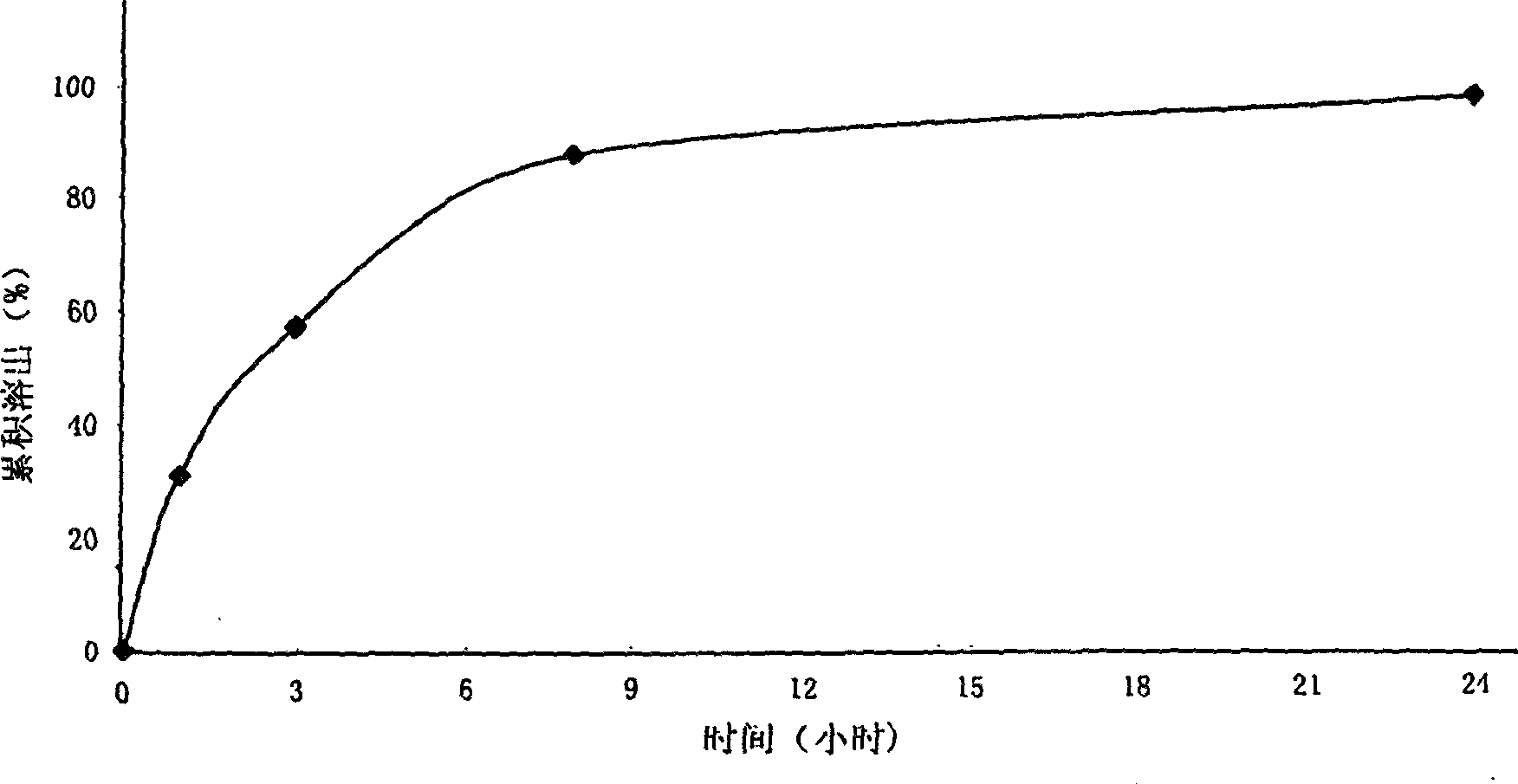
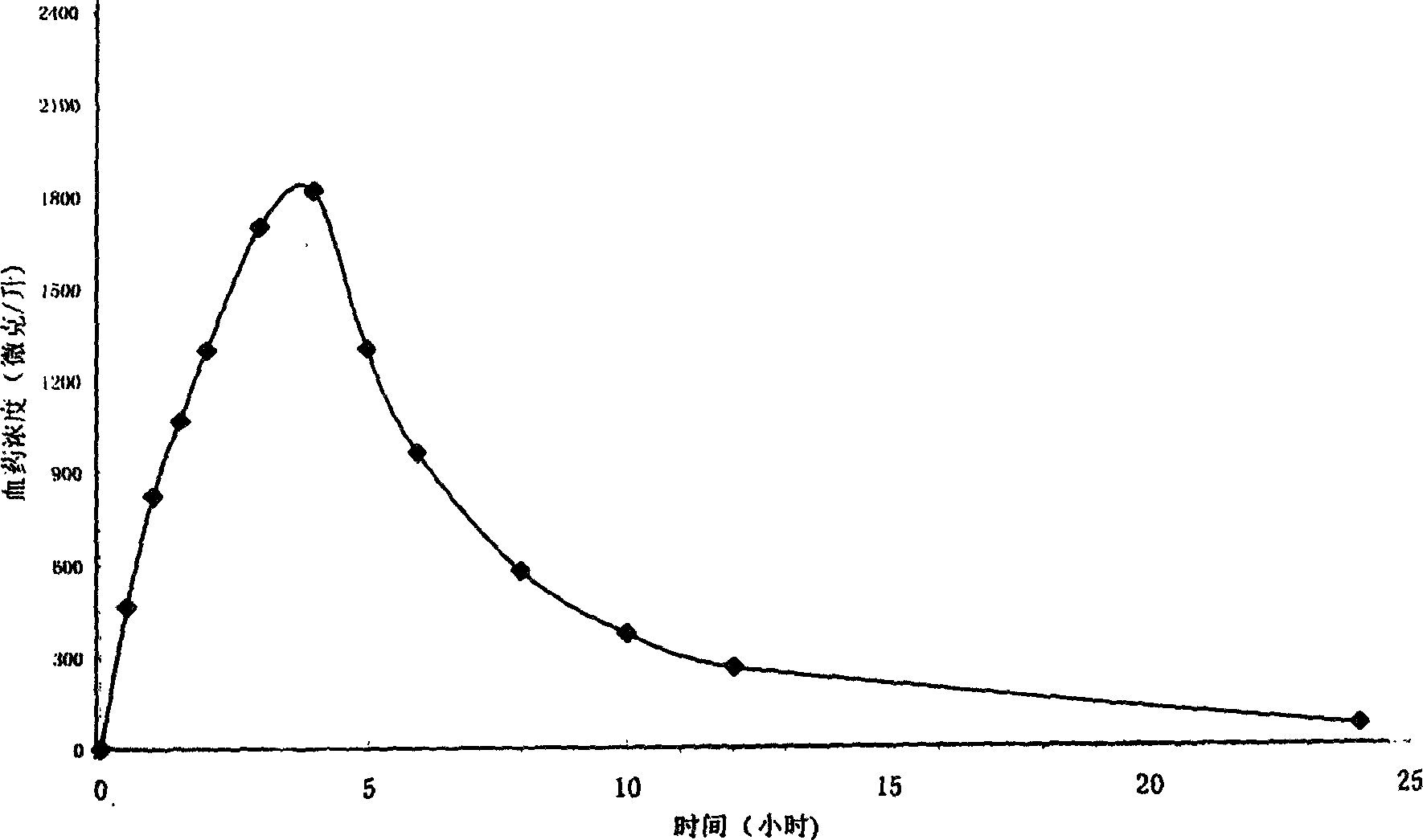
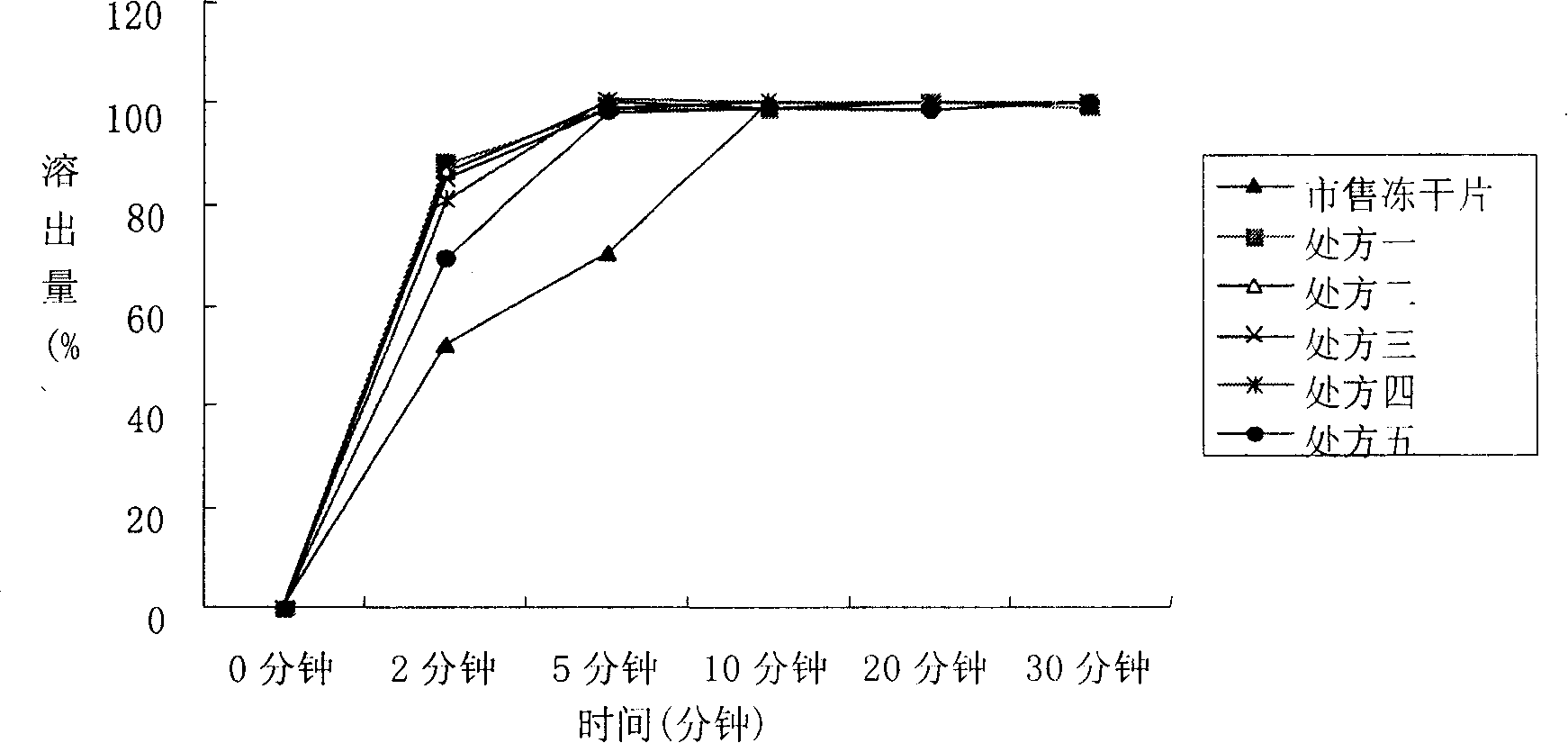
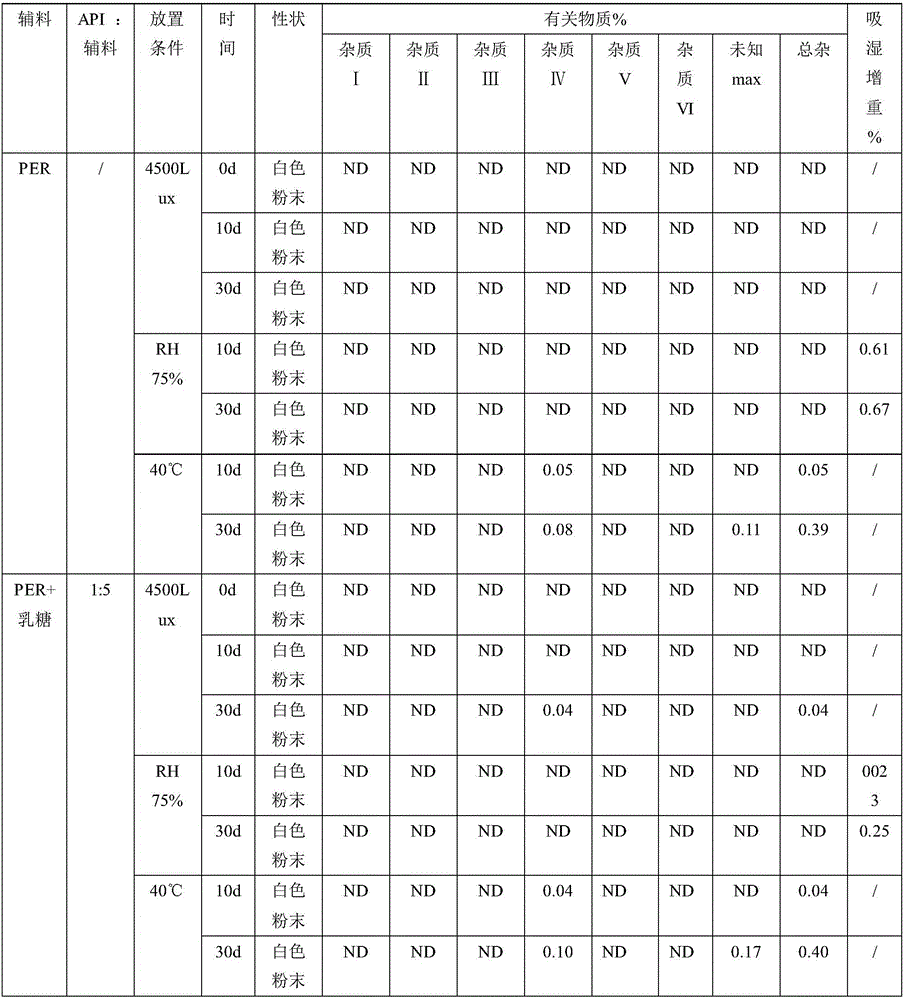
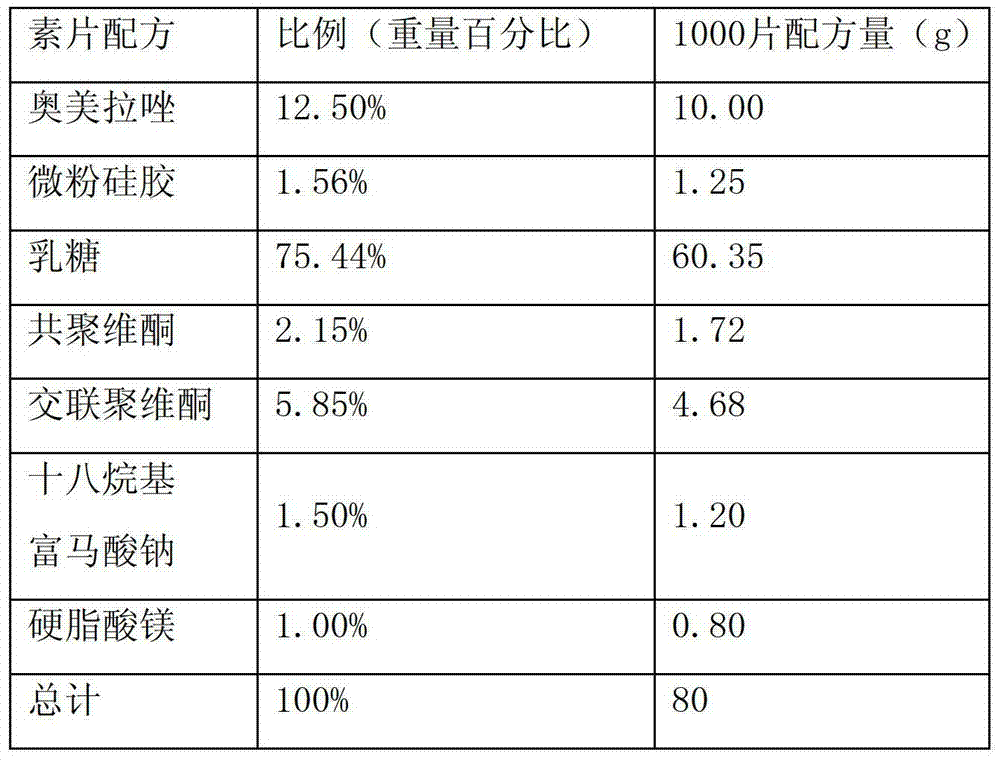
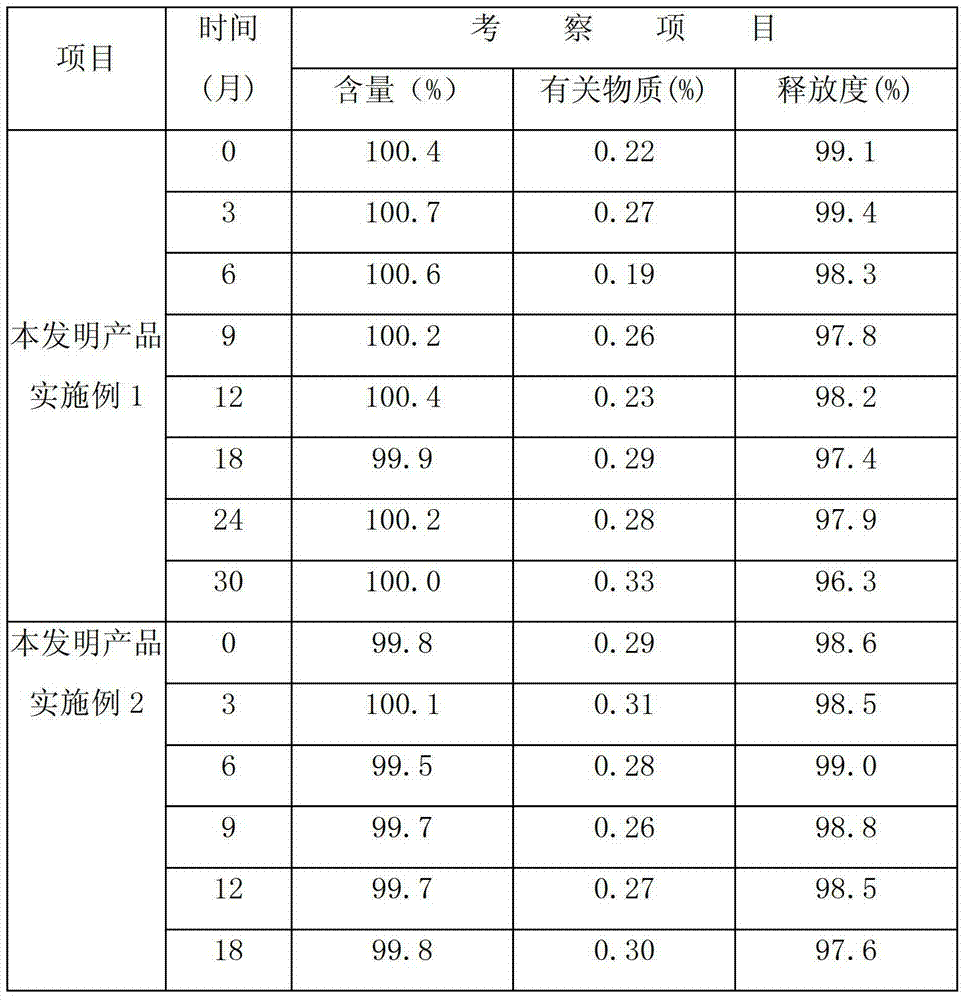
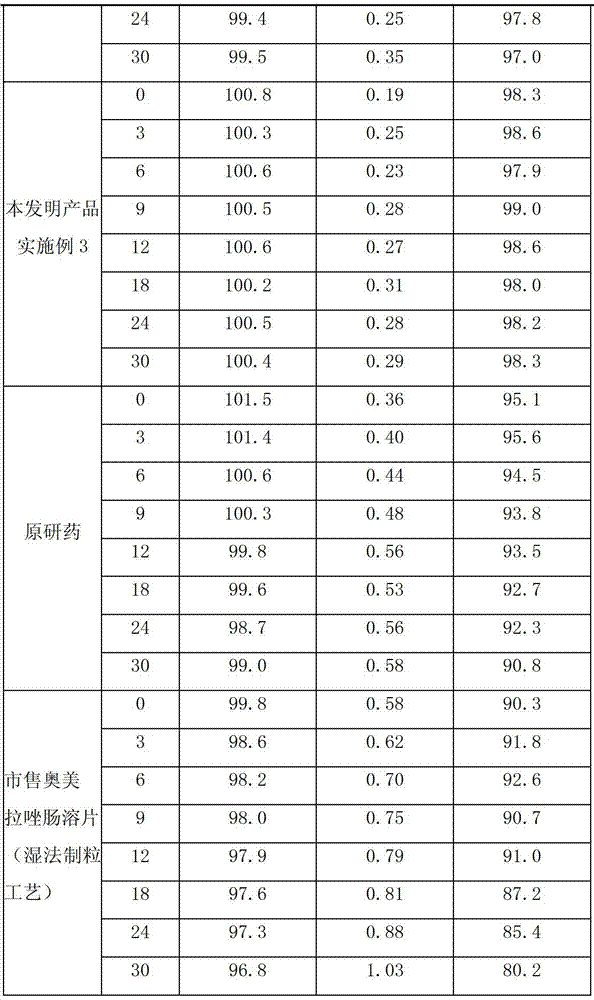
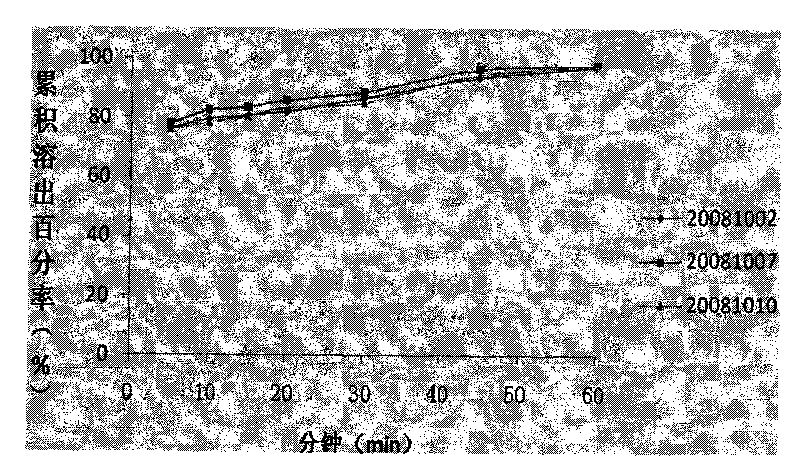
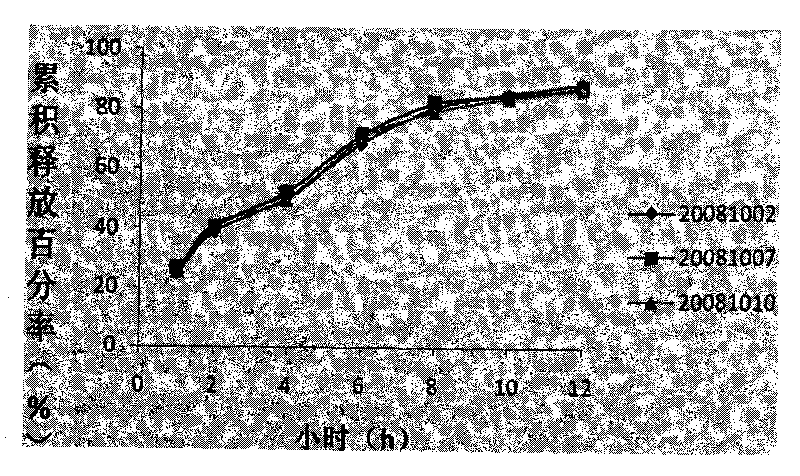
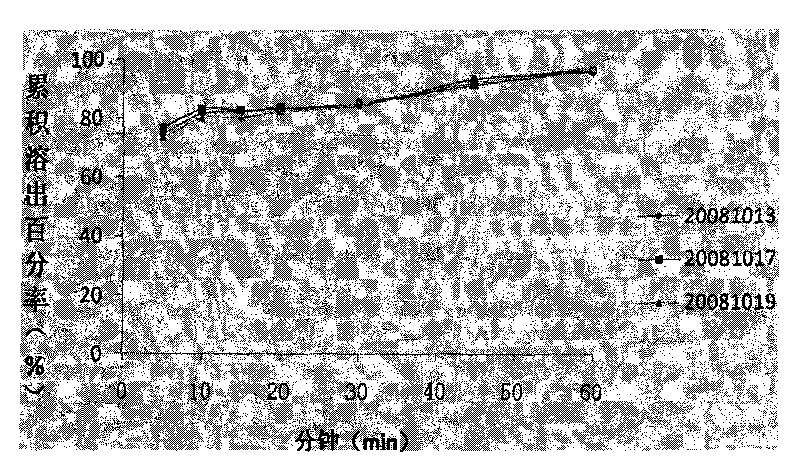
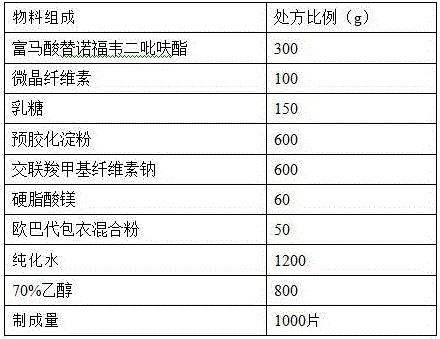
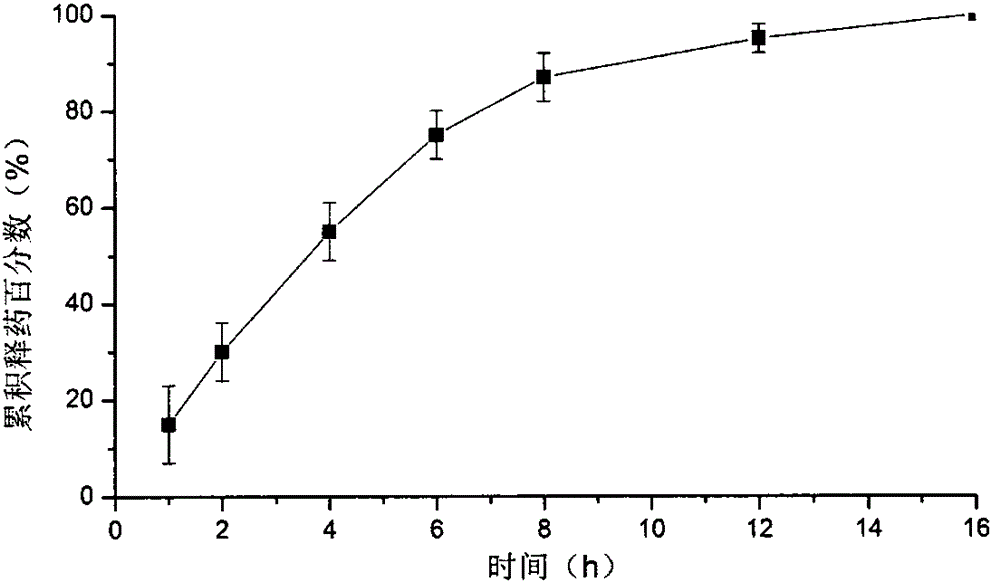
