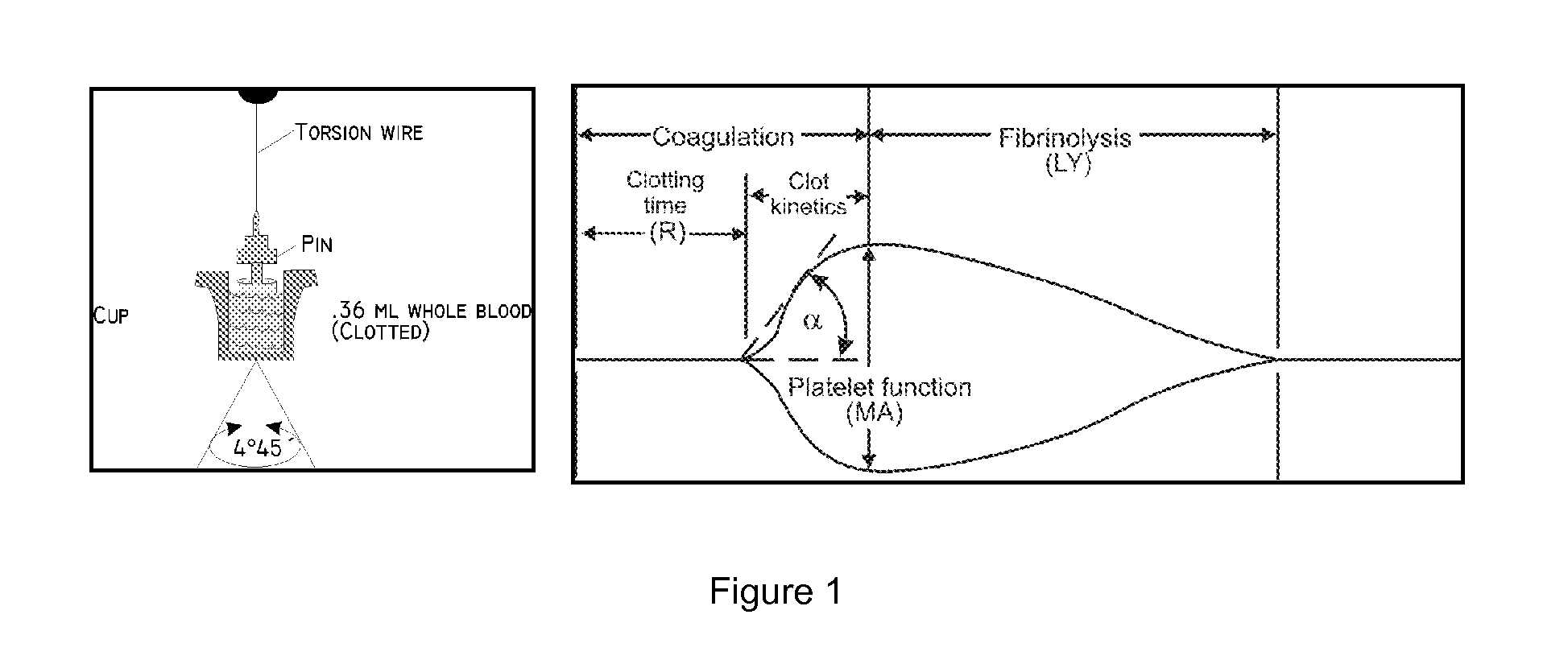
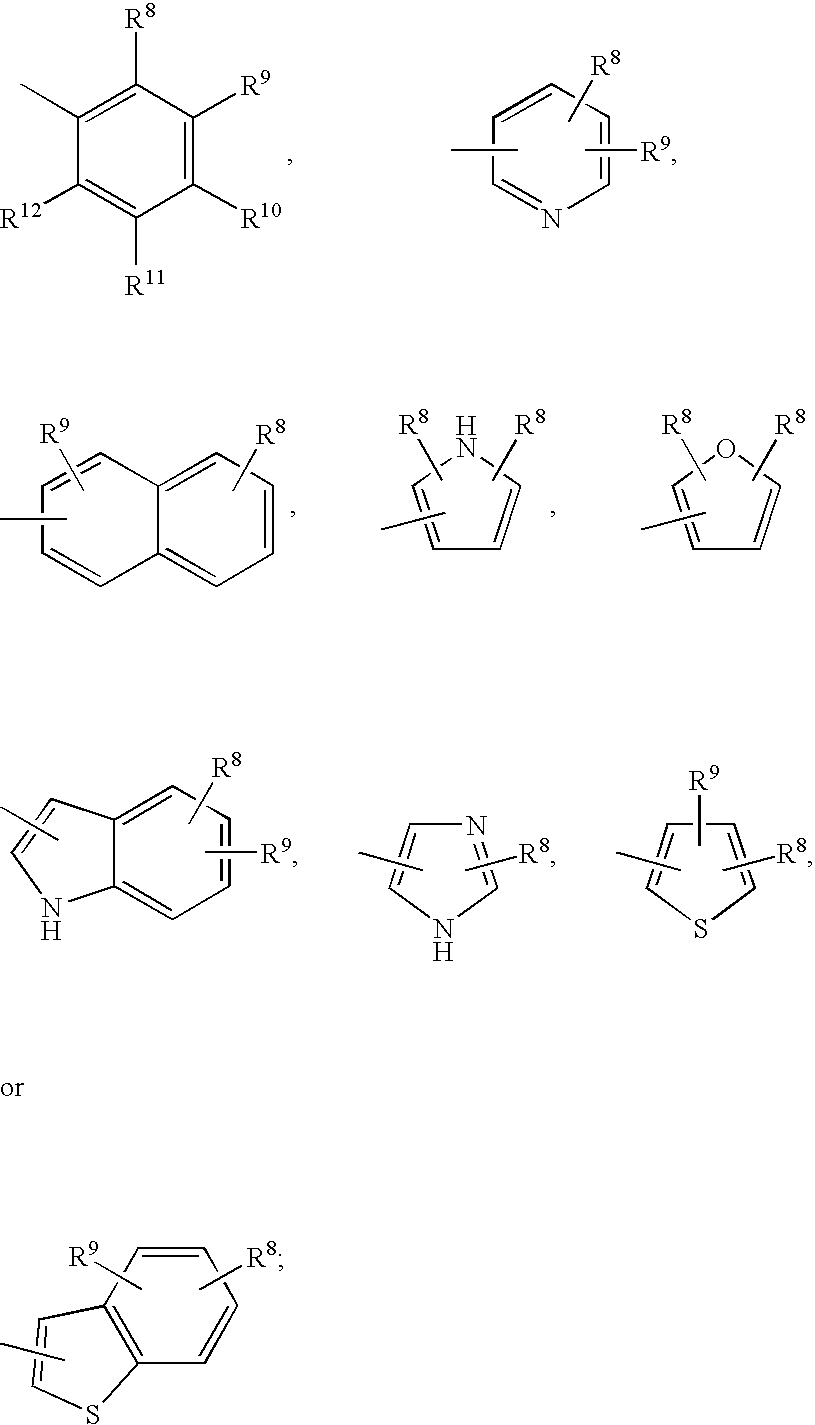
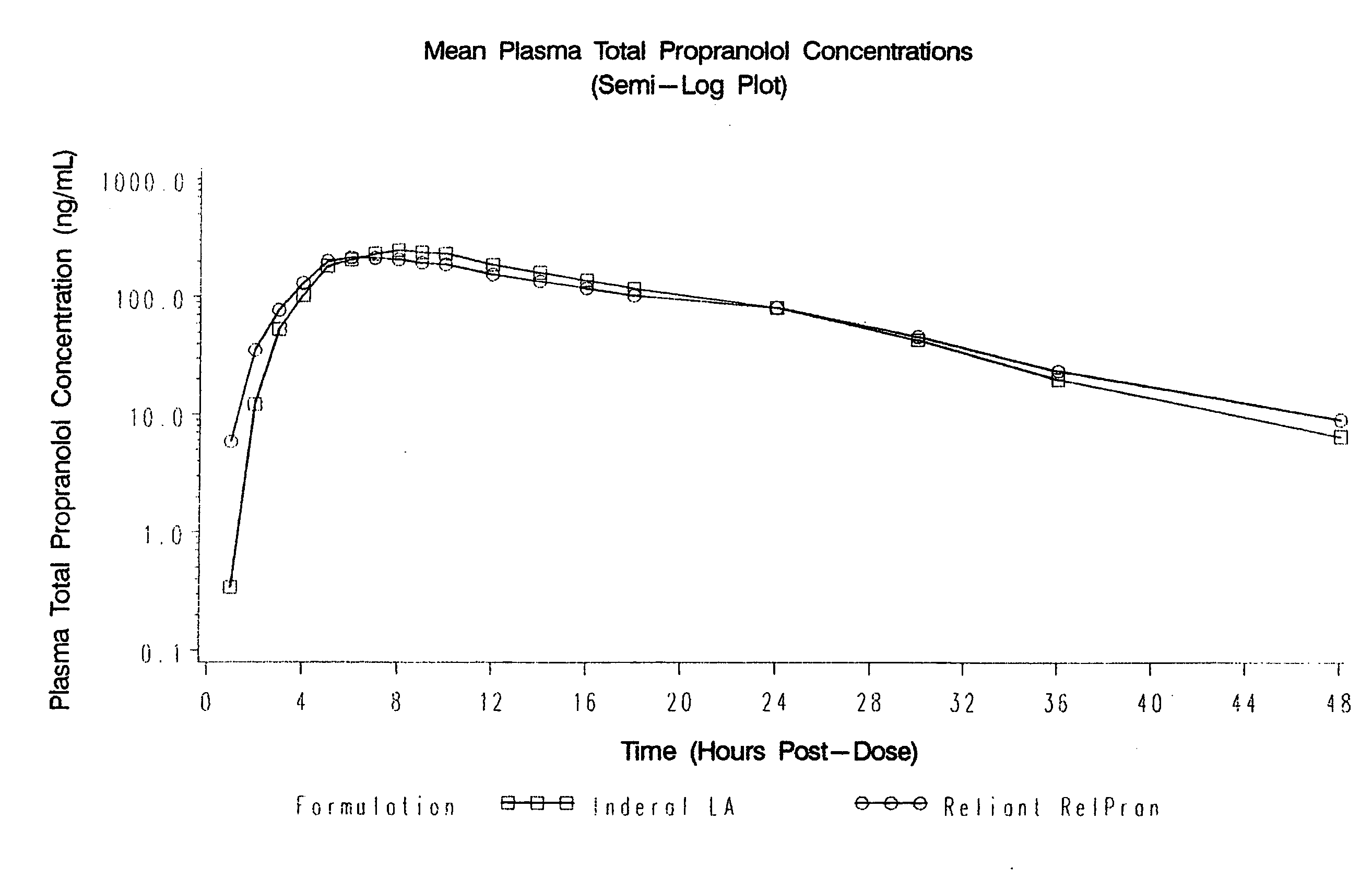
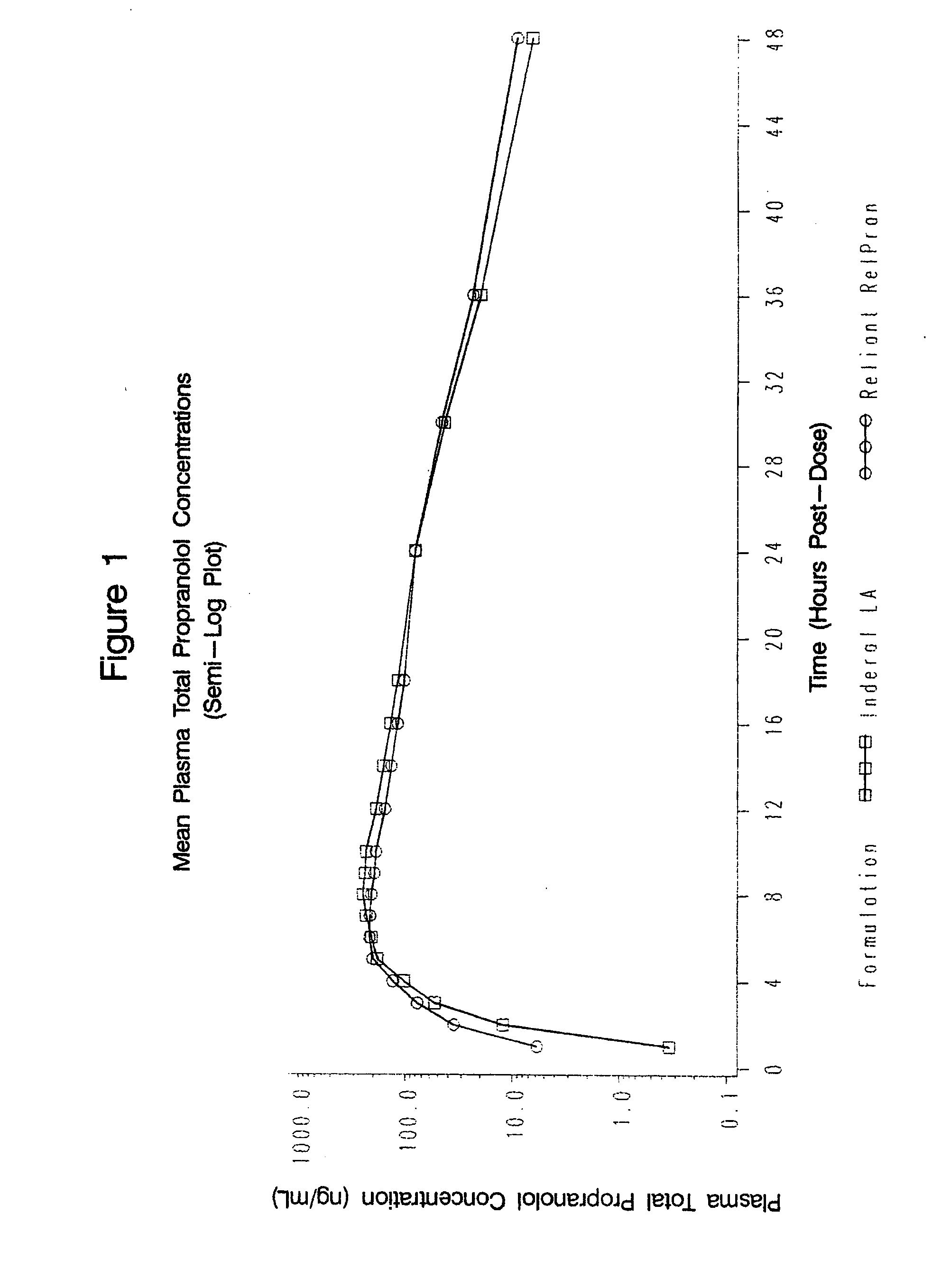
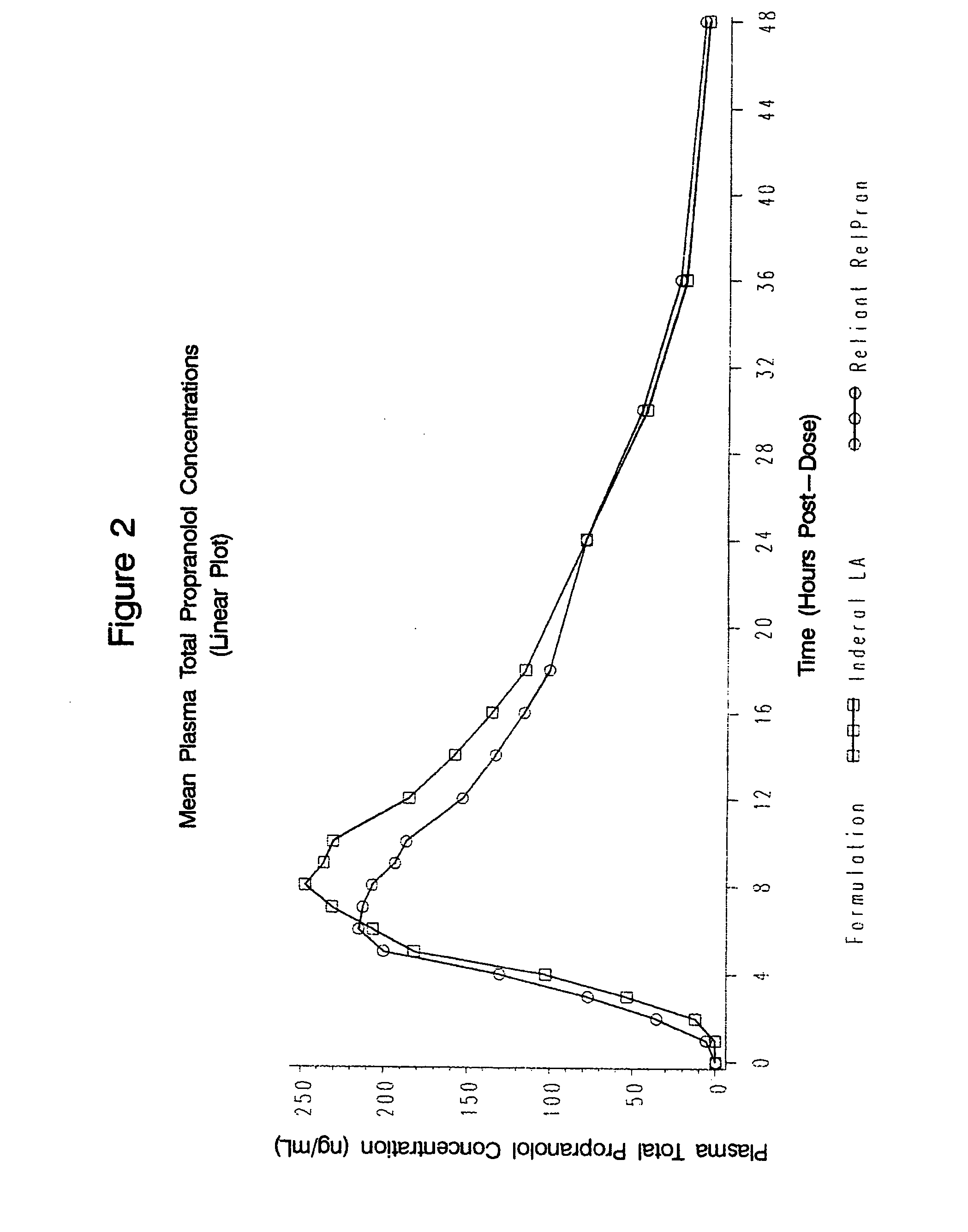
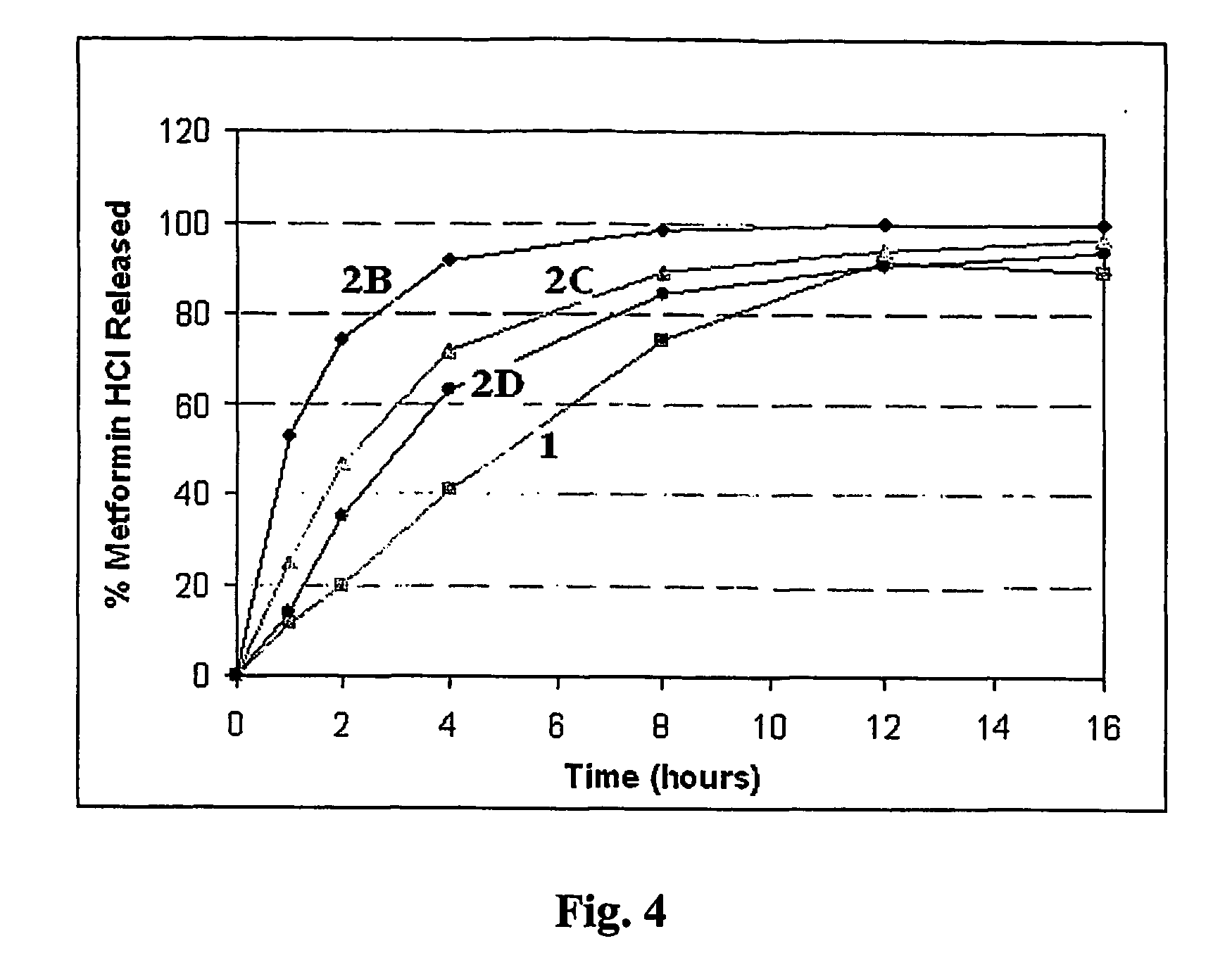
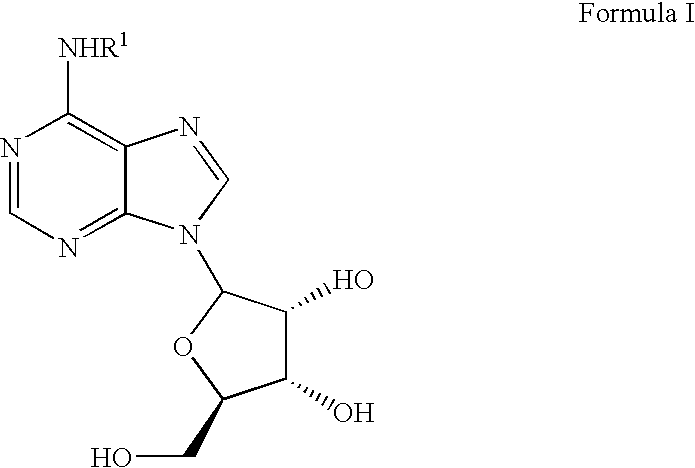
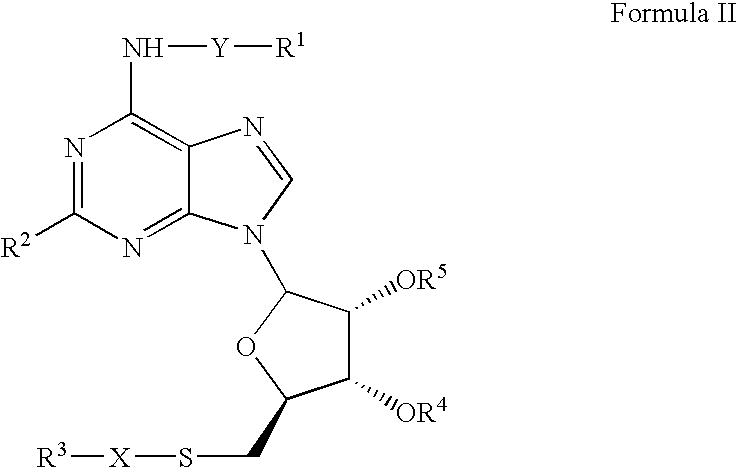
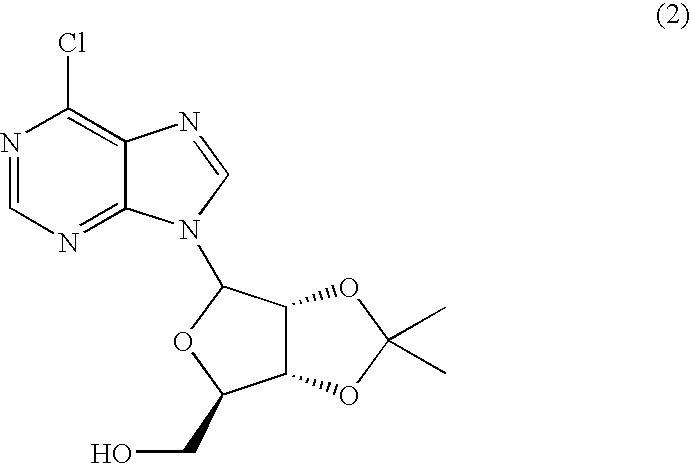
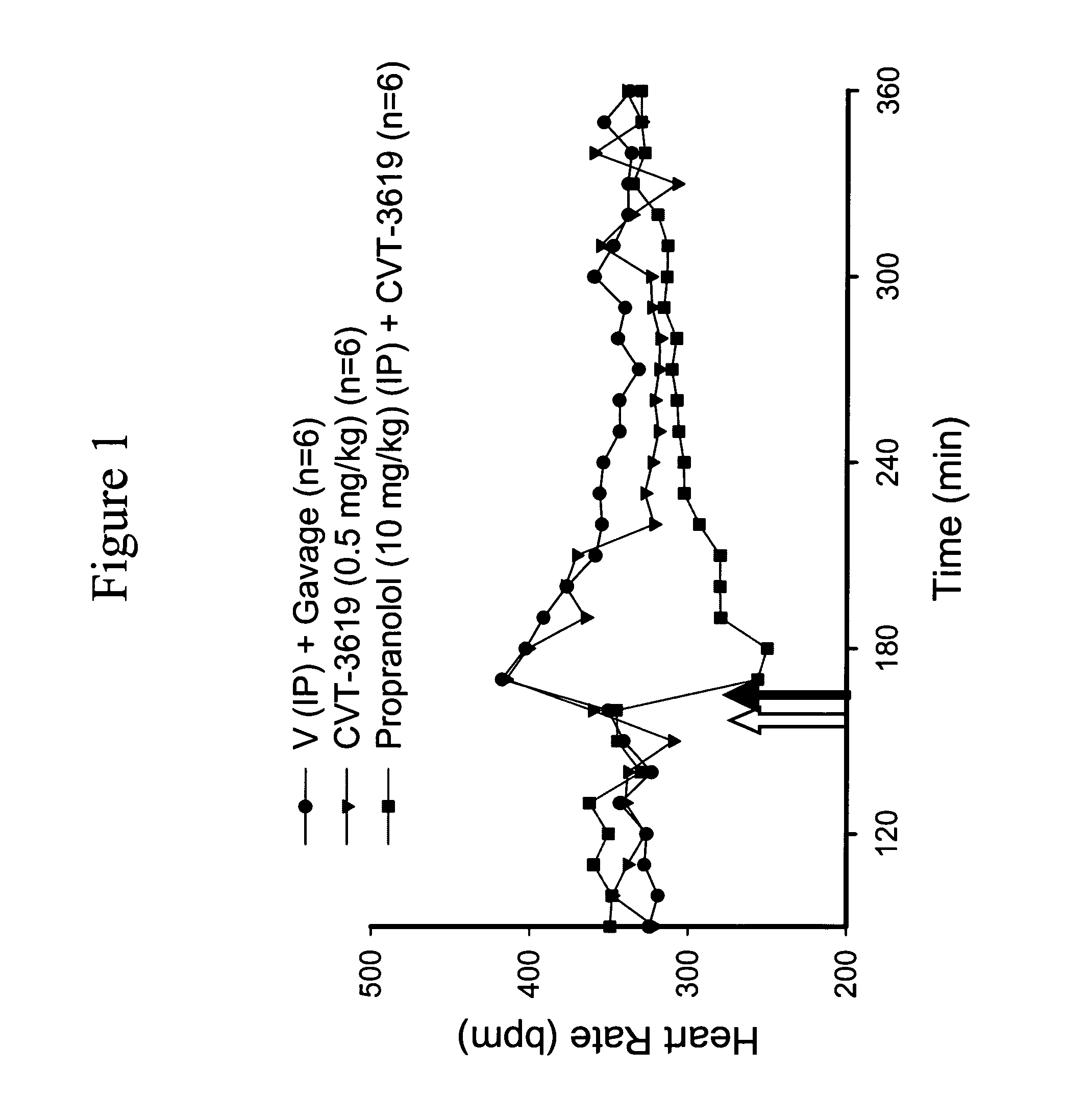
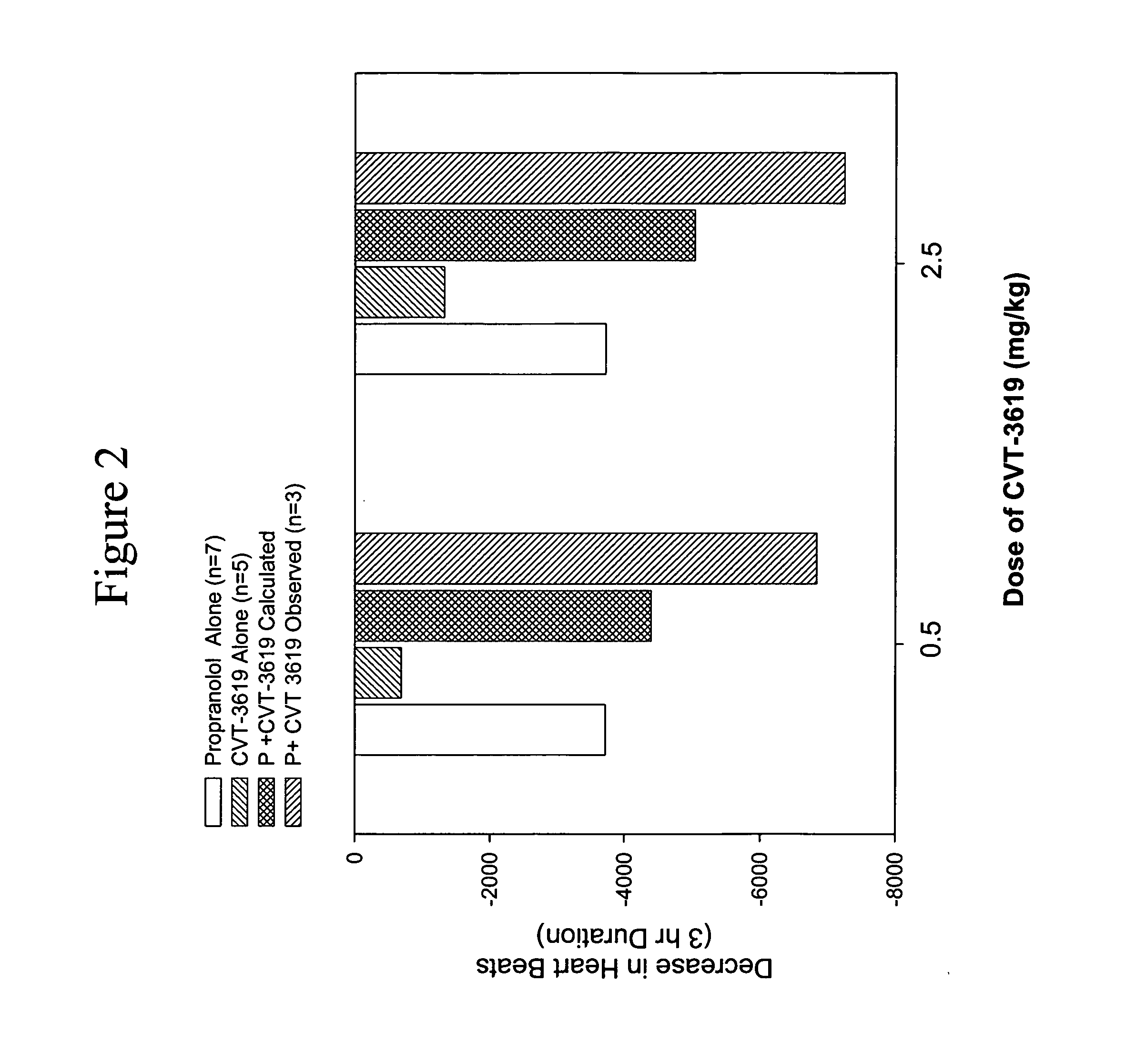
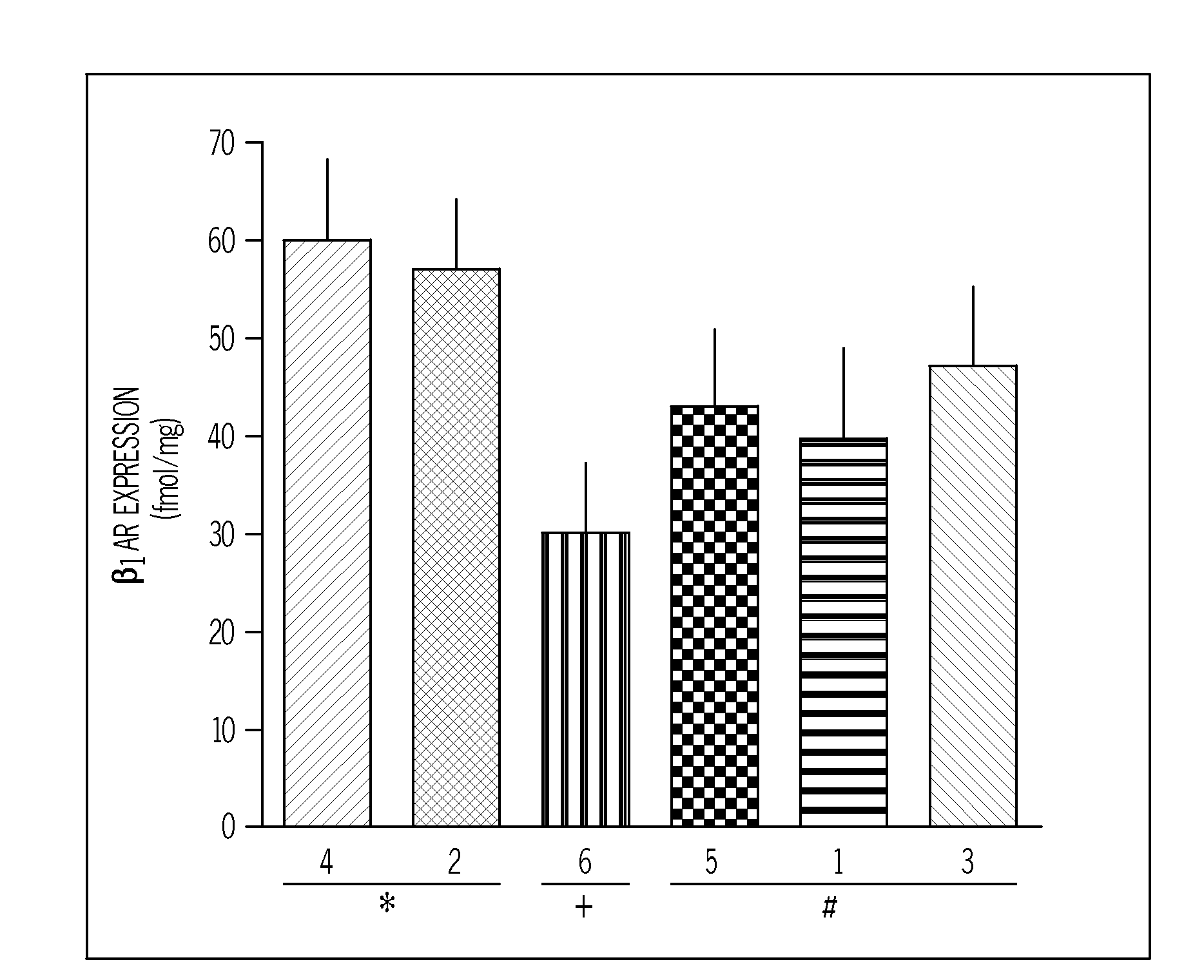
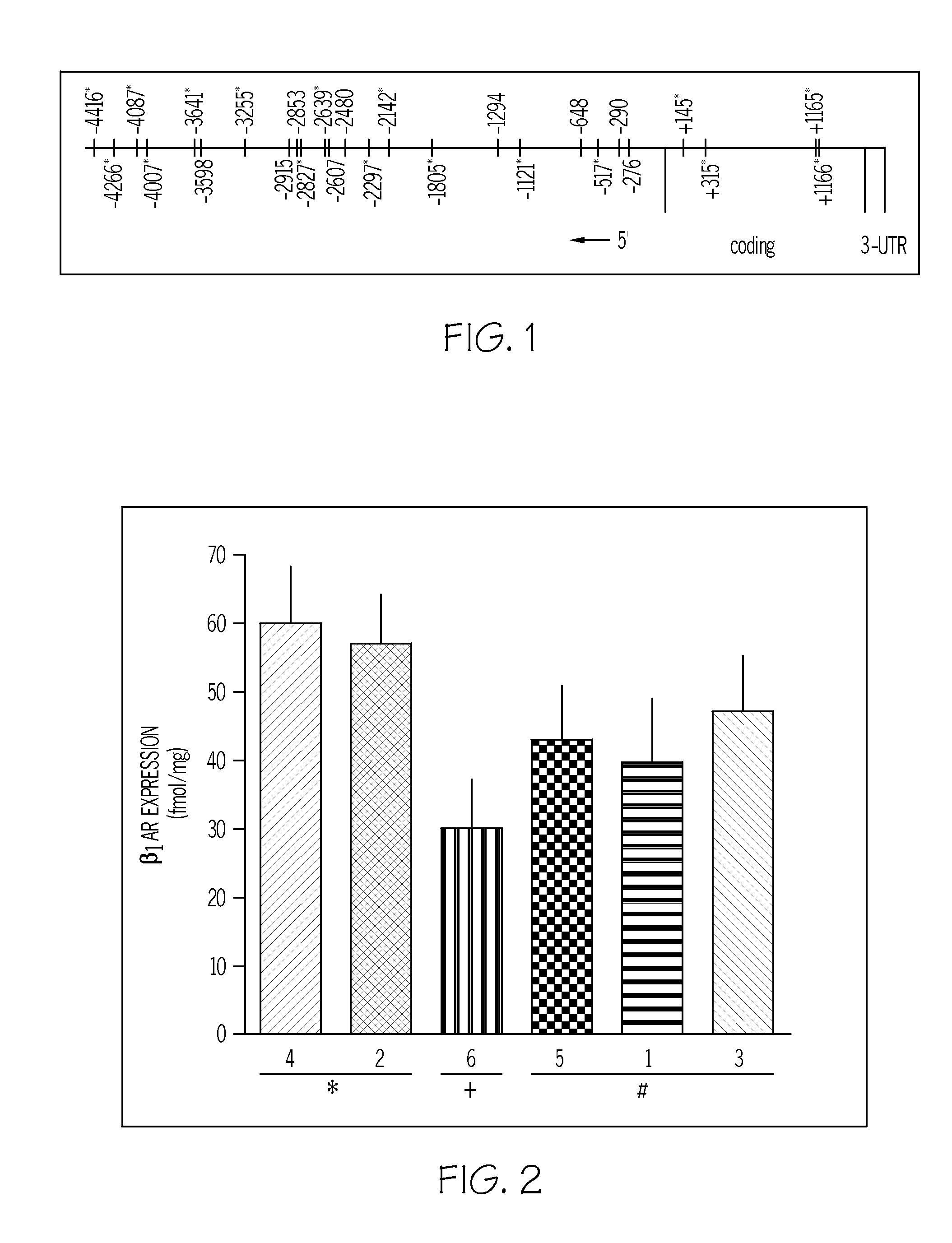
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
97 results about "Beta blocker" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
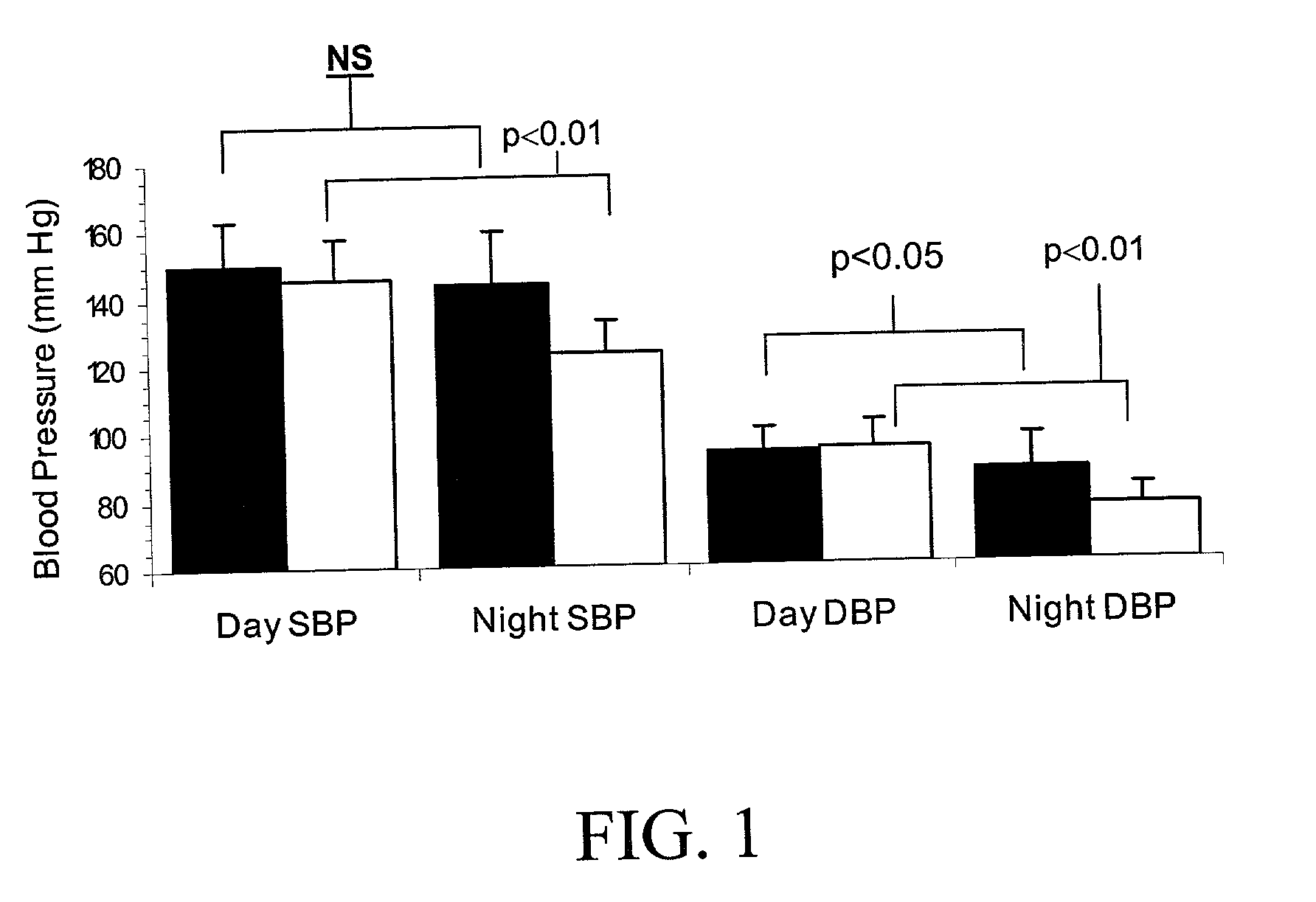
Beta blockers (beta-blockers, β-blockers, etc.) are a class of medications that are predominantly used to manage abnormal heart rhythms, and to protect the heart from a second heart attack (myocardial infarction) after a first heart attack (secondary prevention). They are also widely used to treat high blood pressure (hypertension), although they are no longer the first choice for initial treatment of most patients.
Treatment of conditions through pharmacological modulation of the autonomic nervous system
Methods are provided for treating a subject for a condition caused by an abnormality in the subject's autonomic nervous system. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is pharmacologically modulated with at least one beta-blocker in a manner that is effective to treat the subject for the condition. The subject methods find use in the treatment of a variety of different conditions, where such conditions include various disease conditions. Also provided are systems and kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS
Inhalable formulations for treating pulmonary hypertension and methods of using same
InactiveUS20040265238A1Many symptomBiocideDispersion deliveryAngiotensin-converting enzymeBeta blocker
