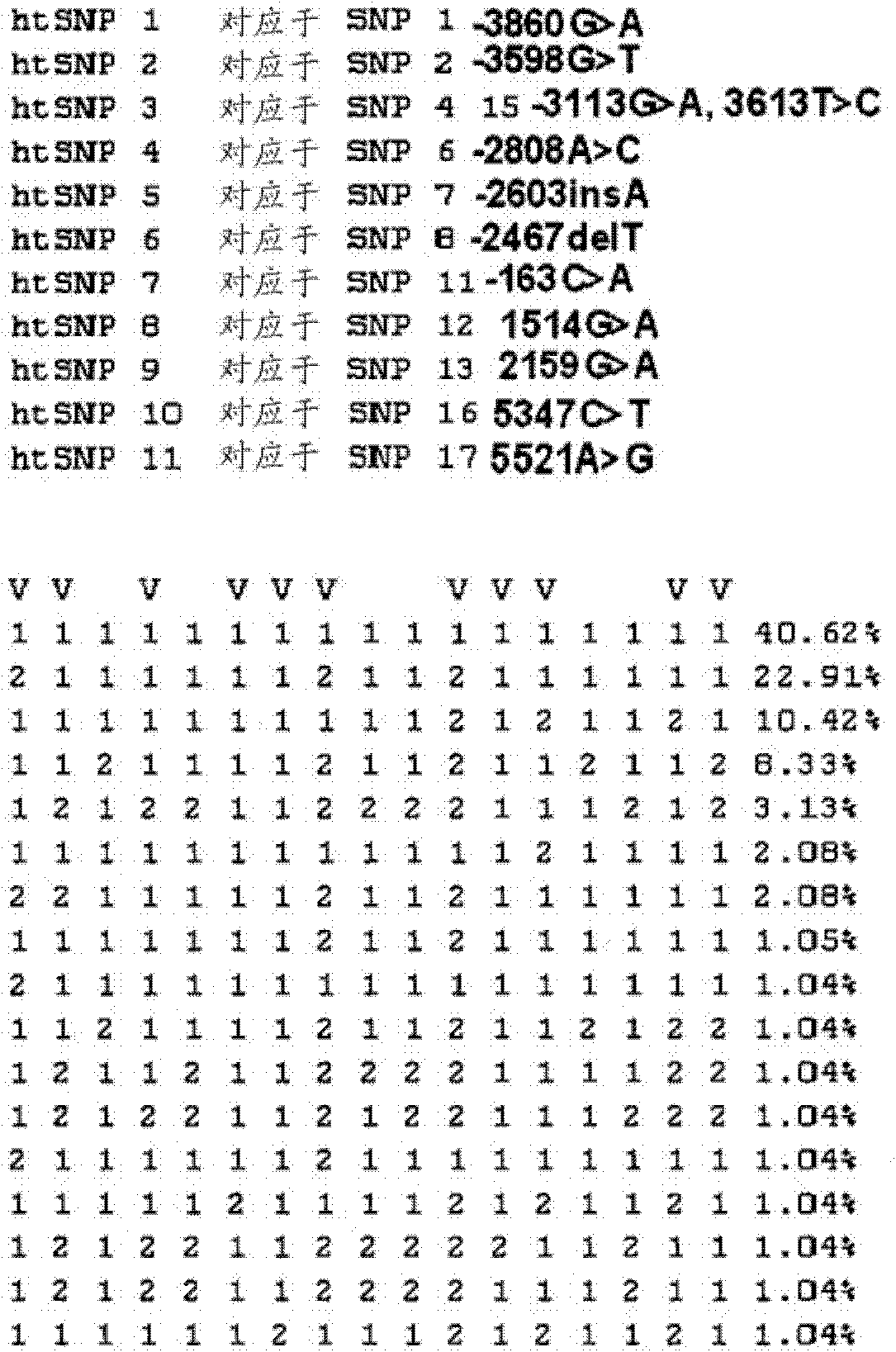
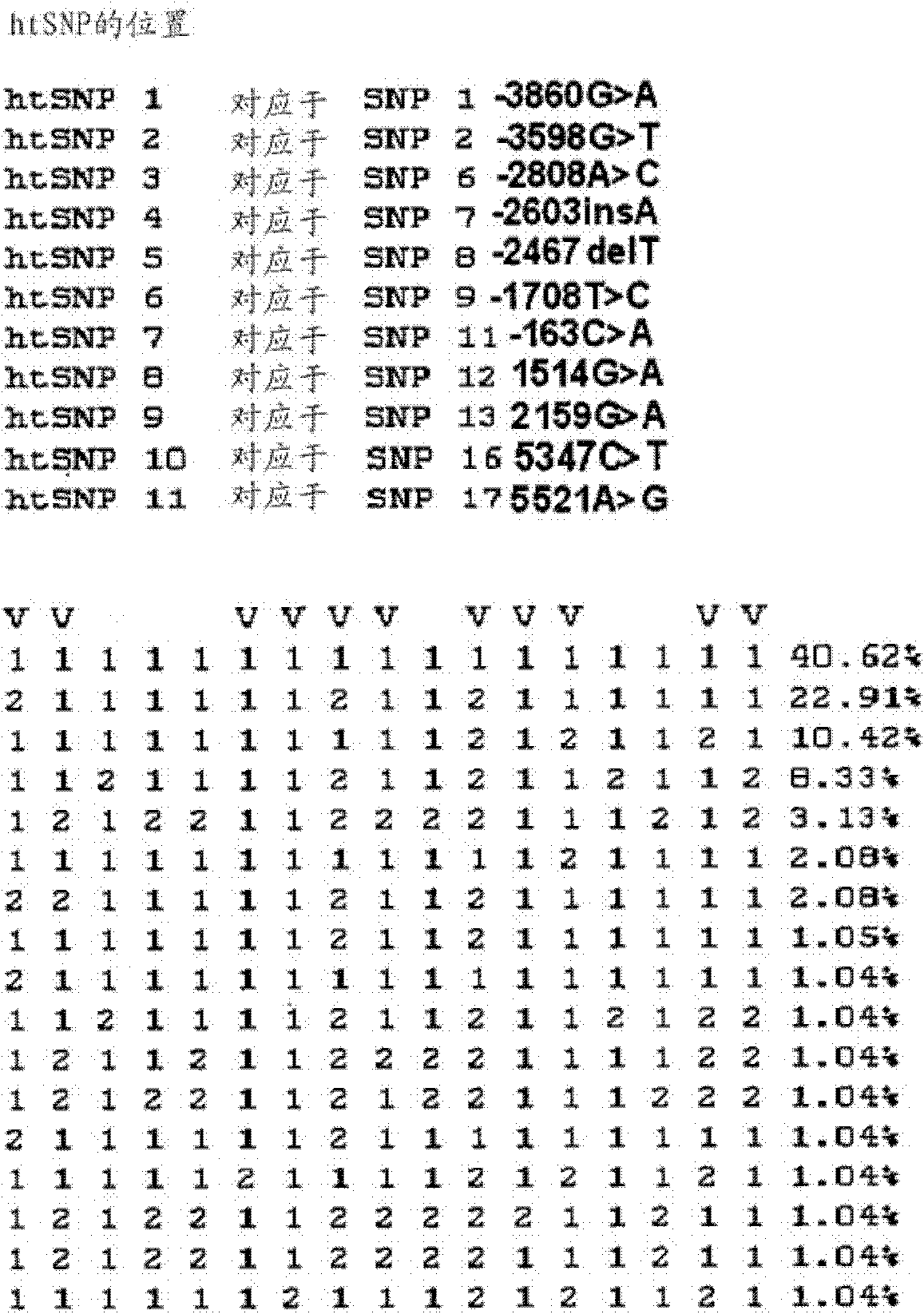
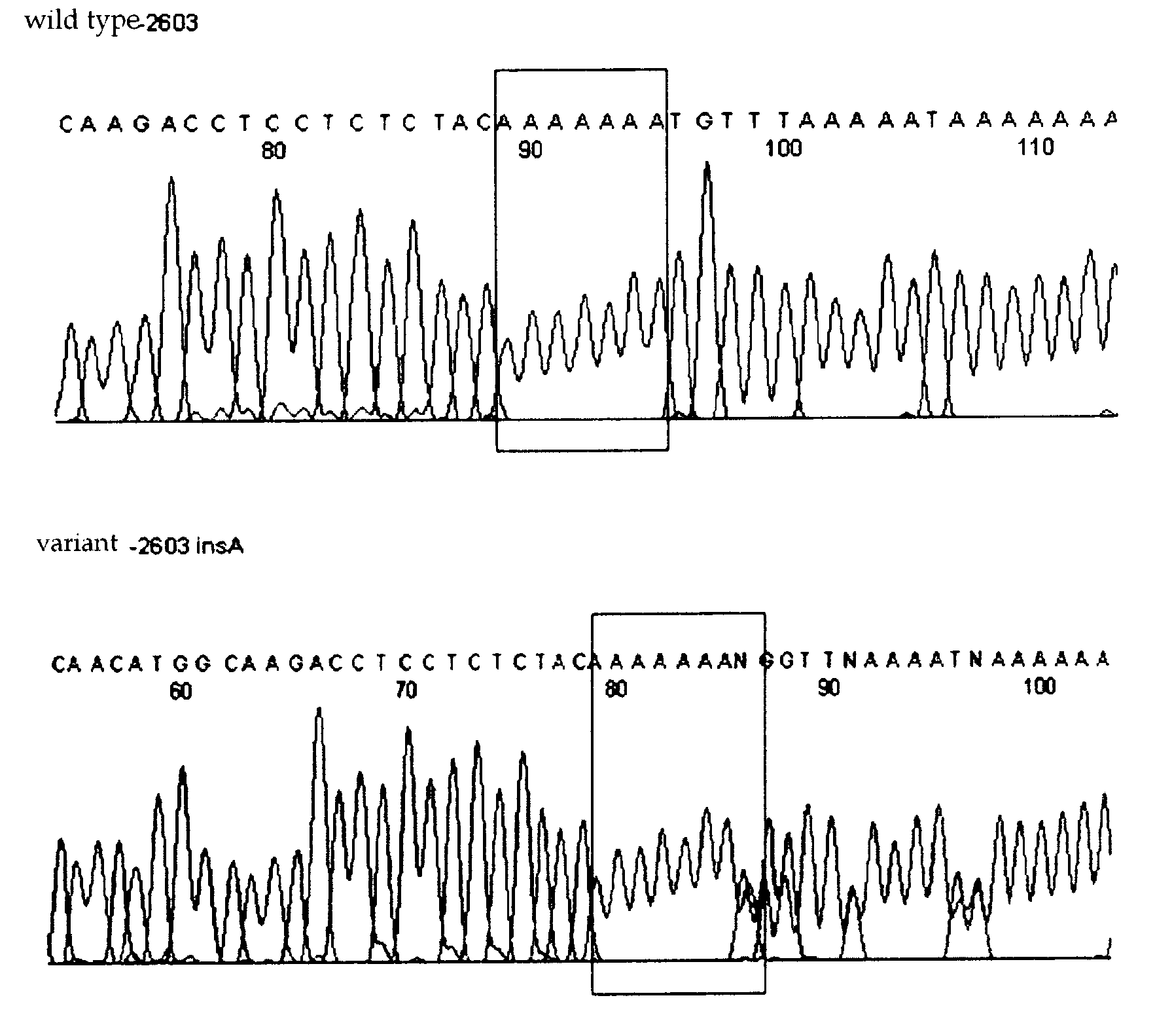
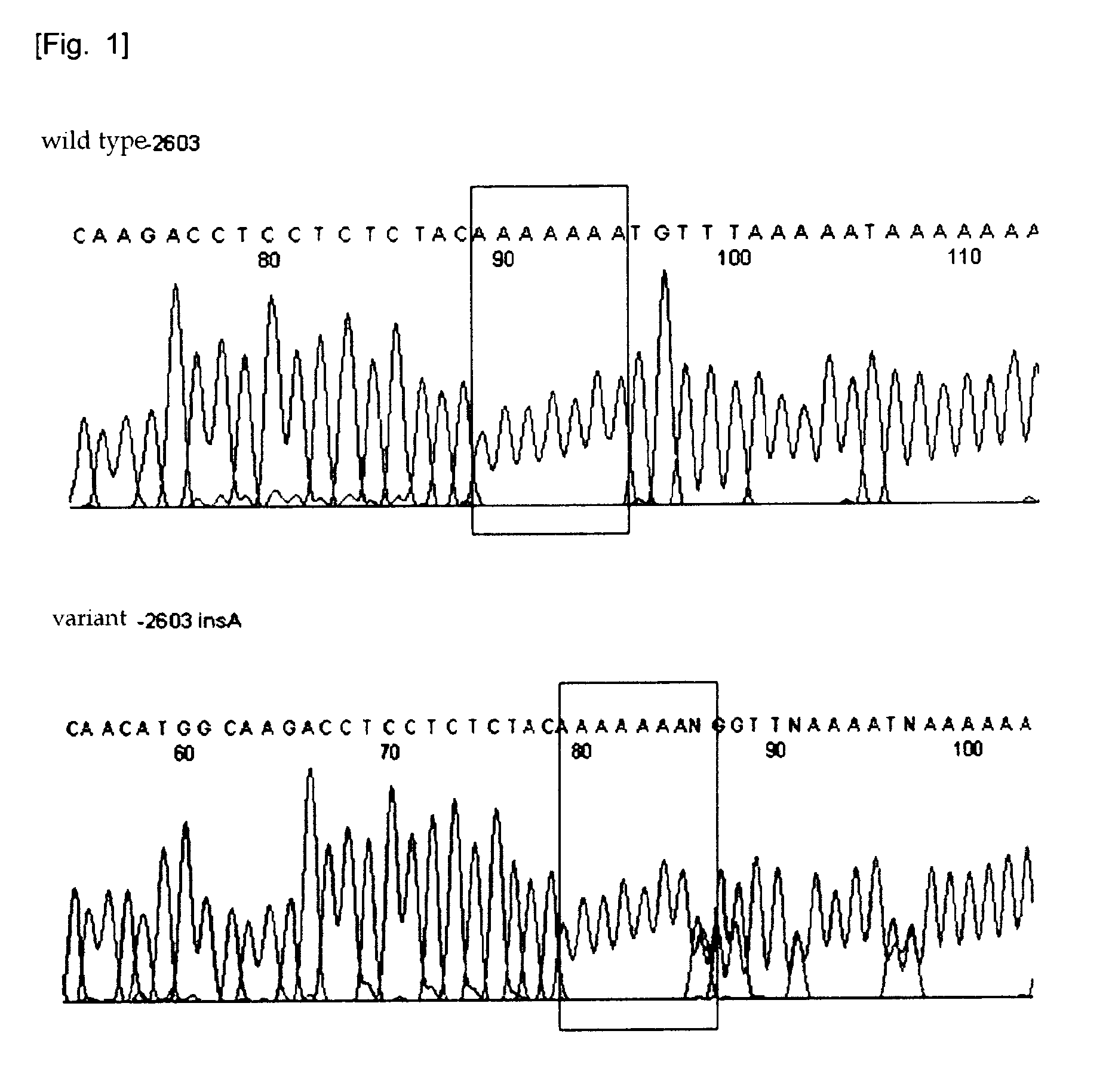
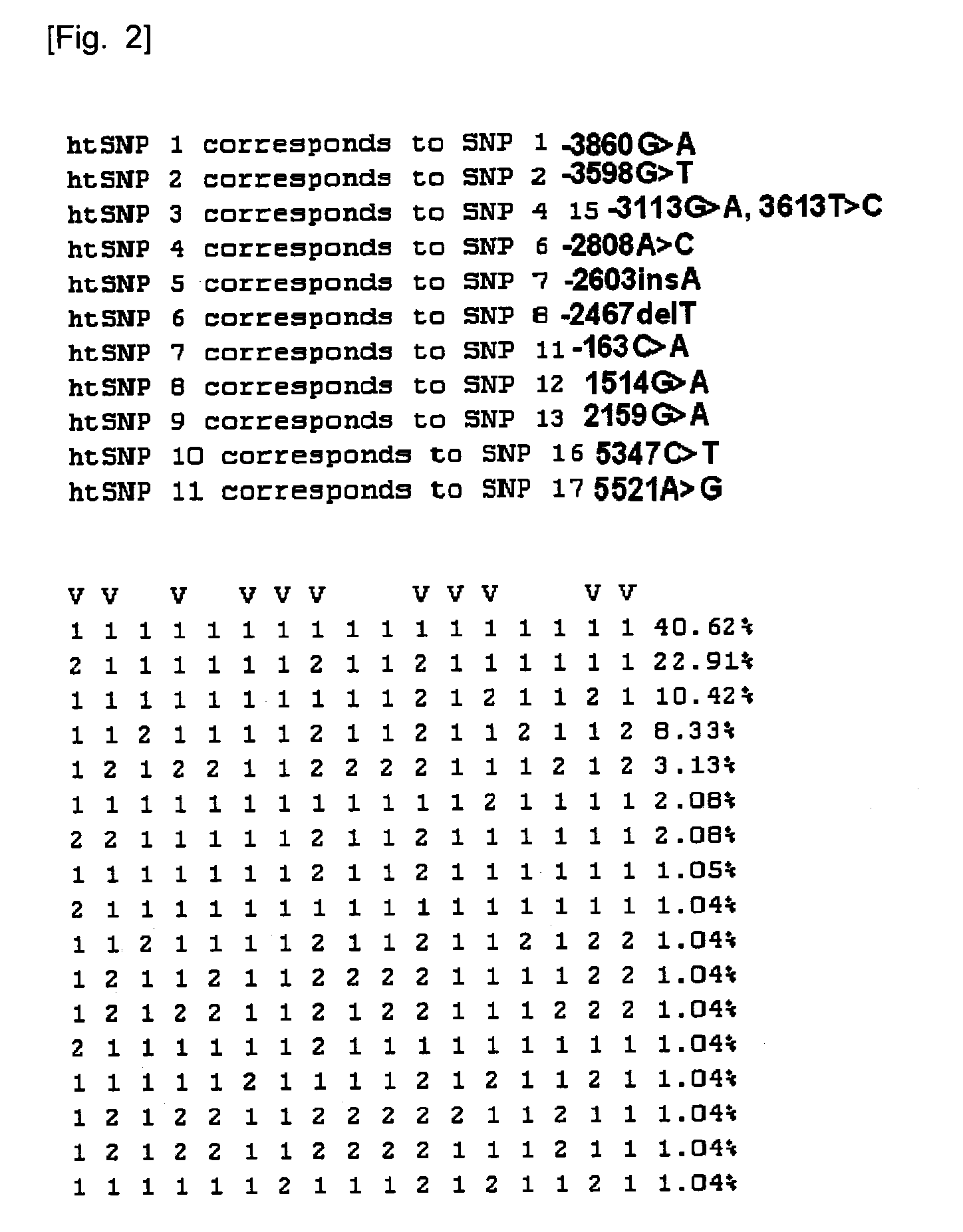
Htsnps for determining a genotype of cytochrome P450 1a2, 2A6 and 2D6, PXR and UPD-glucuronosyltransferase 1A gene and multiplex genotyping methods using thereof
The present invention relates to htSNPs for determining a genotype of cytochrome P450 1A2 (CYP1A2), 2A6 (CYP2A6) and 2D6 (CYP2D6), PXR and UDP- glucuronosyltransf erase Ia (UGTlA) genes and a gene chip using the same, and more particularly, to a selection method of htSNPs for determining a haplotype of human CYP1A2, CYP2A6, CYP2D6, PXR and UGTlA genes, a method of determining a genotype of the genes by using the htSNPs and a gene chip therefor.