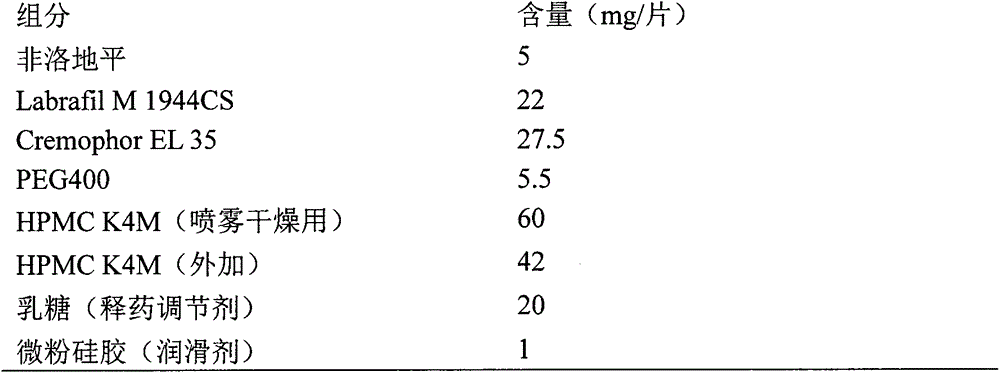
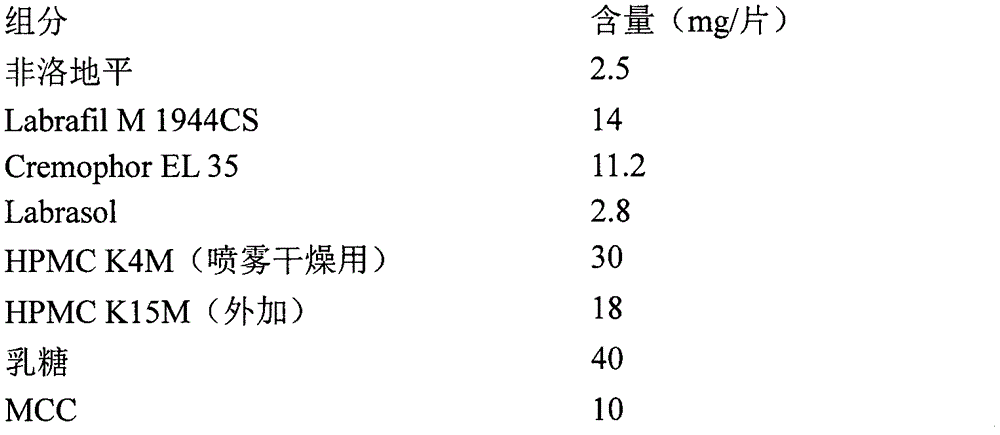
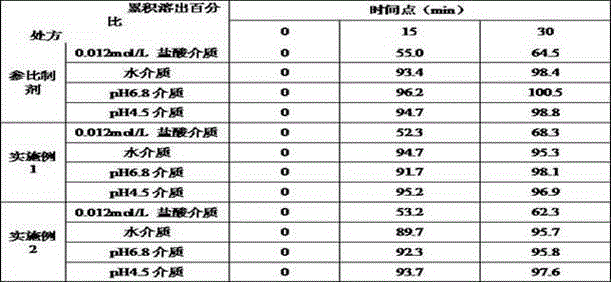
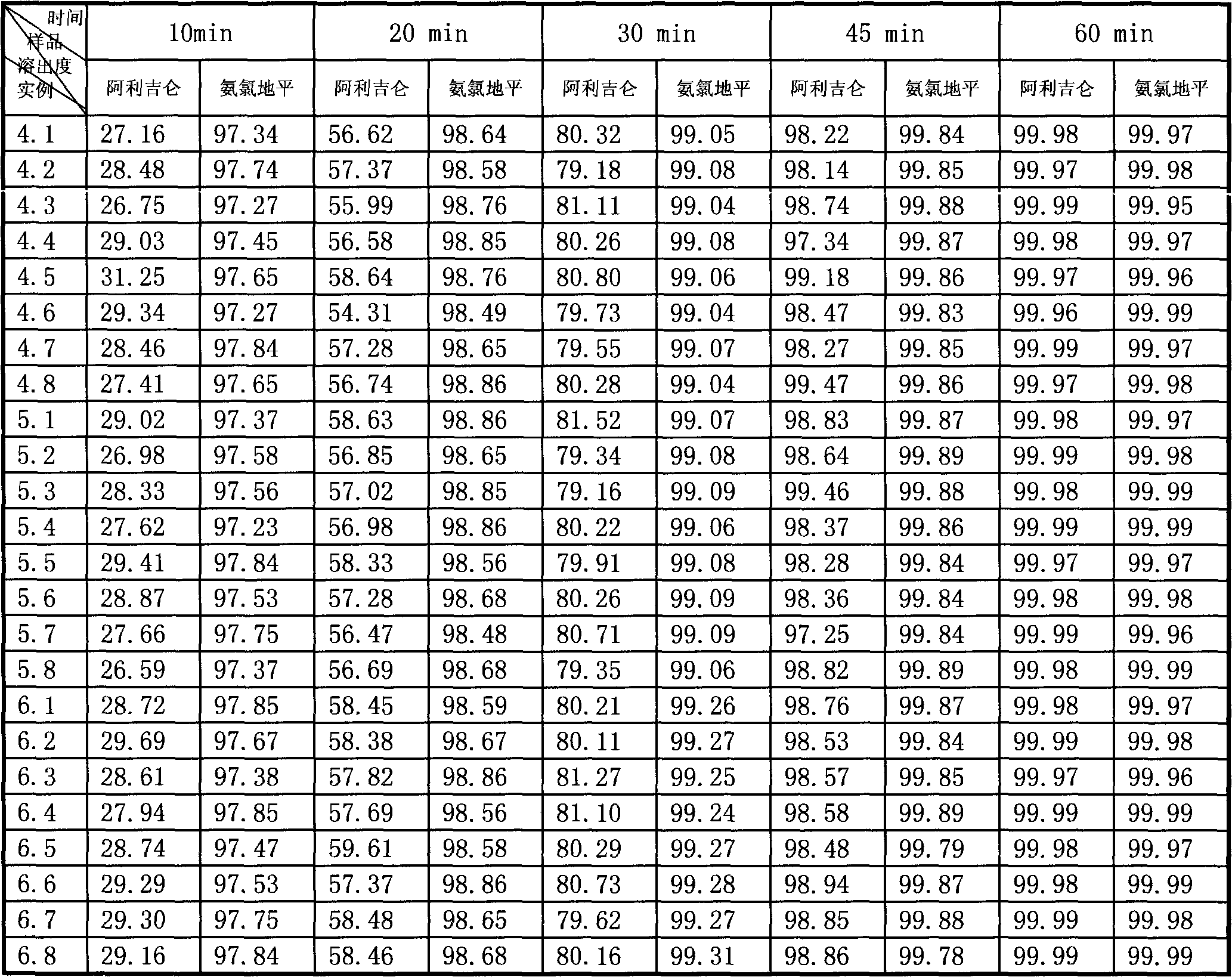
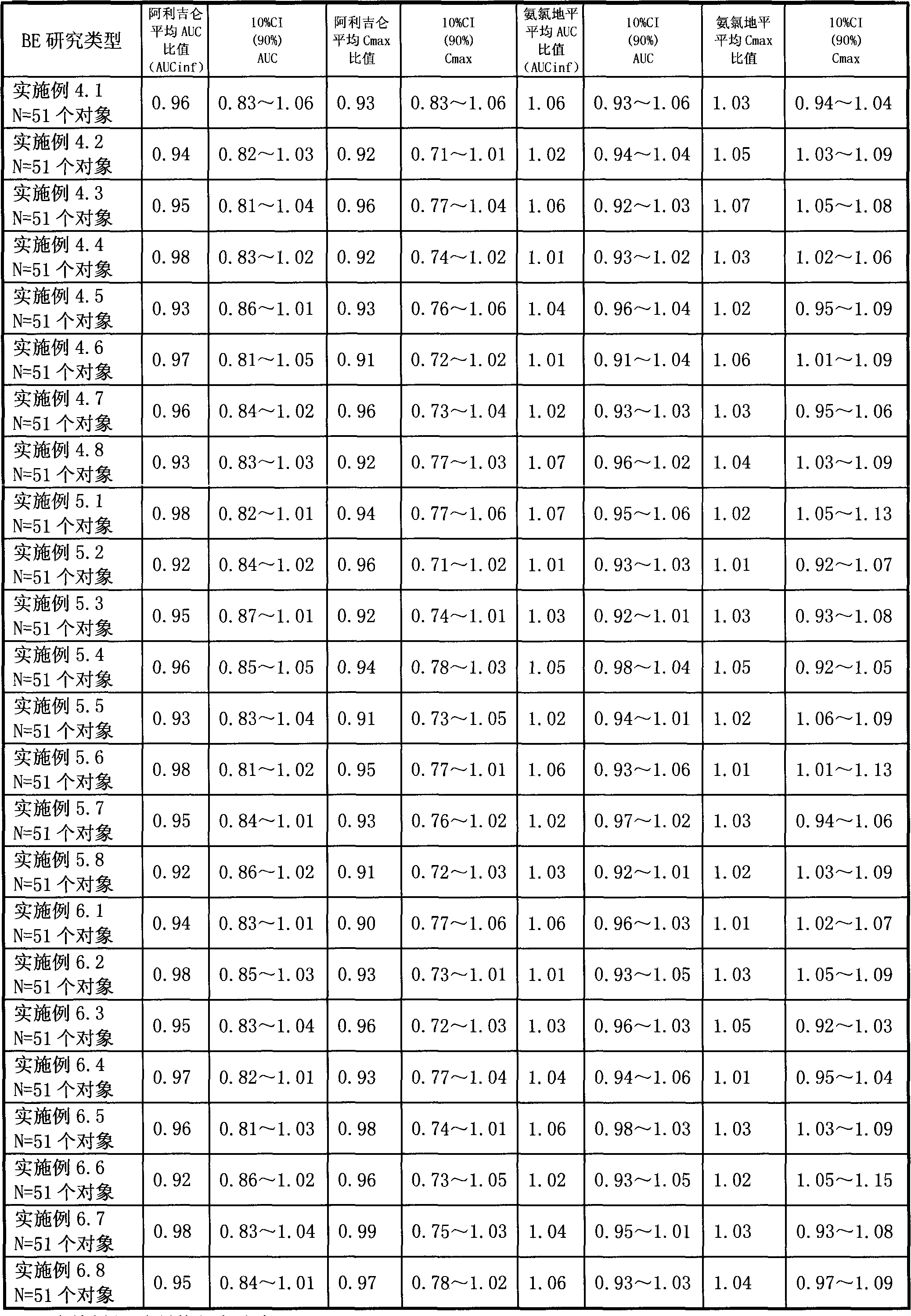
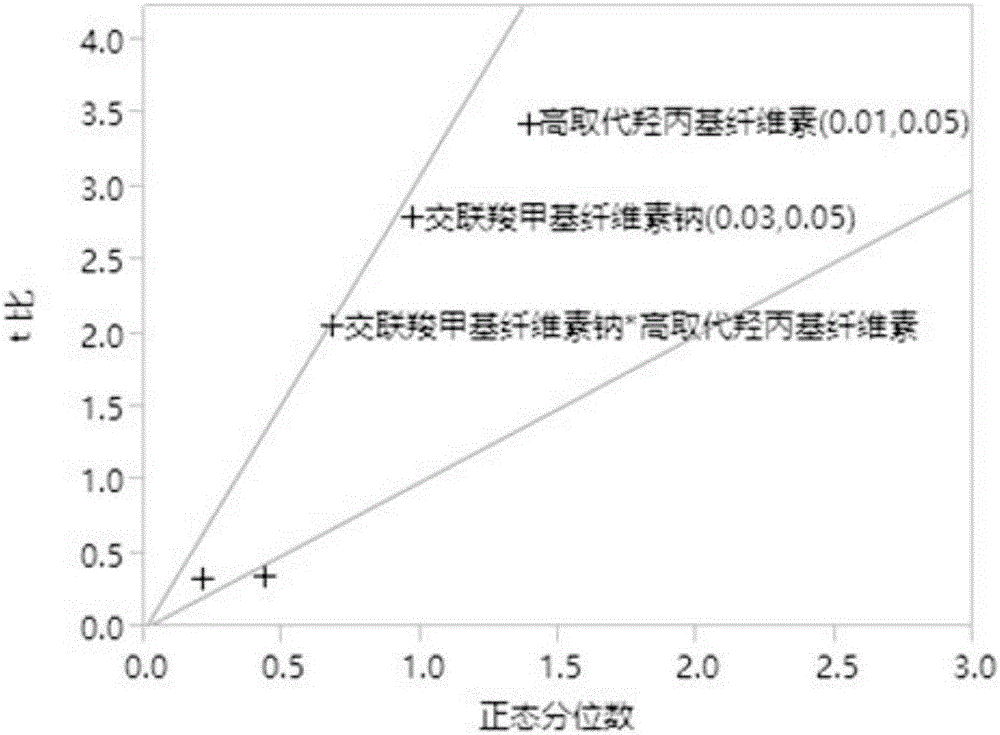
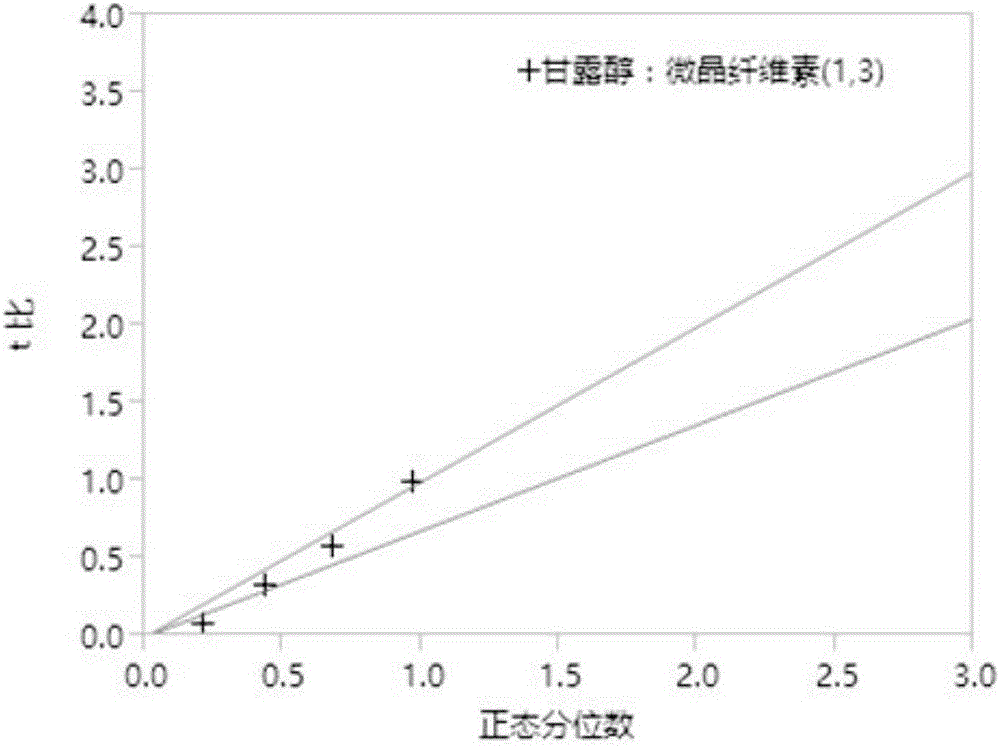
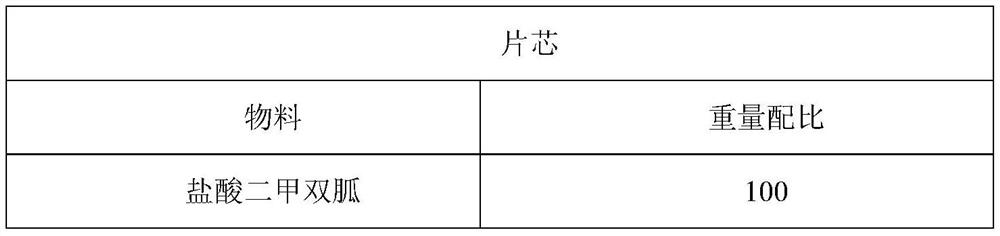
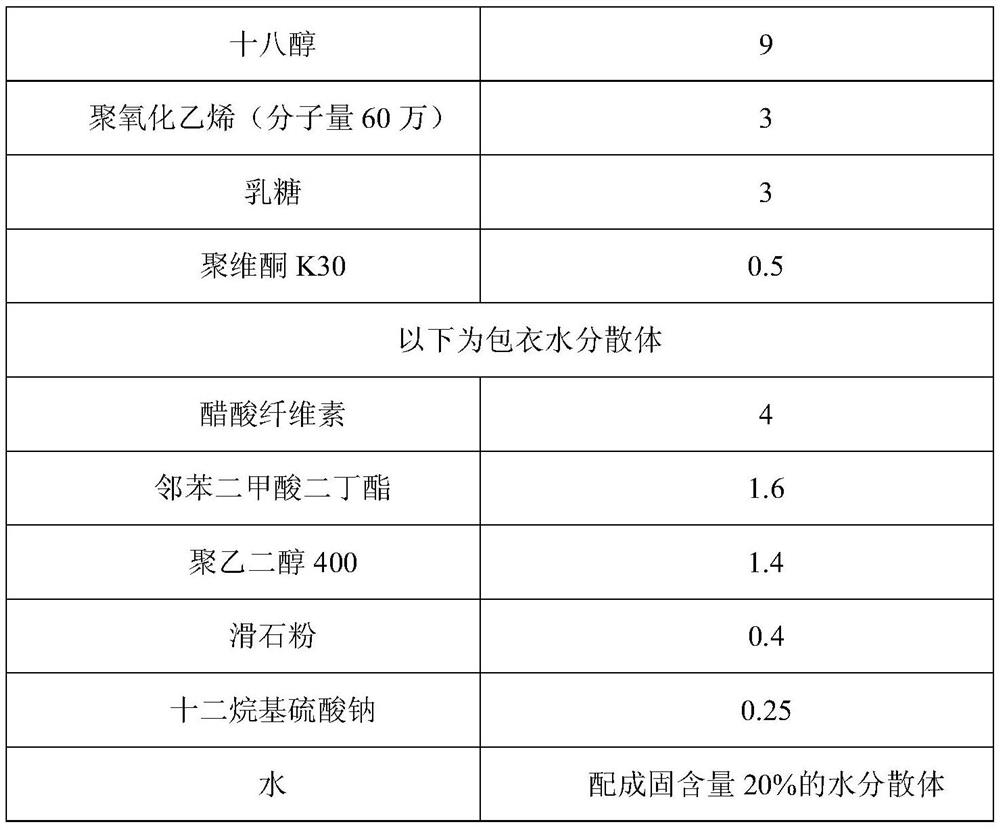
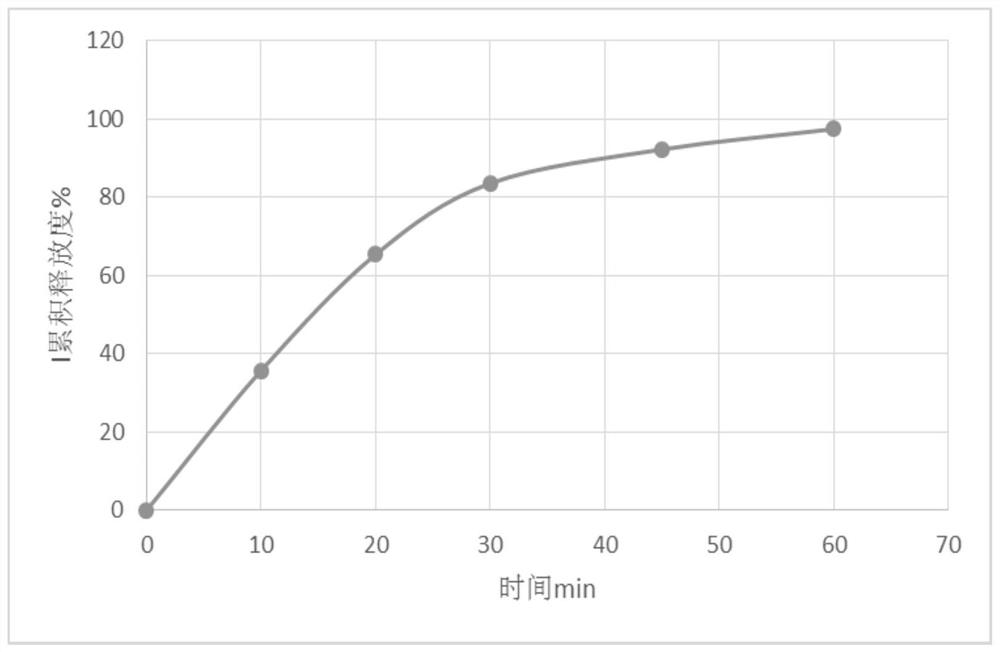
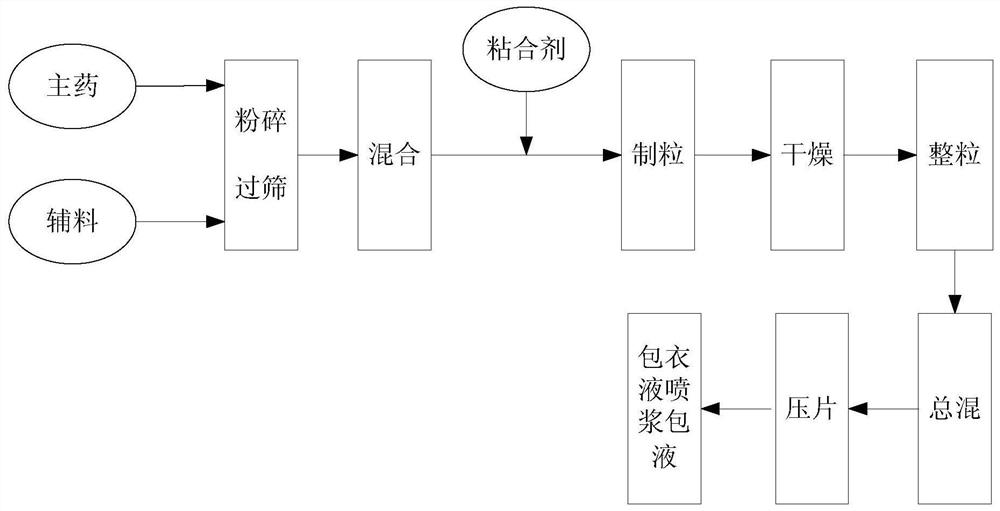
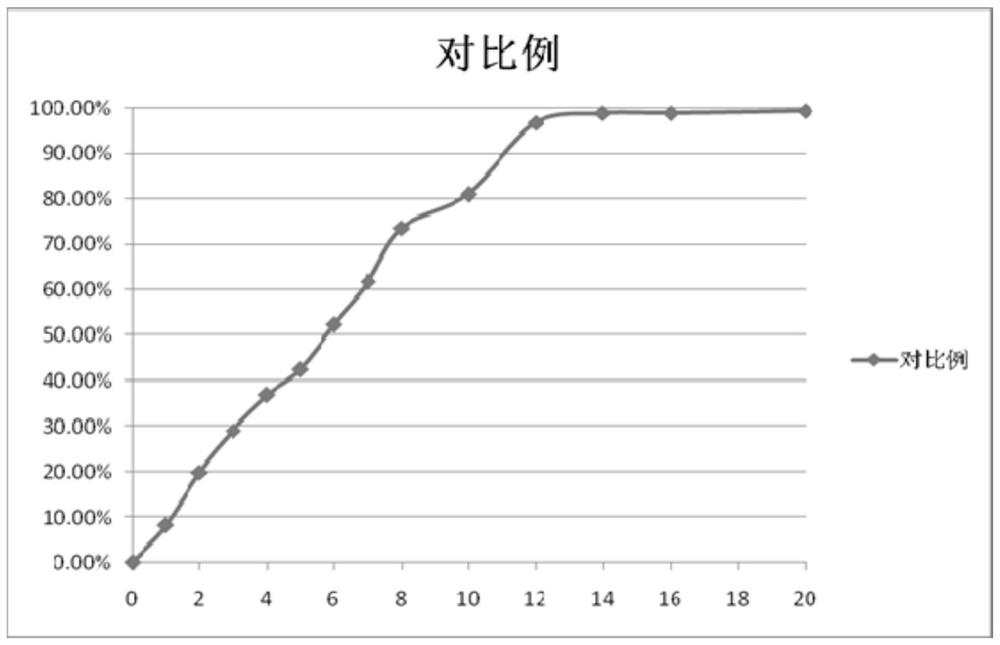
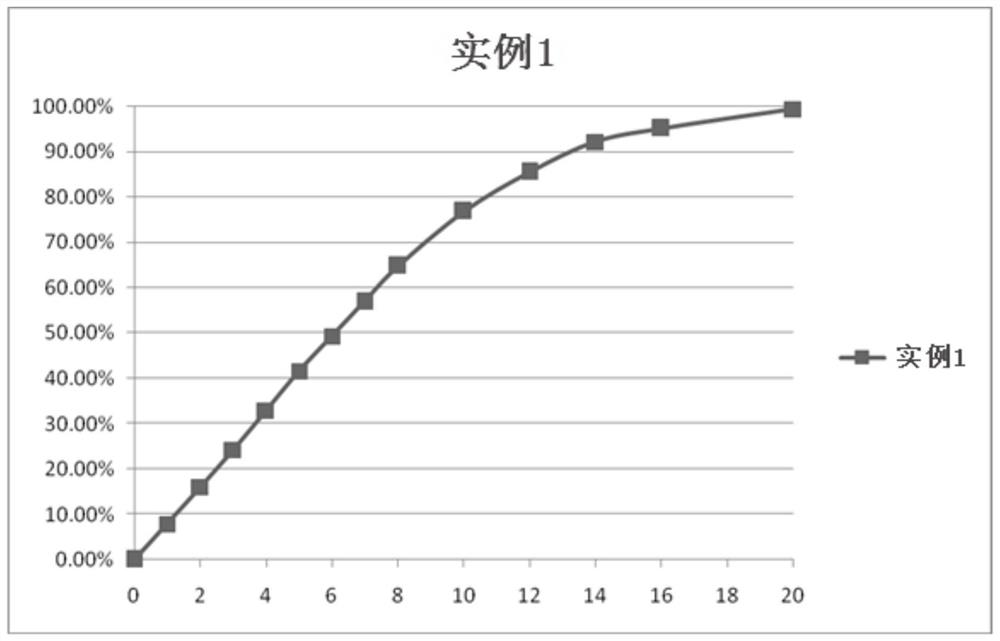