The present invention is directed to an inhalable formulation for the treatment of pulmonary hypertension in a mammal (e.g., humans), wherein the formulation comprises at least one hypertension reducing agent, including but not limited to an angiotensin converting enzyme inhibitor, angiotensin receptor blocker, beta-blocker, calcium-channel blocker or vasodilator, or any combination thereof. The formulations of the present invention may be a solution or suspension, and preferably are suitable for administration via nebulization. The present invention is also directed to a method and kit for treating a mammal suffering from pulmonary hypertension.
Owner:DEY
Methods of treatment of patients at increased risk of development of ischemic events and compounds hereof
InactiveUS20130040898A1Impair thrombus formationIncreased riskElcosanoid active ingredientsInorganic active ingredientsBeta blockerPlatelet inhibitors
The present invention relates to compounds for treatment that protects the endothelium, prevents pathologic thrombus formation in the microcirculation and preserves platelet number and function and thus may be related to treatment or prevention of ischemic events in patients with cardiovascular disease. The present invention is particularly useful for patients having or being at increased risk of development of an ischemic event such as an acute myocardial infarction and / or no-reflow phenomena and / or ischemia-reperfusion injury by administration of agent(s) modulating and / or preserving endothelial integrity. The compounds may be administered in combination with standard treatment of acute cardiovascular ischemic events such as Platelet inhibitors such as aspirin (ASA), Thienopyridins, GPIIb / IIIa inhibitors), Parenteral anticoagulants such as unfractioned heparin (UFH), bivalirudin, enoxaparin, and fondaparinux, Verapamil, Adenosine, Sodium nitroprusside, Nitroglycerin, Epinephrine, Beta-blockers and surgical methods such as percutaneous coronary intervention (PCI), PCI with thrombus aspiration, PCI with stents.