Owner:申载国
Kit and method for detecting tamoxifen personalized medicine genetic polymorphism by use of pyrosequencing technique
InactiveCN102643905AQuick analysisAccurate analysisMicrobiological testing/measurementPersonalized medicinePyrosequencing
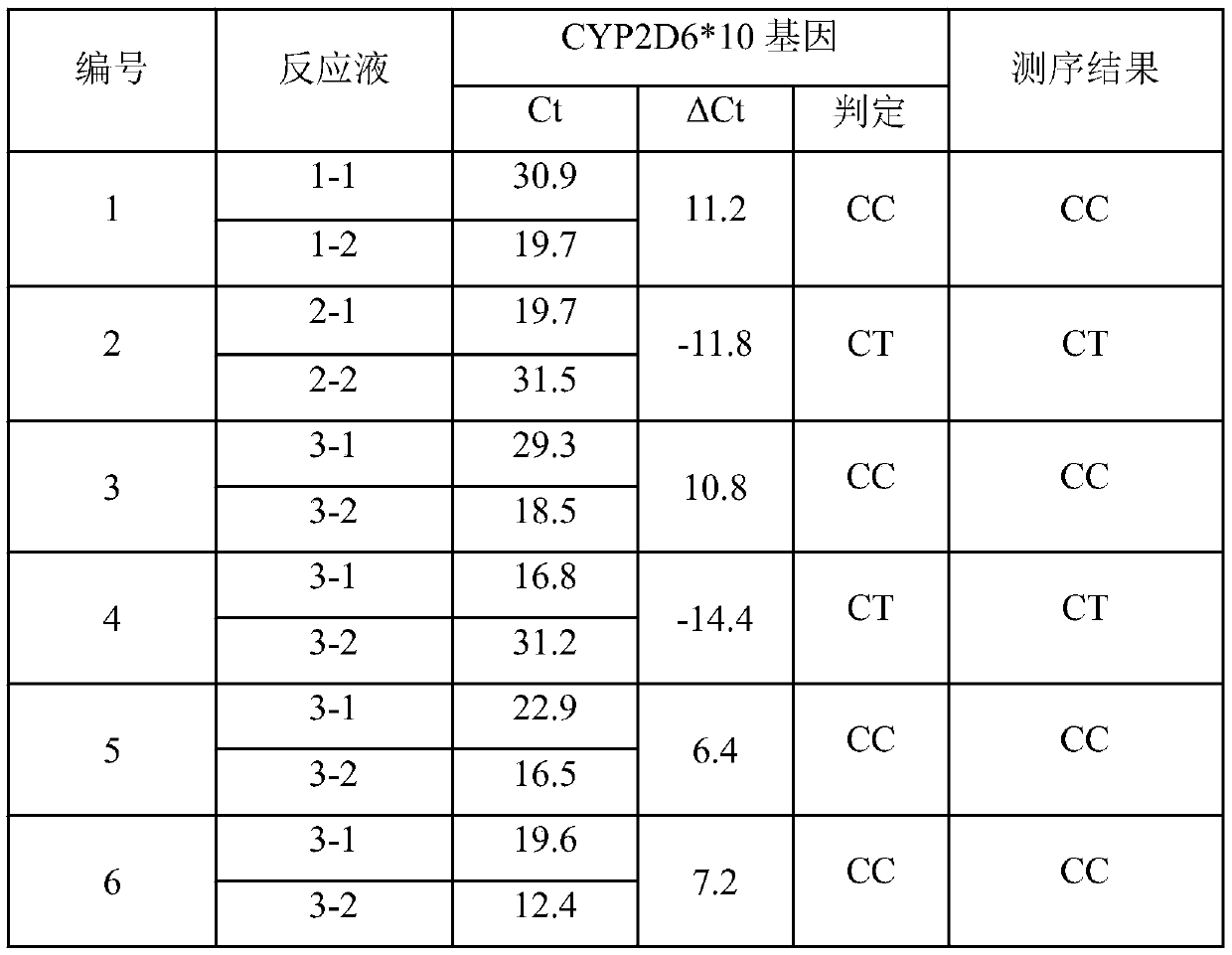
The invention discloses a kit and method for detecting the tamoxifen personalized medicine genetic polymorphism by use of the pyrosequencing technique. The genetic polymorphism specifically refers to the single nucleotide polymorphism of CYP2D6*10(rs1065852) and (SULT1A1*2) (rs9282861). The kit comprises the primer shown by SEQ ID NO.3-8. The kit disclosed by the invention can realize accurate, quick and high-flux detection on CYP2D6*10(rs1065852) and (SULT1A1*2) (rs9282861) so as to realize safe, reasonable and effective personalized administration of the tamoxifen medicine.
Owner:CENT SOUTH UNIV
Method and Composition to Evaluate Cytochrome P450 2D6 Isoenzyme Activity Using a Breath Test
InactiveUS20100329979A1Organic active ingredientsMicrobiological testing/measurementMetaboliteOral medication
The present invention relates, generally to a method of determining and assessing cytochrome P450 2D6 isoenzyme (CYP2D6)-related metabolic capacity in an individual mammalian subject via a breath assay, by determining the relative amount of 13CO2 exhaled by a the subject upon intravenous or oral administration of a 13C-labeled CYP2D6 substrate compound. The present invention is useful as an in vivo phenotype assay for evaluating CYP2D6-related activity using the metabolite 13CO2 in expired breath and to determine the optimal dosage and timing of administration of CYP2D6 substrate compound.
Owner:CAMBRIDGE ISOTOPE LAB
Gene detection method, primer probe combination and kit for precise drug use of depression
InactiveCN109929927AMicrobiological testing/measurementDNA/RNA fragmentationDrug metabolismCurative effect
The invention relates to the field of gene detection, in particular to a gene detection method, a primer probe combination and a kit for precise drug use of depression to overcome the shortcoming thatthe market is short of products for gene detection of the drug use of the depression, only detection of a drug metabolism gene is adopted clinically, and the drug therapeutic effect predication is inaccurate. 11 loci of 5 related genes of CYP2D6, CYP2C19, FKBP5, HTR2A and HTR1A of anti-depression drugs are detected to determine the relevant gene locus types, and the clinical drug use can be guided by analysis.
Owner:广州海思医疗科技有限公司
Individualized medication gene testing reagent kit for beta-receptor blocker
InactiveCN109136360AGood curative effectReduce adverse reactionsMicrobiological testing/measurementBeta blockerCurative effect
The invention belongs to the technical field of biology, and particularly relates to an individualized medication gene testing reagent kit for a beta-receptor blocker. The individualized gene testingreagent kit for the beta-receptor blocker mainly comprises: (1) two pairs of CYP2D6*10 and ADRBB1(1165 G) [C] locus amplification and sequencing primers, (2) PCR amplification reagents, (3) a PCR product purification reagent, and (4) a DNA sequencing reagent. The reagent kit is used for detecting the loci of the beta-receptor blocker drug metabolizing enzyme and the receptor CYP2D6*10 and ADRBB1 (1165 G) [C] of the hypertension patient, uses the key technology for Sanger sequencing (the accuracy rate is more than 99.9%). The genotypes of the two loci are proved to be closely associated with the curative effect of medication of the beta-receptor blocker, so that the gene testing reagent kit can be used for guiding the hypertension patient in using the beta-receptor blocker drug reasonably and safely according to the testing result of the gene testing reagent kit.
Owner:中科基因生物科技(江苏)有限公司
Kit for detecting polymorphism of hypertension medication related genes
The invention relates to the technical field of in vitro diagnosis, in particular to a kit for detecting polymorphism of human CYP2D6, CYP2C9, ADRB1, AGTR1, ACE genes by a multiple fluorescent PCR method. The kit is used to detect CYP2D6*10, CYP2C9*3, ADRB1 (1165G > C), AGTR1 (1166A > C), ACE (I / D) polymorphism sites. The primer and the probe have high sensitivity and high specificity, and can accurately detect genomic DNA as low as 0.1 ng / <mu>L. The kit is easy to operate, and can cooperate with an automated instrument for detection.
Owner:AUTOBIO DIAGNOSTICS CO LTD
htsnp for determining the genotype of the ugt1a gene and its method for multiplex genotyping
The present invention relates to htSNPs for determining a genotype of cytochrome P450 1A2 (CYP1A2), 2A6 (CYP2A6) and 2D6 (CYP2D6), PXR and UDP-glucuronosyltransferase Ia (UGT1A) genes and a gene chip using the same, and more particularly, to a selection method of htSNPs for determining a haplotype of human CYP1A2, CYP2A6, CYP2D6, PXR and UGT1A genes, a method of determining a genotype of the genes by using the htSNPs and a gene chip therefor.
Owner:申载国
Test chipe of cytochrome P450 gene hereditary variation and its application
InactiveCN1912139AGuide rational drug useImprove throughputMicrobiological testing/measurementTherapeutic effectCYP2C9
The invention discloses a cytochrome P450 gene genetic variation detecting chip. It includes the probe fixed on the solid phase carrier to hybridize with the cytochrome P450 gene nucleotide sequence and / or complementation sequence. The invention also discloses the used chip detecting method. The detecting chip can effectively detect subtype genetic variation of CYP2C9, CYP2C19, CYP2D6, CYP3A5, forecast the therapeutic effectiveness of over 40% clinic common medicine to realize individualization medical treatment.
Owner:SHANGHAI BIOCHIP
HtSNPs FOR DETERMINING A GENOTYPE OF CYTOCHROME P450 1A2, 2A6 AND 2D6, PXR AND UDP-GLUCURONOSYLTRANSFERASE 1A GENE AND MULTIPLEX GENOTYPING METHODS USING THEREOF
InactiveUS20100159454A1Microbiological testing/measurementMaterial analysisHaplotypeMultiplex genotyping
The present invention relates to htSNPs for determining a genotype of cytochrome P450 1A2 (CYP1A2), 2A6 (CYP2A6) and 2D6 (CYP2D6), PXR and UDP-glucuronosyltransferase Ia (UGT1A) genes and a gene chip using the same, and more particularly, to a selection method of htSNPs for determining a haplotype of human CYP1A2, CYP2A6, CYP2D6, PXR and UGT1A genes, a method of determining a genotype of the genes by using the htSNPs and a gene chip therefor.
Owner:INJE UNIV IND ACADEMIC COOP FOUND
Primer probe combination, kit and method for detecting copy number variation and genotyping of human CYP2D6
PendingCN113186267AEasy to operatePrevent false positive amplificationMicrobiological testing/measurementDNA/RNA fragmentationHuman DNA sequencingPsychoactive drug
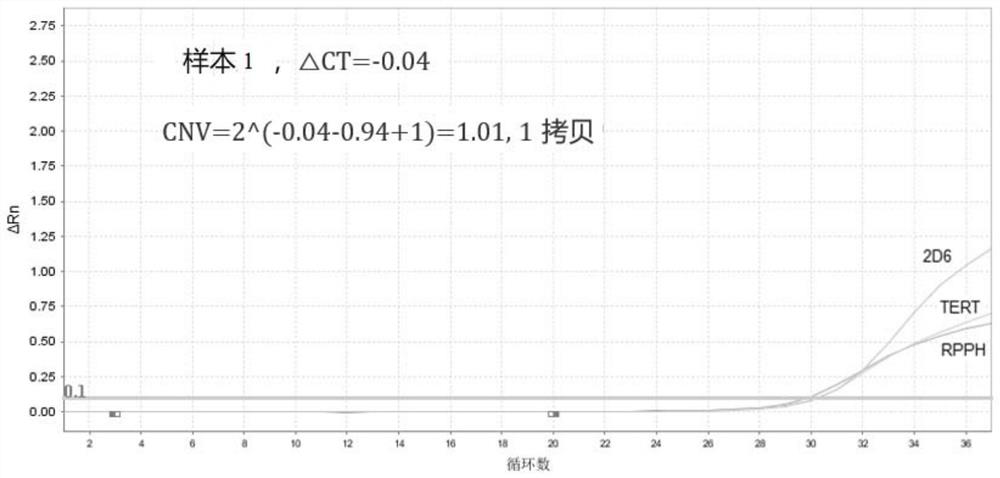
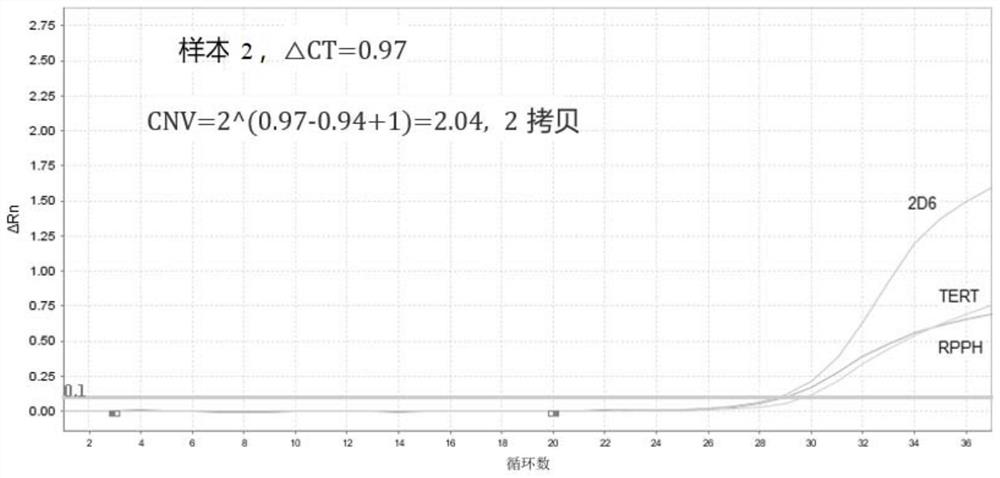
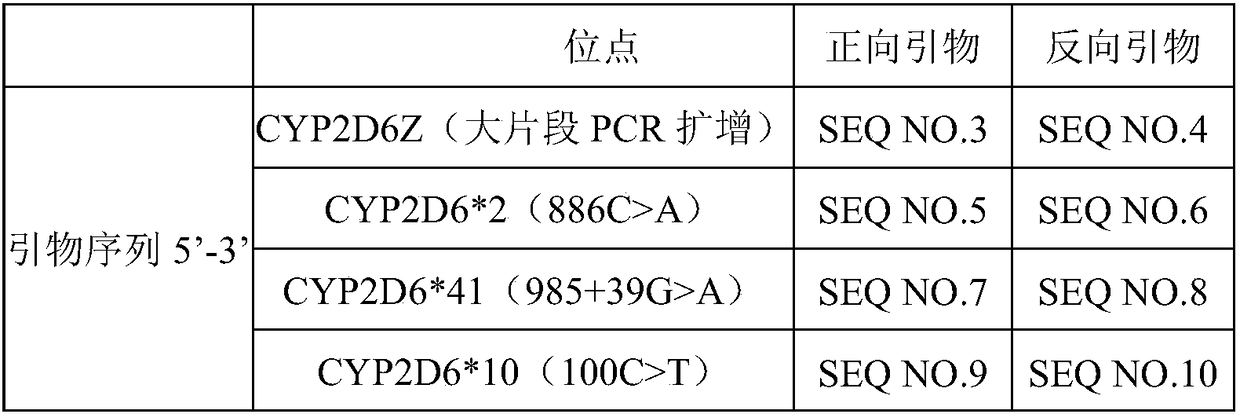
The invention provides a primer probe combination, a kit and a method for detecting copy number variation and genotyping of human CYP2D6. The nucleotide sequences of the primer probe combination for detecting the copy number variation of the human CYP2D6 are as shown in SEQ ID No.1 to SEQ ID No.9, and the nucleotide sequences of the primer probe combination for detecting the genotyping of the human CYP2D6 are as shown in SEQ ID No.10 to SEQ ID No.17. The primer probe combination can be used for in-vitro qualitative detection of CYP2D6 copy number variation and * 10 and * 41 genotypes in human genome DNA, realizes simultaneous detection of * 10 and * 41 genotypes in a single tube on imported / domestic fluorescent quantitative PCR equipment without mutual interference, has the advantages of high accuracy, good stability and excellent detection limit, and has a wide application prospect. According to the detection result, clinical selection of antipsychotic drugs can be assisted to realize medication guidance for mental / depression patients, the curative effect is ensured, and toxic and side effects are reduced.
