Bortezomib freeze-dried powder injection and preparation method thereof
A technology of bortezomib and freeze-dried powder injection, which is applied in the field of medicine, can solve the problems of increased impurities and poor clarity of bortezomib preparations, and achieve the effects of good resolubility and stability, small total impurities, and avoiding enlargement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
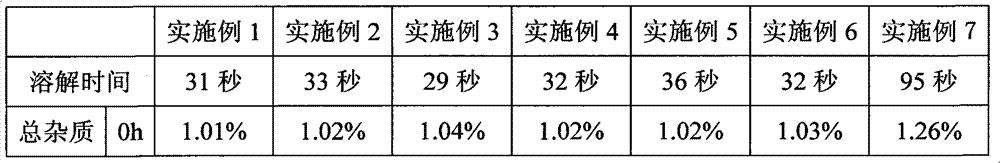
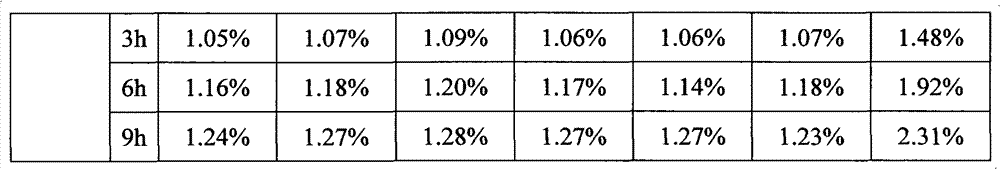
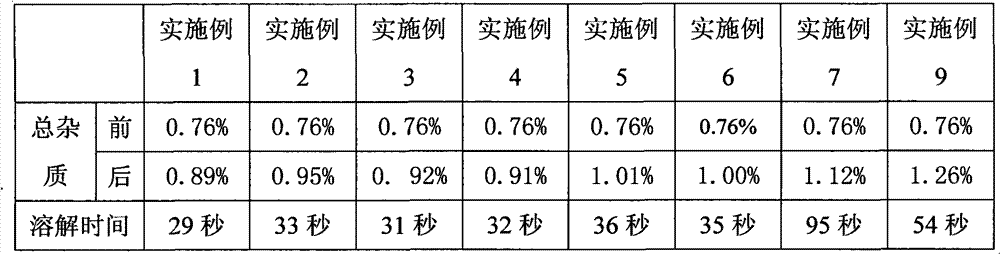
[0023] Embodiment 1, bortezomib freeze-dried powder injection
[0024] Weigh 3.5g of bortezomib, 105g of mannitol, and 0.7g of sodium bisulfite, mix and grind for 8 minutes, add 2700mL of water for injection, stir until completely dissolved, add 2.7g of activated carbon for needles, stir for 15 minutes, decarbonize and filter. Add water for injection to the filtrate to 3500mL, stir evenly, and adjust the pH to 6.0 with 1mol / L sodium hydroxide solution. The medicinal solution was sterilized and filtered through a 0.22 μm microporous membrane, filled into 7 mL vials, each bottle filled with a volume of 3.5 mL, half-filled with a rubber stopper, and placed in a lyophilizer for lyophilization.
[0025] First lower the temperature to -8°C and keep it warm for 90 minutes, then quickly drop the temperature to -40°C and keep it warm for 180 minutes, start vacuuming after the cold trap cools down to -40°C, slowly increase the temperature of the shelf to -10°C, and keep it warm for 18 h...
Embodiment 2
[0026] Embodiment 2, bortezomib freeze-dried powder injection
[0027] Weigh 3.5g of bortezomib, 157.5g of dextran, and 3.2g of sodium metabisulfite, mix and grind for 5 minutes, add 2700mL of water for injection, stir until completely dissolved, add 2.7g of activated carbon for needles, stir for 15 minutes, decarbonize and filter. Add water for injection to the filtrate to 3500mL, stir evenly, and adjust the pH to 6.5 with 1mol / L sodium hydroxide solution. The medicinal solution was sterilized and filtered through a 0.22 μm microporous membrane, filled into 7 mL vials, each bottle filled with a volume of 3.5 mL, half-filled with a rubber stopper, and placed in a lyophilizer for lyophilization.
[0028] First lower the temperature to -8°C and keep it warm for 90 minutes, then quickly drop the temperature to -40°C and keep it warm for 120 minutes, start vacuuming after the cold trap cools down to -40°C, slowly increase the temperature of the shelf to -10°C, and keep it warm for...
Embodiment 3
[0029] Embodiment 3, bortezomib freeze-dried powder injection
[0030]Weigh 3.5g bortezomib, 280g excipient (the excipient is a mixture of mannitol and dextran), 2.6g sodium bisulfite, mix and grind for 8 minutes, add 2700mL water for injection, stir until completely dissolved, add 2.7g Activated carbon was used for the needle, stirred for 15 minutes, and decarbonized and filtered. Add water for injection to the filtrate to 3500 mL, stir evenly, and adjust the pH to 6.0 with 0.5 mol / L sodium carbonate solution. The medicinal solution was sterilized and filtered through a 0.22 μm microporous membrane, filled into 7 mL vials, each bottle filled with a volume of 3.5 mL, half-filled with a rubber stopper, and placed in a lyophilizer for lyophilization.
[0031] First lower the temperature to -8°C and keep it warm for 90 minutes, then quickly drop the temperature to -40°C and keep it warm for 200 minutes, start vacuuming after the cold trap cools down to -40°C, slowly increase the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com