Owner:THROMBOLOGIC
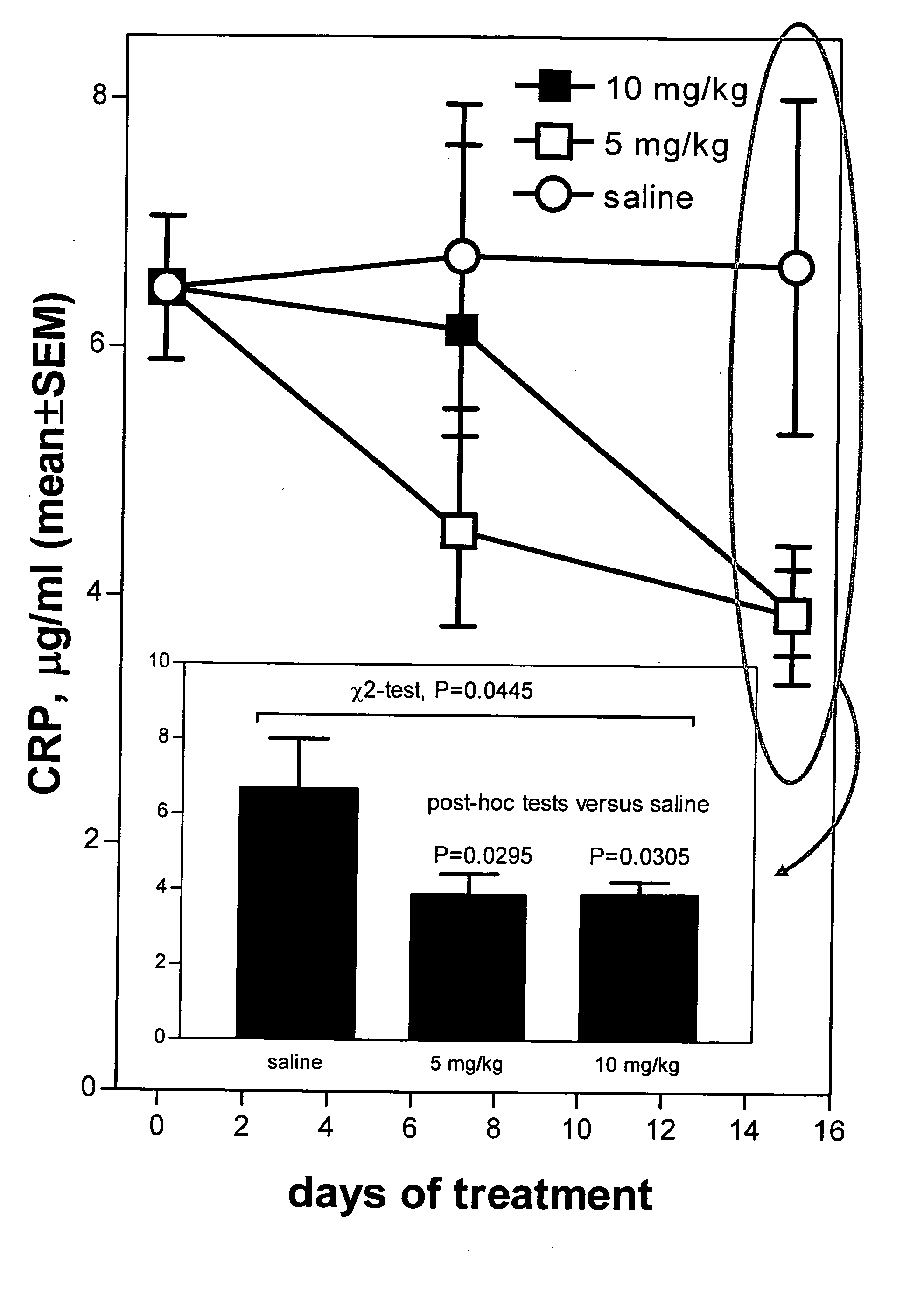
Method of reducing C-reactive protein using growth hormone secretagogues
The present invention relates to a method of reducing C-reactive protein in a subject in need of treatment thereof, wherein the subject is at risk of having or the subject has already had a vascular event or suffering from an inflammatory disease or disorder. In one embodiment, the vascular event is a cardiovascular event (e.g., myocardial infarction). In another embodiment, the vascular event is a cerebrovascular event (e.g., stroke (such as transient ischemic attacks (TIAs)). In yet another embodiment the vascular event is a peripheral vascular event (e.g., intermittent claudication). The method comprises administering a therapeutically effective amount of at least one growth hormone secretagogue compound or a pharmaceutically acceptable salt, hydrate or solvate thereof. The growth hormone secretagogue can be coadministered with a second growth hormone secretagogue, HMG CoA reductase inhibitor, an ACAT inhibitor, a CETP inhibitor, an anti-inflammatory agent, an ACE inhibitor, a Beta blocker, a cholesterol absorption inhibitor, a nicotonic acid, a fibric acid derivative, a bile acid sequestering agent or a combination thereof.
Owner:HELSINN THERAPEUTICS (US) INC
Time-sustained-release formulations comprising a beta-blocker
InactiveUS20080131517A1Providing therapyAvoid problemsPowder deliveryOrganic active ingredientsCarteololBeta blocker
The present invention relates to compositions and methods of treating human subjects with a beta-adrenergic receptor blocking agent (“beta-blocker”) provided in a time-sustained-release delivery system. The time-sustained-release drug delivery systems includes at least three populations of beads, where each population of beads includes a beta-blocker. The beads may be selected from immediate-release beads, enteric-release beads, sustained-release beads, and time-sustained-release beads. The beta-blocker may be selected from acebutolol, atenolol, betaxolol, bisoprolol, esmolol, metoprolol, nebivolol, butoxamine, carteolol, carvedilol, labetalol, nadolol, oxprenolol, penbutolol, propranolol, pindolol, sotalol, and timolol. According to presently preferred embodiments, the beta-blocker is propranolol. The dosage forms of the present invention are useful for treating conditions including hypertension, angina pectoris due to coronary atherosclerosis, hypertrophic subaortic stenosis, congestive heart failure, arrhythmias, angina, anxiety, glaucoma, migraines, esophageal varices, alcohol withdrawal syndrome, irregular heartbeat, tachycardia, tremor, and neuroleptic-induced akathisia. They are also useful in the prophylaxis of migraine headaches.
Owner:RELIANT PHARMACEUTICALS INC
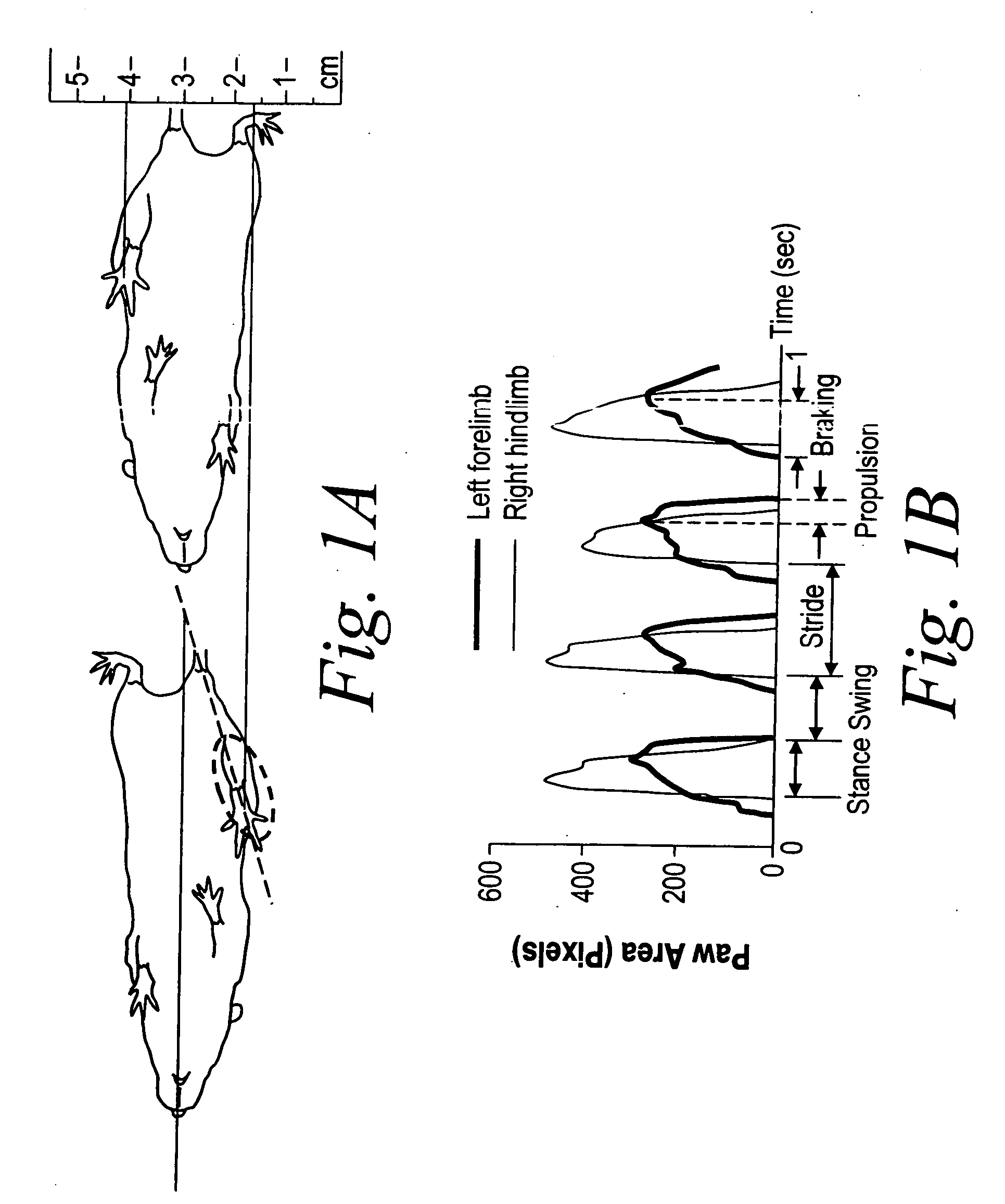
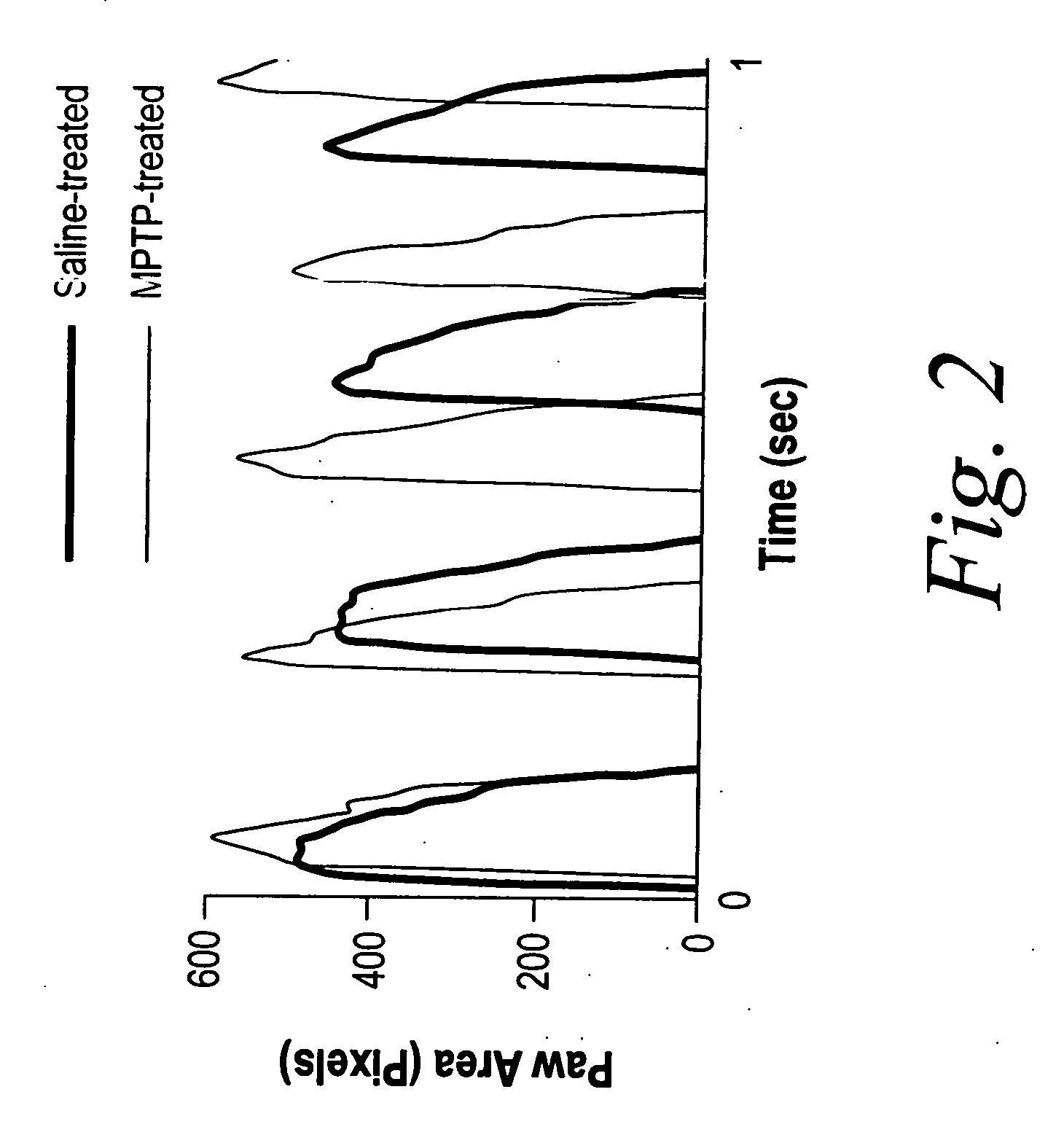
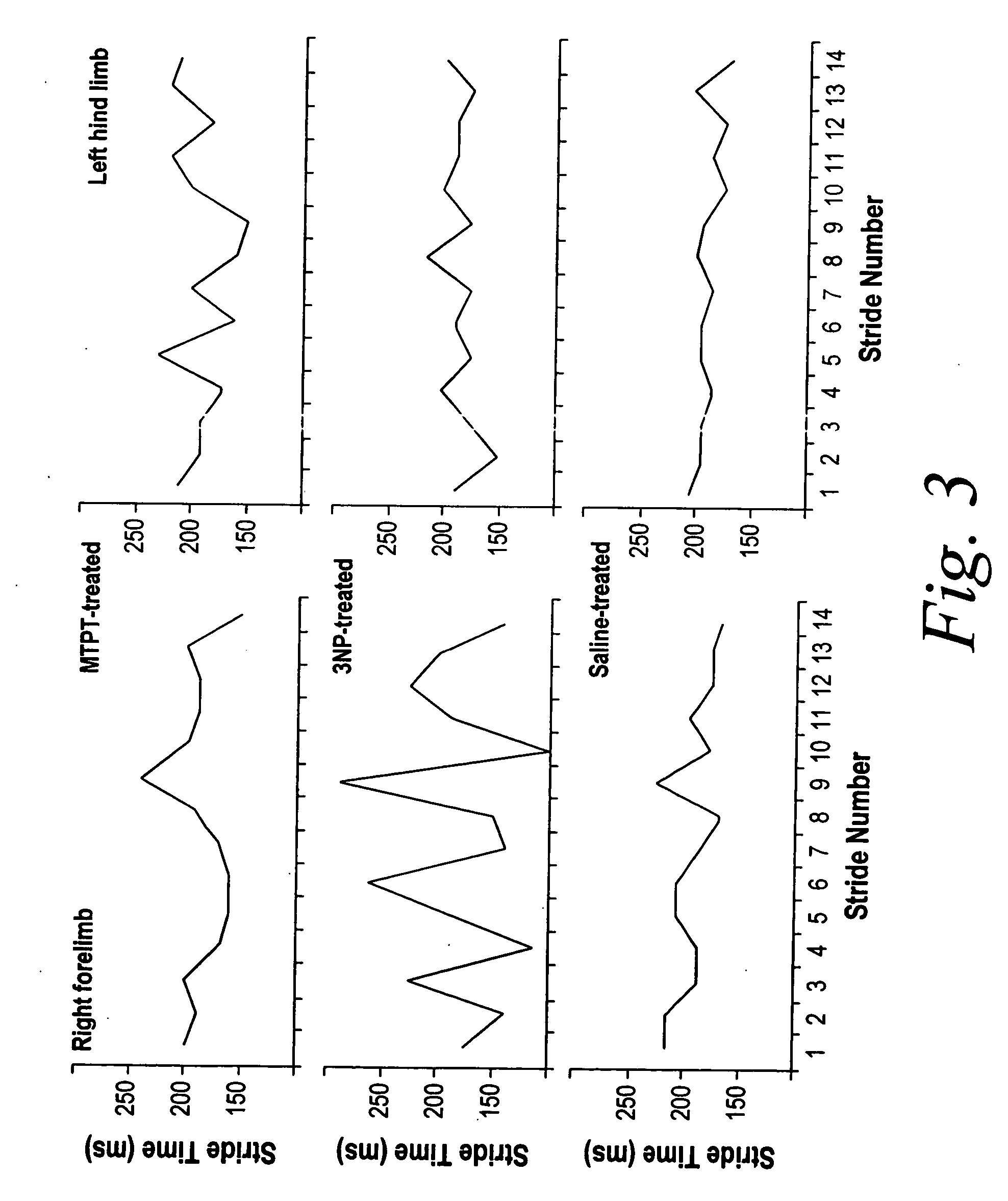
Measurement of gait dynamics and use of beta-blockers to detect, prognose, prevent and treat amyotrophic lateral sclerosis
InactiveUS20070021421A1Prevent weight lossPreventing and delaying and mitigating symptomCompounds screening/testingBiocideBeta blockerAdrenergic
The present invention, at least in part, provides methods of improved early diagnosis of neurodegenerative disease, e.g., ALS, in a subject via measurement of the gait dynamics of the subject (e.g., via the exemplary ventral plane videography methods disclosed herein). The present invention also provides for administration of a beta-adrenergic blocking agent (beta-blocker) to a subject at risk of developing ALS (e.g., a SOD1 G93A mouse) and / or having early stages of ALS, to modulate supranormal gait characteristics and to prevent, treat and / or ameliorate the onset, advancement, severity or effects of a neurodegenerative disease, e.g., ALS, in the subject.
Owner:THE CURAVITA CORP
Co-formulations or kits of bioactive agents
Provided, among other things, is a formulation or kit comprising: (a) a pharmaceutically effective dosage of one or more a glucose-level-controlling bioactive agents selected from an α-glucodase inhibitor, sulfonylurea, meglitinide, thiazolidinediones, biguanide, insulin, dual PPARα / γ agonist, PPARγ agonist or insulin secretagogue; and (b) a pharmaceutically effective dosage of (i) one or more of an antihypertensive bioactive agent selected from an ACE inhibitor, calcium channel blocker, beta blocker, angiotension II receptor antagonist or diuretic, or (ii) one or more of an anti-dyslipidemia bioactive agent selected from a HMG-CoA reductase inhibitor, bile acid sequestrant, fibric acid derivative, sterol, cholesterol absorption inhibitor, MTP inhibitor or nicotinic acid derivative; wherein: in the case of (i) a combination of a first bioactive agent of group (a) that is metformin with a second bioactive agent of group (b), or (ii) a combination of a first bioactive agent of group (a) that is a thiazolidinedione or dual PPARα / γ agonist with an angiotension II receptor antagonist, one or more of the following applies: (I) one of the first bioactive agent or the second bioactive agent is formulated for sustained release, and the other is formulated for immediate release, each formulated for once-a-day dosing; or (II) the co-formulation or kit comprises (A) a biguanide and a thiazolidinedione and (B) one or more group (b) bioactive agents.
Owner:ABEILLE PHARMA
Method for treating arrhythmias
Methods are provided for treating arrhythmia in a manner that minimizes undesirable side effects, comprising administration of a therapeutically effective minimal dose of an A1 adenosine receptor agonist with a therapeutically effective minimal dose of a beta blocker, calcium channel blocker, or a cardiac glycoside.