Owner:上海康黎诊断技术有限公司
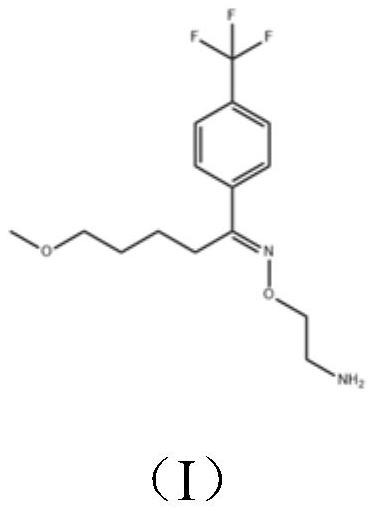
Targeted drug rescue with novel compositions, combinations, and methods thereof
ActiveUS20200069674A1Improve efficiencyIncidence of adverseOrganic active ingredientsMetaboliteReceptor
Compounds of Formula I, pharmaceutically acceptable salts thereof, enantiomers thereof, metabolites thereof, derivatives thereof, prodrugs thereof, acid addition salts thereof, pharmaceutically acceptable salts thereof, or N-oxides thereof; or a combination thereof; processes and intermediates for preparation thereof, compositions thereof, and uses thereof; are provided. Pharmaceutical compositions comprising a compound of Formula I, or enantiomers thereof, metabolites thereof, derivatives thereof, prodrugs thereof, acid addition salts thereof, pharmaceutically acceptable salts thereof, or N-oxides thereof; or a combination thereof; wherein the compound is a double and / or triple agent or ligand for CYP2D6, 5-HT2A, and / or 5HT2C receptors, and / or acetylcholinesterase are provided.
Owner:VEPACHEDU SREENIVASARAO +1
Clozapine individualized drug gene detection kit
PendingCN109207582AGood curative effectSmall toxicityMicrobiological testing/measurementSide effectLarge fragment
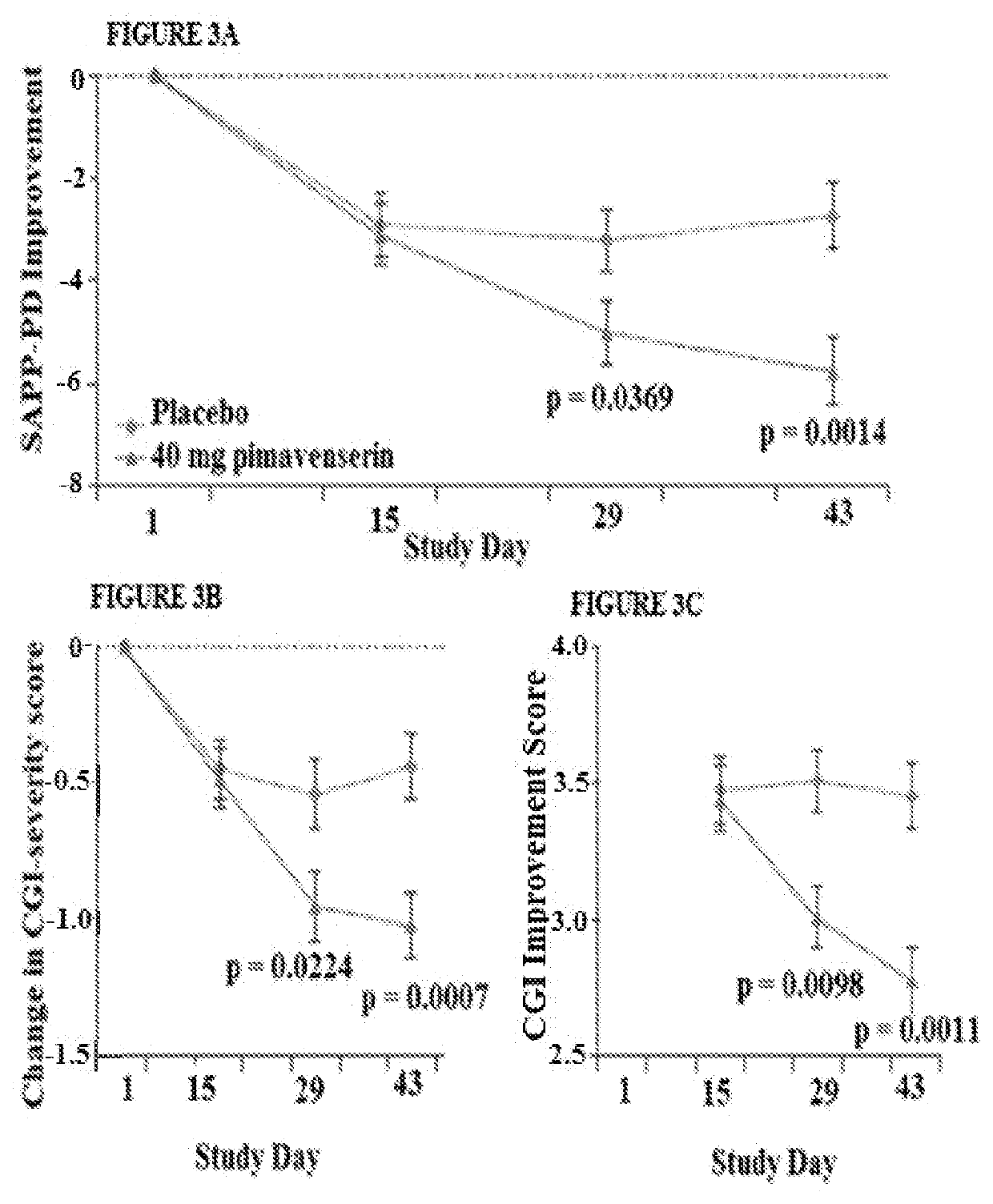
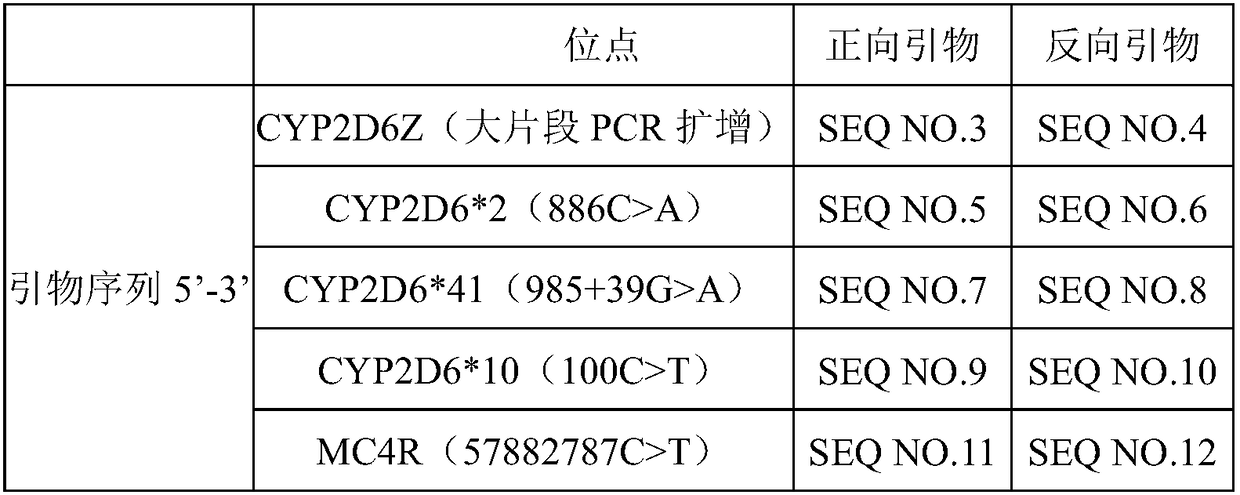
A clozapine individualized drug gene detection kit comprises the following components: CYP2D6*2 (886C) A, CYP2D6*41 (985+39G) A, CYP2D6*10 (100C) T and MC4R (57882787C) T; 4 pair of site amplificationand sequencing prim, 1 pair of CYP2D6Z large fragment PCR amplification primers, PCR amplification reagent, PCR product purification reagent and DNA sequencing reagent; as shown in Table 1 of that prim sequence. The invention has the following technical effects: a gene detection kit for individualized clozapine medication for Chinese people is provided, and the sensitivity is high and the accuracy is high. The kit is used for CYP2D6*2 (886C) A), CYP2D6*41(985+39G)A), CYP2D6*10 (100C) T) and MC4R (57882787C) T). The genetic difference between individuals can be found through the test of this item, and the drug constitution (slow metabolism or normal metabolism) can be defined, the relevant gene information can be interpreted, and the physiological status or drug reaction can be known in time, so that the drug efficacy can be improved, the toxic and side effects of drugs can be reduced, and the medical cost can be reduced.