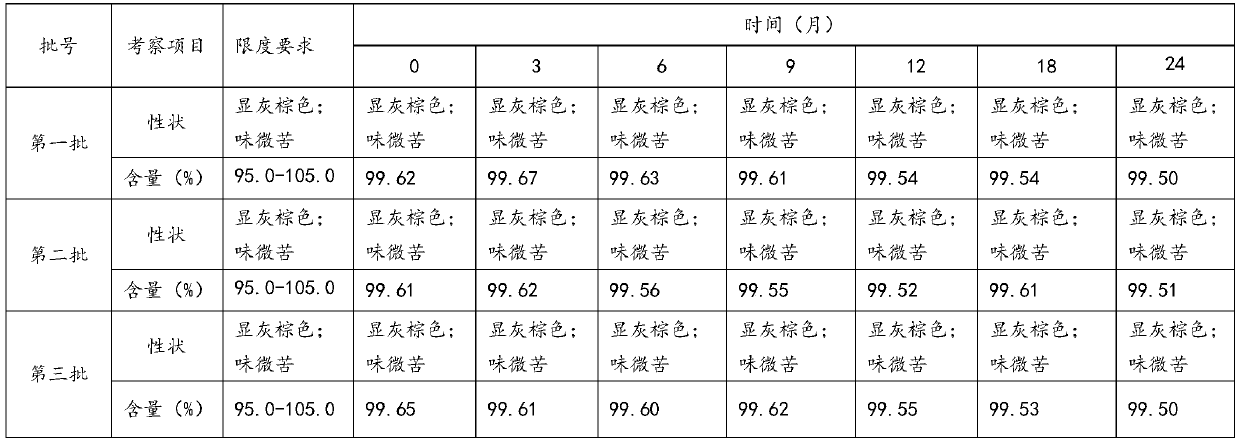
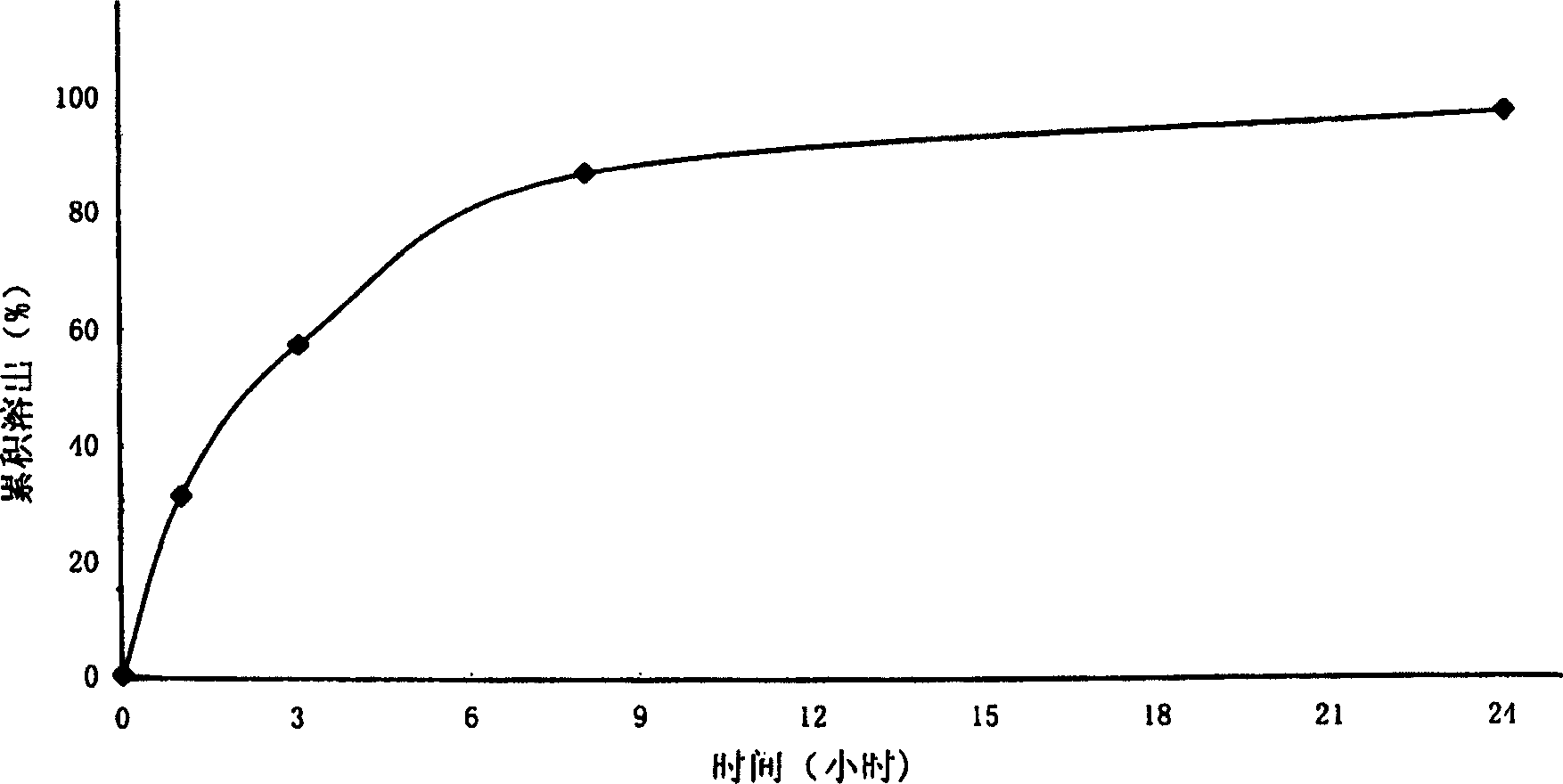
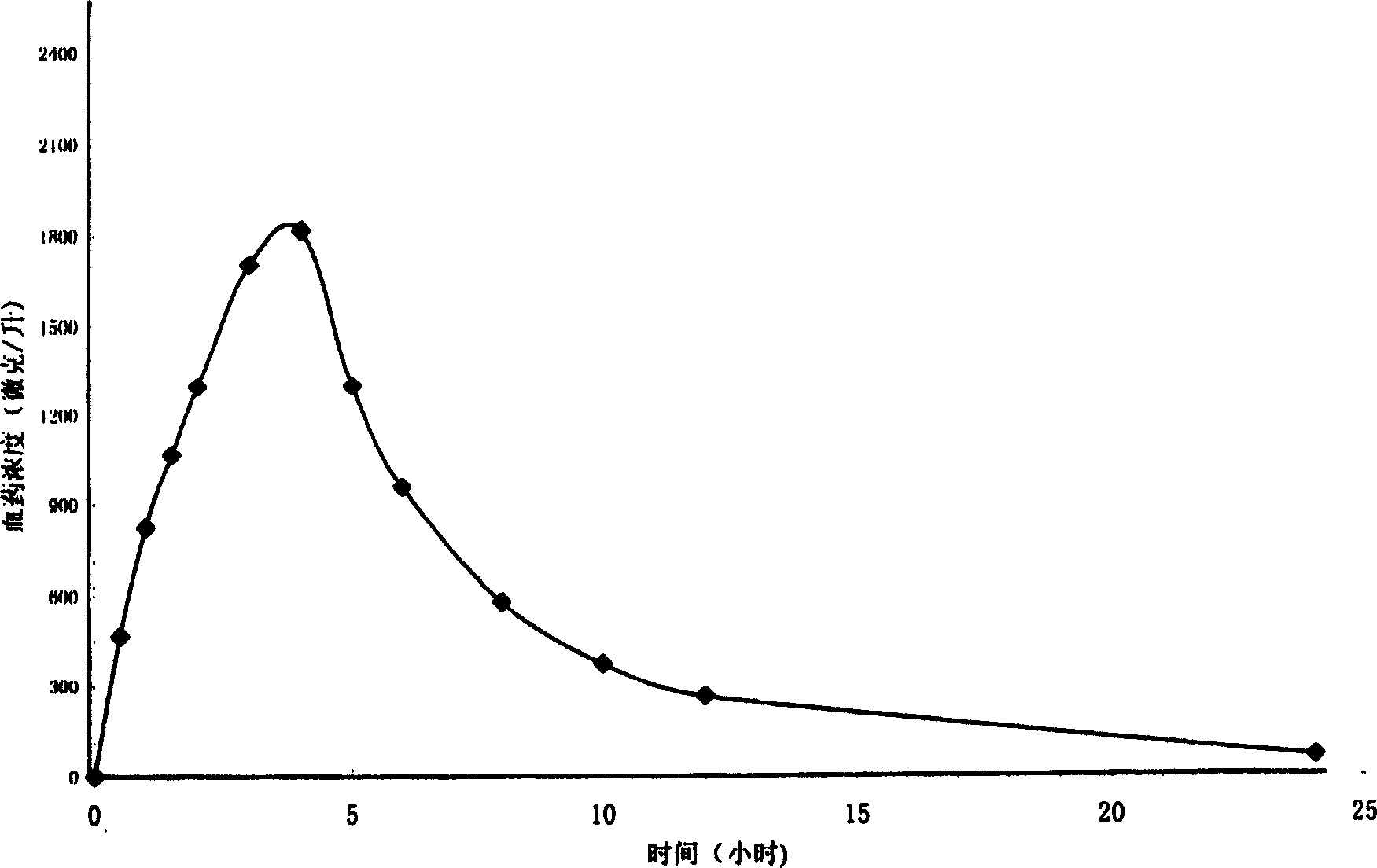
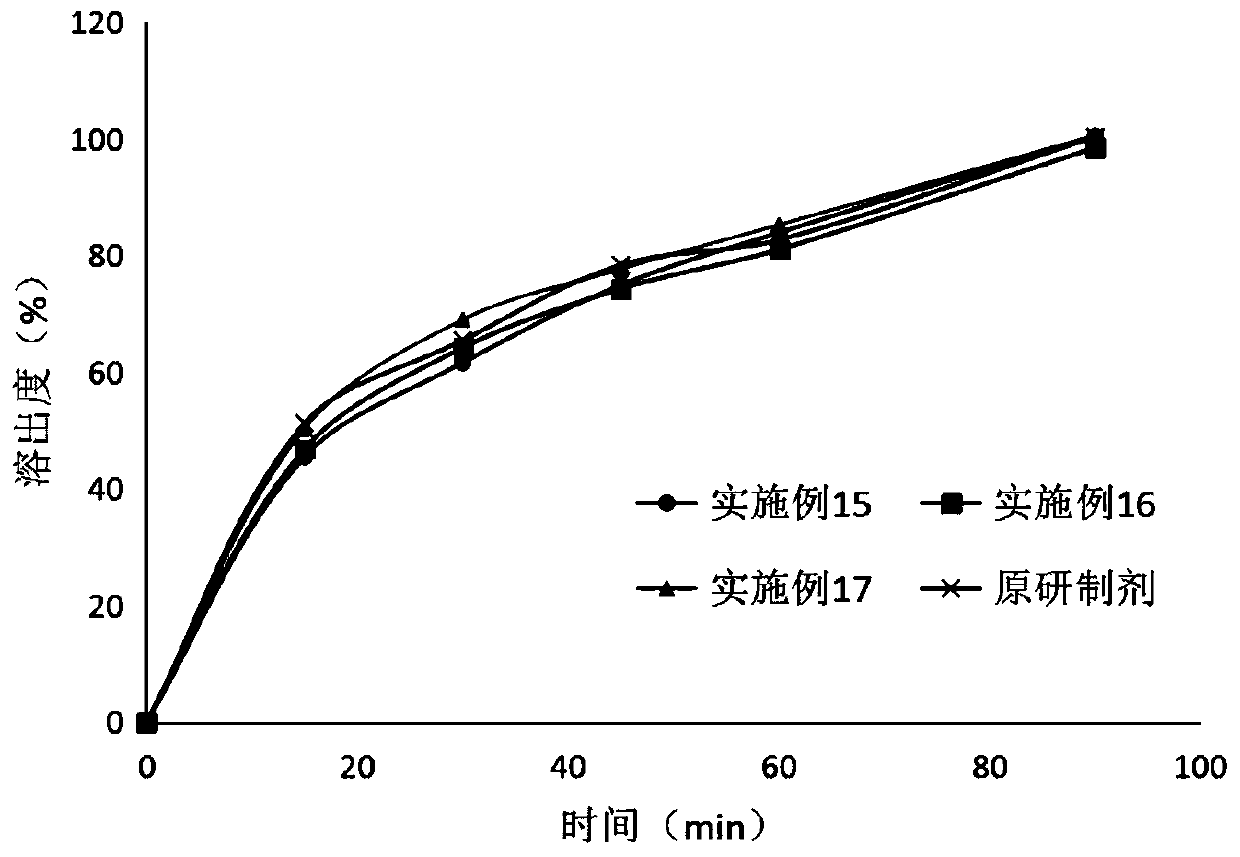
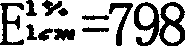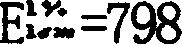Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
58results about How to "Piece weight small" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Palbociclib gastric-floating tablet and preparation method thereof