Owner:CV THERAPEUTICS INC
Ophthalmic composition
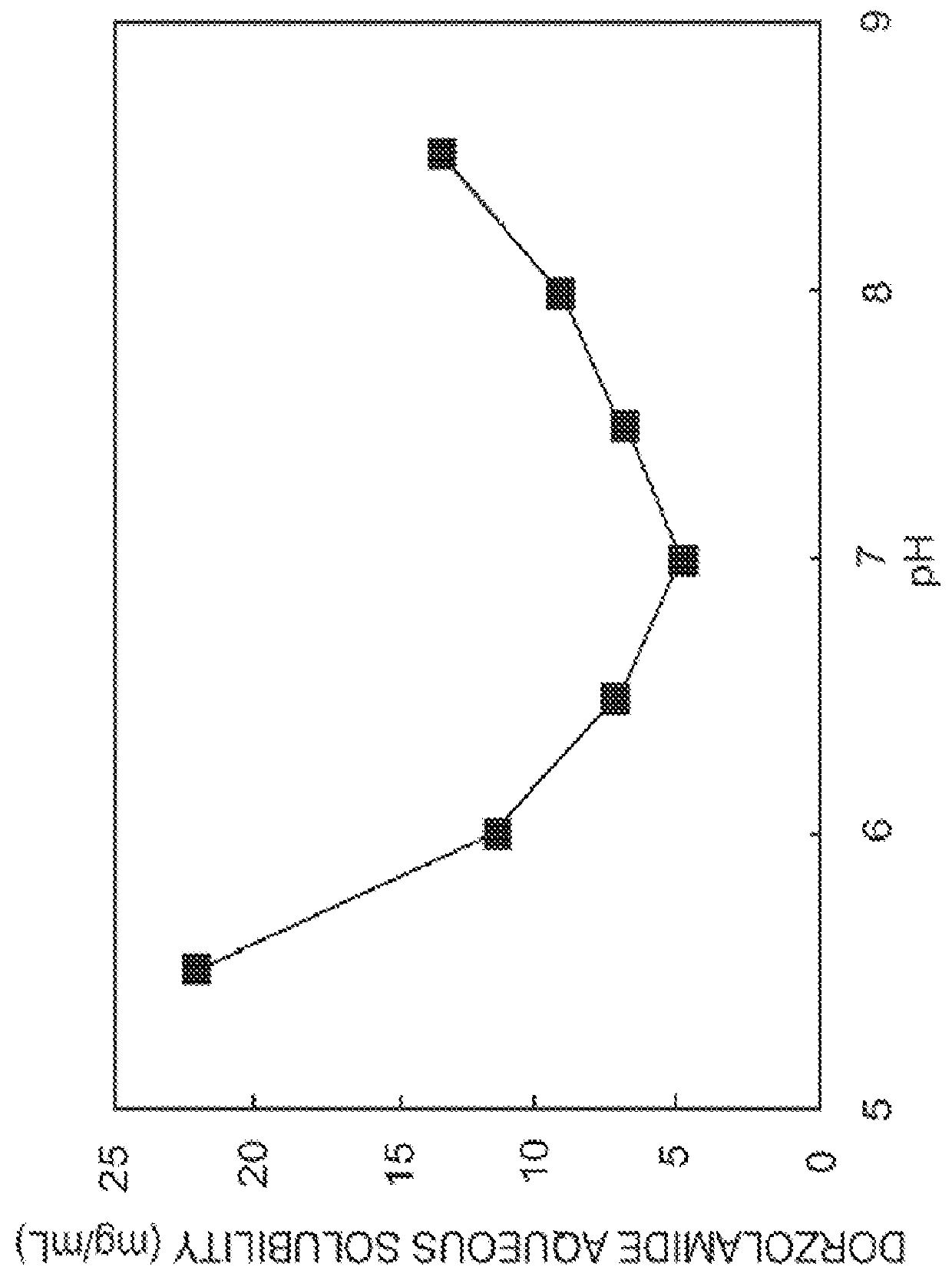
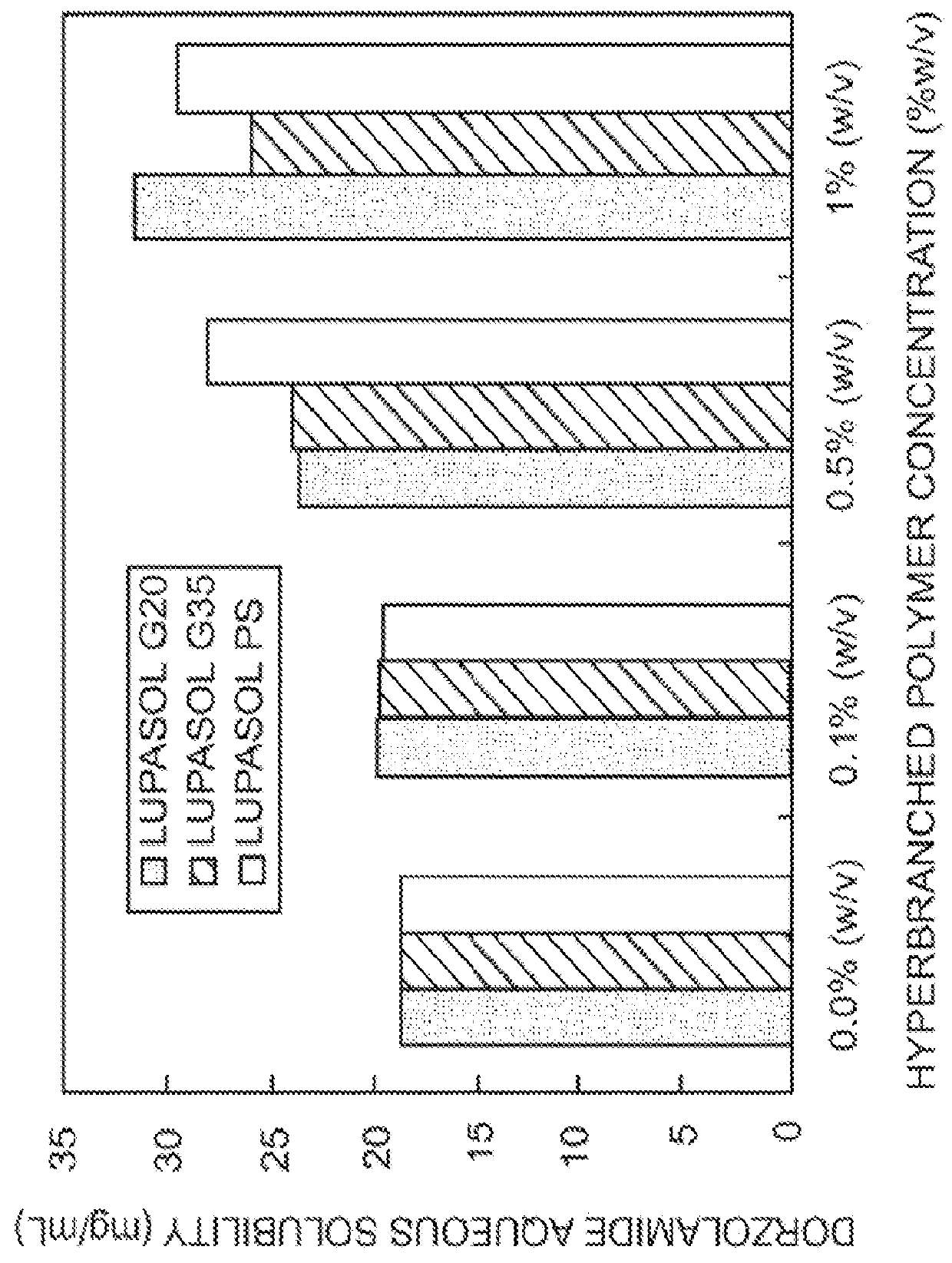
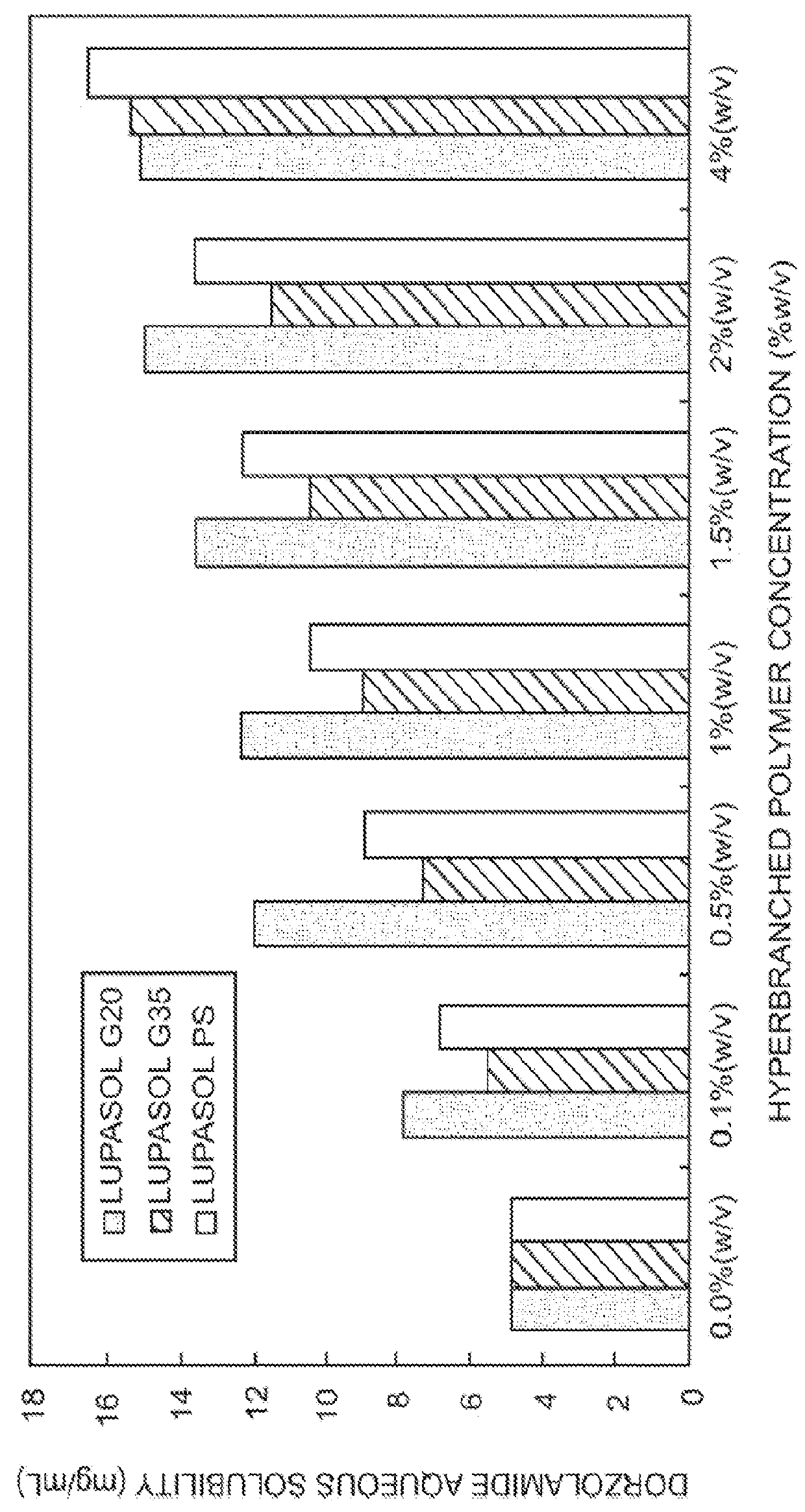
InactiveUS20130053374A1Good water solubilitySafely employedBiocideSenses disorderCarteololBeta blocker
The present invention provides an ophthalmic composition comprising a hyperbranched polyester. The ophthalmic compositions may also comprise carbonic anhydrase inhibitors, wherein the hyperbranched polyester increases the aqueous solubility of the carbonic anhydrase inhibitor, and increases corneal permeation of the active agent. The ophthalmic compositions may also comprise non-ionic surfactants, such as PEG, Polysorbate, HPMC or HEC, and beta-blockers, such as Carteolol, Levobunolol, Betaxolol, Metipranolol, Timolol or Propranolol. The concentration of the hyperbranched polyester in the ophthalmic formulation should be less than or equal to 4% (w / v) in order to avoid any cytotoxic effects on human corneal cells and thus the eye irritation.
Owner:SENJU USA
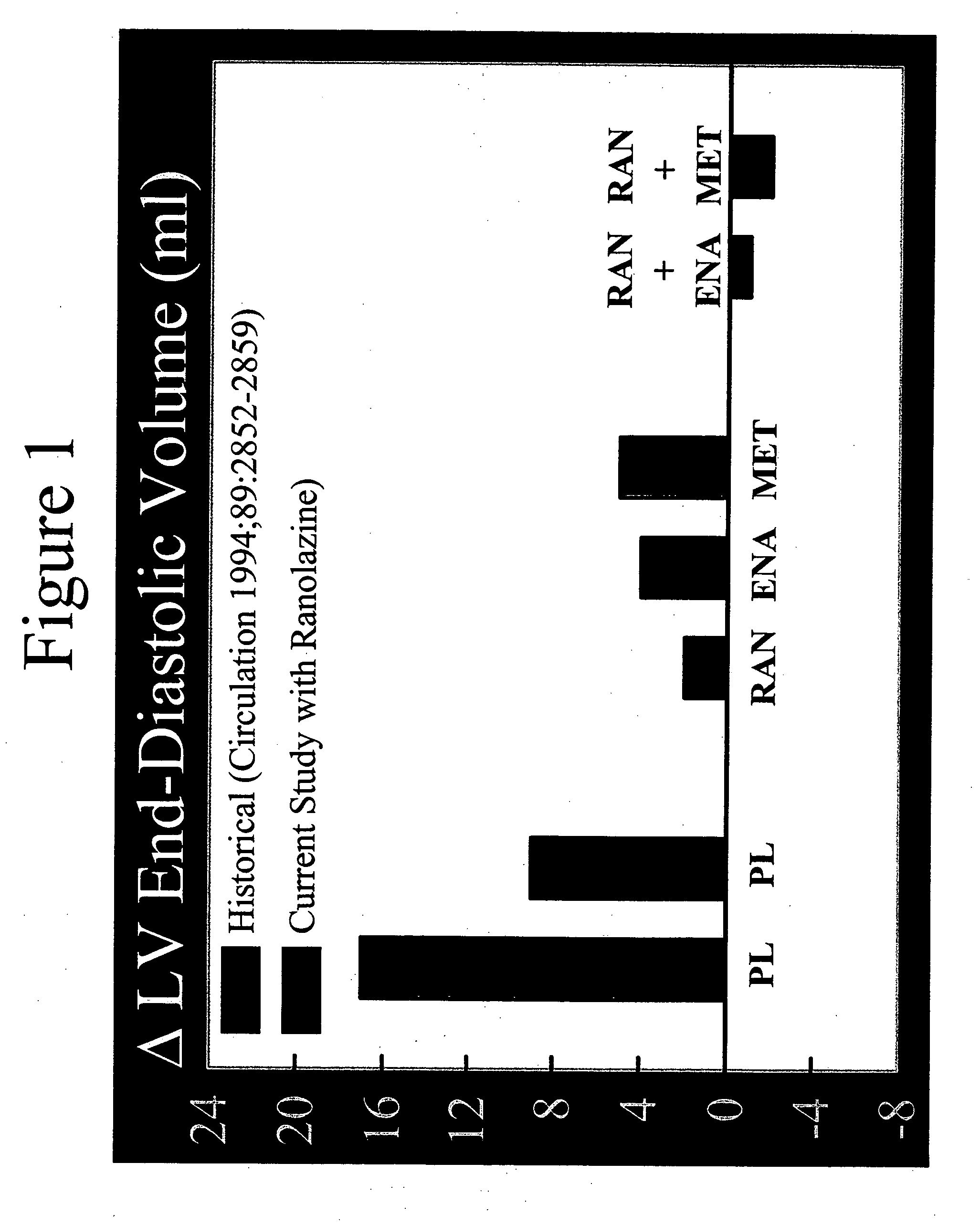
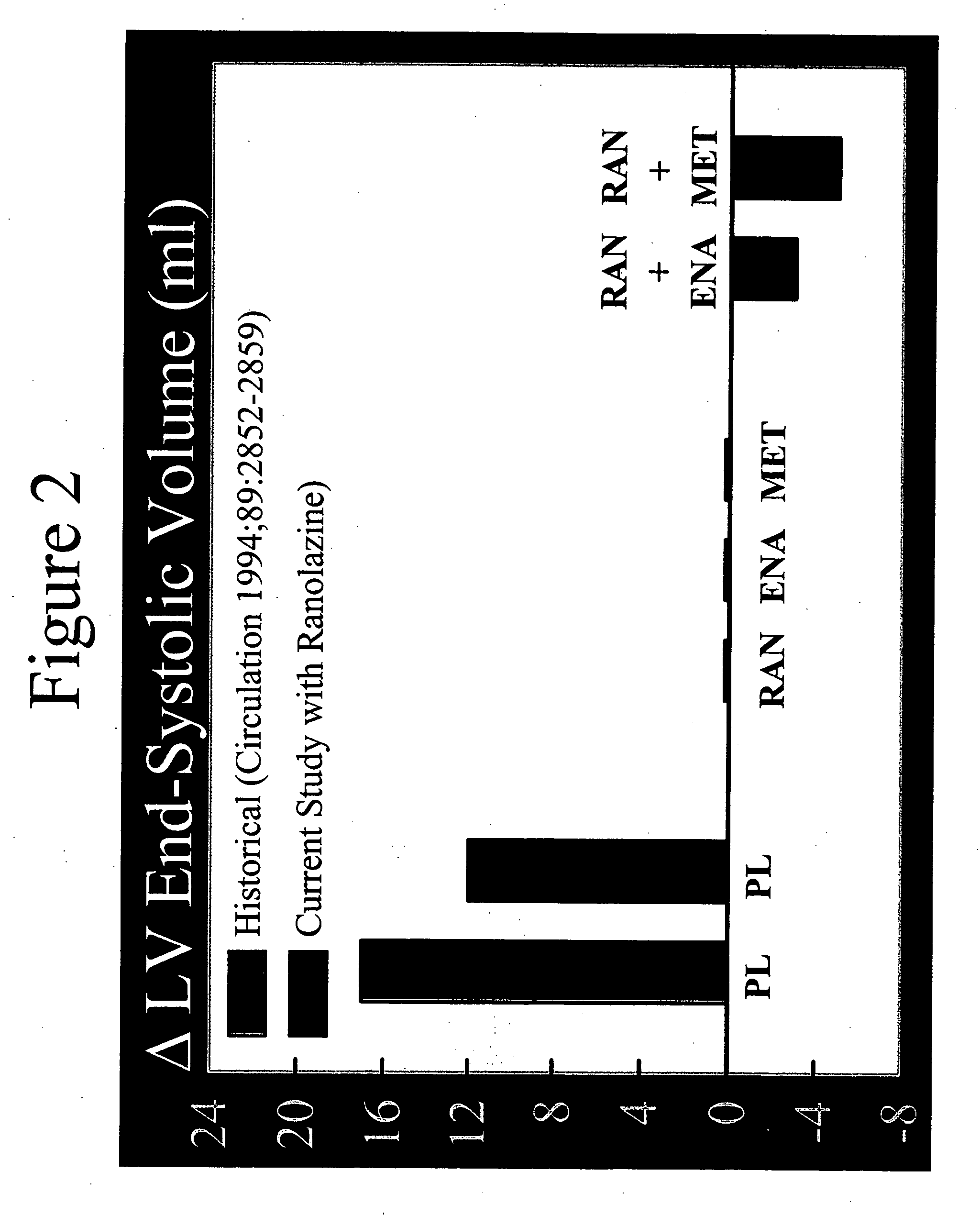
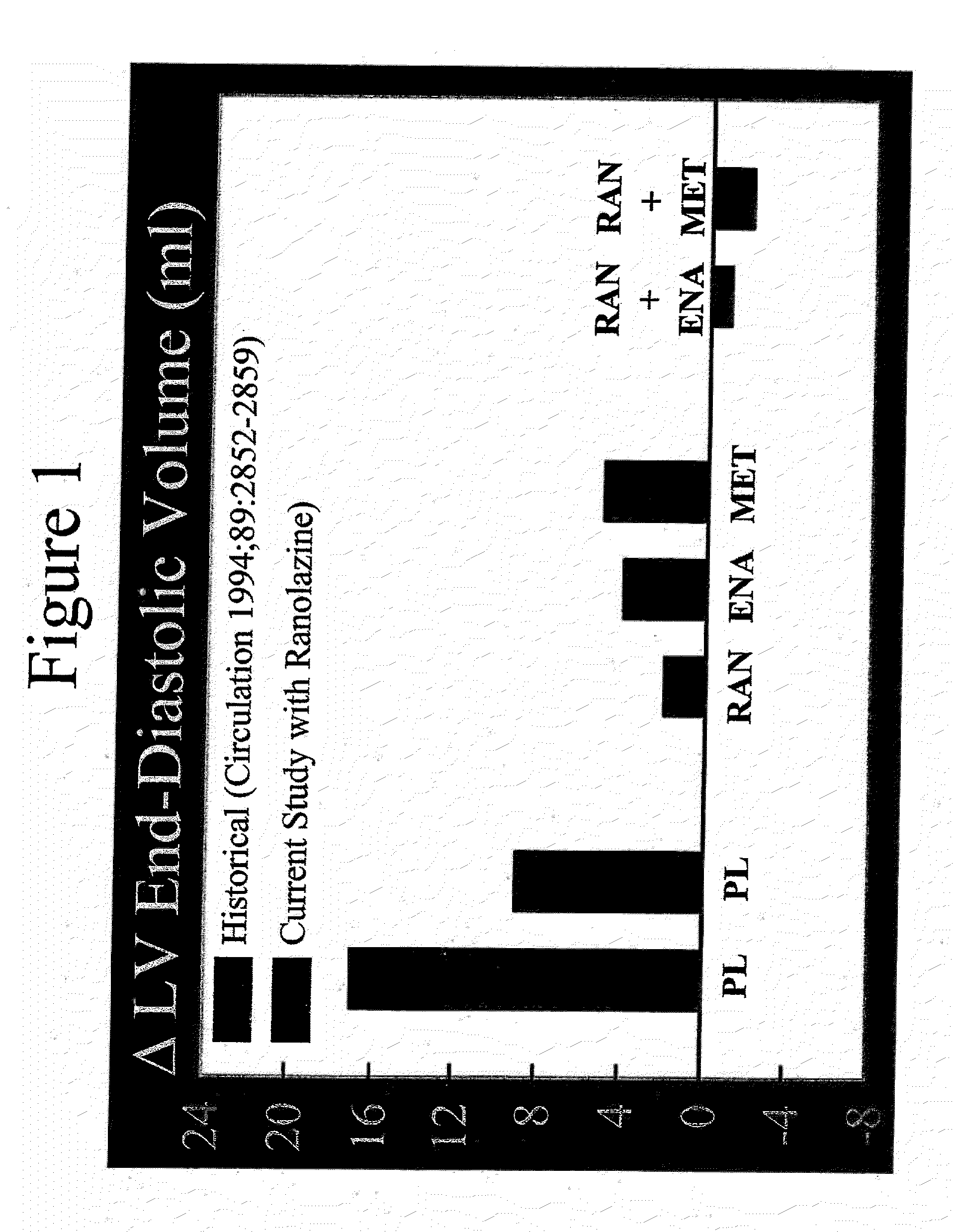
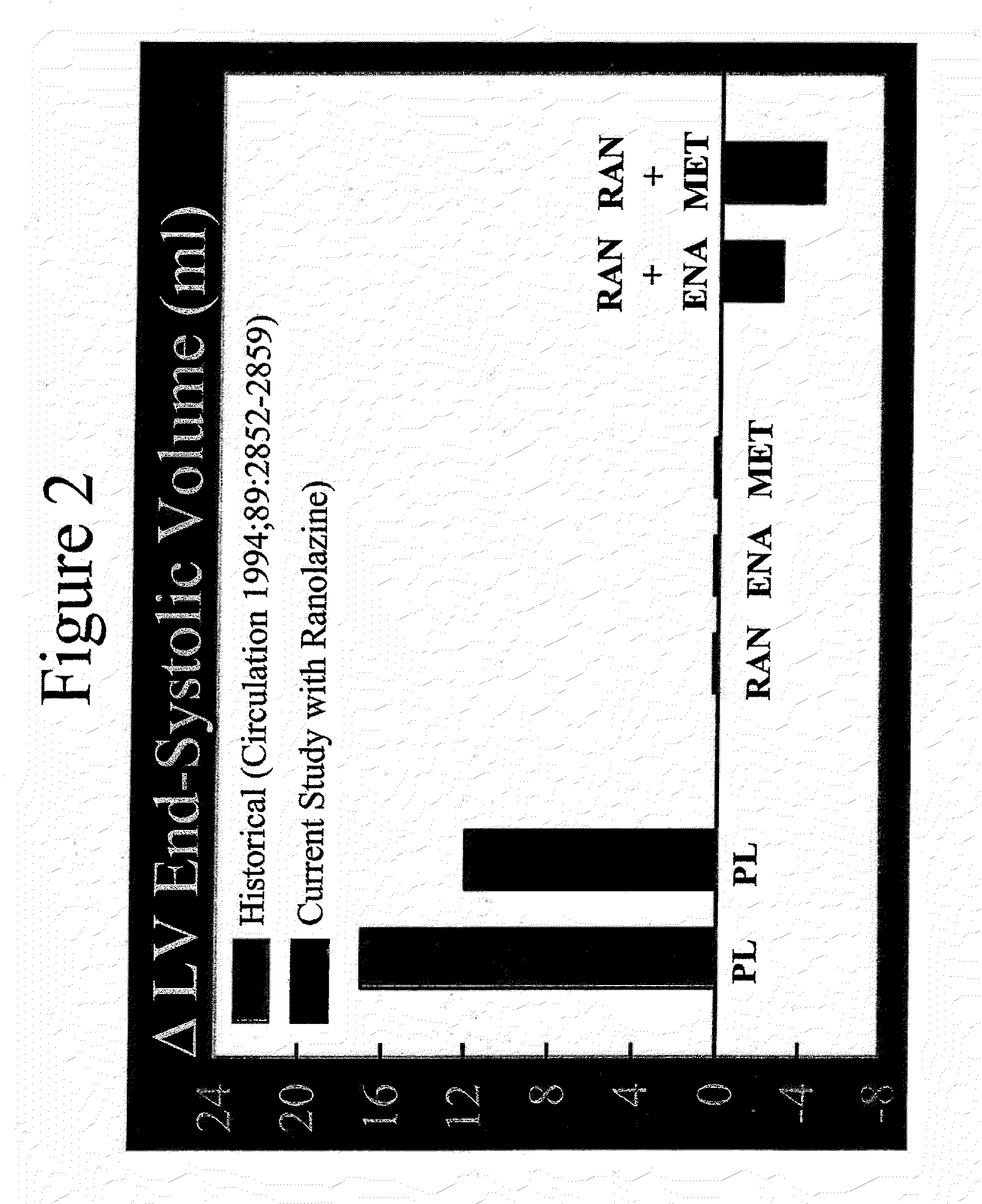
Method of reversing left ventricle remodeling
InactiveUS20060111361A1Reduction in LV volumeCardiac outputBiocideAnimal repellantsBeta blockerRanolazine
The present invention relates to method of reversing left ventricle remodeling by combined administration of therapeutically effective amounts ranolazine and at least one co-remodeling agent, which may be an ACE inhibitor, an ARB, or a beta-blocker. The method finds utility in the treatment of heart failure. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
Inhalable formulations for treating pulmonary hypertension and methods of using same
The present invention is directed to an inhalable formulation for the treatment of pulmonary hypertension in a mammal (e.g., humans), wherein the formulation comprises at least one hypertension reducing agent, including but not limited to an angiotensin converting enzyme inhibitor, angiotensin receptor blocker, beta-blocker, calcium-channel blocker or vasodilator, or any combination thereof. The formulations of the present invention may be a solution or suspension, and preferably are suitable for administration via nebulization. The present invention is also directed to a method and kit for treating a mammal suffering from pulmonary hypertension.