Owner:南通中科医学检验实验室有限公司
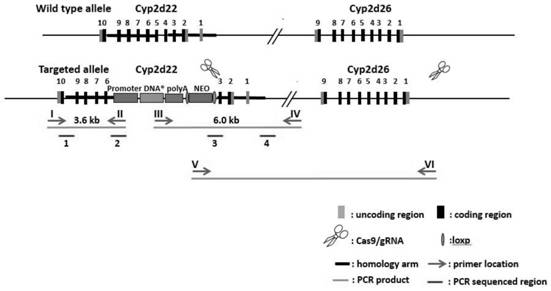
Construction method of humanized CYP2D6*10 transgenic mouse model
PendingCN112626119AOvercoming problems with certain differencesNucleic acid vectorOxidoreductasesCYP2D6Cell
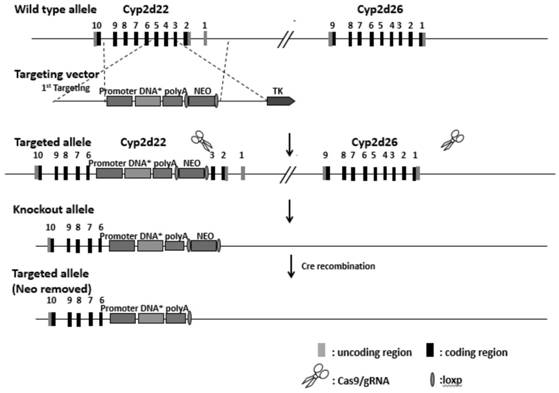
The invention discloses a construction method of a humanized CYP2D6*10 transgenic mouse. According to the method, the homologous recombination principle is utilized, an ES targeting mode is adopted, an expression cassette of hCYP2D6*10 is knocked into an exon4-5 site of the Cyp2d22 gene in a fixed-point mode, and then a Cyp2d gene cluster is knocked out in a Crispr / Cas9 mode; firstly, a targeting vector is prepared, and ES cells are electrically transfected after the vector is linearized; through long-fragment PCR identification, positive clones of correct homologous recombination are obtained; and the positive ES cell clone is amplified to be injected into a blastocyst of a C57BL / 6J mouse to obtain a chimeric mouse. Compared with a non-transgenic mouse, the humanized CYP2D6*10 transgenic mouse bred by the invention can express a human CYP2D6*10 type gene (dominant proportion allele of Chinese population), and can replace human beings to be used for developing pharmacokinetic and pharmacodynamic related research of substrate drugs.
Owner:WENZHOU MEDICAL UNIV
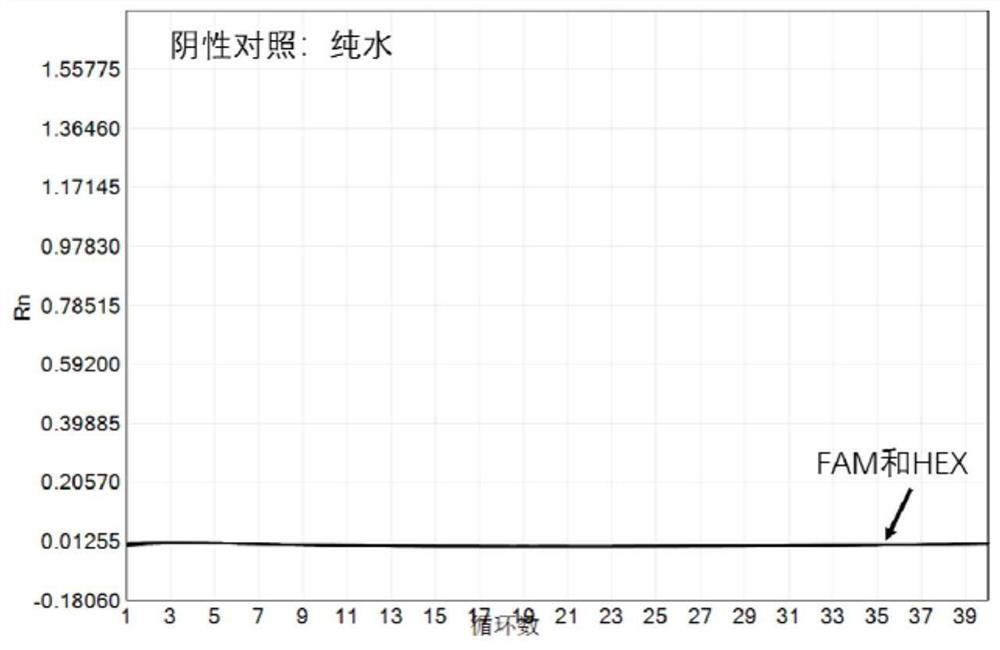
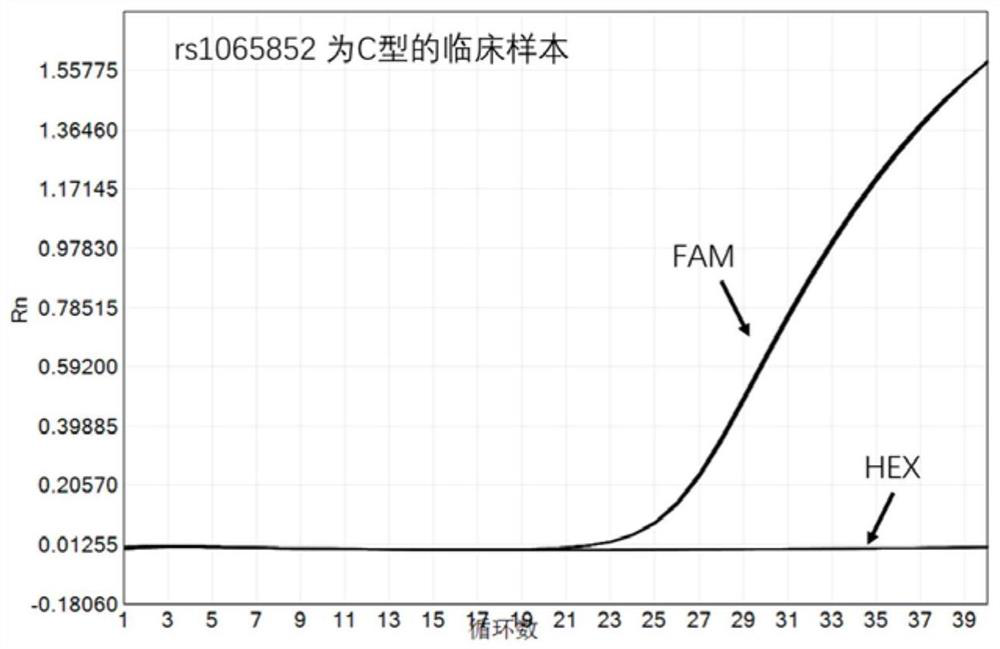
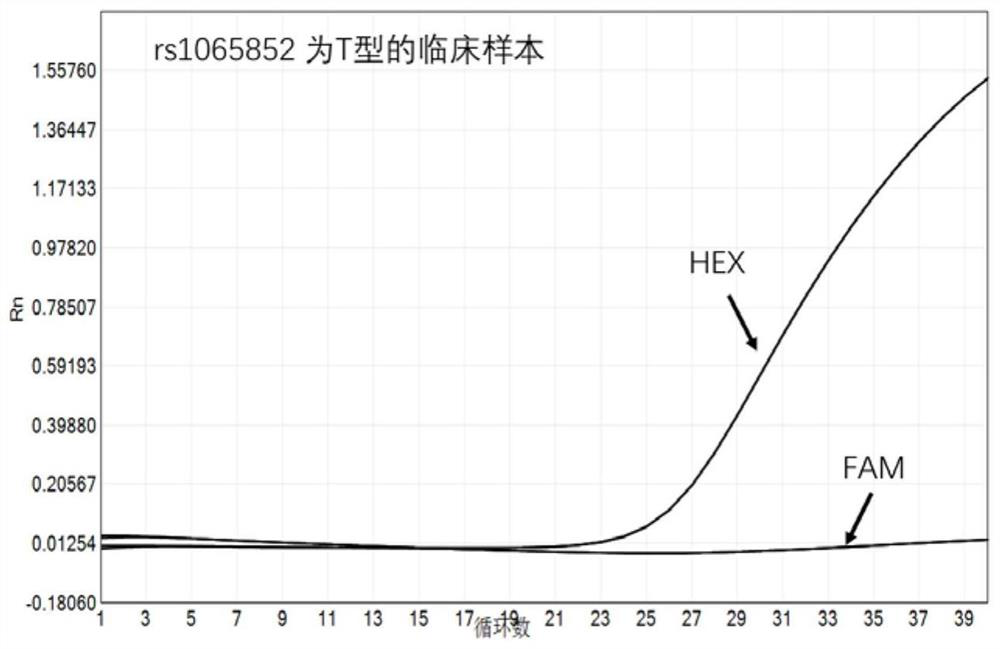
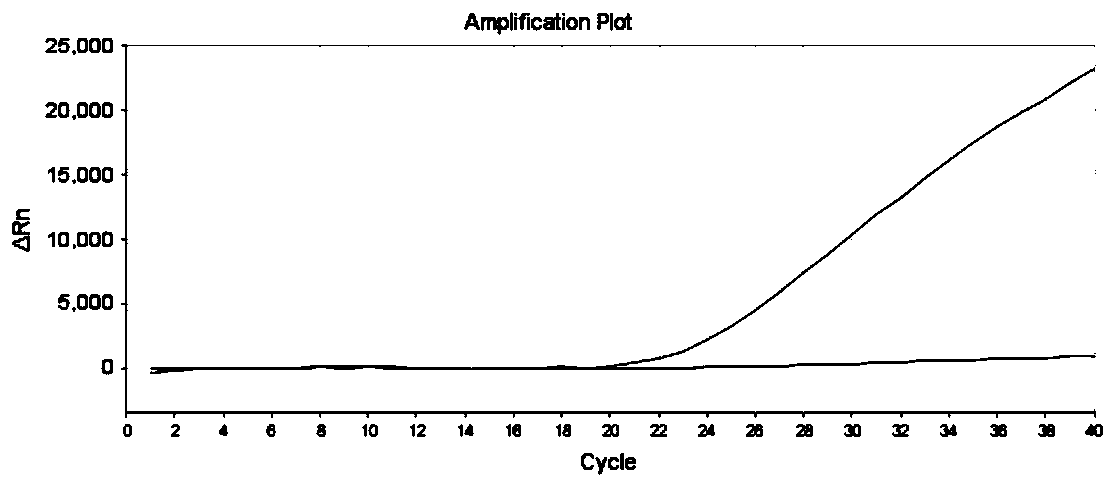
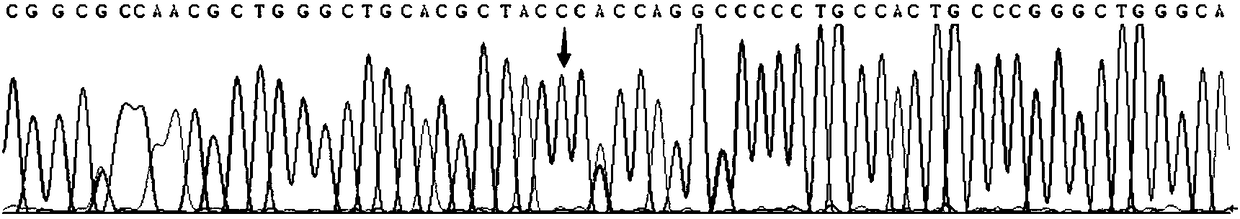
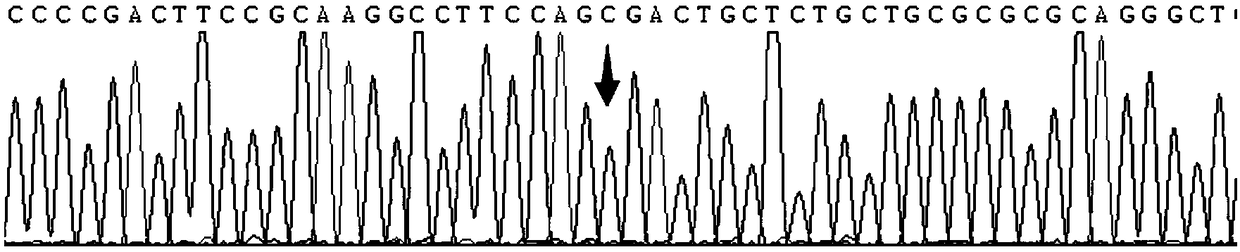
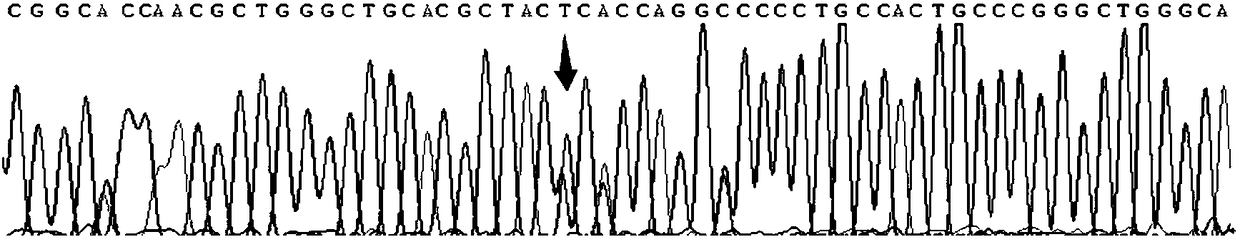
Gene detection kit used for [beta] receptor antagonist medication, and detection method and application of gene detection kit
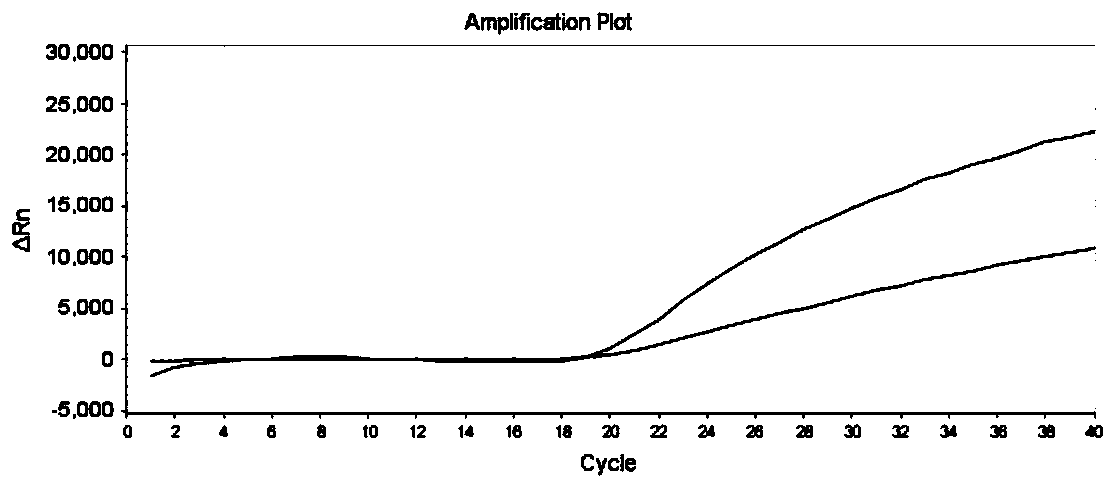
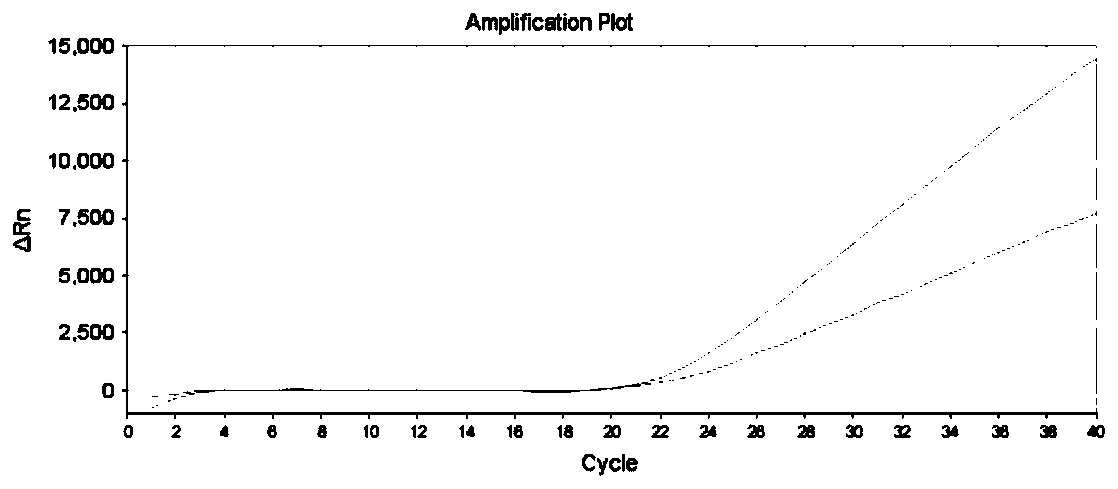
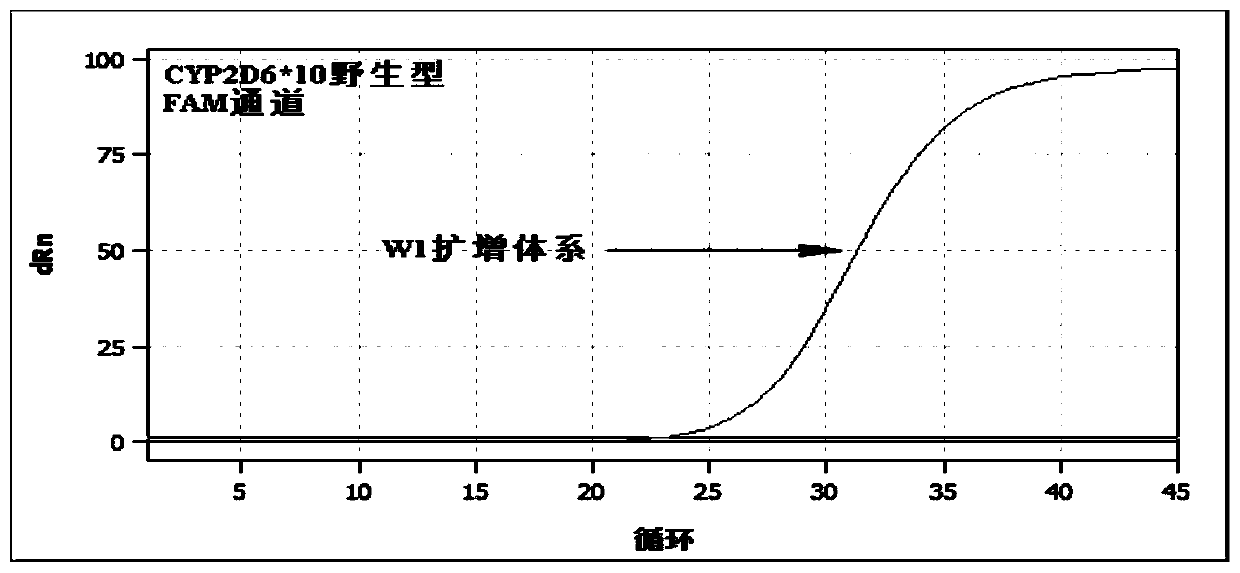
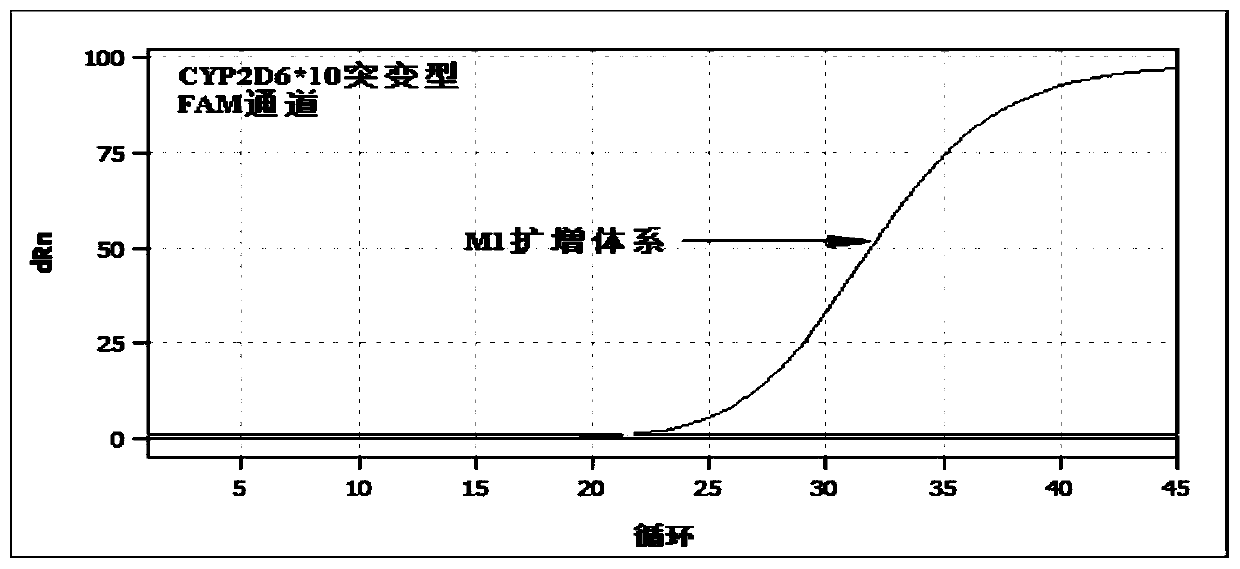
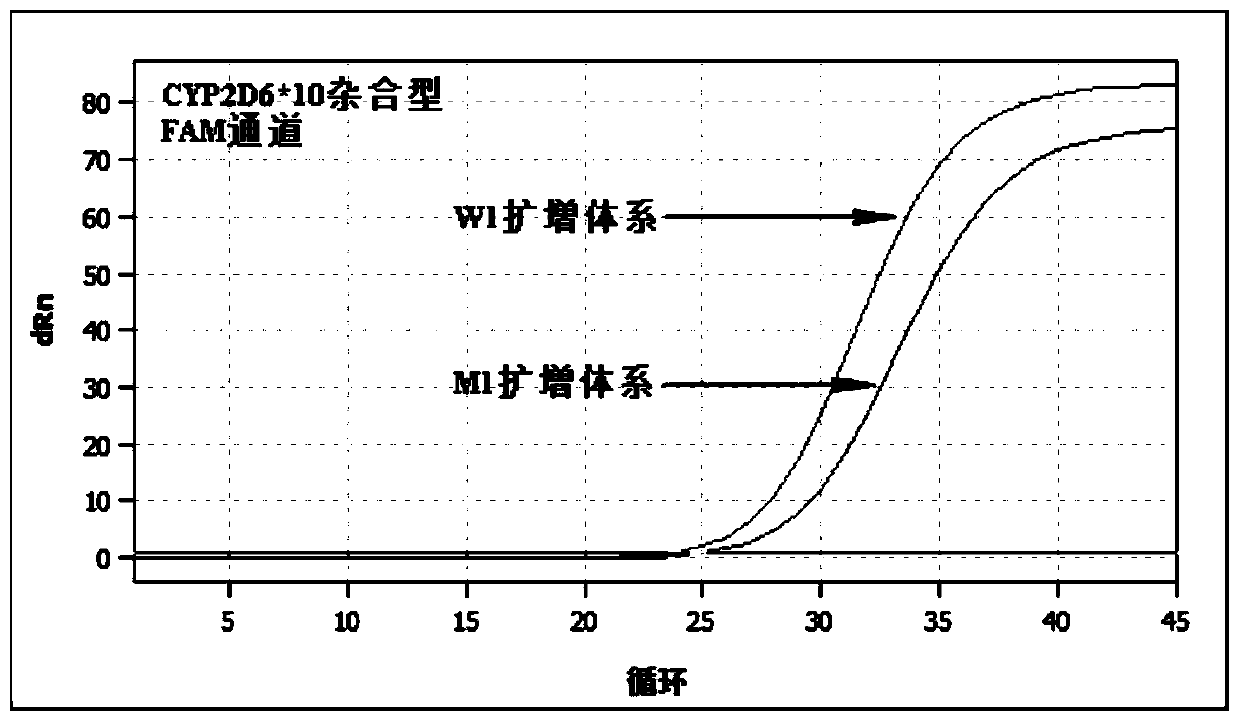
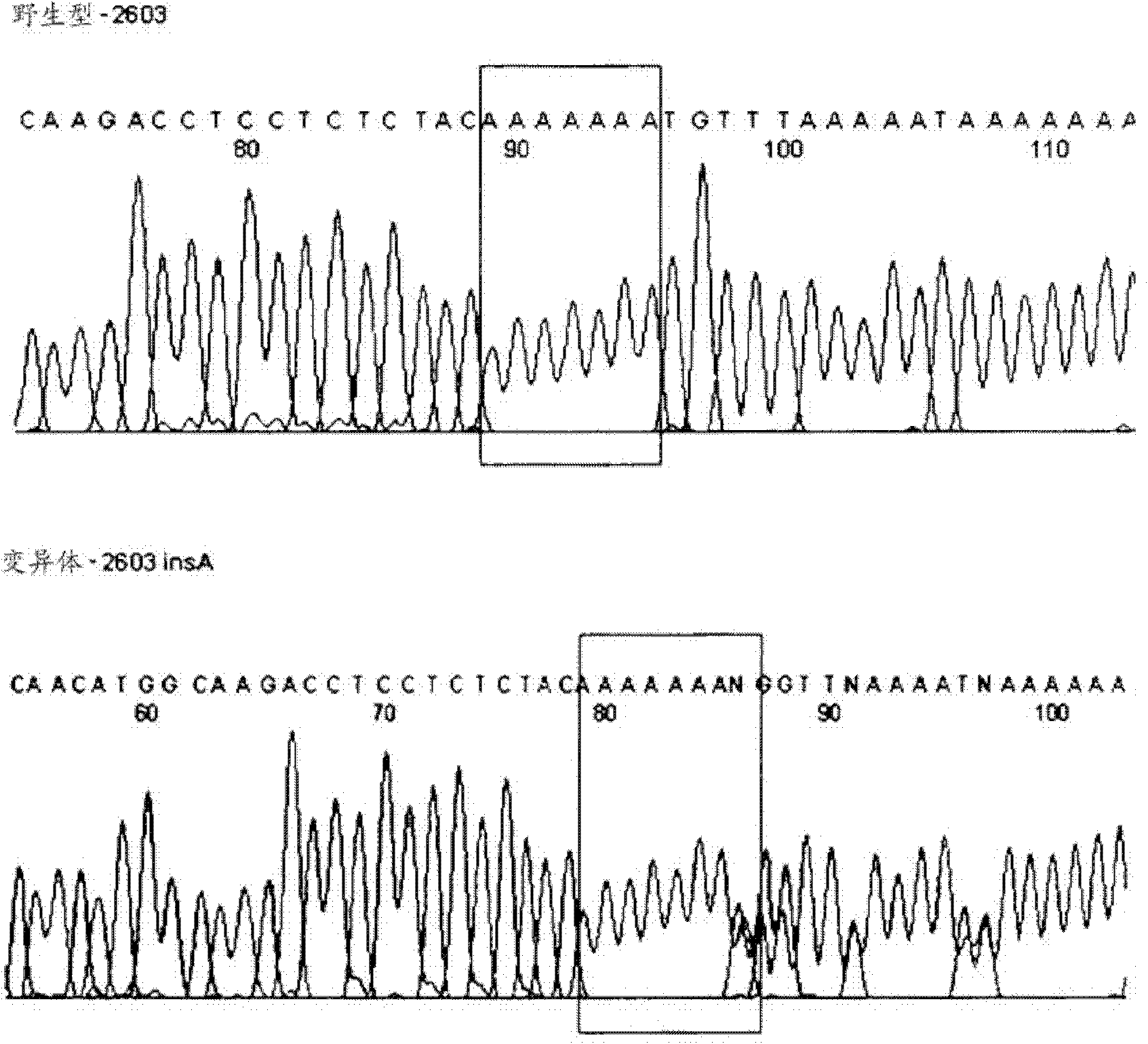
PendingCN113151441AAvoid interferenceAvoid damageMicrobiological testing/measurementDNA/RNA fragmentationMultiplexPseudogene
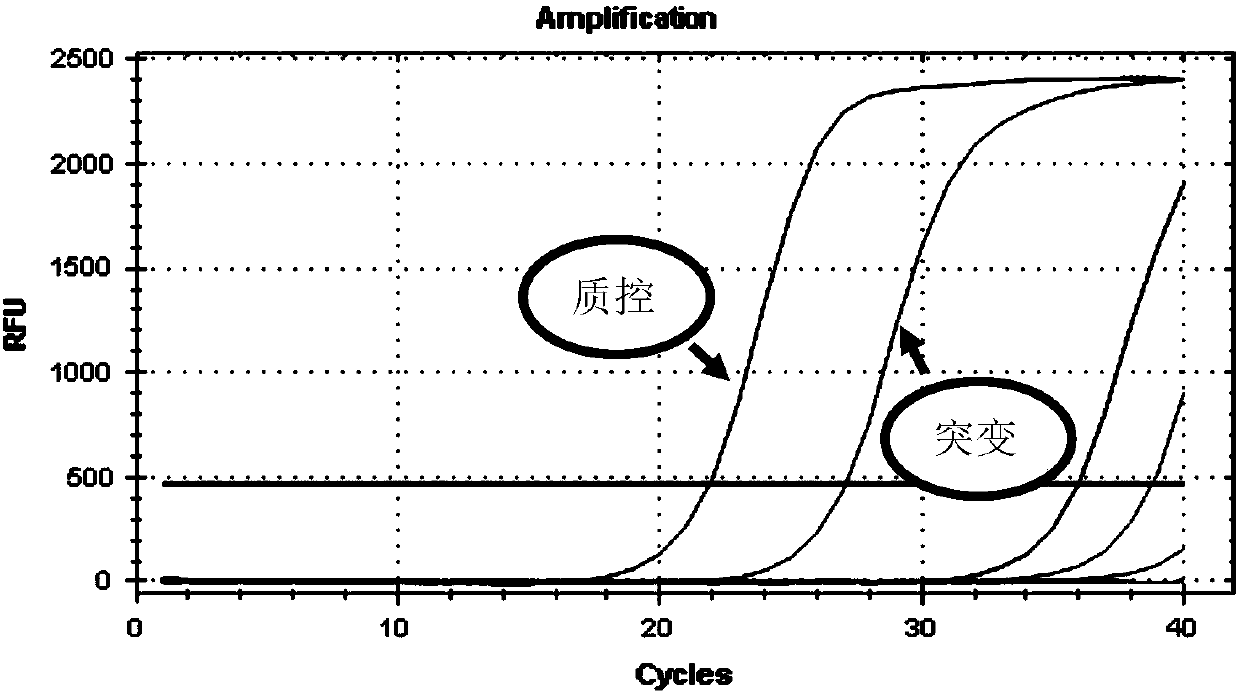
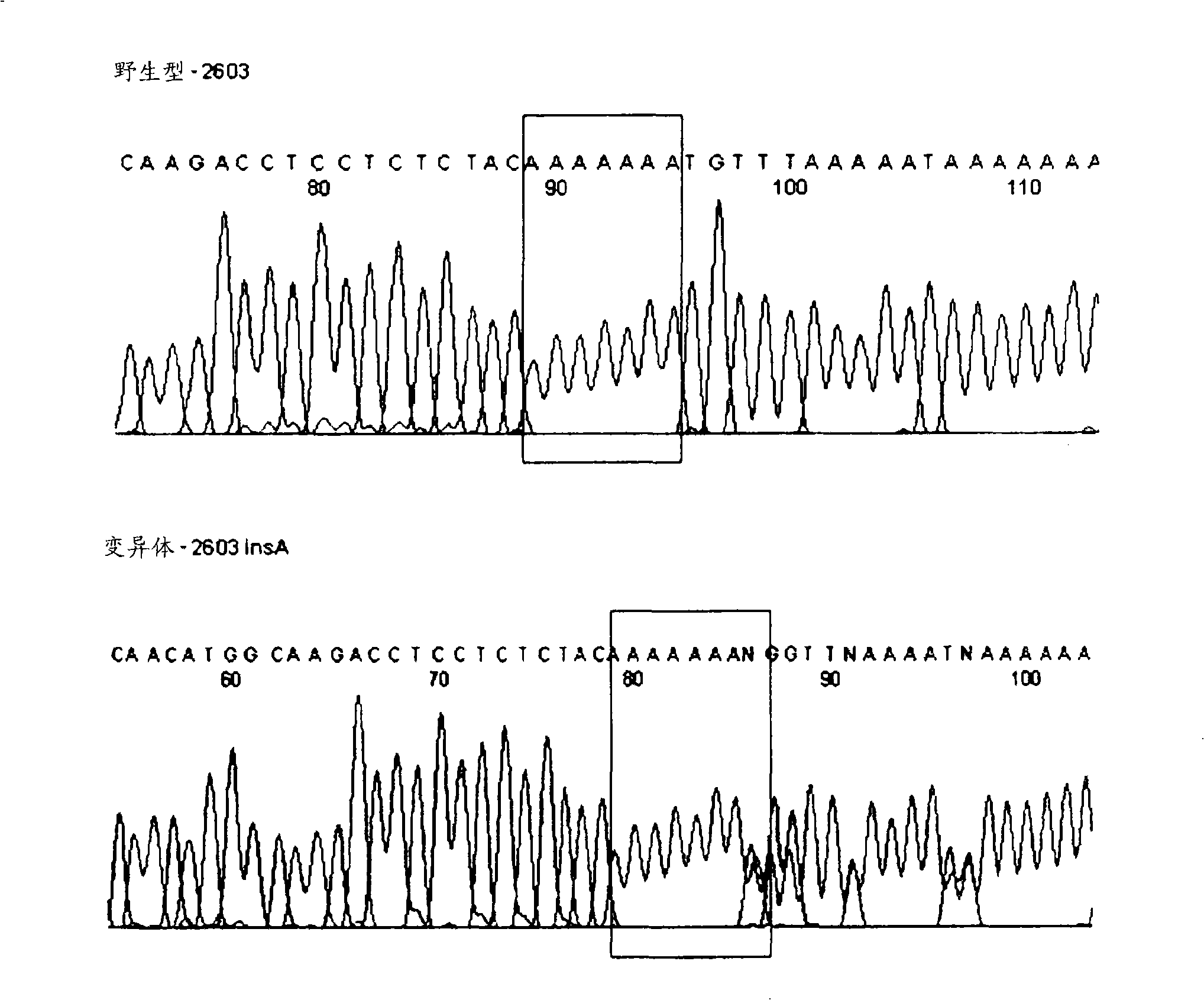
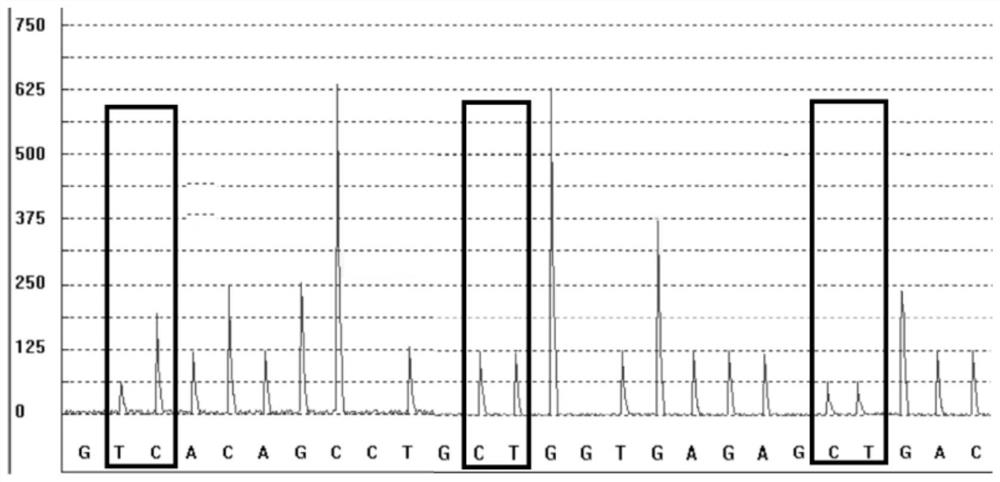