ActiveCN104887641ALow drift timeReduce dosageOrganic active ingredientsPill deliveryUse medicationPharmaceutical drug
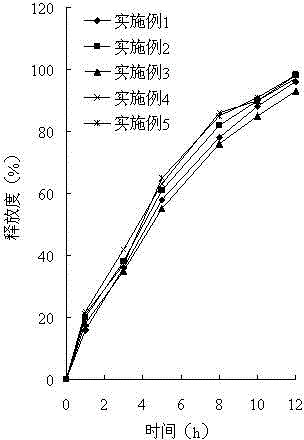
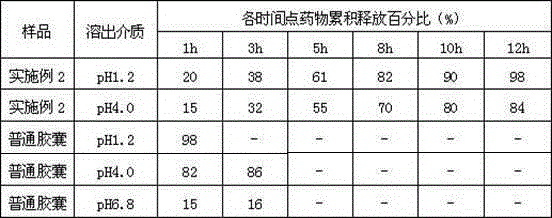
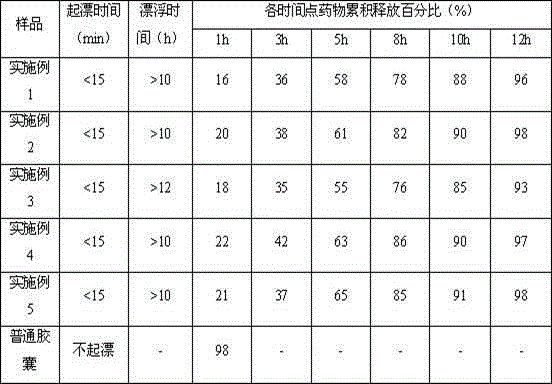
The invention belongs to the technical field of medicine, and relates to a palbociclib gastric-floating tablet and a preparation method thereof. The palbociclib gastric-floating tablet comprises, by mass, 10%-30% of palbociclib, 20%-50% of hydroxypropyl methylcellulose, 20%-40% of bleaching auxiliaries, 2%-10% of foaming agents, 0%-25% of microcrystalline cellulose and 0.5%-3% of magnesium stearate. A dry granulating technology or a wet granulating technology can be used as the preparation technology. The palbociclib gastric-floating tablet is high in bioavailability, has a slow release tendency, and effectively lowers the total dosage. The palbociclib gastric-floating tablet and the preparation method thereof have the unique advantages that two different mechanisms are used for preparing the gastric-floating tablet, and accordingly the prepared tablet can keep floating in gastric juice by more than 10 hours and continuously release drugs in the hydrochloric acid solution with the pH being 1.2; the problem that the bioavailability is low due to the fact that drugs are extremely difficult to dissolve after the pH is higher than four is effectively solved; the medicine taking frequency is reduced; toxic and side effects are lightened; and the complaisance of a patient is effectively improved.