Owner:MYLAN SPECIALTY
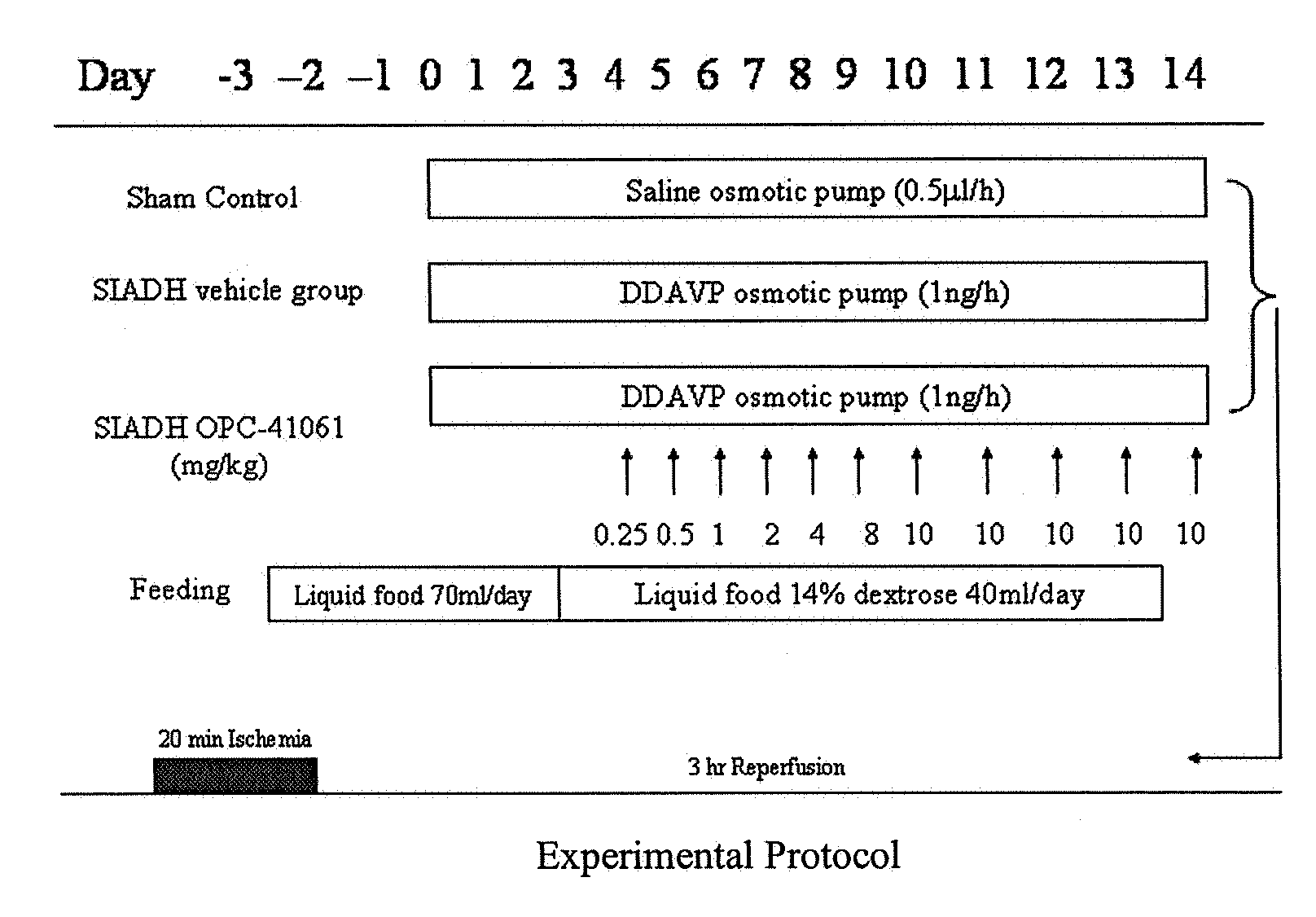
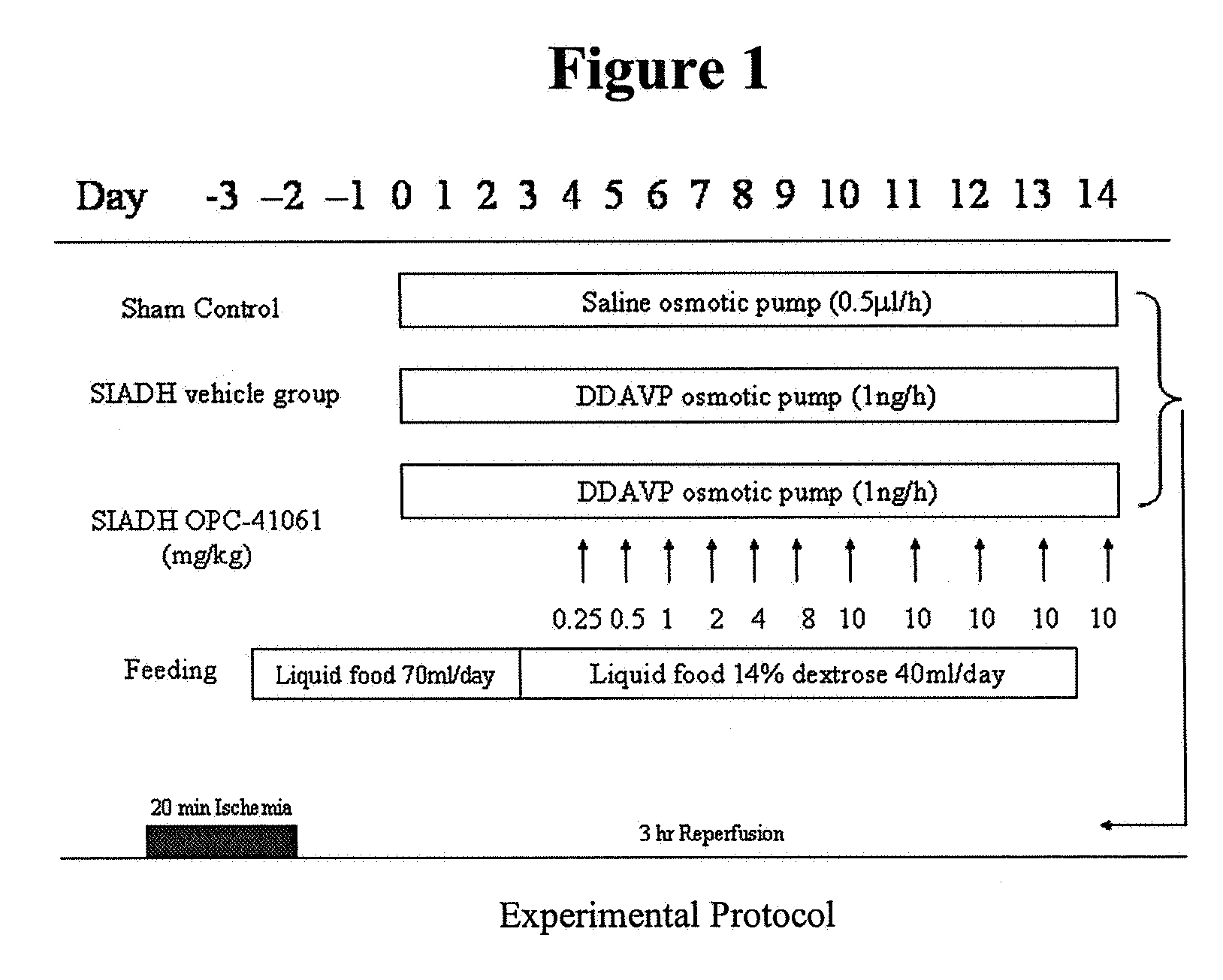
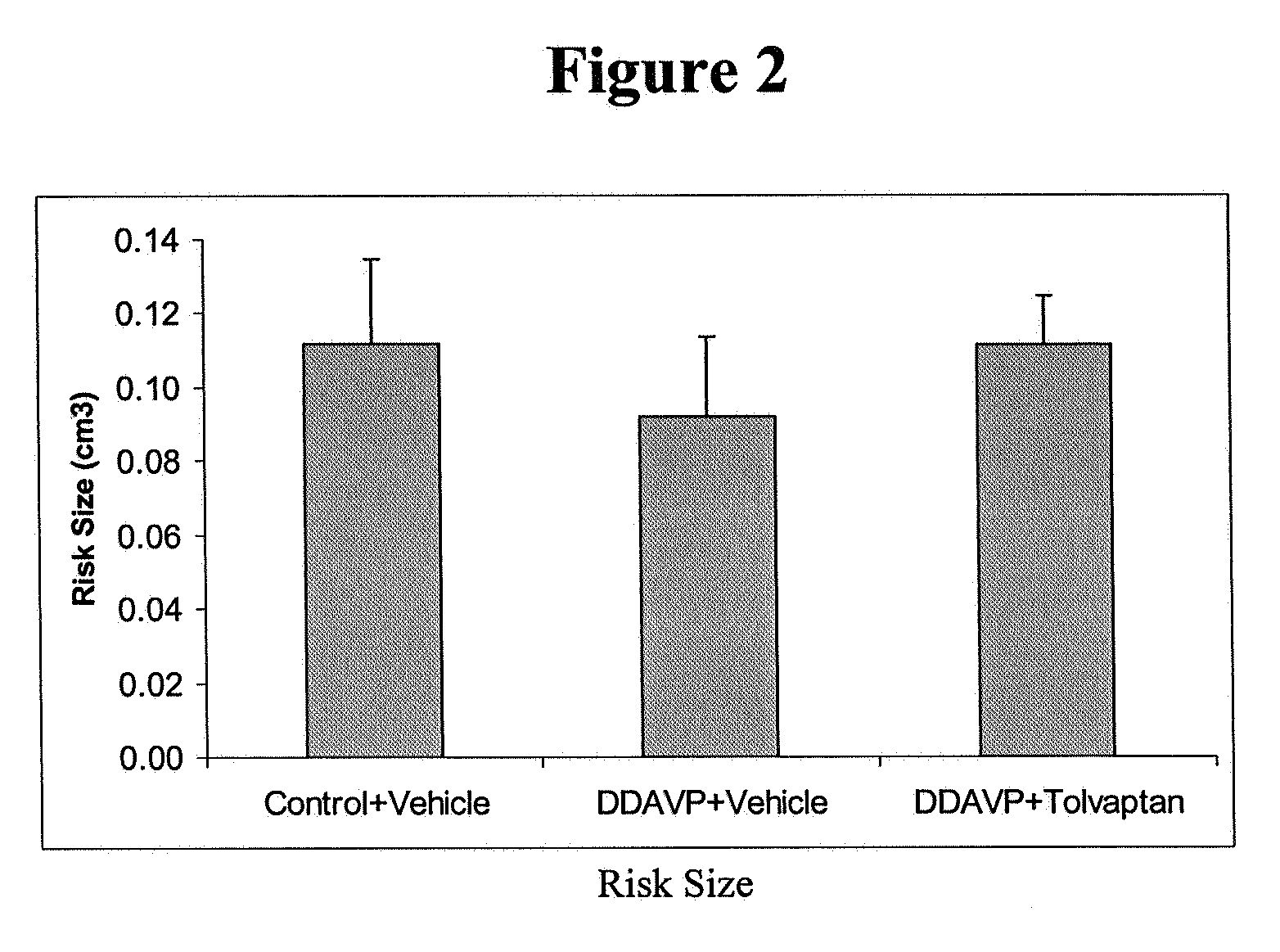
Method for reducing infarction using vasopressin antagonist compounds, and compositions and combinations therefor
InactiveUS20080221084A1Reduce infarctionMyocardial infarctionBiocideAnimal repellantsDiseaseDecreased sodium
The present invention relates to a method for reducing infarction comprising administering to a patient ion need thereof a therapeutically effective amount of a composition comprising as an active ingredient a vasopressin antagonist compound and to a composition useful therefor. The present invention also relates to a method for reducing infarction comprising administering to a patient in need thereof a therapeutically effective amount of a combination of a vasopressin antagonist compound and a beta-blocker and to combinations useful therefor. The methods, compositions and combinations of the present invention can be used for reducing infarction in the heart (myocardial infarction) and the brain (stroke). The methods, compositions and combinations of the present invention can also be used for the treatment and / or prevention of hypertension, edema, ascites, heart failure, renal function disorder, vasopressin inappropriate secretion syndrome (SIADH), hepatocirrhosis, hyponatremia, hypokalemia, polycystic kidney disease, diabetes, or circulation disorder.
Owner:OTSUKA PHARM CO LTD
Treatment of Conditions Through Pharmacological Modulation of the Autonomic Nervous System
Methods are provided for treating a subject for a condition caused by an abnormality in the subject's autonomic nervous system. In accordance with the subject methods, at least a portion of a subject's autonomic nervous system is pharmacologically modulated with at least one beta-blocker in a manner that is effective to treat the subject for the condition. The subject methods find use in the treatment of a variety of different conditions, where such conditions include various disease conditions. Also provided are systems and kits for use in practicing the subject methods.
Owner:PALO ALTO INVESTORS LP
Beta-adrenoceptor genetic polymorphisms and obesity
InactiveUS20020187491A1Reducing trial and error approachShorten the time limitBiocideOrganic active ingredientsHypertension medicationsBeta blocker
The present invention provides a method for identifying a subject having a risk of developing obesity, coronary microvascular dysfunction, or hypertension, comprising detection of the presence of a single nucleotide polymorphism (SNP) in a nucleic acid encoding an element of at least one beta-adrenergic receptor from the subject. The presence of the SNP is correlated with obesity, coronary microvascular dysfunction, or hypertension, and thereby identifies the subject as having a risk of developing obesity, coronary microvascular dysfunction, or hypertension. The subject invention also provides methods of identifying patients likely to benefit from the prescription of beta blocker hypertension medications. In various embodiments, the nucleic acids detected include those genes encoding ADRB1 (beta1AR), ADRB2 (beta2AR), ADRB3 (beta3AR), GNB3 (G protein beta3 subunit), or GNAS1 (GS protein alpha subunit). Methods of treating identified individuals are also provided.
Owner:FLORIDA UNIV OF A FLORIDA
Method of reversing left ventricular remodeling
InactiveUS20090176772A1Improve actionReduce releaseCardiovascular disorderHeterocyclic compound active ingredientsBeta blockerLeft ventricular size
The present invention relates to method of reversing left ventricle remodeling by combined administration of therapeutically effective amounts ranolazine and at least one co-remodeling agent, which may be an ACE inhibitor, an ARB, or a beta-blocker. The method finds utility in the treatment of heart failure. This invention also relates to pharmaceutical formulations that are suitable for such combined administration.
Owner:GILEAD SCI INC
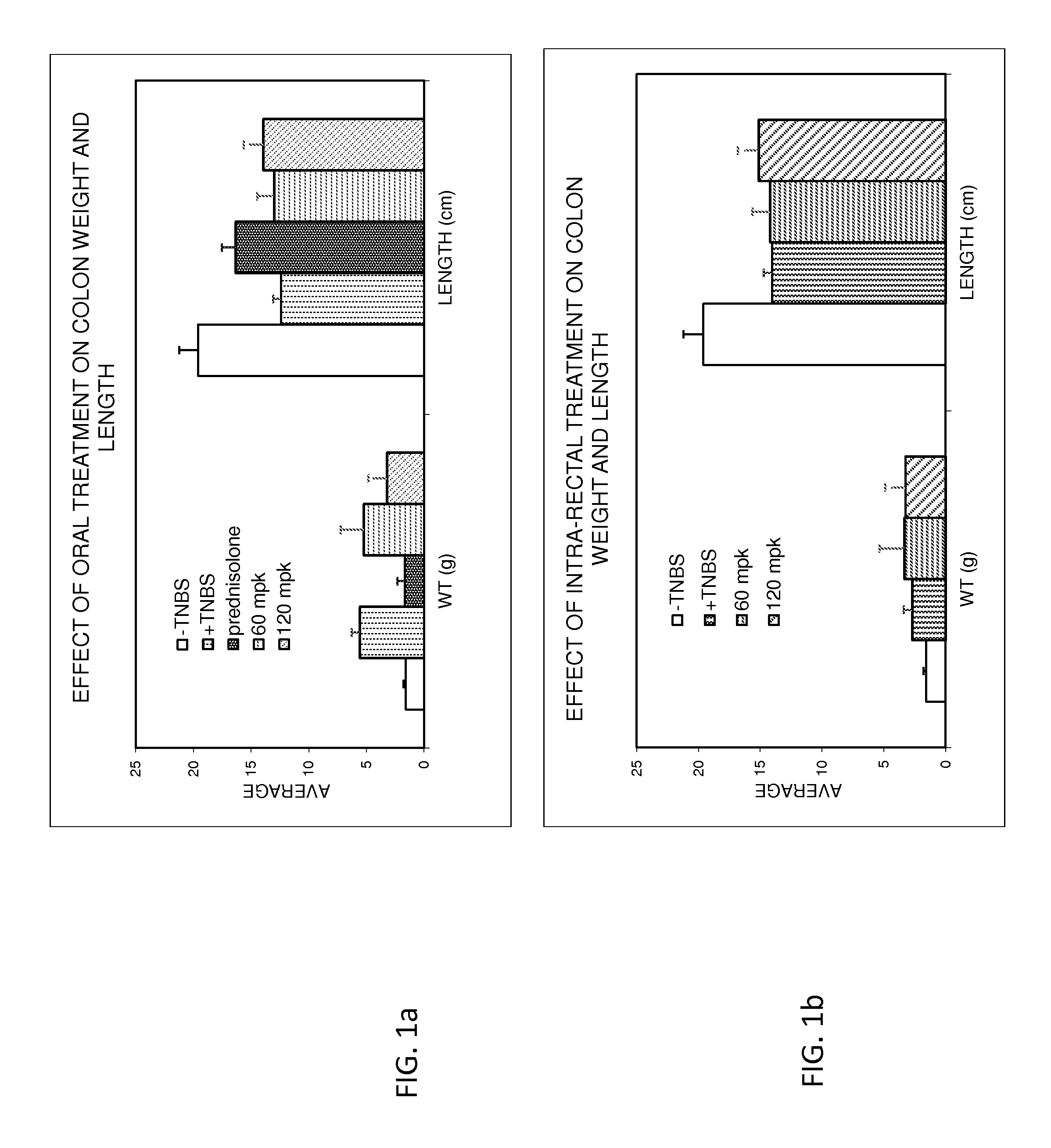
Treatment of gastrointestinal and other disorders
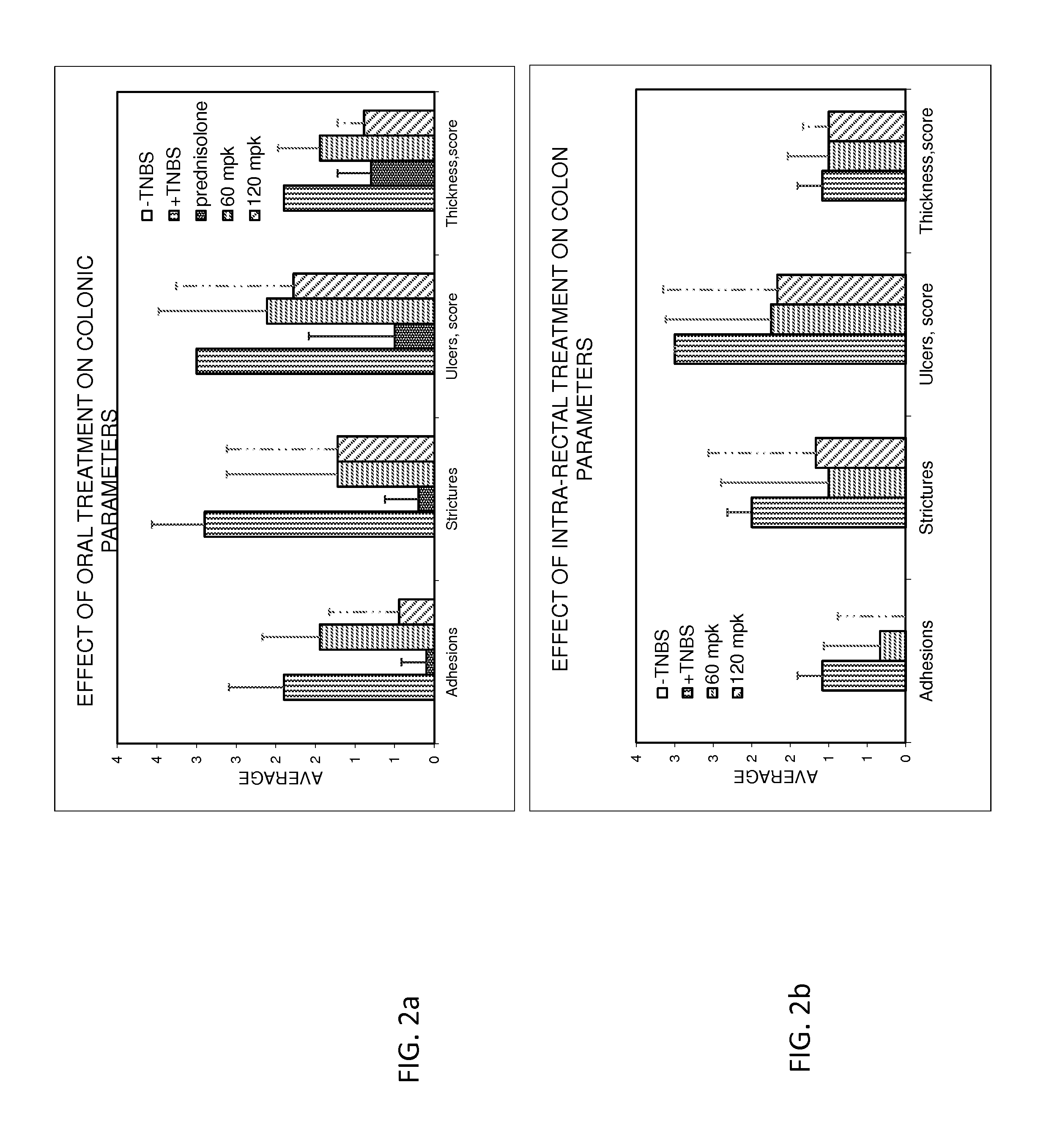
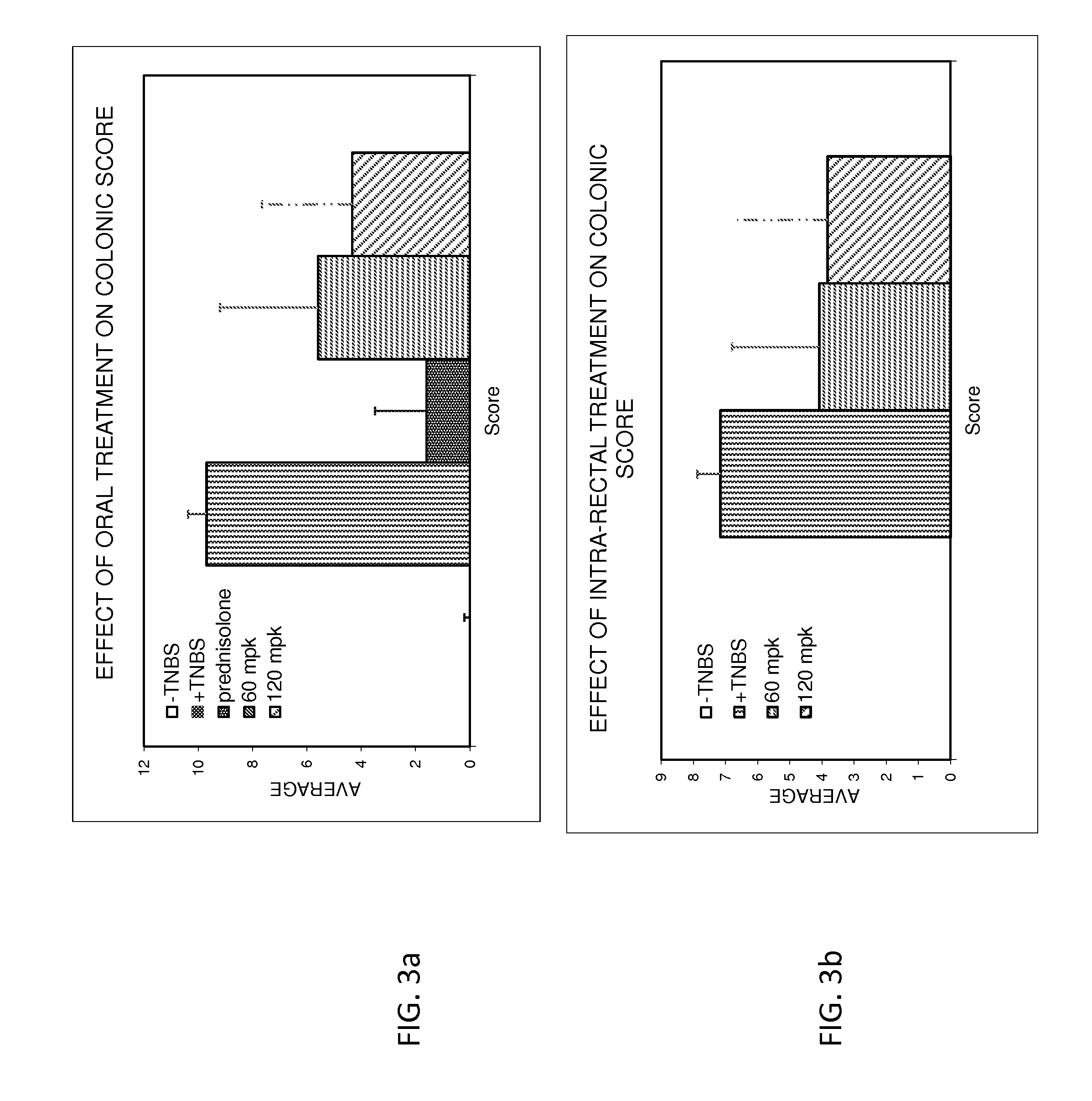
InactiveUS20160303133A1Easy to optimizeLower Level RequirementsOrganic active ingredientsDispersion deliveryBeta blockerCrohn's disease
Provided herein are methods and compositions for treating gastrointestinal disorders using beta blockers. Compositions comprising Beta blockers such as Timolol and Nadolol are used to treat diseases including ulcerative colitis, IBD and Crohn's disease. The Compositions are administered orally or rectally, at doses that provide a peak plasma concentration of less than 10 ng / ml.