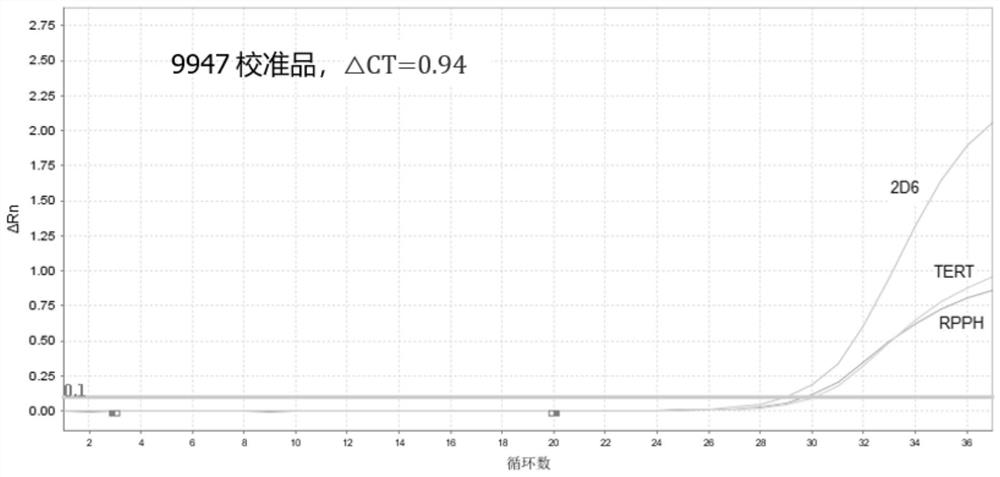
The invention discloses a gene detection kit used for [beta] receptor antagonist medication, and a detection method and application of the gene detection kit. The detection kit designs specific amplification primers and sequencing primers by aiming at the polymorphism and the CYP2D6 effective copy number of two genes, including CYP2D6C100T and ADRB1G1165C. The kit comprises the following ingredients: amplification reaction liquid, a CYP2D6C100T sequencing primer, an ADRB1G1165C sequencing primer, a CYP2D6 effective copy number sequencing primer and a positive control. The gene detection kit adopts asymmetric multiplex PCR one-tube amplification on CYP2D6(C100T), ADRB1(G1165C and the CYP2D6 effective copy number, a great quantity of biotin-labeled single-strand DNA is generated, in a single-strand amplification process, a CYP2D7-PNA blocking probe blocks binding of a biotin-labeled probe and a pseudogene CYP2D7 so as to prevent the pseudogene from disturbing a sequencing result, the biotin-labeled single-strand DNA carries out binding with streptavidin, after washing is carried out, the sequencing primers and a sequencing raw material are added, pyrophosphoric acid sequencing is carried out, injuries to an amplified fragment by a strong basicity reagent are reduced, sequencing procedures are simplified, and sequencing time is shortened.
Owner:湖南菲思特精准医疗科技有限公司
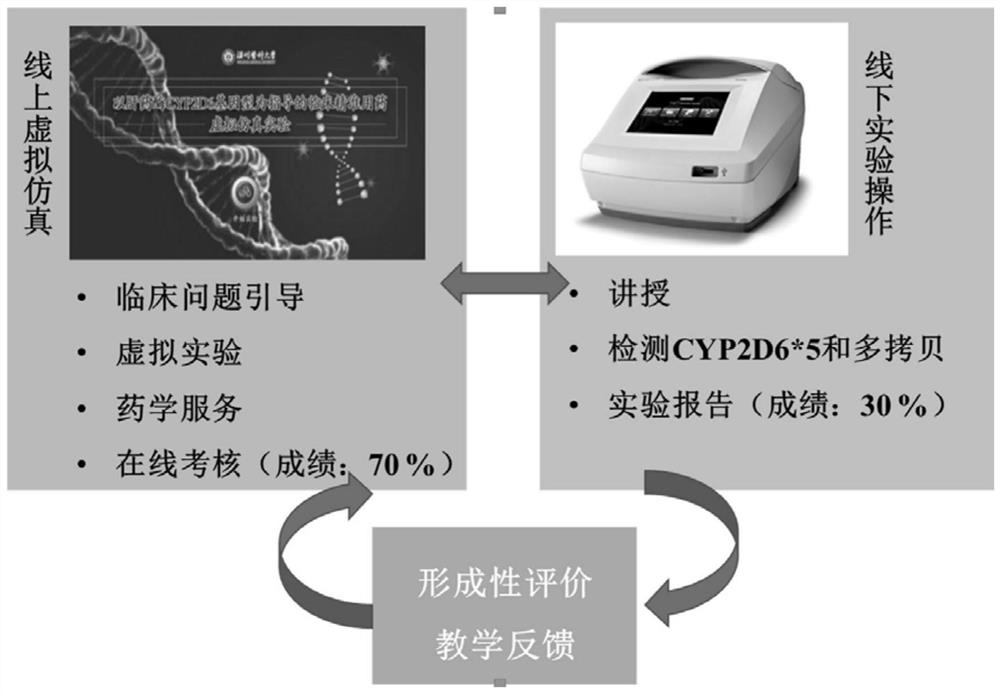
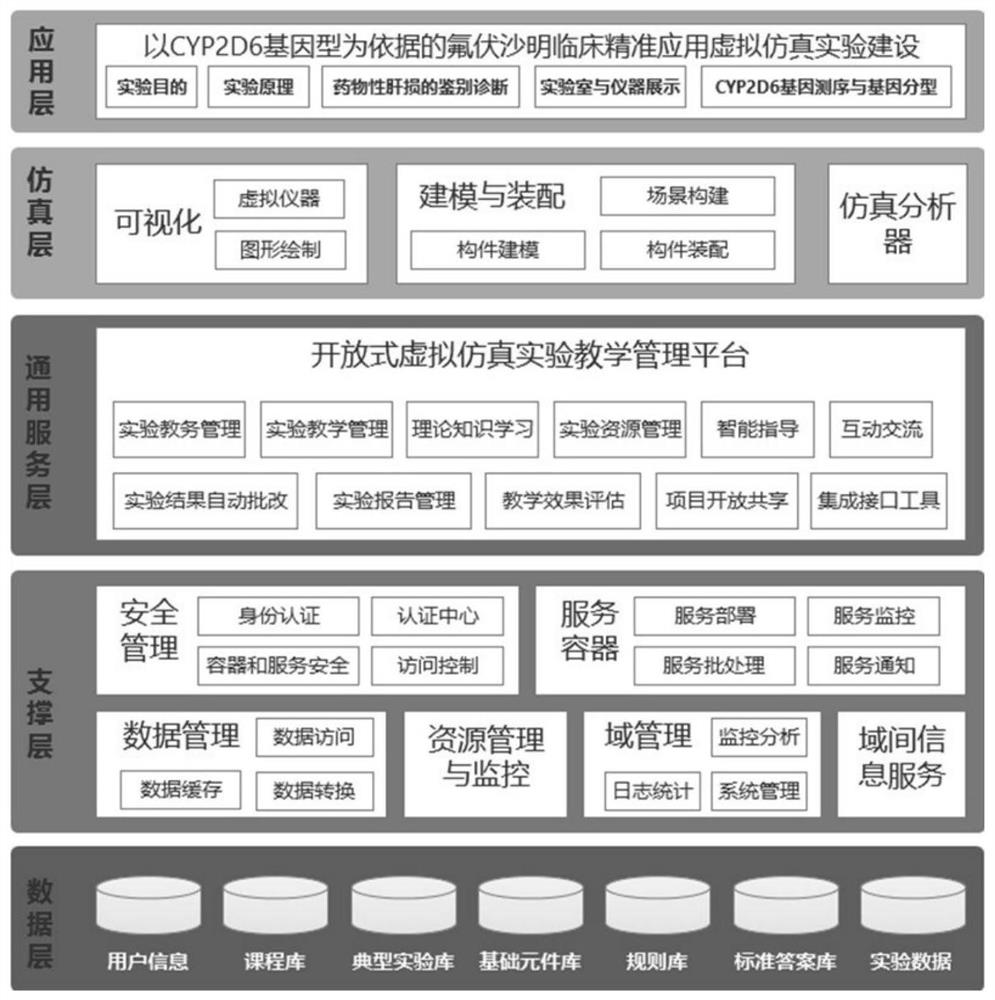
Fluvoxamine clinical precise application virtual simulation experiment system based on CYP2D6 genotype
PendingCN113270140AKeep abreast of learning effectsEasy to understandDesign optimisation/simulationProteomicsExperimental laboratoryEnzyme Gene
The invention discloses a fluvoxamine clinical precise application virtual simulation experiment platform based on a CYP2D6 genotype, the platform is oriented to clinical problems, a complete knowledge system of laboratory research and pharmaceutical service is built, students' understanding of precise medication is cultivated, and specifically, according to the project, students are guided to understand and master gene sequencing and analysis according to the ideas of drug monitoring, drug enzyme gene detection, metabolic type judgment, guide searching and individualized drug administration scheme making of special crowds.