Owner:上海润泰医药科技有限公司
Secnidazole tablet and its prepn process
InactiveCN1973838AHigh dose of main drugPiece weight smallAntibacterial agentsOrganic active ingredientsCarboxymethyl celluloseMagnesium stearate
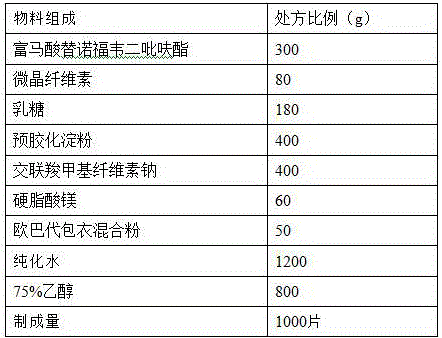
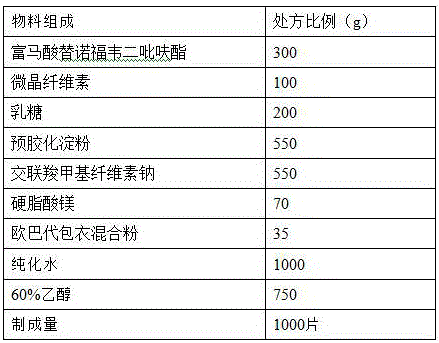
The secnidazole tablet contains secnidazole 50-90 wt%, microcrystalline cellulose, starch, pre-gelatinized starch and / or lactose 0.5-40 wt%, cross-linked sodium carboxymethyl cellulose and / or cross-linked polyvinyl pyrrolidone 0.5-9 wt%, polyvinyl pyrrolidone or hydroxypropylmethyl cellulose 0.1-2.5 wt%, magnesium stearate 0.5-1 wt%, and talcum powder and / or fine silica gel powder 0.5-1 wt%. The preparation process of the secnidazole tablet includes the steps of mixing the said materials, palletizing and tabletting. The secnidazole tablet has high effective component content, easy swallowing, no bitter taste and fast leaching.
Owner:湖北科益药业股份有限公司
Metformin hydrochloride sustained-release tablet and method for preparing the same
ActiveCN1543937AConvenient amountReduce dosageOrganic active ingredientsMetabolism disorderSustained Release TabletMetformin Hydrochloride
The invention provides a Metformin Hydrochloride slow release tablet and process for preparation, wherein the slow release tablet comprises metformin hydrochloride 46.5-70%, hydroxypropylcellulose 13.5-33.0%, micronization ethyl cellulose 10.0-14.0%, filling agent 1.3%-9.4%, and lubricating agent 1.2-1,5%. The preparation process comprises processing the prescribed raw material of metformin hydrochloride, cellulose glycollic ether, and the filling agent through the conventional tablet production process of granulation, drying, granulating, charging micronization ethyl cellulose, mixing homogenously with lubricant and tabletting.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
Medical coating powder containing nano material
InactiveCN1616105AOvercome the defects that the quality is difficult to guarantee, etc.Simple and fast operationPharmaceutical non-active ingredientsDrageesUltimate tensile strengthMaterials science
The medicinal coating powder containing nanometer material consists of hydromellose 55-65 wt%, copolymer of vinyl pyrrolidone and vinyl acetate 8 wt%, glycerin 15 wt%, Span 8 wt%, coloring agent 2-4 wt%, and nanometer titania 2-10 wt%. The medicinal coating powder containing nanometer material has simple production process, wide application, high performance / cost ratio, no physiological toxicity and many other advantages, and may meet the requirement of coating various solid Chinese medicine preparation.
Owner:GUANGDONG GUOFANG MEDICAL TECH
Spaston orally disintegrating tablets and preparation thereof
InactiveCN101209249AReduce absorptionImprove bioavailabilityHydroxy compound active ingredientsAntipyreticPatient complianceFreeze-drying
The invention provides a phloroglucinol oral disintegrating tablet, which takes the phloroglucinol as the active ingredient, and disintegrating agents, fillers, lubricants, flavoring agents, binding agents and effervescent agents are added for preparation. The invention further provides a preparation method of the phloroglucinol oral disintegrating tablet. The phloroglucinol oral disintegrating tablet of the invention not only has the rapid disintegration, good taste and no sense of gravel, but can also can be taken under the condition of no water and have the rapid action and more convenient taking, so the invention can improve the compliance of the patients and the efficacy of the drug and other features of the orally disintegrating tablet, at the same time, the invention has light tablet weight, small volume, no powder falling, good brittleness and better dissolution effect than the freeze-dried orally disintegrating tablet. The production cost is low, the dissolution is faster, the invention is more beneficial to the absorption, and the invention can play the effect of pain reliving faster. The invention has the advantage that the invention can carry out the preparation by only adopting the ordinary pressing equipments and the simple technique without the need of special production equipments and conditions.
Owner:HAINAN JINRUI PHARMA
Medicine for treating hypertension and its preparing process
InactiveCN1768803AImprove bioavailabilityReduced onset time of drug effectsPill deliveryCardiovascular disorderTraditional medicineCyathula
The invention relates to a medicament for treating hypertension and its preparing process, wherein the medicament is prepared from astragalus root, eucommia bark, dried rehmannia root, cornus officinalis, kudzu vine root, root bark of tree peony, abalone shell, hooked uncaria, wild chrysanthemum flower, Ligusticum wallichii, achyranthes and cyathula root, poria cocos, poria with hostwood, arborviate seed and red sage root.
Owner:重庆茂霖农业生物技术有限公司
Spleen-invigorating bolus (pellet) for tonifying qi and invigorating spleen
ActiveCN103550164APrevent volatilizationWell mixedNervous disorderDigestive systemAstragalusPharmaceutic Adjuvant
The invention relates to a pellet preparation, in particular to a spleen-invigorating bolus (pellet) for tonifying qi and invigorating spleen; the spleen-invigorating bolus (pellet) disclosed by the invention is composed of the following components in parts by weight: 66 parts of codonopsis pilosula, 131 parts of fried bighead atractylodes rhizome, 66 parts of astragalus membranaceus baked with honey, 33 parts of liquorice baked with honey, 131 parts of poria cocos, 131 parts of prepared polygala tenuifolia, 66 parts of fried spina date seed, 131 parts of longan aril, 131 parts of Chinese angelica, 33 parts of elecampane, and 33 parts of denucleated Chinese-date; the spleen-invigorating bolus (pellet) contains 10-500 parts by weight of pharmaceutic adjuvants, preferably 20-100 parts by weight. The invention further relates to a preparation method of the spleen-invigorating bolus (pellet); the traditional spleen-invigorating bolus is prepared into the spleen-invigorating bolus (pellet), the bolus diameter of which is 3.5-4 mm, and therefore, the spleen-invigorating bolus (pellet) has the advantages of being steady in quality, obvious in pharmaceutical effect and good in fluidity and dissolution rate and is very applied to clinical use.
Owner:ZHEJIANG WECOME MEDICINE IND
Stable perindopril indapamide tablet and preparation technology
ActiveCN106620644AReduce sizePiece weight smallOrganic active ingredientsDipeptide ingredientsDissolutionSilicon dioxide
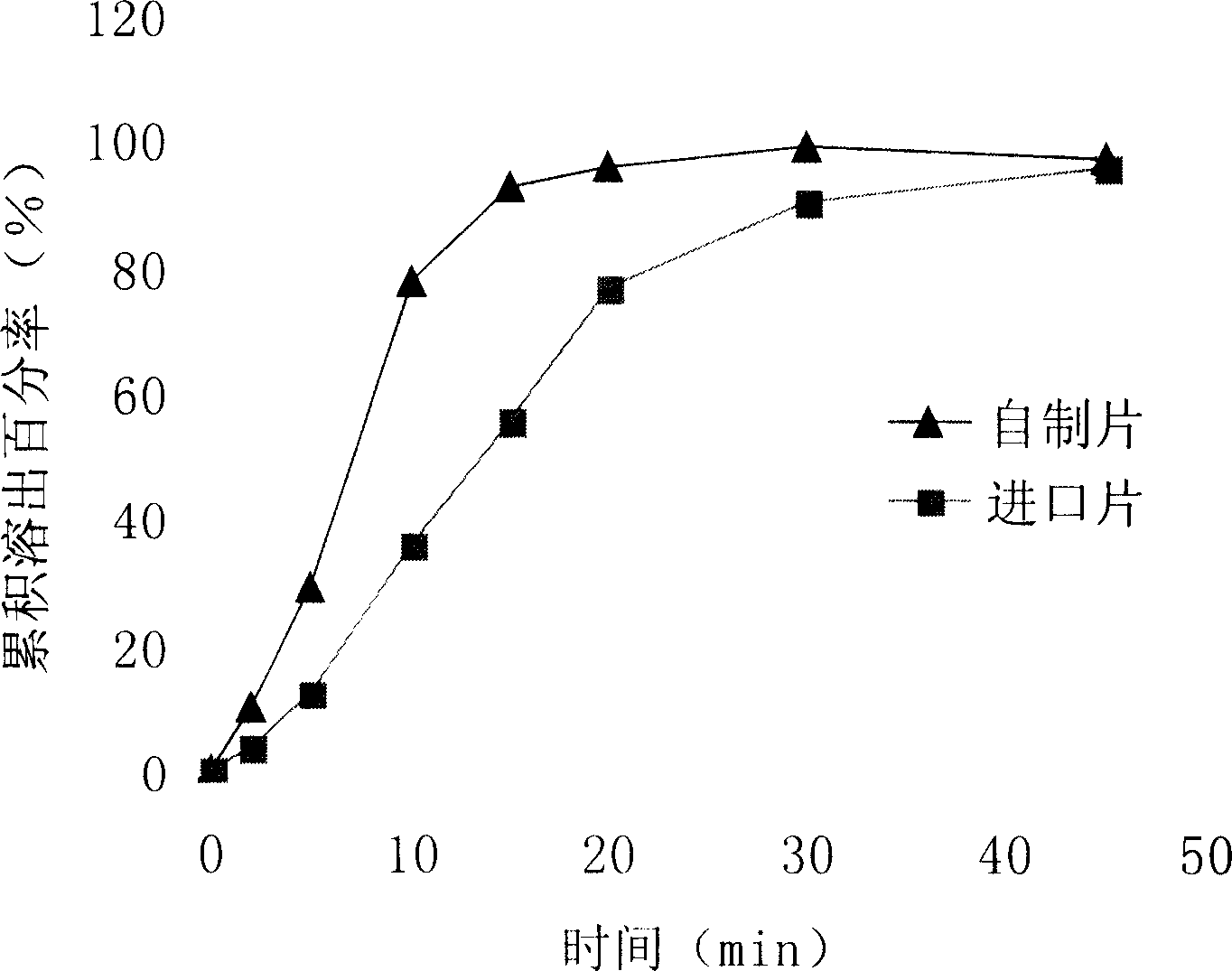
The invention discloses a stable perindopril indapamide tablet and a preparation technology. The pharmaceutical composition is composed of the following components: perindopril tert-butylamine, indapamide, lactose, hydroxypropyl cellulose, sodium carboxymethyl starch, silicon dioxide, and magnesium stearate. Powder is directly pressed to prepare the pharmaceutical composition. The properties, dissolution rate, water content, content, crystal form, and dissolving-out behavior in different medium with different pH values of the prepared perindopril indapamide tablet are similar with those of commercial products. Furthermore, the dissolution curve shows that the difference of tablets in a batch and the difference of tablets in different batches is prominently less than that of commercial products. Compared with the commercial products, the stability of the provided tablet is better in production and storage. The clinical effectiveness and safety are guaranteed. Moreover, the preparation technology is simple, and the production cost is reduced.