Owner:NUMEDII
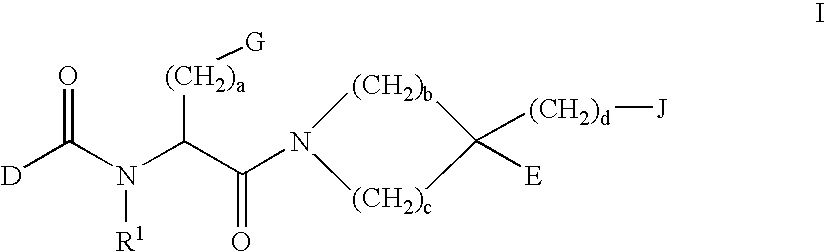
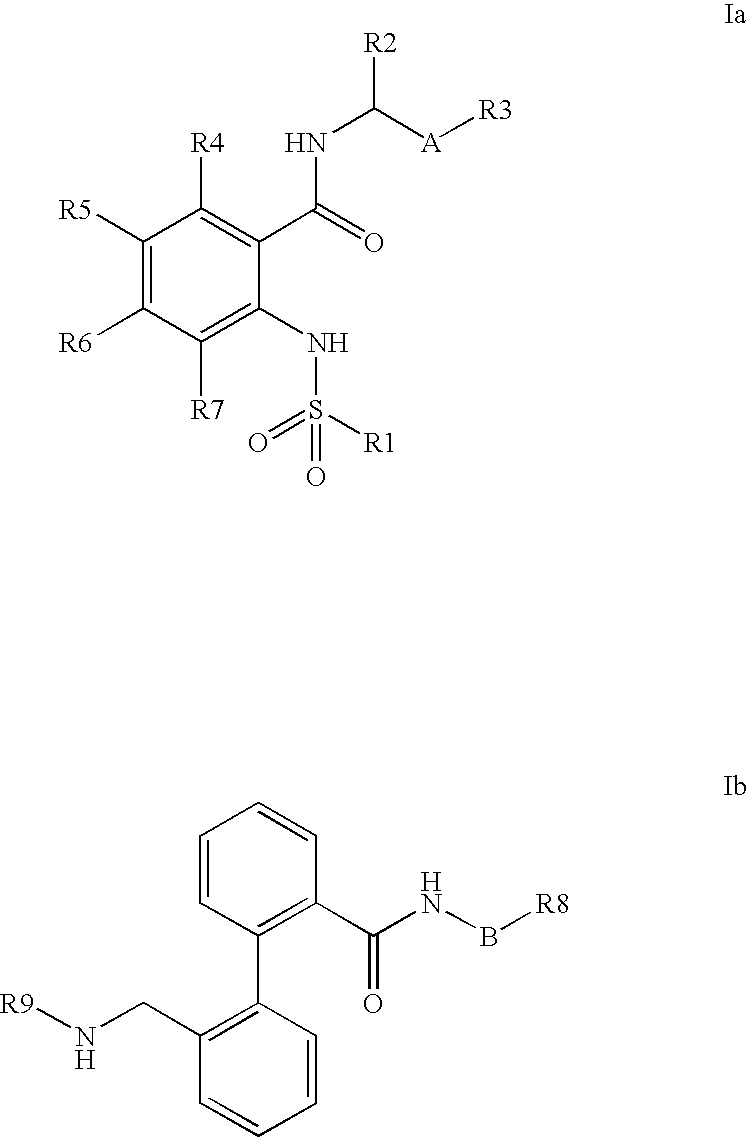
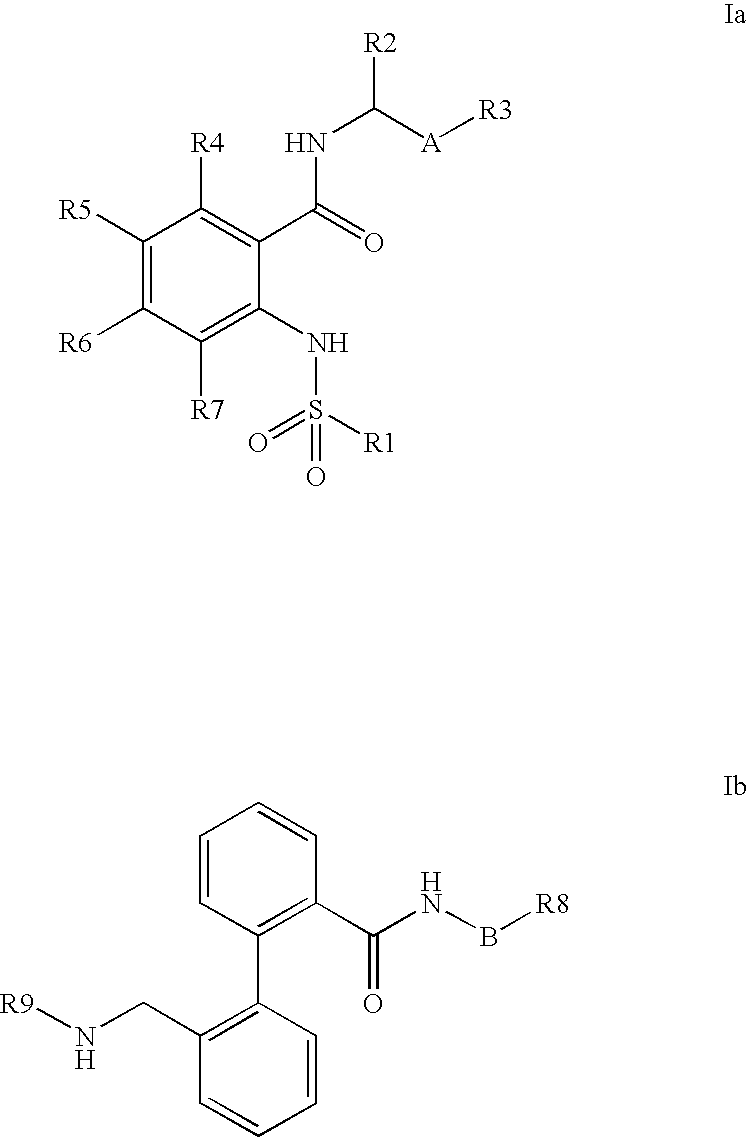
Combination of phenylcarboxylic acid amides with beta-adrenoreceptor blockers and their use for the treatment of atrial arrhythmias
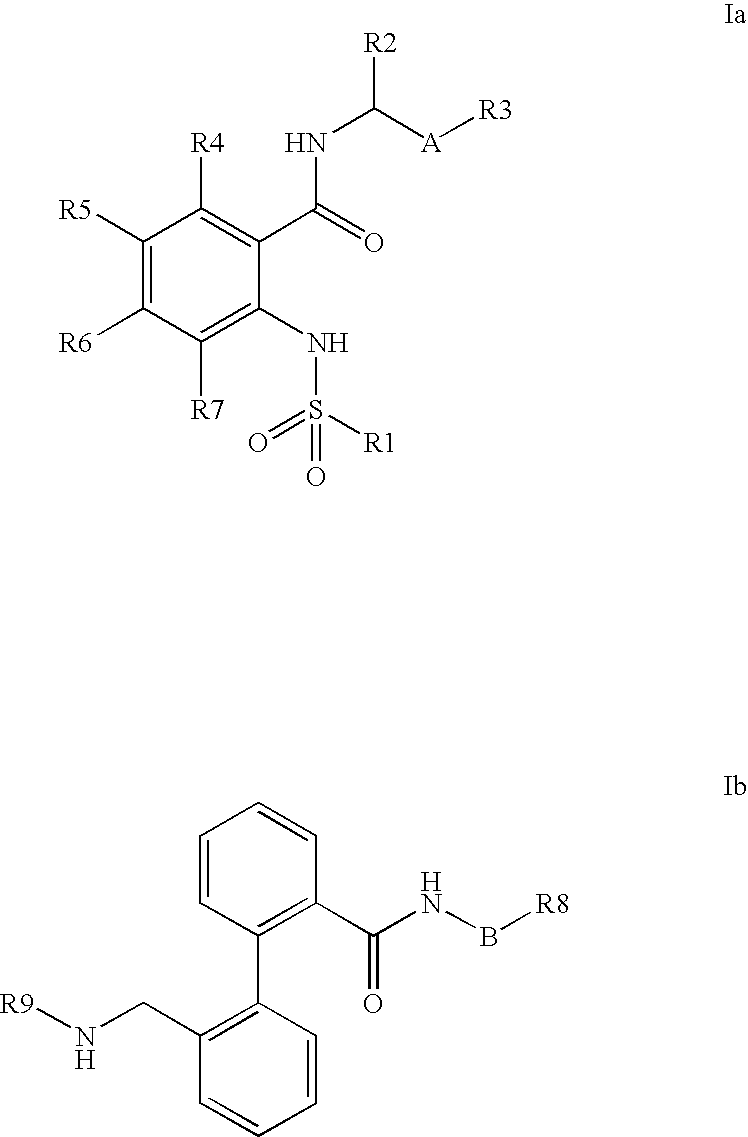
The invention relates to the combination of one or more beta-blockers and of one or more Kv1.5 blockers, in particular phenylcarboxamides of the formula la and / or lb and / or physiologically tolerable salts thereof, and the use of the combination for the treatment or prophylaxis of atrial arrhythmias.
Owner:SANOFI AVENTIS DEUT GMBH
Pharmaceutical Composition for Treatment of Diabetic Complications
ActiveUS20110021526A1High activityImprove the level ofBiocideSenses disorderCollagen accumulationBeta blocker
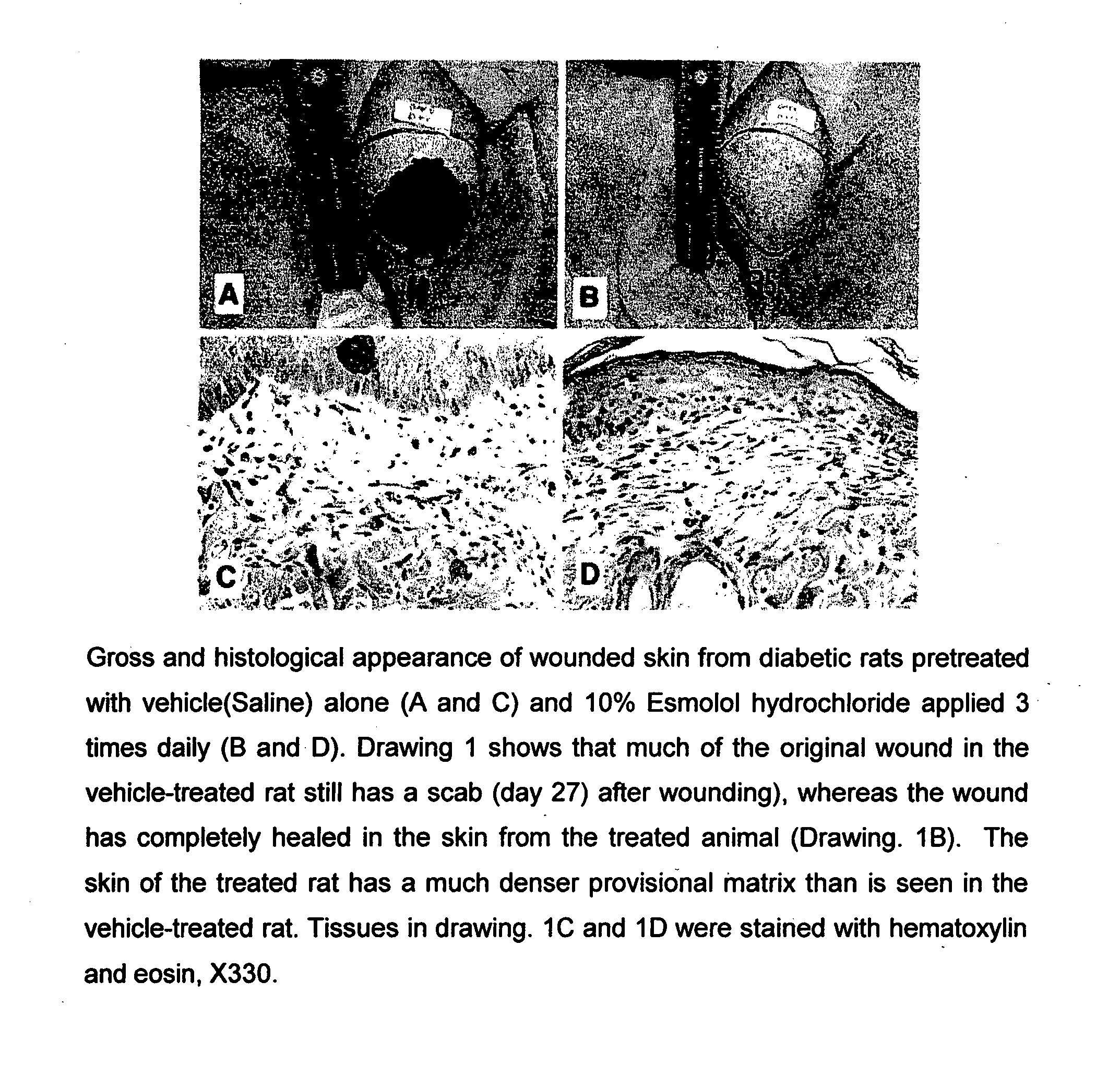
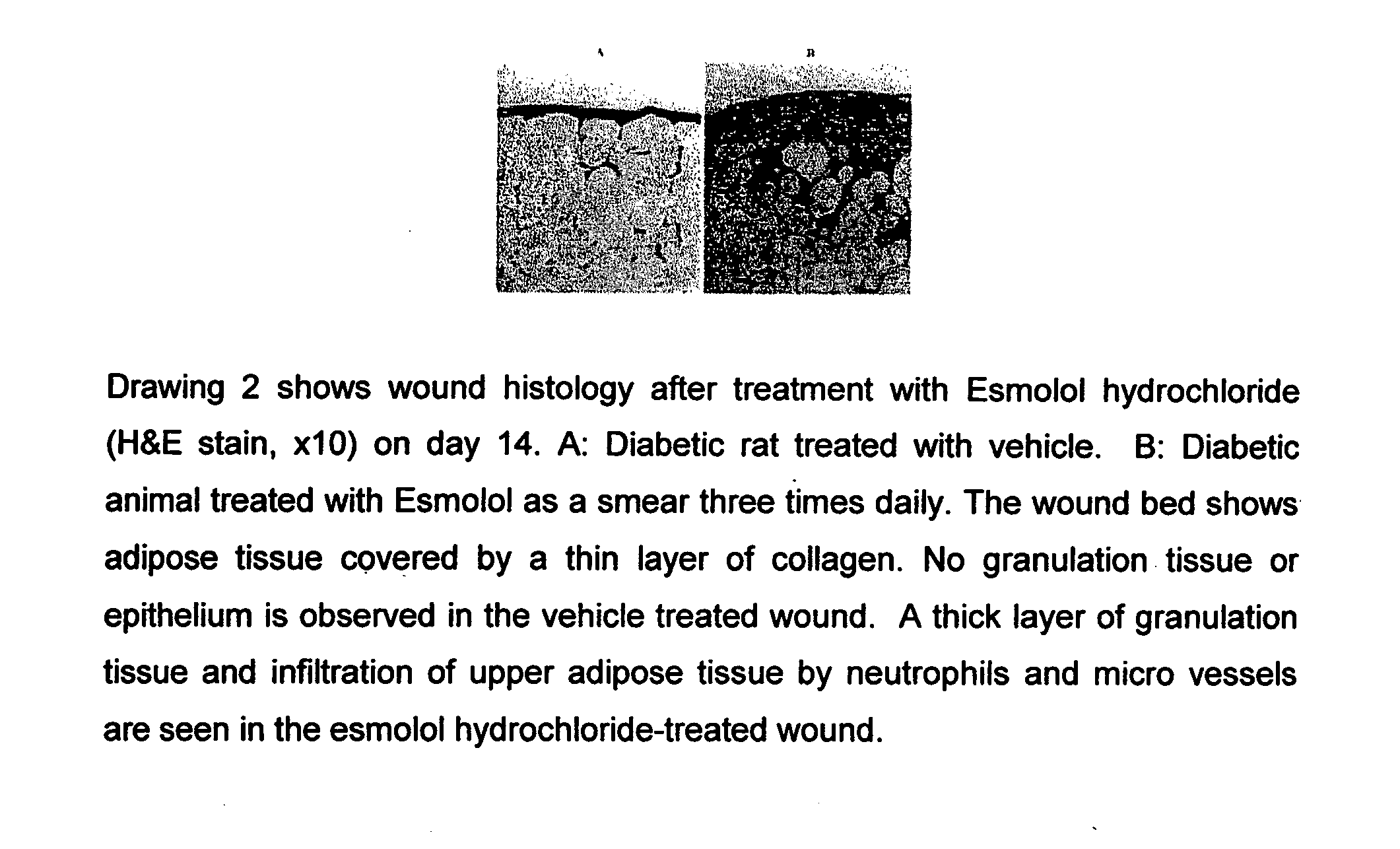
A method for treating diabetic complications by administration of a beta-blocker is disclosed. Diabetic complications arise from diabetes and have few or no existing treatment options. The present invention describes the use of a beta-blocker in the treatment of a diabetic. The present invention also describes the inhibition of aldose reductase, one of the chief causative factors of diabetic complications. Also provided are methods of diabetic wound healing. Compositions for treating diabetic complications, such as diabetic wounds, are disclosed. The present invention includes employing a topical formulation of a beta-blocker, having substantially no antibacterial activity, to improve the process of diabetic wound healing. The present invention also involves increasing the rate of collagen accumulation of the healing epithelialized tissue in the wound of a diabetic individual.