Owner:WENZHOU MEDICAL UNIV
Targeted drug rescue with novel compositions, combinations, and methods thereof
ActiveUS11478467B2Good curative effectImproving antitussive propertyOrganic active ingredientsMetaboliteReceptor
Compounds of Formula I, pharmaceutically acceptable salts thereof, enantiomers thereof, metabolites thereof, derivatives thereof, prodrugs thereof, acid addition salts thereof, pharmaceutically acceptable salts thereof, or N-oxides thereof; or a combination thereof; processes and intermediates for preparation thereof, compositions thereof, and uses thereof; are provided. Pharmaceutical compositions comprising a compound of Formula I, or enantiomers thereof, metabolites thereof, derivatives thereof, prodrugs thereof, acid addition salts thereof, pharmaceutically acceptable salts thereof, or N-oxides thereof; or a combination thereof; wherein the compound is a double and / or triple agent or ligand for CYP2D6, 5-HT2A, and / or 5HT2C receptors, and / or acetylcholinesterase are provided.
Owner:VEPACHEDU SREENIVASARAO +1
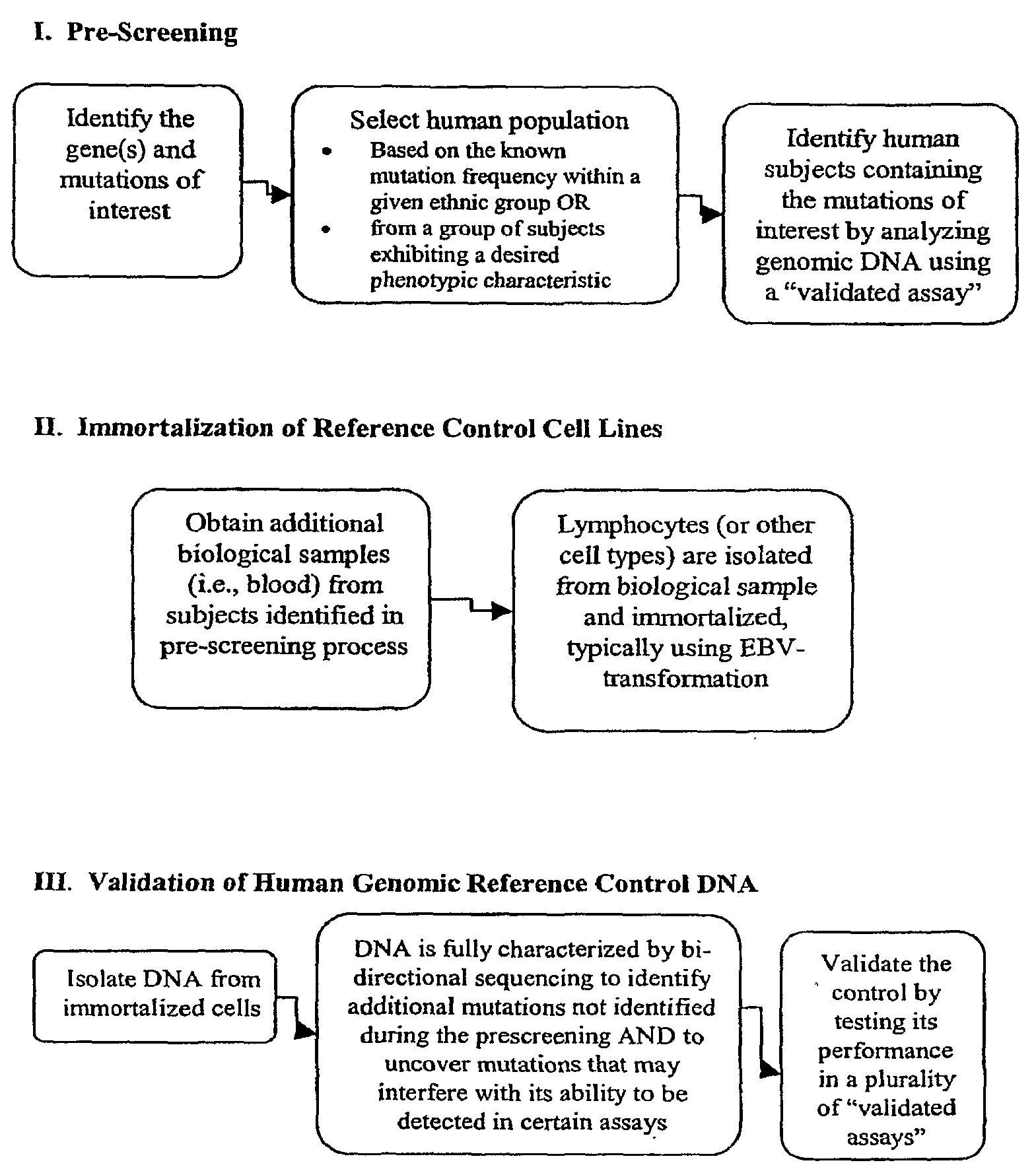
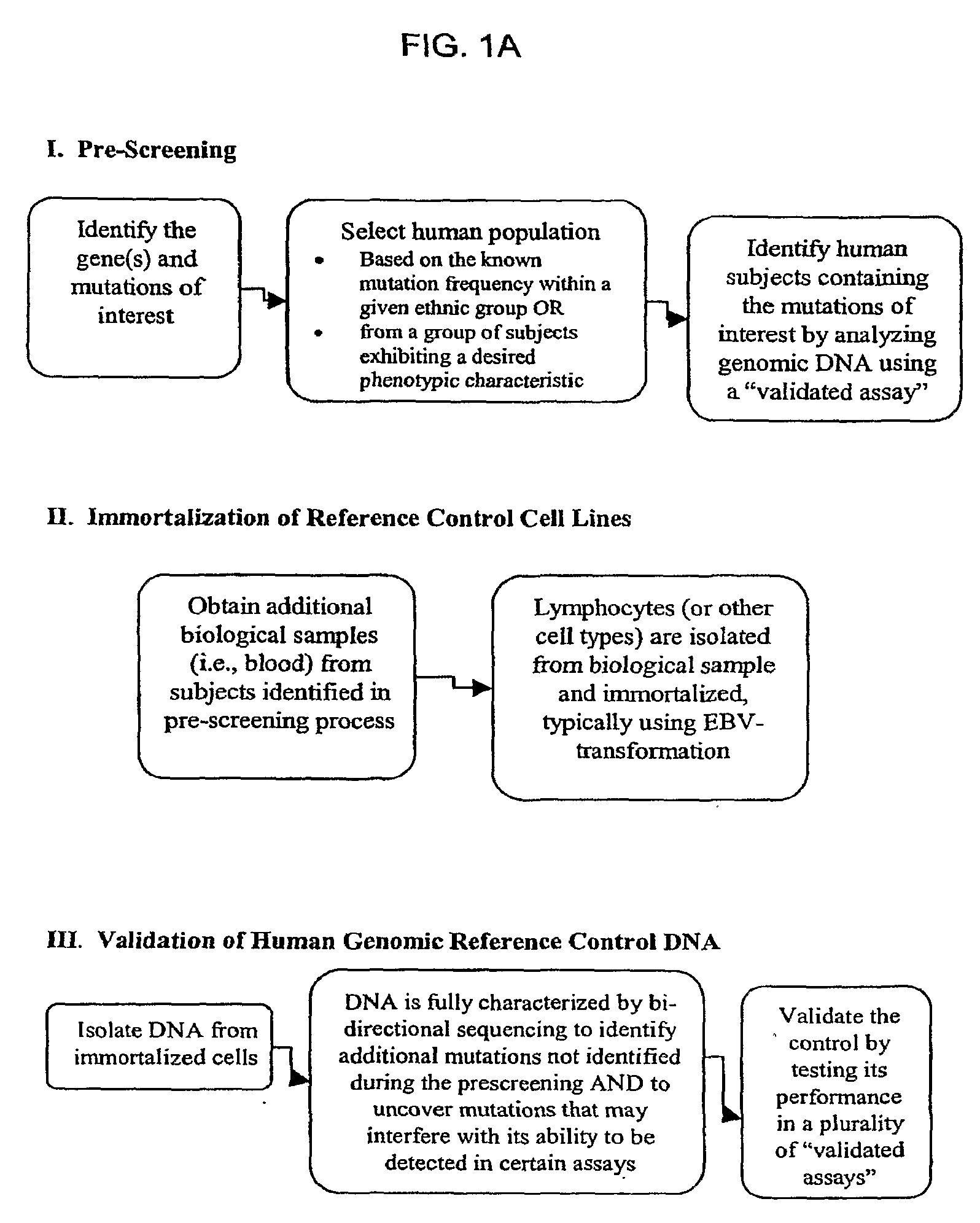
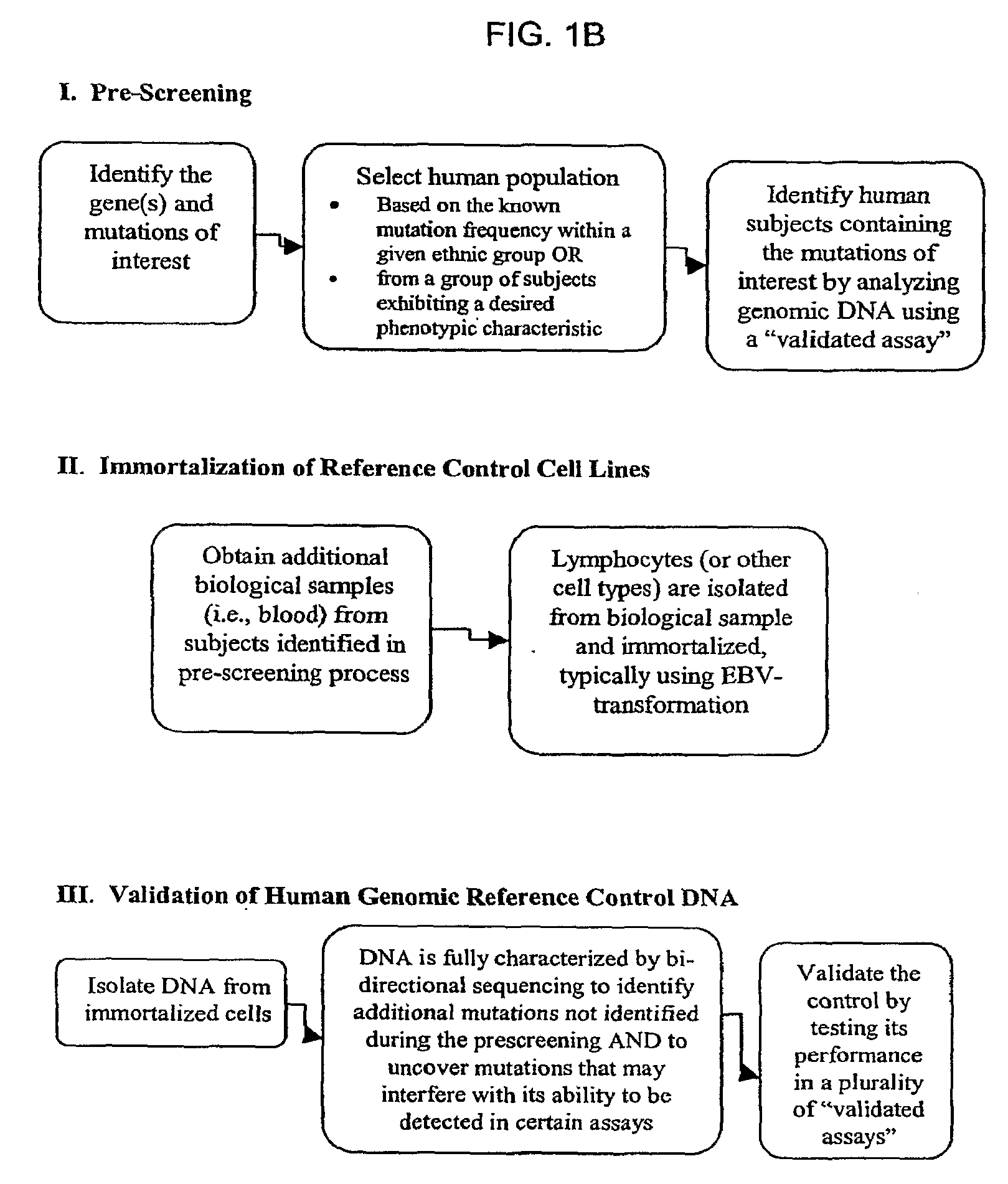
Method for Generating Reference Controls for Pharmacogenomic Testing
InactiveUS20090197945A1Accurate identificationAccurate doseBiocideNervous disorderGenomicsGenome human
Reference controls for use with pharmacogenomic testing, and methods for their identification, preparation, and use, are disclosed. The reference controls can confirm that pharmacogenomic testing correctly identifies individuals that do or do not have the mutation of interest, in both clinical trial and patient treatment settings. The reference controls can be selected to include one or more mutations to be identified, and prescreened to confirm that they bind to one or more of the primers used in the pharmacogenomic testing. The reference controls are human genomic DNA that includes certain identified polymorphisms (mutations) of interest, ideally derived from individuals, pre-selected and optionally properly consented, which have one or more of the polymorphism(s) of interest. The reference controls can be prepared by targeted pre-screening of human patients, by examining the genotype or genetic profile of the patients, isolating cells with the desired mutation, optionally immortalizing the cells, and obtaining DNA from the cells. The prescreening of prospective donors can be targeted based on any of a number of factors, such as genes of interest, mutations within the genes of interest, and membership in a specific ethnic or disease state population. The genomic DNA can be pre-screened for its ability to be detected, using a standard pharmacogenomic test, as including a specific mutation. Examples of mutations of interest include those present in a Phase I or Phase II metabolic enzyme such as CYP2D6, CYP2C19, CYP2C9, CYP2C8, and CYP3A5, CYP3A4, CYP2A6, CYP2B6, UGT1A1, DPD, ERCC1, MDR1, ADH2, NAT1 and NAT2 or any other metabolic or disease gene.
Owner:CATALYST ASSETS LLC
CYP2D6*10 genetic polymorphism detection kit capable of distinguishing CYP2D7P and CYP2D8P
ActiveCN112695097AHigh sensitivitySingle amplificationMicrobiological testing/measurementDNA/RNA fragmentationGenotypingMolecular medicine
The invention discloses a CYP2D6*10 genetic polymorphism detection kit capable of distinguishing CYP2D7P and CYP2D8P, and belongs to the technical field of molecular medicine. The kit can utilize a highly specific primer pair CYP2D6 (rs1065852) to realize accurate target amplification; and through the combination of the advantages of a Taqman probe of being high in specificity, fast and simple, the genotyping of the CYP2D6 (rs1065852) can be realized.
Owner:XIAMEN UNIV
Enhanced detoxification function gene fragment and modified hepg2 cells
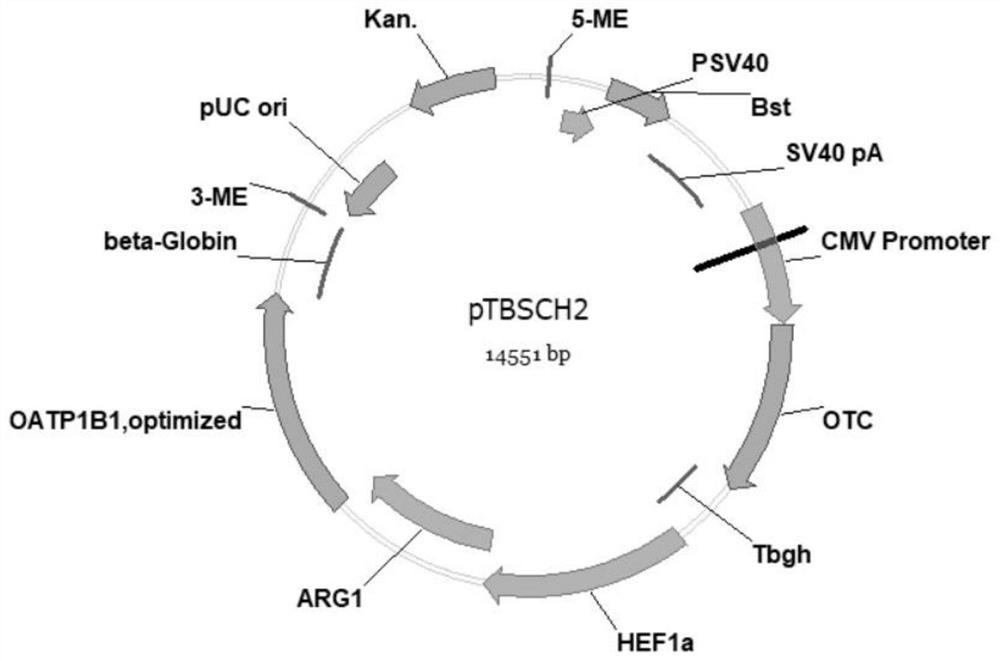
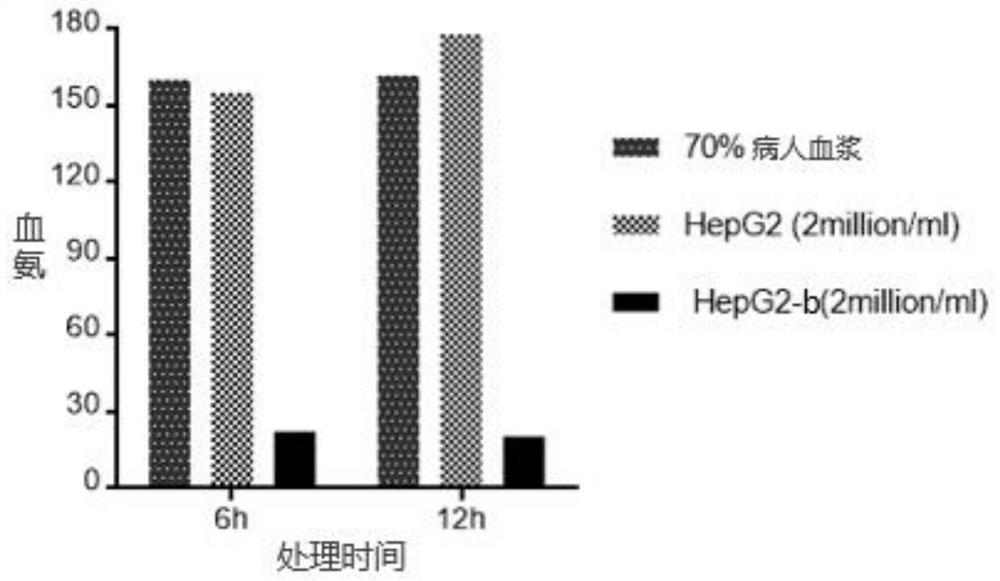
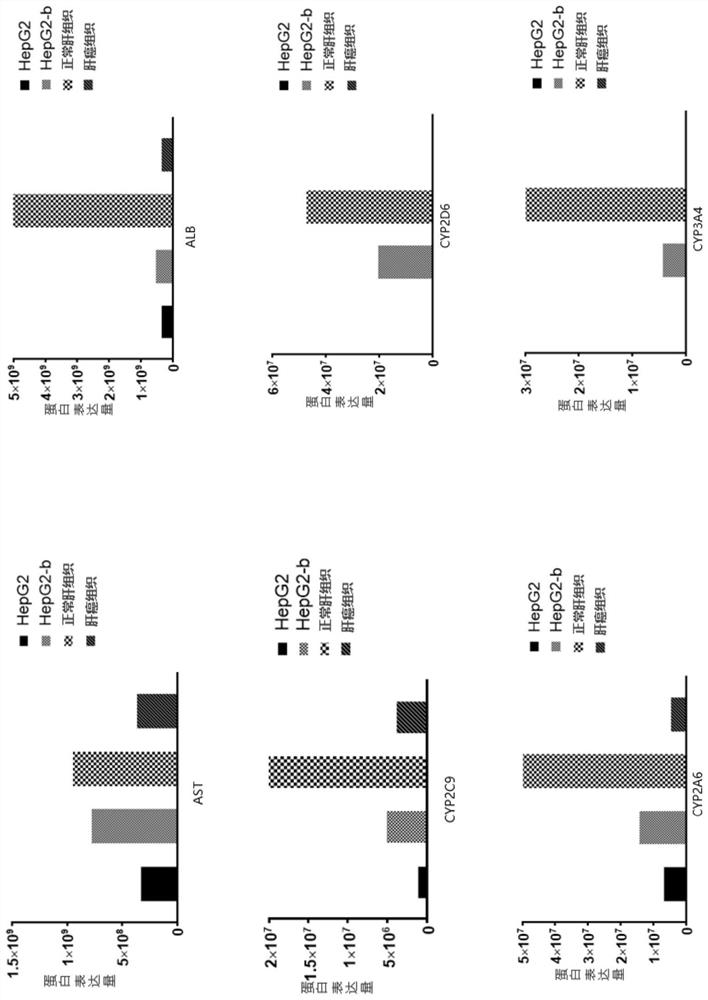
ActiveCN111778268BIncreased blood ammonia degradation rateEnhance detoxification abilityHydrolasesAntibody mimetics/scaffoldsCYP2D6Endogeny
The invention discloses a gene fragment for enhancing detoxification function and a transformed HepG2 cell, wherein the gene fragment for enhancing detoxification function is composed of three gene fragments of OTC, ARG1 and OATP1B1 in sequence. In HepG2 cells transformed with this gene fragment, the expression of albumin was doubled; the expression of aspartate aminotransferase was increased by 10%; the secretion of key proteins related to endogenous detoxification ability in the cytochrome P450 family was increased, and the detoxification ability was improved: the secretion of CYP2C9 was increased by 5 times, CYP2D6 secretion increased by 50 times, CYP2A6 secretion increased by 2 times, and CYP3A4 secretion increased by 100 times.
Owner:WUHAN TOGO MEDITECH CO LTD
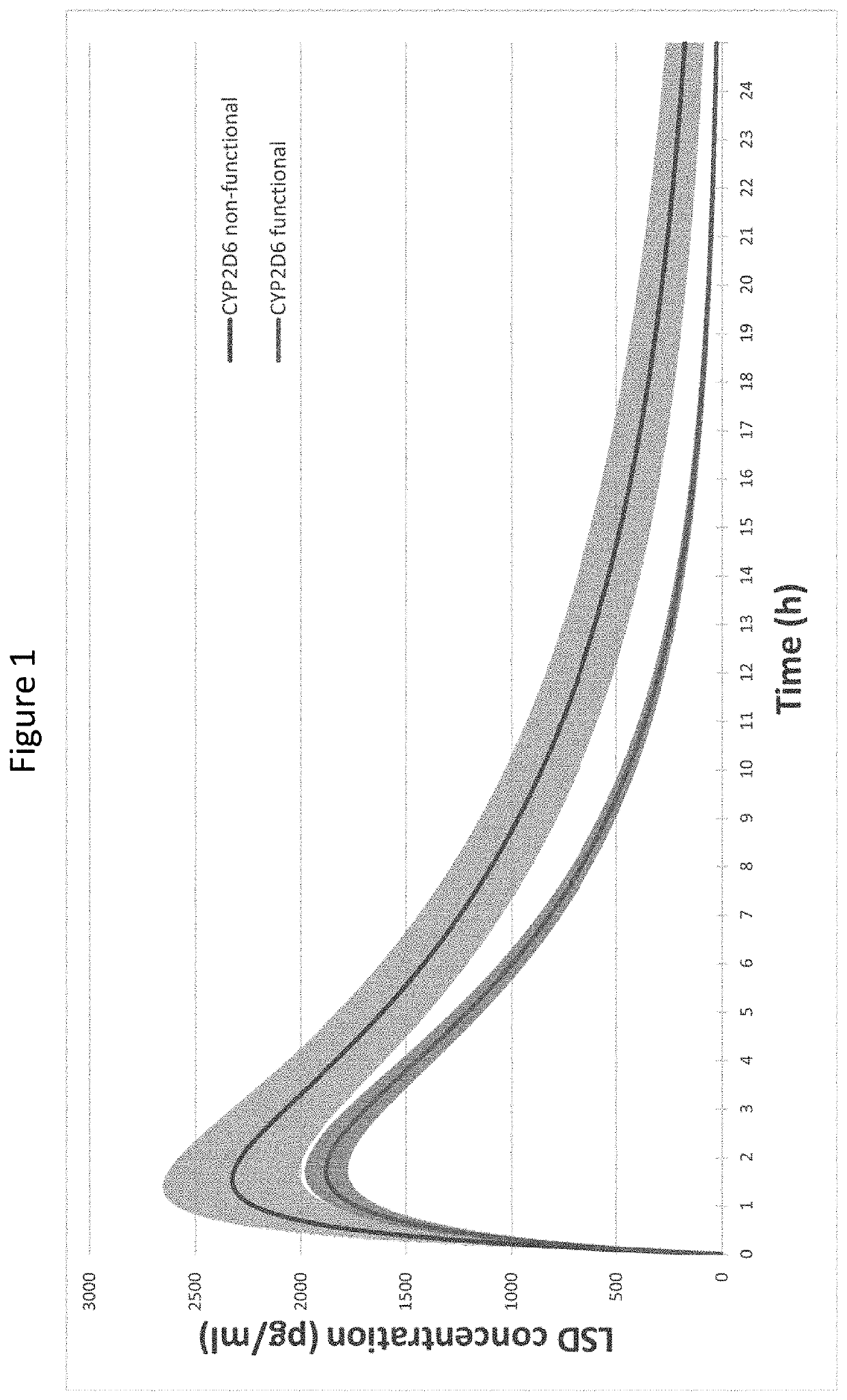
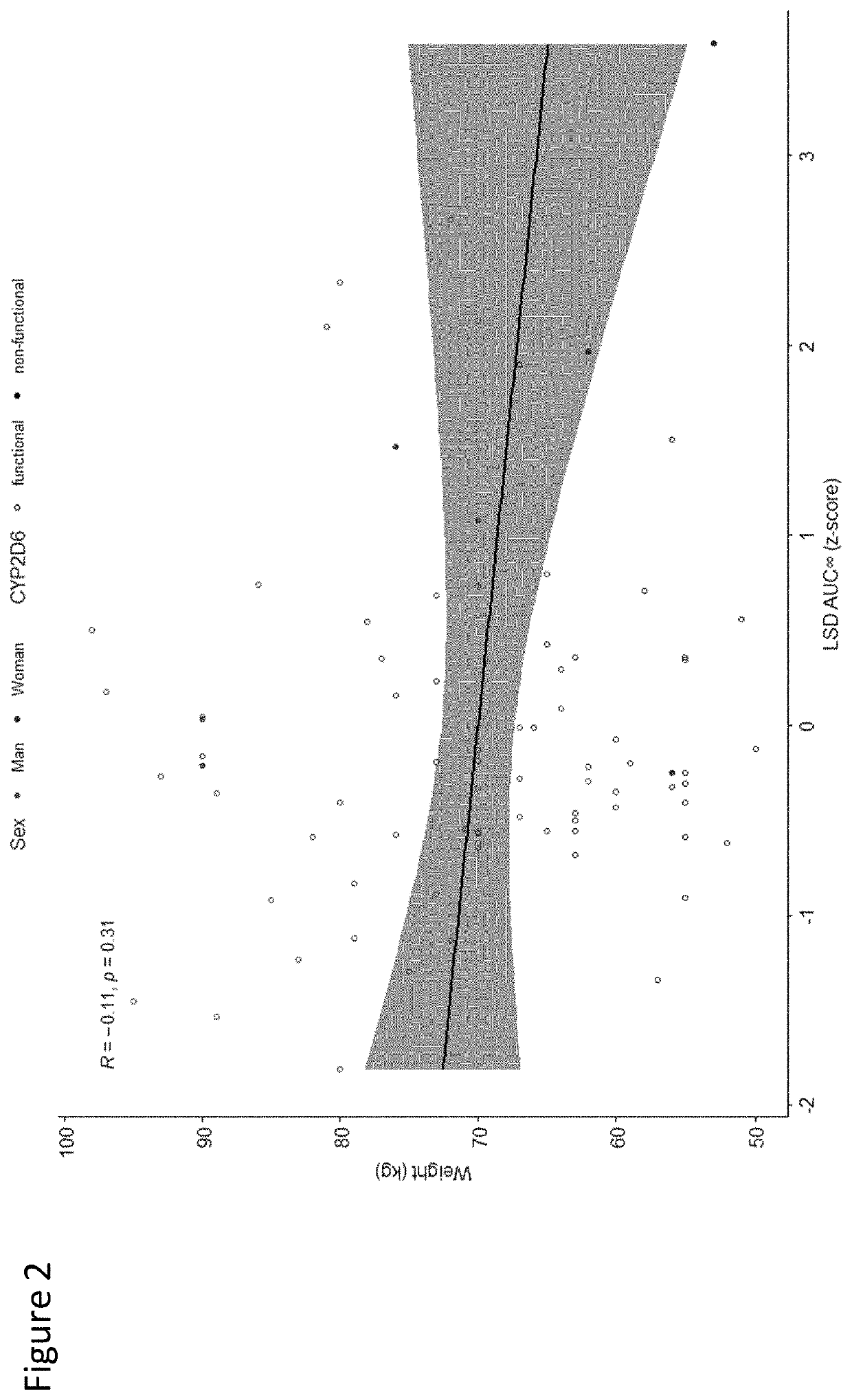
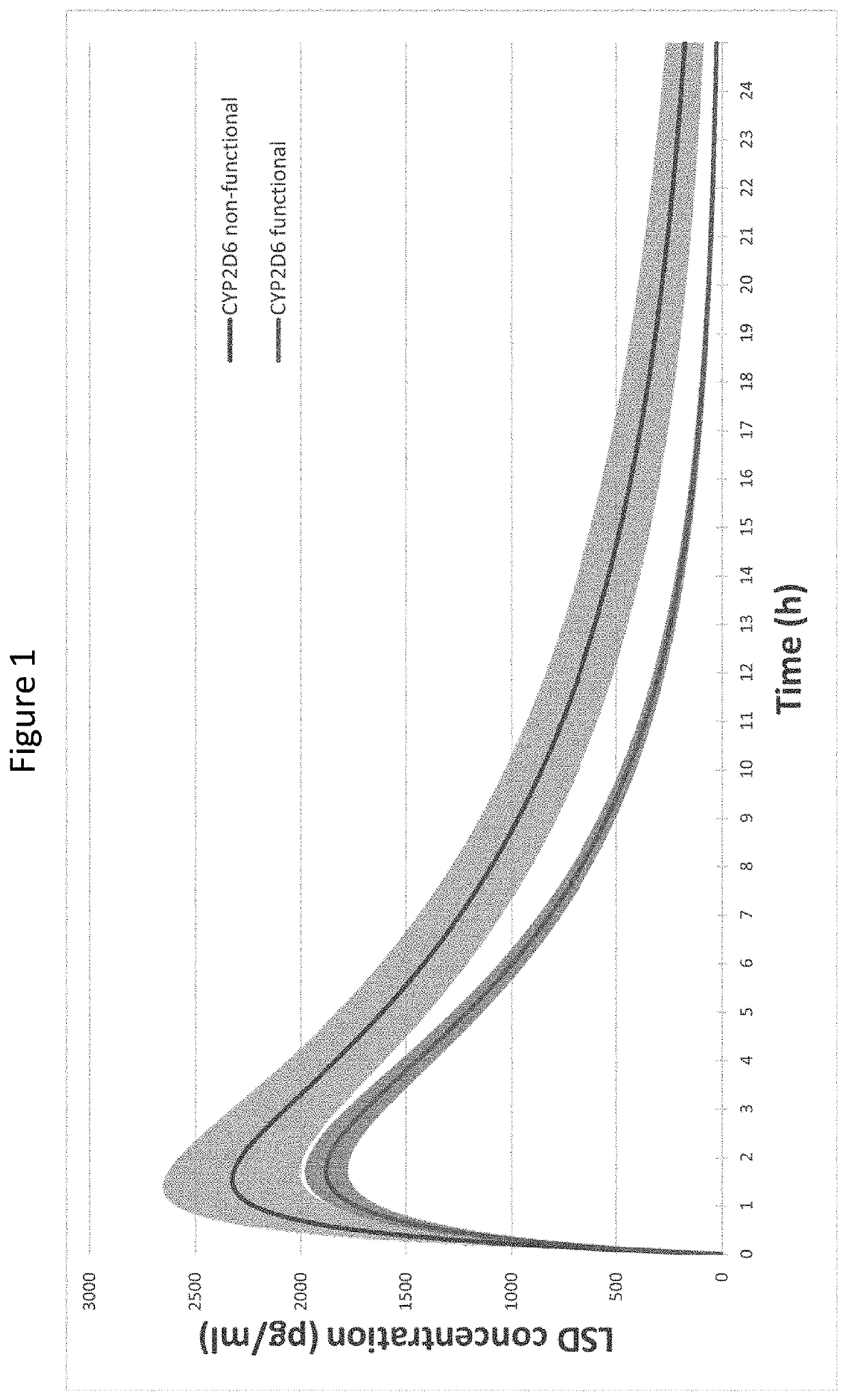
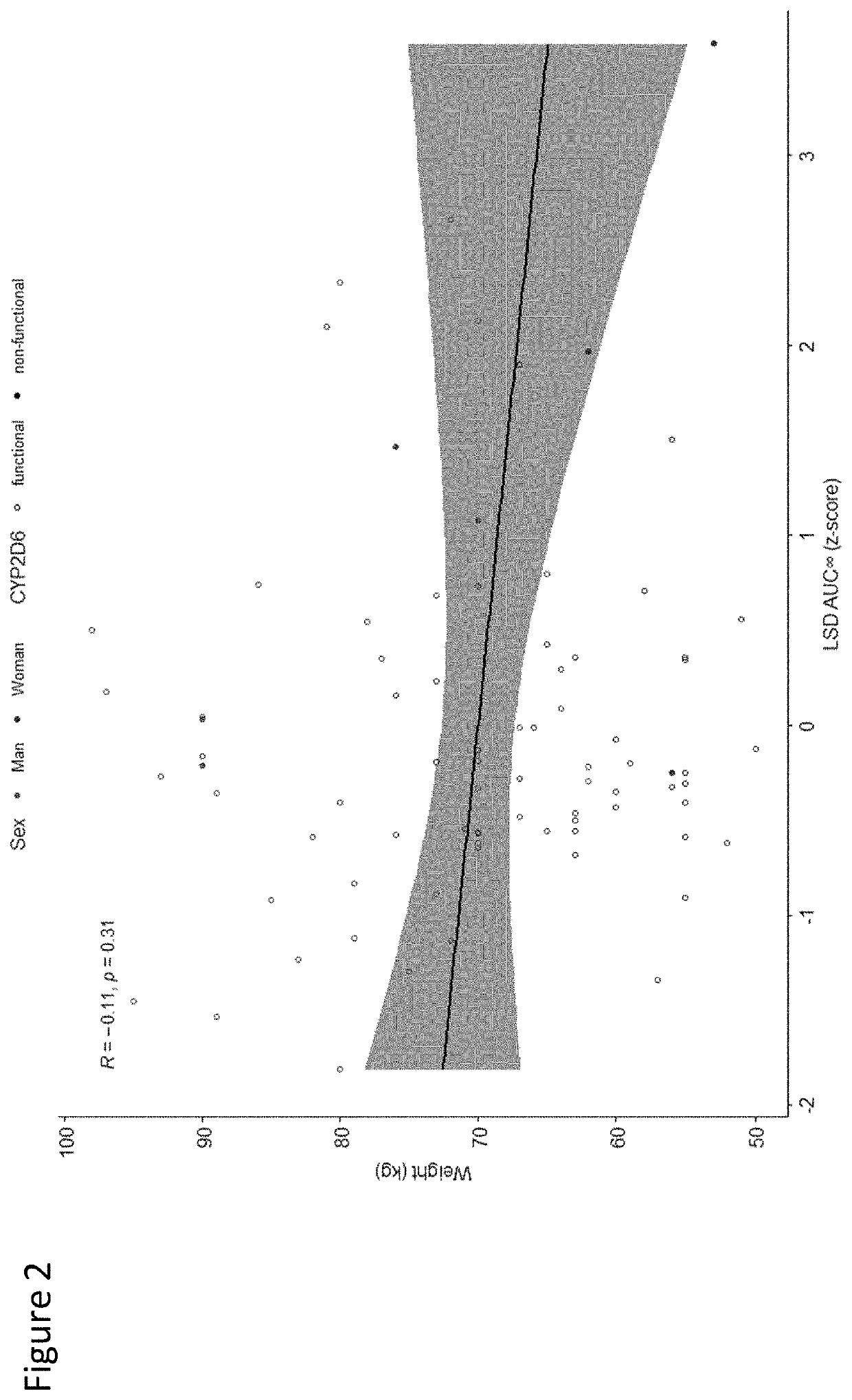
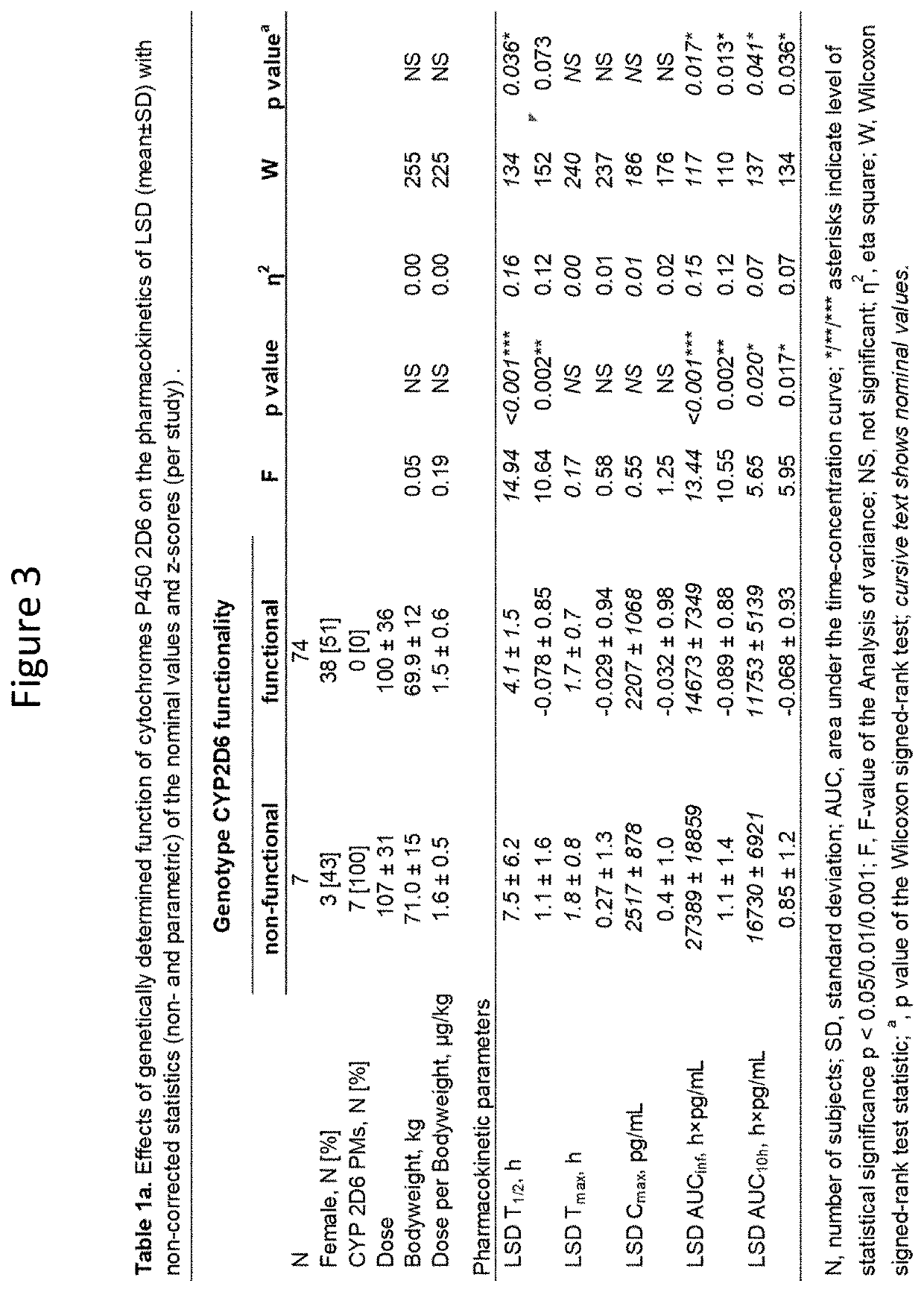
Using geno- or phenotyping to adjust lsd dosing
PendingUS20220347169A1Subject to effectOrganic active ingredientsNervous disorderDosage adjustmentAnxiety reduction
A method of dosing LSD in treating patients, by assessing genetic characteristics in the patient by identifying polymorphisms of CYP2D6 before use of a composition chosen from the group consisting of LSD, analogs thereof, derivatives thereof, and salts thereof, administering the composition to the patient based on the patient genetics, wherein a 50% dose is administered in a patient with non-functional CYP2D6 compared to a dose in functional CYP2D6 individuals, and producing maximum positive subjective acute effects in the patient and / or reducing anxiety and negative effects. A method of determining a preferred dose of LSD.
Owner:UNIVSSPITAL BASEL
Tamoxifen treatment effect-related gene (CYP2D6, CYP3A5 and RRAS2) polymorphism detection kit and tamoxifen treatment effect-related gene (CYP2D6, CYP3A5 and RRAS2) polymorphism detection method
InactiveCN107841546AReduce pollutionMicrobiological testing/measurementCurative effectQuality control
The invention provides a drug tamoxifen treatment effect-related gene (CYP2D6, CYP3A5 and RRAS2) polymorphism detection kit and a drug tamoxifen treatment effect-related gene (CYP2D6, CYP3A5 and RRAS2) polymorphism detection method, wherein the kit comprises a PCR buffer solution, specific ARMS detection primers and quality control primers. The present invention further provides the tamoxifen treatment effect-related gene (CYP2D6, CYP3A5 and RRAS2) polymorphism detection method. According to the present invention, with the method, the tamoxifen treatment effect-related gene (CYP2D6, CYP3A5 andRRAS2) polymorphism can be rapidly and accurately detected; and the kit has characteristics of high sensitivity, strong specificity, simple method, accurate result and the like.
Owner:宁波美丽人生医药生物科技发展有限公司
Individual genetic test reagent kit of medicines for relieving fever and alleviating pain
The invention belongs to the field of biological technology, relates to a genetic test reagent kit, in particular to an individual genetic test reagent kit of medicines for relieving fever and alleviating pain. The reagent kit comprises the main components of: (1) 4 pairs of CYP2D6*10, CYP2C9*3 and CYP2C19*2 / *3 gene PCR amplification and sequencing primers; (2) preparing a PCR amplification reagent; (3) preparing a PCR product purifying reagent; and (4) preparing a DNA sequencing reagent. The reagent kit can be used for detecting the metabolic capability of the medicines for relieving fever and alleviating pain, and understanding the metabolic capability of the medicines in bodies of children, can effectively and objectively assess the treatment effect and adverse reactions of the medicines to the children, and is favorable for reduction of damage of the medicines to the children, so that the treatment effect is maximized.
Owner:山东中科翰康医学检验有限公司 +2
Htsnps for determining a genotype of cytochrome P450 1a2, 2A6 and 2D6, PXR and UPD-glucuronosyltransferase 1A gene and multiplex genotyping methods using thereof
The present invention relates to htSNPs for determining a genotype of cytochrome P450 1A2 (CYP1A2), 2A6 (CYP2A6) and 2D6 (CYP2D6), PXR and UDP- glucuronosyltransf erase Ia (UGTlA) genes and a gene chiThe present invention relates to htSNPs for determining a genotype of cytochrome P450 1A2 (CYP1A2), 2A6 (CYP2A6) and 2D6 (CYP2D6), PXR and UDP- glucuronosyltransf erase Ia (UGTlA) genes and a gene chip using the same, and more particularly, to a selection method of htSNPs for determining a haplotype of human CYP1A2, CYP2A6, CYP2D6, PXR and UGTlA genes, a method of determining a genotype of the genp using the same, and more particularly, to a selection method of htSNPs for determining a haplotype of human CYP1A2, CYP2A6, CYP2D6, PXR and UGTlA genes, a method of determining a genotype of the genes by using the htSNPs and a gene chip therefor.es by using the htSNPs and a gene chip therefor.
Owner:申载国
Detection kit for metabolic markers of ondansetron and tropisetron, detection method of detection kit and application of detection kit and detection method
The invention discloses a detection kit for metabolic markers of ondansetron and tropisetron, a detection method of the detection kit and application of the detection kit and the detection method. The detection kit is used for detecting gene polymorphism of the metabolic markers CYP2D6C100T, CYP2D6G1846A and CYP2D6*5 of the ondansetron and the tropisetron. According to the kit, specific amplification primers and sequencing primers are designed according to the polymorphism of the three genes CYP2D6C100T, CYP2D6G1846A and CYP2D6*5. The kit comprises the following components of an amplification reaction solution, the CYP2D6C100T sequencing primer, the CYP2D6G1846A sequencing primer, the CYP2D6*5 sequencing primer and a positive control. According to the invention, blood direct expansion, rapid amplification and optimized pyrosequencing technologies are combined to detect gene polymorphism related to prediction of drug effects and adverse reactions of the ondansetron and the tropisetron, and suggestions from the gene perspective are provided for clinical use of the ondansetron and the tropisetron.