Owner:VLIFE SCI TECH PVT
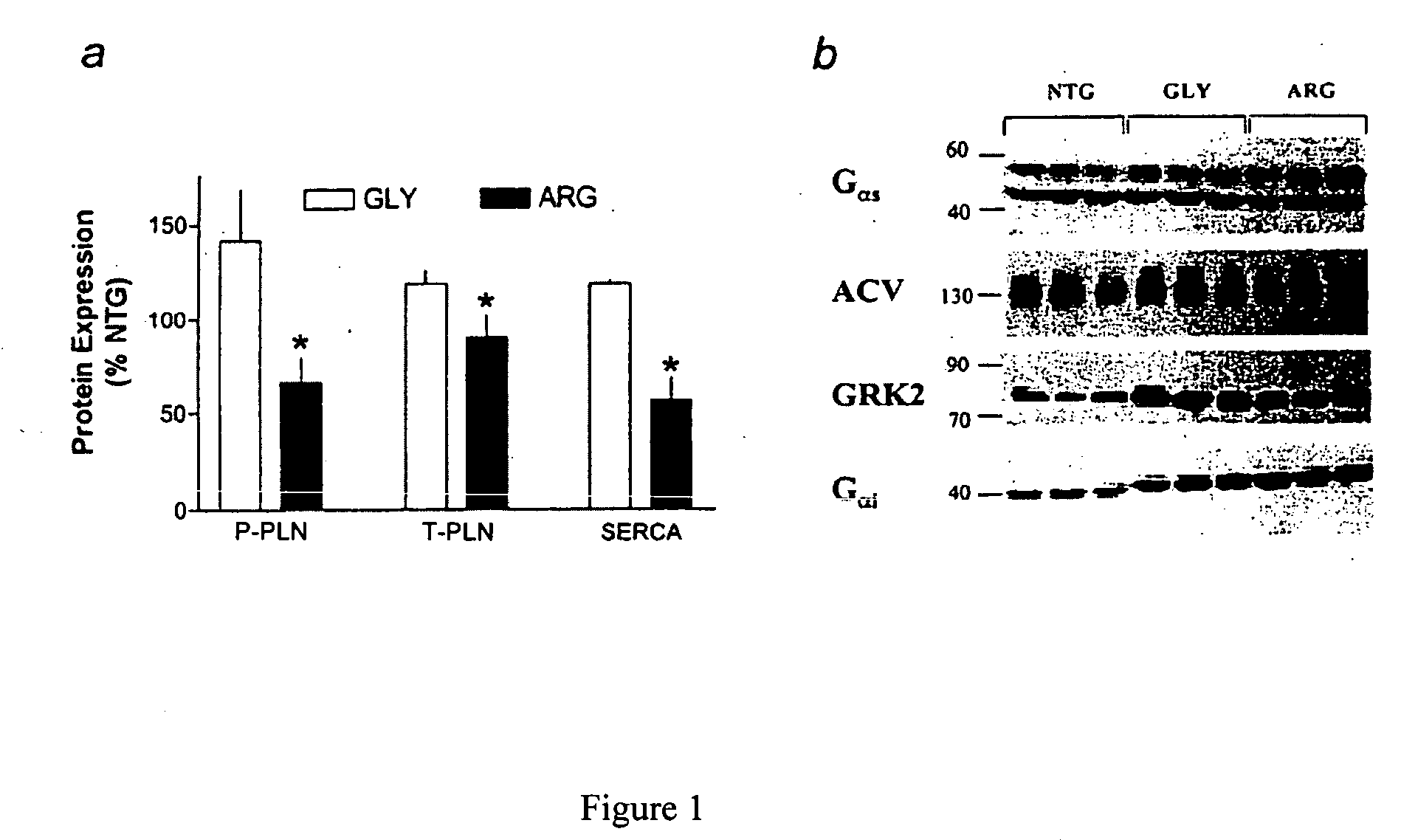
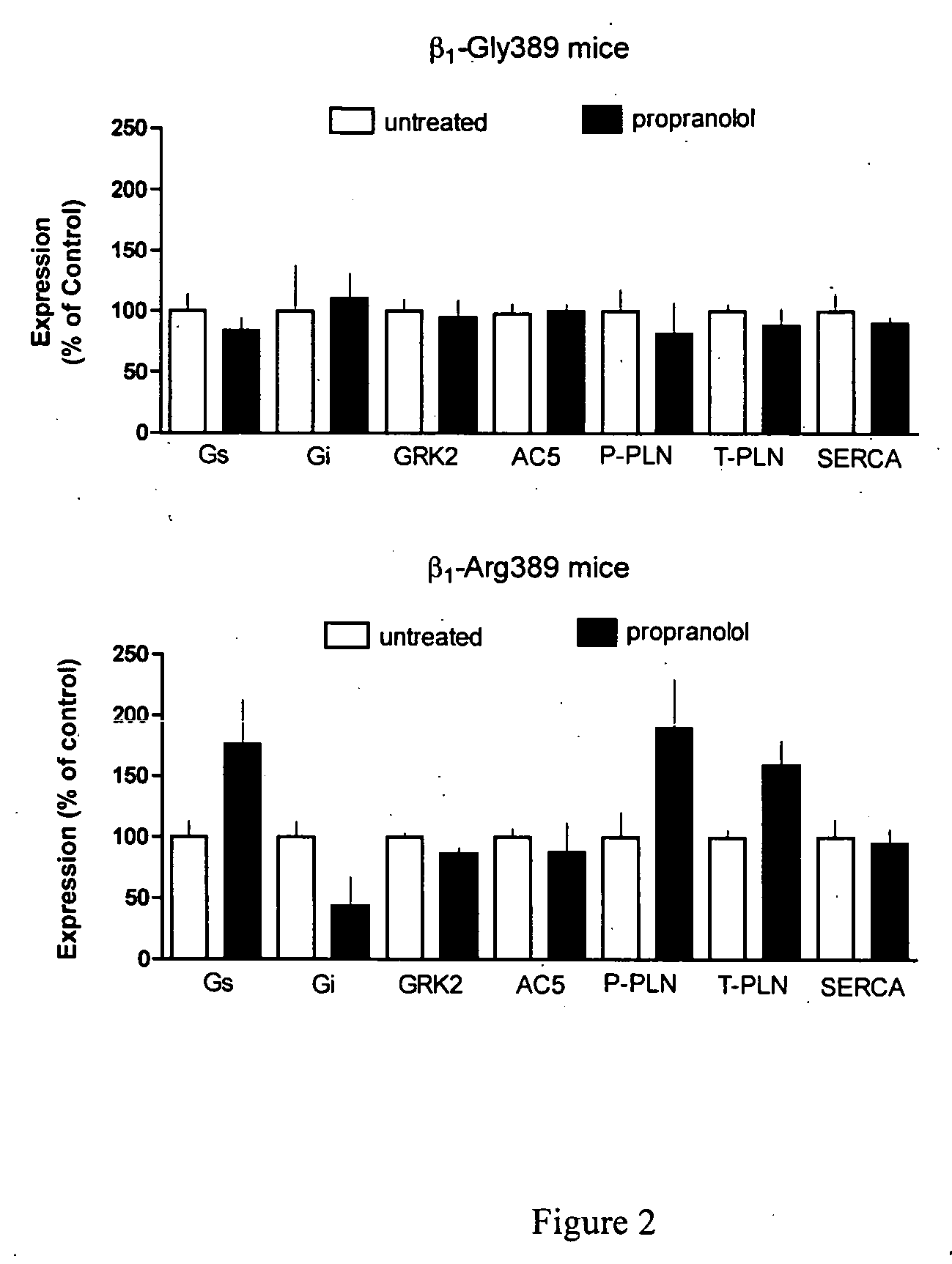
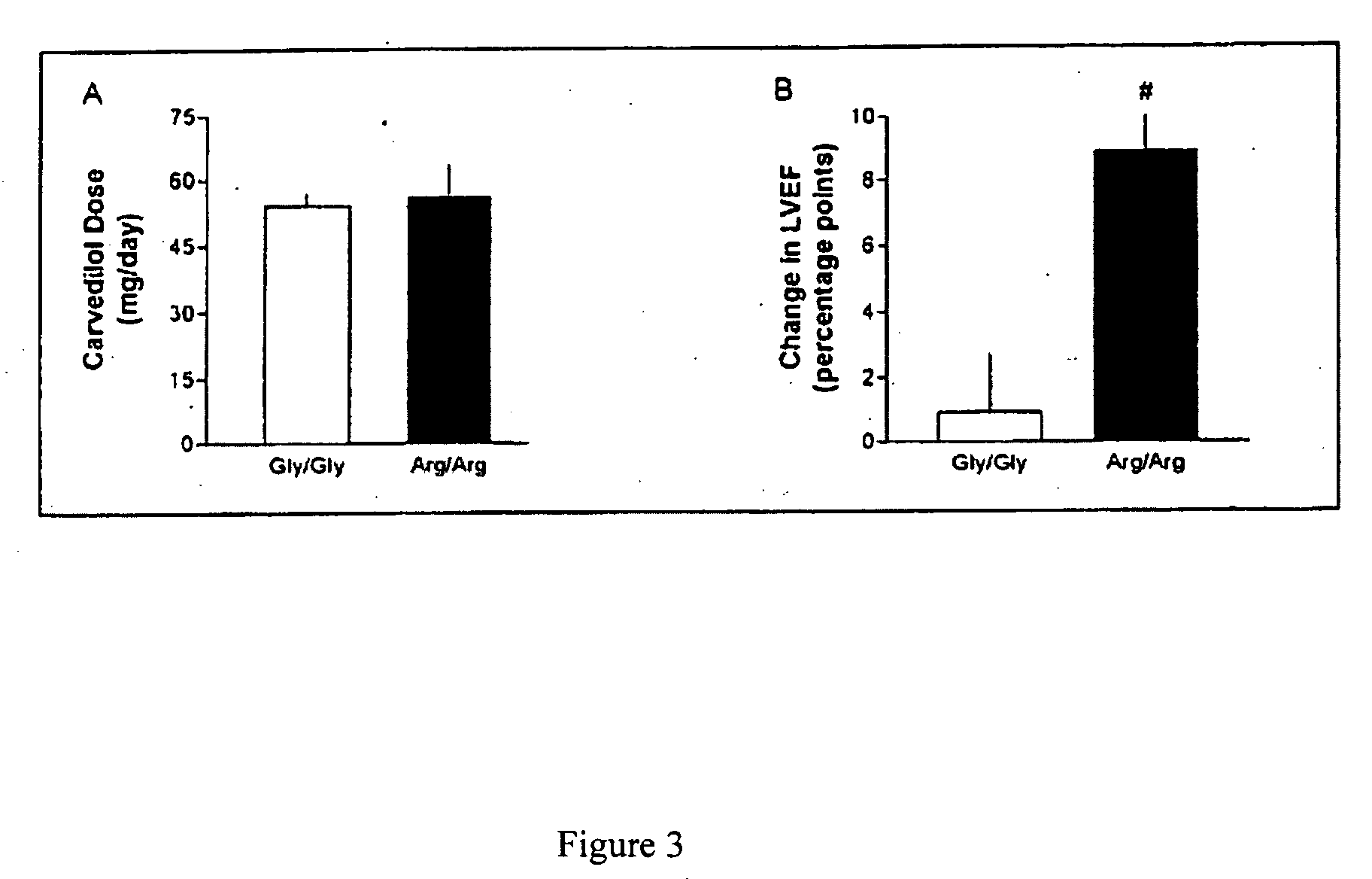
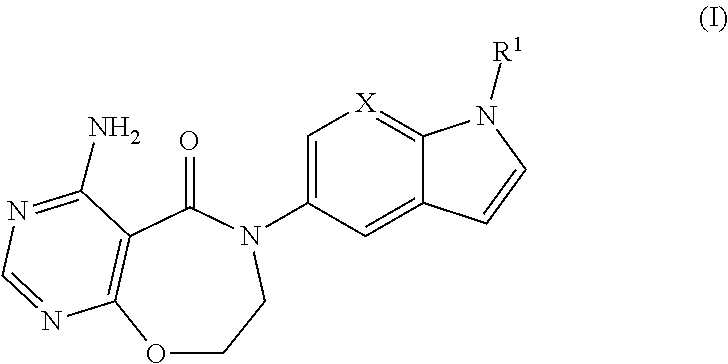
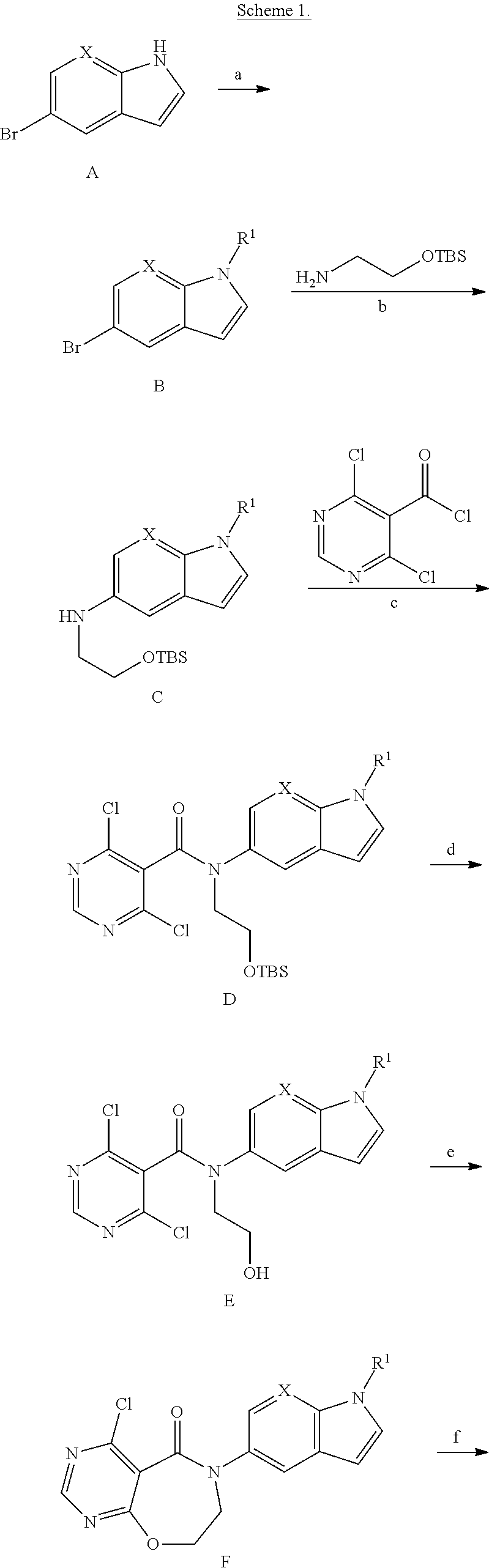
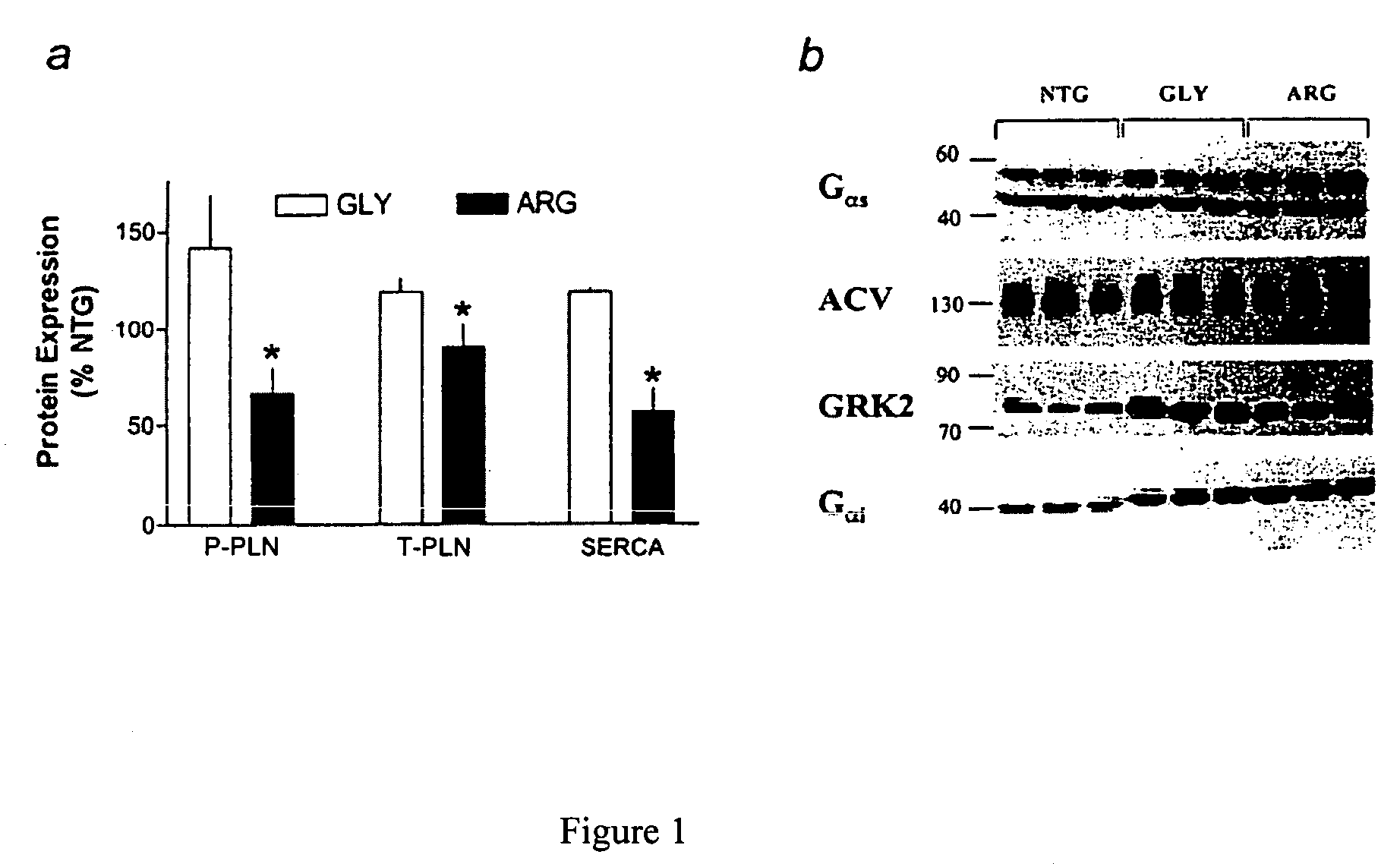
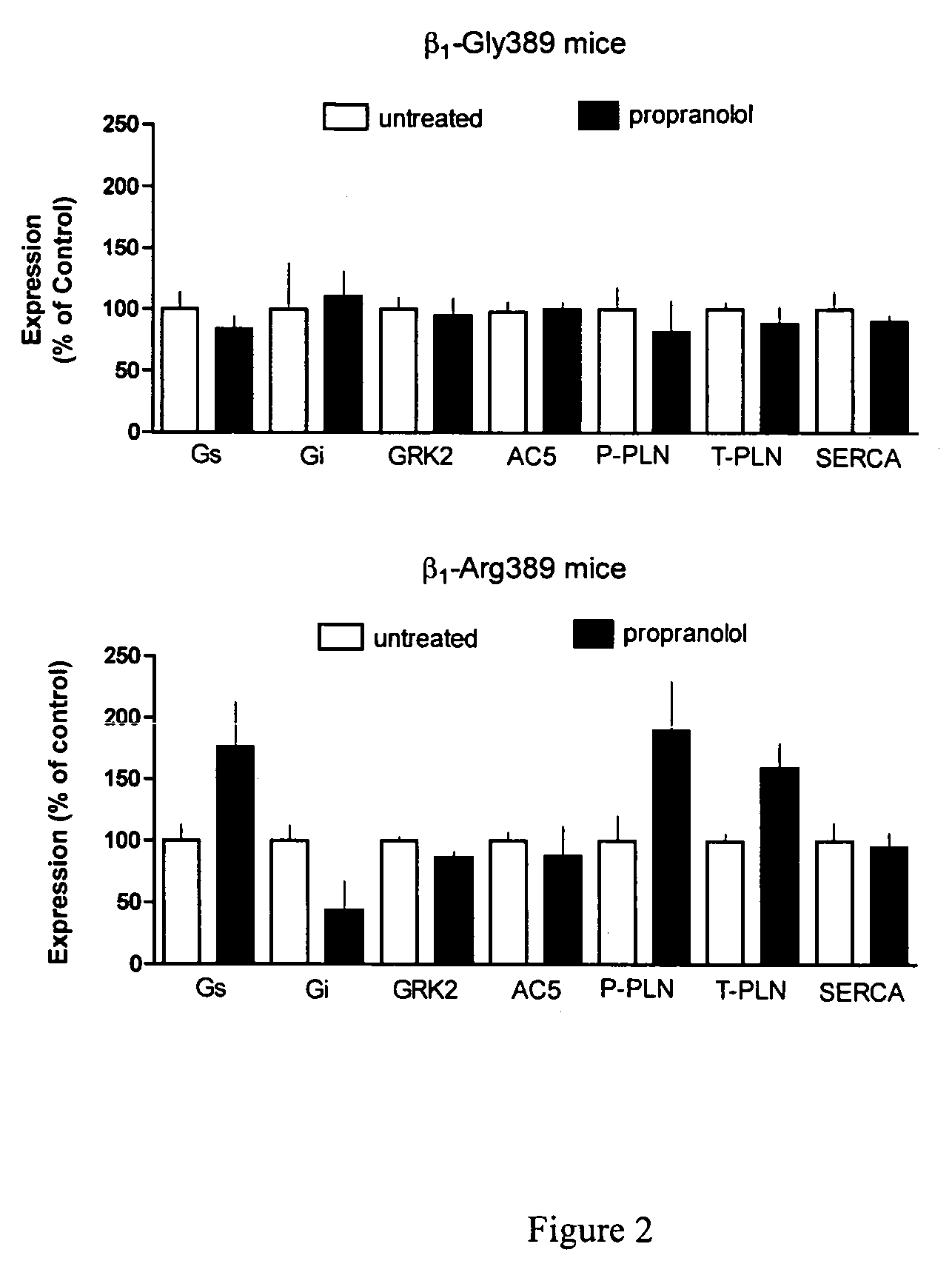
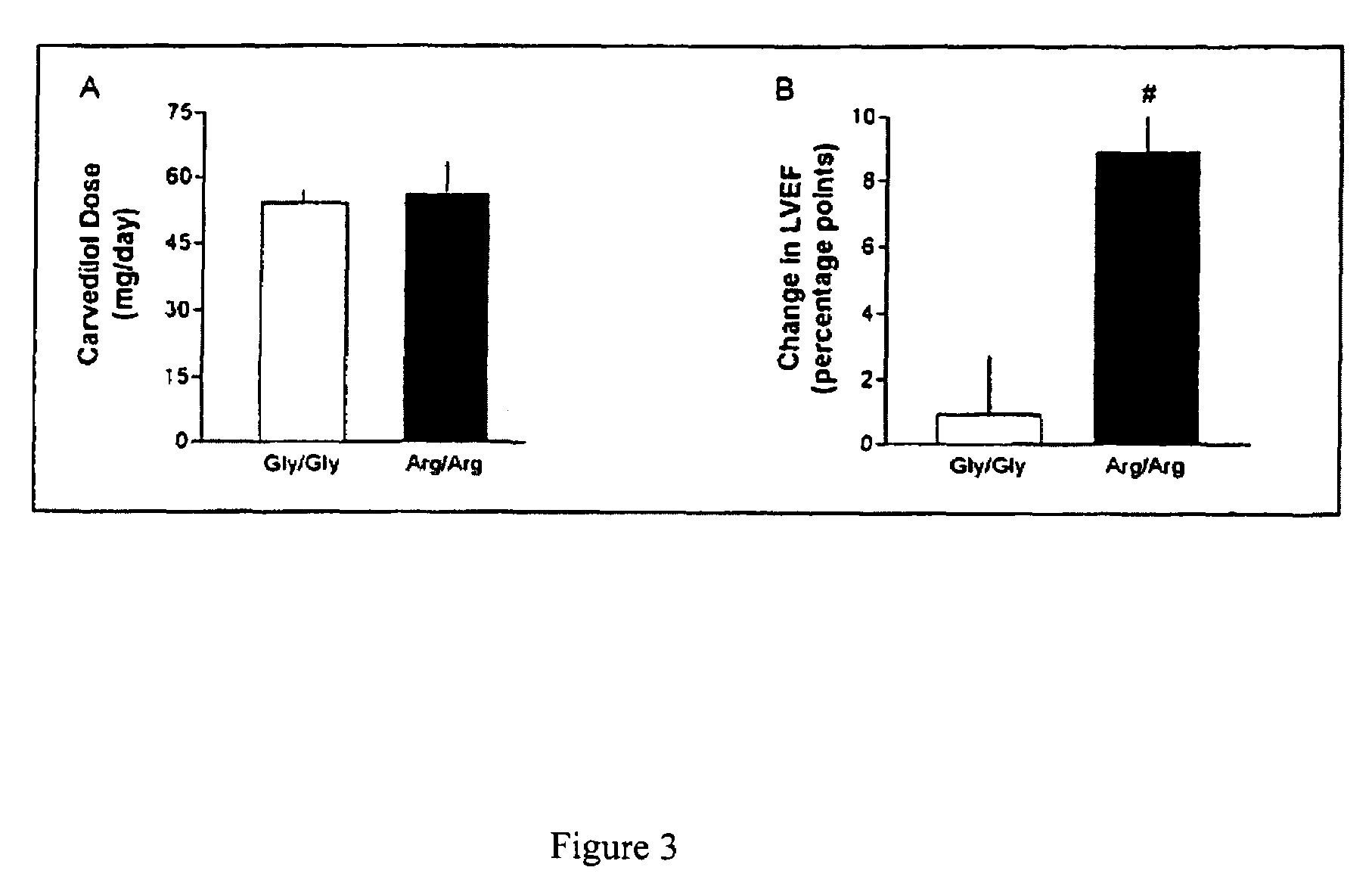
Methods for diagnosis, prediction and treatment of heart failure and other cardiac conditions based on beta1-adrenergic receptor polymorphism
Methods and compositions for the detection, diagnosis, and prevention of cardiac conditions are provided. Polymorphisms of β1-adrenergic receptor are provided. The Gly389 β1-adrenergic receptor variants are not as responsive to treatment β blockers such as carvedilol, metoprolol or bisoprol. Thus, genotyping β1-adrenergic receptor polymorphisms is useful for predicting relative responsiveness to treatment with beta blockers. The Gly389 polymorphism also may be used, alone or in conjunction with other adrenergic receptor polymorphisms, to predict relative risk of developing cardiovascular diseases such as heart failure or to predict relative survival rate in patients with heart failure or other cardiovascular diseases. Also provided are transgenic mice and transgenic cells expressing the β1-adrenergic receptor polymorphisms, and their use in identifying therapeutic agents.