Owner:上海普然生物科技有限公司
Targeted drug rescue with novel compositions, combinations, and methods thereof
Compounds of Formula I, pharmaceutically acceptable salts thereof, enantiomers thereof, metabolites thereof, derivatives thereof, prodrugs thereof, acid addition salts thereof, pharmaceutically acceptable salts thereof, or N-oxides thereof; or a combination thereof, processes and intermediates for preparation thereof, compositions thereof, and uses thereof, are provided. Pharmaceutical compositions comprising a compound of Formula I, or enantiomers thereof, metabolites thereof, derivatives thereof, prodrugs thereof, acid addition salts thereof, pharmaceutically acceptable salts thereof, or N-oxides thereof; or a combination thereof, wherein the compound is double and / or triple agent or ligand for CYP2D6, 5-HT2A, and / or 5HT2C receptors, and / or acetylcholinesterase are provided.
Owner:エクシーバゲーエムベーハー +1
Detection system for detecting CYP2D6*10 gene mutation and kit of detection system
PendingCN108796051ATo achieve the purpose of specific bindingReduce testing costsMicrobiological testing/measurementNucleotideTrue positive rate
The invention relates to a detection system for detecting CYP2D6*10 gene mutation and a kit of the detection system. The detection system comprises digital PCR (polymerase chain reaction) premixed solution and primer probe mixed solution, the primer probe mixed solution comprises an upstream primer, a downstream primer and a peptide nucleic acid probe which are used for detecting CYP2D6*10 sites of CYP2D6 genes, a nucleotide sequence of the upstream primer is as shown in SEQ ID No.1, a nucleotide sequence of the downstream primer is as shown in SEQ ID No.2, a nucleotide sequence of the peptidenucleic acid probe and CYP2D6*10 mutant gene sites are completely complemented, the nucleotide sequence of the peptide nucleic acid probe is as shown in SEQ ID No.3, and the 5' end of the peptide nucleic acid probe is connected with a strong chelation group formed by four amino acids. According to the detection system for detecting CYP2D6*10 gene mutation and the kit of the detection system, sensitivity and specificity are high, needed samples are less, and quantitative PCR detection can be performed rapidly, conveniently and accurately.
Owner:上海赛安生物医药科技股份有限公司
Using geno- or phenotyping to adjust lsd dosing
PendingUS20220280501A1Subject to effectReduce negative impactOrganic active ingredientsNervous disorderDosage adjustmentAnxiety reduction
A method of dosing LSD in treating patients, by assessing genetic characteristics in the patient before LSD use, administering LSD to the patient based on the patient genetics, and producing maximum positive subjective acute effects in the subject and / or reducing anxiety and negative effects. A method of determining a preferred dose of LSD, by determining metabolic and genetic markers in a patient (such as by assessing CYP2D6 activity and / or assessing 5HTR1A rs6295 and 5HTR2A rs6313 genotypes in a patient), adjusting a dose of LSD based on the metabolic activity and genetic profile, administering the dose of LSD to the patient, and producing maximum positive subjective acute effects in the subject and / or reducing anxiety and negative effects. A method of determining a dose of LSD based on an assessment of the presence of CYP2D6 inhibitors.
Owner:UNIVSSPITAL BASEL
SYBR Green I detection primer of CYP2D6*10 gene, kit and detection method thereof
PendingCN110819722AHigh sensitivityStrong specificityMicrobiological testing/measurementDNA/RNA fragmentationDiseaseElectrophoreses
The invention provides an SYBR Green I detection primer of a CYP2D6 * 10 gene, a kit and a detection method. Wherein the detection primer comprises a CYP2D6*10 wild type upstream primer, a CYP2D6*10 mutant type upstream primer, a CYP2D6*10 wild type universal downstream primer and a CYP2D6*10 mutant type universal downstream primer; the nucleotide sequence of the CYP2D6*10 wild type upstream primer is shown as SEQ ID NO: 1, the nucleotide sequence of the CYP2D6*10 mutant type upstream primer is shown as SEQ ID NO: 2, and the nucleotide sequence of the universal downstream primer is shown as SEQ ID NO: 3. According to the application, the detection primers and the kit containing the primers are used; a fluorescence signal value in the PCR process is directly explored, so that the detectionresult is obtained, PCR post-treatment or electrophoresis detection is not needed, the technical problems of easy pollution and false positive of the conventional PCR technology are solved, the problem of non-specific amplification can be effectively avoided, and the method is suitable for detection of large-batch samples. The method has the advantages of high sensitivity and strong specificity, and can guide patients with various diseases to realize individualized medication according to individual genome information.
Owner:爱尔生基因医学科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com







![Gene detection kit used for [beta] receptor antagonist medication, and detection method and application of gene detection kit Gene detection kit used for [beta] receptor antagonist medication, and detection method and application of gene detection kit](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5d327bfc-18e9-4732-9226-0f28fd6f25ca/HDA0003016366650000011.png)
![Gene detection kit used for [beta] receptor antagonist medication, and detection method and application of gene detection kit Gene detection kit used for [beta] receptor antagonist medication, and detection method and application of gene detection kit](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5d327bfc-18e9-4732-9226-0f28fd6f25ca/HDA0003016366650000012.png)
![Gene detection kit used for [beta] receptor antagonist medication, and detection method and application of gene detection kit Gene detection kit used for [beta] receptor antagonist medication, and detection method and application of gene detection kit](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/5d327bfc-18e9-4732-9226-0f28fd6f25ca/HDA0003016366650000021.png)