Owner:UNIVERSITY OF CINCINNATI
Treatment of cutaneous hemangioma
InactiveUS20120058056A1Small sizeEffective treatmentBiocidePeptide/protein ingredientsBeta blockerHemangioma
This invention concerns a method of treating hemangiomas with a beta blocker by applying the beta blocker onto the hemangiomas directly. The invention also concerns a combination therapy by using a beta blocker along with a corticosteroid or an alpha adrenergic receptor agonist for the treatment of hemangiomas.
Owner:RUTGERS THE STATE UNIV
Novel compounds as diacylglycerol acyltransferase inhibitors
This invention relates to novel compounds which are inhibitors of acyl coenzyme A: diacylglycerol acyltransferase 1 (DGAT-1), to pharmaceutical compositions containing them, to processes for their preparation, and to their use in therapy, alone or in combination with weight management therapies or other triglyceride lowering therapy for the prevention or treatment of diseases related to DGAT-1 dysfunction or where modulation of DGAT-1 activity may have therapeutic benefit including but not limited to obesity, obesity related disorders, genetic (Type 1, Type 5 hyperlipidemia) and acquired forms of hypertriglyceridemia or hyperlipoproteinemia-related disorders, caused by but not limited to lipodystrophy, hypothyroidism, medications (beta blockers, thiazides, estrogen, glucocorticoids, transplant) and other factors (pregnancy, alcohol intake), hyperlipoproteinemia, chylomicronemia, dyslipidemia, non-alcoholic steatohepatitis, diabetes, insulin resistance, metabolic syndrome, cardiovascular outcomes, angina, excess hair growth (including syndromes associated with hirsutism), nephrotic syndrome, fibrosis such as myocardial, renal and liver fibrosis, hepatitis C virus infection and acne or other skin disorders.
Owner:GLAXO SMITHKLINE LLC
Method for delivering ophthalmic drugs
This invention relates to a method of delivering ophthalmic drugs, specifically prostaglandins and prostamides. This method may also be applied to other types of intraocular pressure (IOP)-lowering drugs (e.g. carbonic anhydrase inhibitors, beta blockers, alpha-adrenergic agonists, and parasympathomimetics) as well as other types of ophthalmic drugs for other indications (e.g. dry eye, inflammation, and infection).
Owner:JOHNSON & JOHNSON VISION CARE INC
Methods for predicting relative efficacy of a beta blocker therapy based on a B1-adrenergic receptor polymorphism
InactiveUS7449292B2Sugar derivativesMicrobiological testing/measurementBeta blockerRelative efficacy
Methods and compositions for the detection, diagnosis, and prevention of cardiac conditions are provided. Polymorphisms of β1-adrenergic receptor are provided. The Gly389 β1-adrenergic receptor variants are not as responsive to treatment β blockers such as carvedilol, metoprolol or bisoprol. Thus, genotyping β1-adrenergic receptor polymorphisms is useful for predicting relative responsiveness to treatment with beta blockers. The Gly389 polymorphism also may be used, alone or in conjunction with other adrenergic receptor polymorphisms, to predict relative risk of developing cardiovascular diseases such as heart failure or to predict relative survival rate in patients with heart failure or other cardiovascular diseases. Also provided are transgenic mice and transgenic cells expressing the β1-adrenergic receptor polymorphisms, and their use in identifying therapeutic agents.
Owner:UNIVERSITY OF CINCINNATI
Beta-adrenoceptor genetic polymorphisms and obesity
InactiveUS20040033524A1Reducing trial and error approachShorten the time limitBiocideOrganic active ingredientsHypertension medicationsBeta blocker
The present invention provides a method for identifying a subject having a risk of developing obesity, coronary microvasular dysfunction, or hypertension, comprising detection of the presence of a single nucleotide polymorphism (SNP) in a nucleic acid encoding an element of at least one beta-adrenergic receptor from the subject. The presence of the SNP is correlated with obesity, coronary microvascular dysfunction, or hypertension, and thereby identifies the subject as having a risk of developing obesity, coronary microvascular dysfunction, of hypertension. The subject invention also provides methods of identifying patients likely to benefit from the prescription of beta blocker hypertension medications. In various embodiments, the nucleic acids detected include those genes encoding ADRB1 (beta1AR), ADRB2 (beta2AR), ADRB3 (beta3AR), GNB3 (G protein beta3 subunit), or GNAS1 (Gs protien alpha subunit). Methods of treating identified individuals are also provided.
Owner:FLORIDA UNIV OF A FLORIDA
Use of a beta blocker for the manufacture of a medicament for the treatment of hemangiomas
ActiveUS8987262B2Reduce expressionPrevents cardiac hypertrophyBiocideUrea derivatives preparationBeta blockerBETA BLOCKING AGENTS
The present technology relates the use of a beta blocker for the manufacture of a medicament for the treatment of hemangiomas, for example of infantile hemangiomas. The beta blocker may be a non-selective beta-blocker, for example propranolol. The present technology provides an alternative to the known compounds, e.g. corticosteroïds, interferon or vincristine, generally used for the treatment of hemangiomas.
Owner:UNIV DE BORDEAUX +1
Method for treating arrhythmias
Methods are provided for treating arrhythmia in a manner that minimizes undesirable side effects, comprising administration of a therapeutically effective minimal dose of an A1 adenosine receptor agonist with a therapeutically effective minimal dose of a beta blocker, calcium channel blocker, or a cardiac glycoside.
Owner:CV THERAPEUTICS INC
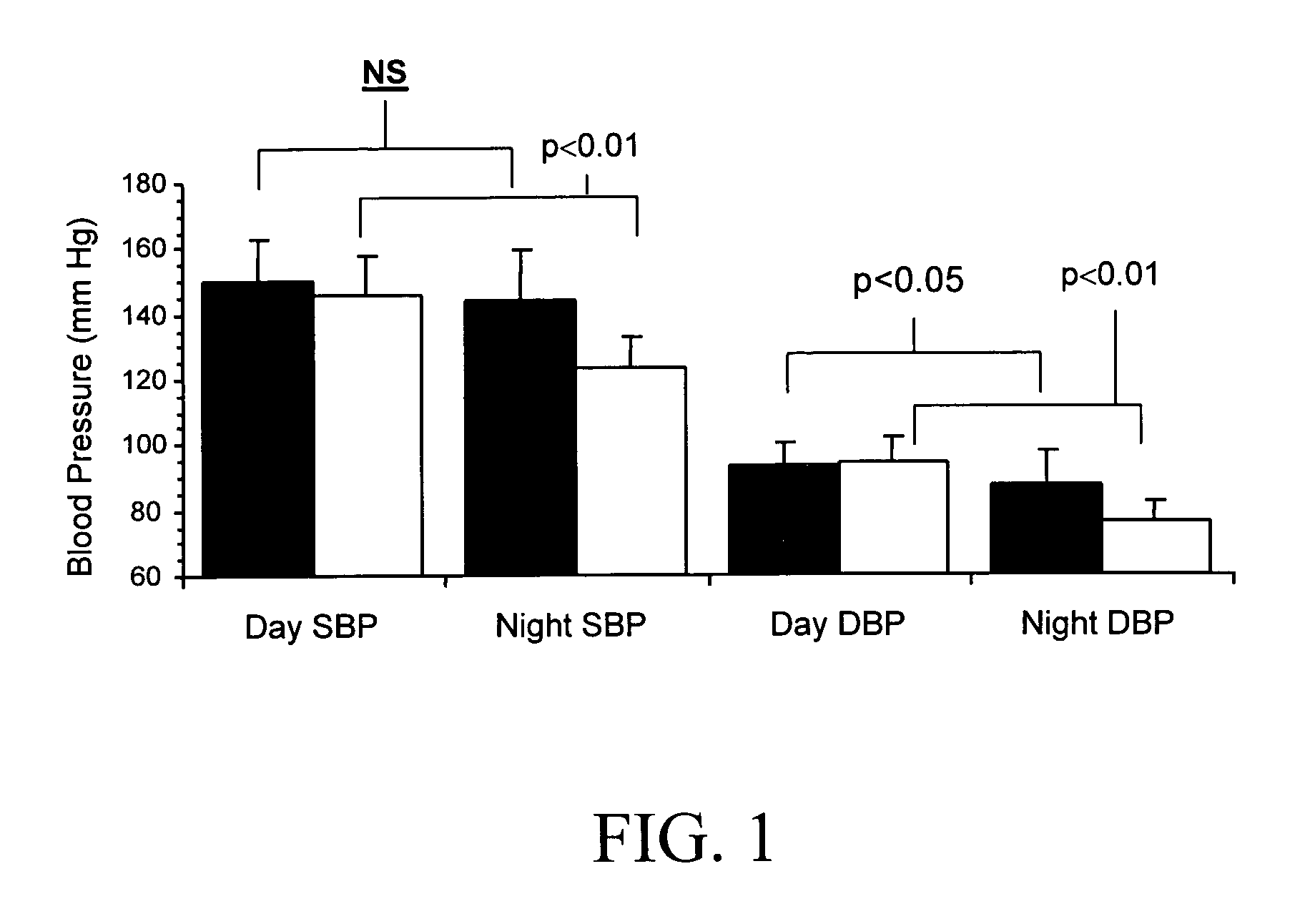
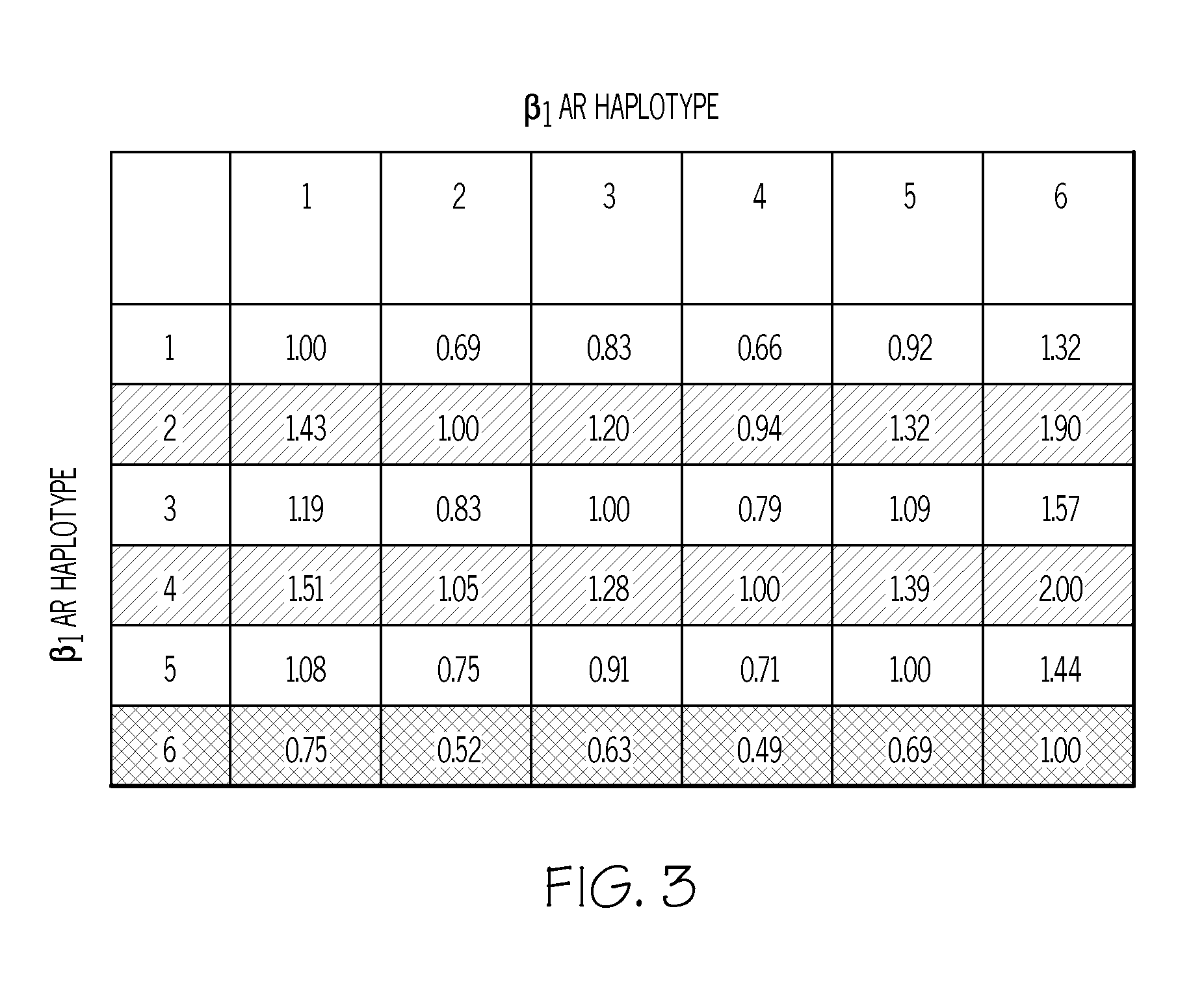
Methods for Individualizing Cardiovascular Disease Treatment Protocols Based on Beta-1 Adrenergic Receptor Haplotype
A method is provided for determining whether a treatment protocol for a human patient who is suffering from heart failure, ischemic heart disease, cardiac arrhythmias, or hypertension includes administration of a beta blocker, the method including obtaining a biological sample from the patient, determining a β1-adrenergic receptor (β1AR) sequence of a β1AR gene from the biological sample, identifying locations of any polymorphisms in the β1AR sequence, assigning a haplotype to the β1AR sequence based on the locations identified, and determining whether the treatment protocol includes administration of a beta blocker to the patient based on the haplotype assigned.
Owner:UNIVERSITY OF CINCINNATI
Methods of Treatment
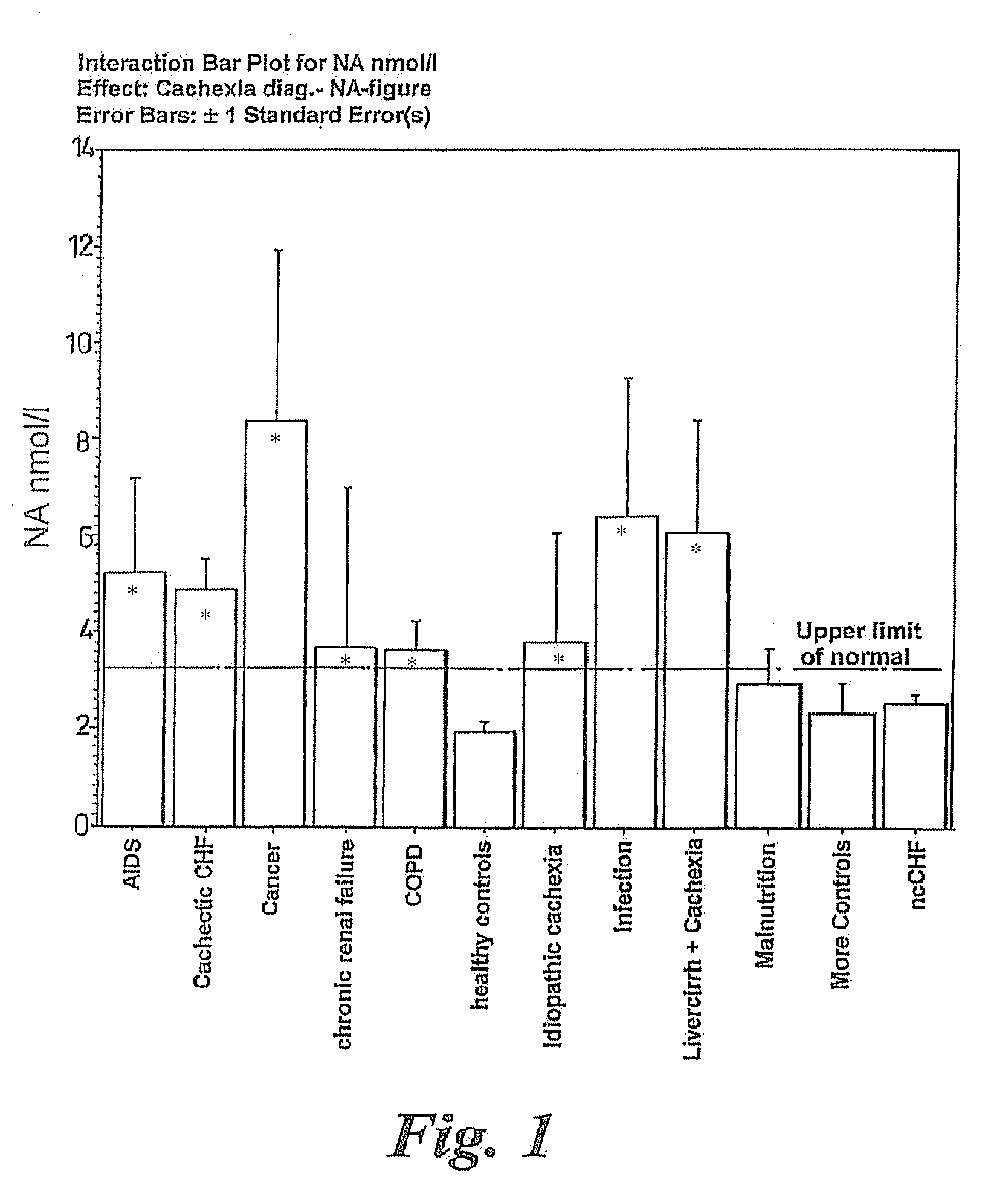
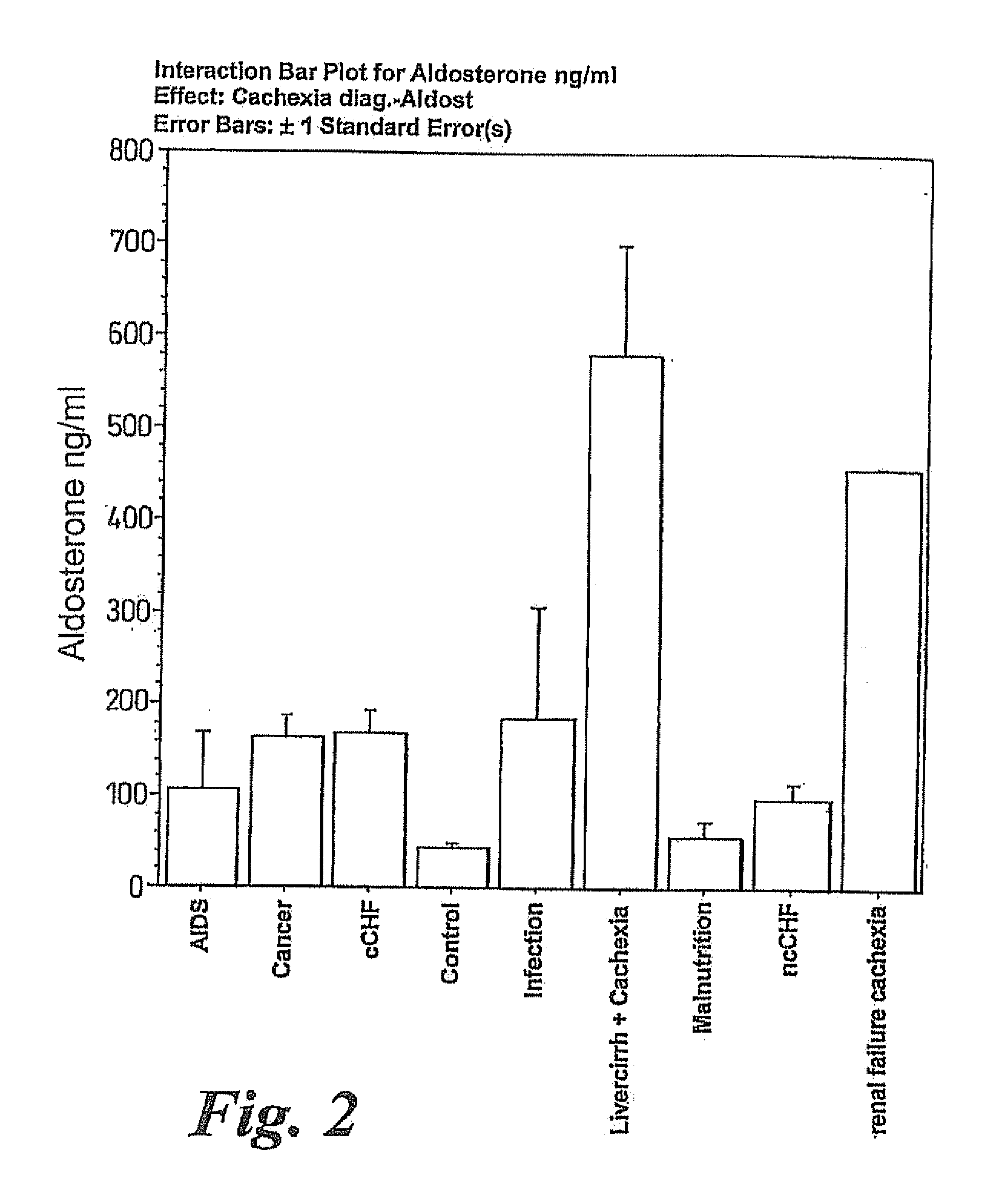
A method of treating weight loss due to underlying disease in a patient, the method comprising administering to the patient an effective amount of an agent which reduces sympathetic nervous system activity. A method of treating weight loss due to underlying disease in a patient the method comprising administering to the patient an effective amount of any one or more of the following: a compound which inhibits the effect of aldosterone such as an aldosterone antagonist; a chymase inhibitor; a cathepsin B inhibitor; a β receptor blocker; an imidazoline receptor antagonist; a centrally acting α receptor antagonist; a peripherally acting α receptor antagonist; a ganglion blocking agent; a drug that has an effect on cardiovascular reflexes and thereby reduces SNS activity such as an opiate; scopolamine; an endothelin receptor antagonist; and a xanthine oxidase inhibitor. The methods are particularly useful in treating cardiac cachexia.
Owner:IMPERIAL INNOVATIONS LTD
Method of treating arrhythmias
Methods are provided for treating arrhythmia in a manner that minimizes undesirable side effects, comprising administration of a therapeutically effective minimal dose of an Al adenosine receptor agonist with a therapeutically effective minimal dose of a beta blocker, calcium channel blocker, or a cardiac glycoside.
Owner:CV THERAPEUTICS INC
Method of determining the weight of the coating to be applied to form a controlled release dosage form
A method of coating spherical particles of a beta blocker compound which is based on(a) determining the surface area of a weighed sample of said spherical particles;(b) determining the weight of coating material per surface area unit of spherical particles that will provide a desired release profile for said beta-blocker;(c) determining the surface area of a subsequent batch of spherical particles containing the same beta;(d) coating the subsequent batch of spherical particles with the coating applied in step (b) to provide a coating having substantially the same weight of coating material per surface area unit of spherical particles as determined in step (b).
Owner:GLATT AIR TECHN
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com