Owner:杭州新诺华医药有限公司
Preparation method for omeprazole enteric coated tablet
ActiveCN103479593ASolve industrial production problemsPiece weight smallOrganic active ingredientsDigestive systemCrospovidonesLactose
The invention relates to a preparation method for an omeprazole enteric coated tablet. The components comprise in percents by weight: 10-50% of omeprazole, 1.25-6.25% of superfine silica powder, 30-78% of lactose, 2-3% of copolyvinylpyrrolidone, 5-12% of crospovidone, 1.5-2.0% of sodium octadecyl fumarate and 0.5-1.0% of magnesium stearate. The preparation method comprises: uniformly mixing micronized omeprazole and superfine silica powder, sieving; sieving copolyvinylpyrrolidone, crospovidone, lactose and sodium octadecyl fumarate, uniformly mixing with omeprazole and superfine silica powder, adding into a drying-method granulator, granulating for 3-4 times, screening out integrated particles with a 60 mesh sieve and obtaining dry particles; blending uniformly the dry particles and magnesium stearate by a three-dimensional mixer, adding into a tablet press for pressing tablets; and then coating to obtain the omeprazole enteric coated tablet. The formula is simple; the preparation method helps to solve the industrialized production problem of omeprazole enteric coated tablet high-specification products such as a product with a specification of 40 mg / tablet; and the preparation is controllable in quality, the product is good in uniformity, and impurity content is low.
Owner:QINGDAO DOUBLE WHALE PHARMA
Compound sustained-release tablet of cetirizine and pseudoephedrine and preparation method thereof
ActiveCN101708178AGood reproducibilitySimple processOrganic active ingredientsRespiratory disorderSustained Release TabletPseudoephedrine
The invention discloses a compound sustained-release tablet of cetirizine and pseudoephedrine and a preparation method thereof. The tablet comprises cetirizine or pharmaceutically acceptable cetirizine salt and pseudoephedrine or pharmaceutically acceptable pseudoephedrine salt. The preparation method comprises the steps of: preparing the pseudoephedrine or the pharmaceutically acceptable pseudoephedrine salt into a sustained-release tablet core; uniformly dispersing the cetirizine or the pharmaceutically acceptable cetirizine salt in a coating solution to coat the surface of the tablet core. Two active materials with different doses are prepared into the compound sustained-release tablet by a coating method. The preparation method solves the problems of the quick release of the cetirizine and the sustained release of the pseudoephedrine, has convenient operation and easy quality control, and is suitable for industrial production. In the tablet, more than 85% of the cetirizine is dissolved within 30 minutes, 90% of the cetirizine is dissolved out within 1 hour, and the pseudoephedrine releases medicaments in a sustained mode within 12 hours or 24 hours. The tablet is taken once or twice a day, and can reduce the administration time, better stabilize the concentration of blood medicaments and reduce adverse effect.
Owner:YANGTZE RIVER PHARM GRP CO LTD +1
Preparation method of tenofovir disoproxil fumarate oral tablets
InactiveCN105663077AReduce sizeImprove bioavailabilityOrganic active ingredientsAntiviralsMedicineDissolution
The invention discloses a preparation method of tenofovir disoproxil fumarate oral tablet, which comprises micronization of raw and auxiliary materials, preparation of pregelatinized starch suspension and fluidized bed granulation and tableting process technical processing, thereby reducing particle size, tablet weight, and difficulty in swallowing, making it easier for patients to accept, and at the same time speeding up its disintegration rate, and improving oral administration of tenofovir disoproxil Bioavailability of esters.
Owner:广东京豪生物制药有限公司
Mycophenolate mofetil dispersible tablet
ActiveCN104382873APiece weight smallDisintegrates quicklyOrganic active ingredientsPill deliverySulfateDissolution
The invention discloses a mycophenolate mofetil dispersible tablet. The tablet consists of mycophenolate mofetil, calcium sulfate, glycerin monostearate, a disintegrating agent and an adhesive. Compared with the prior art, the mycophenolate mofetil dispersible tablet prepared by the invention is small in tablet weight and rapid in dissolution and disintegration and does not need lots of disintegrating agents, the disintegrated granules can smoothly pass through a No.2 sieve, and the mycophenolate mofetil dispersible tablet is suitable for industrial production.
Owner:SHANDONG NEWTIME PHARMA
Brand-new oral solid pharmaceutical composition and preparation method thereof
ActiveCN102335178AReduce dosageSolving Quality Control IssuesOrganic active ingredientsOrganic chemistryOlmesartanLevamlodipine
The invention discloses a brand-new oral solid pharmaceutical composition. The pharmaceutical composition is an oral preparation prepared from hydrochlorothiazide, l-amlodipine, olmesartan medoxomil and pharmaceutically acceptable auxiliary materials, and the oral preparation comprises but is not limited to tablets or capsules. The composition comprises the following raw materials in parts by weight: 5-25 parts of hydrochlorothiazide, 2.5-5 parts of l-amlodipine, 20-40 parts of olmesartan medoxomil, 40-120 parts of microcrystalline cellulose, 30-90 parts of pregelatinized starch, 15-40 parts of low-substituted hydroxypropyl cellulose, 10-45 parts of crosslinked polyvinylpyrrolidone, 3-8 parts of silica and 1-2 parts of magnesium stearate. The pharmaceutical composition disclosed by the invention has the advantages of scientific and reasonable prescription, low auxiliary material content and high bioavailability, and is a drug of first choice for treating hypertension.
Owner:HAINAN JINRUI PHARMA CO LTD
Indobufen-containing pharmaceutical composition and preparation method thereof
ActiveCN114225042AReduce dosageLess varietyOrganic active ingredientsPill deliveryPolyethylene glycolEngineering
The invention relates to the technical field of pharmaceutical preparations, in particular to a pharmaceutical composition containing indobufen and a preparation method thereof.The pharmaceutical composition is prepared from, by weight, 50-200 parts of indobufen, 100 parts of polyethylene glycol, 20 parts of xylitol and 30-50 parts of pregelatinized starch. The tablet has the advantages of small dosage of auxiliary materials, low production cost, high taking compliance, good dissolution rate effect and suitability for large-scale production.
Owner:杭州沐源生物医药科技有限公司 +1
Indissolvable drug oral sustained-release dry emulsion tablet and preparation method thereof
InactiveCN105168163ADissolution medium volume is smallIncrease speedOrganic active ingredientsPharmaceutical delivery mechanismBlood concentrationDissolution
The invention belongs to the field of medicinal preparations, and discloses an indissolvable drug oral sustained-release dry emulsion tablet and a preparation method thereof. The preparation method for the oral sustained-release dry emulsion tablet comprises the steps that after an oil phase, a surface active agent and cosurfactant are evenly mixed, an indissolvable drug is added till the drug is dissolved completely, and accordingly a medicine-carried self-emulsifying system is prepared; after the medicine-carried self-emulsifying system and an aqueous solution of a hydrophilic gel framework material are evenly mixed, spray drying is conducted, and sustained-release dry emulsion powder is obtained; after the sustained-release dry emulsion powder and a conventional tablet auxiliary material needed for preparation are mixed, wet granulation is carried out; at last, tabletting is performed. The sustained-release dry emulsion tablet can improve the dissolution and dissolving-out performance of the indissolvable drug, the bioavailability of the indissolvable drug is improved, a stable blood concentration is formed, and the compliance of patients is improved. The sustained-release dry emulsion tablet is not complex in composition of prescription, and the preparation technology is suitable for industrial production.
Owner:CHINA PHARM UNIV
Oral tablet of acotiamide hydrochloride trihydrate and preparation method thereof
ActiveCN105769784AReduce volumeEasy to acceptOrganic active ingredientsDigestive systemPatient complianceAcotiamide Hydrochloride
The invention discloses o an oral tablet of acotiamide hydrochloride trihydrate and a preparation method thereof. The acotiamide hydrochloride trihydrate disclosed by the invention adopts a water insoluble filler as the first filler, and aims to still maintain the original particle shape when drug particles contact a dissolution medium and other solutions so as to prevent acotiamide hydrochloride from gathering, thus achieving a good dissolution effect. At the same time, through additional adding of a second filler and a disintegrating agent, the material is ensured with good compressibility and dissolution performance. According to the acotiamide hydrochloride tablet and the preparation method provided by the invention, the operation is simple, the dosage of auxiliary materials is significantly reduced, the tablet weight is small, and while the dissolution rate is guaranteed, the patient compliance is also improved.
Owner:REGENEX PHARMA LTD
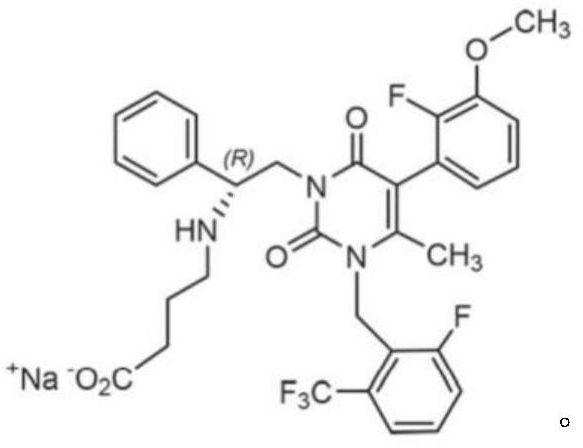
Elagolix freeze-dried tablets and preparation method thereof
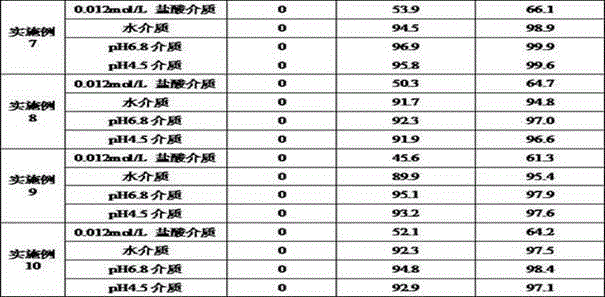
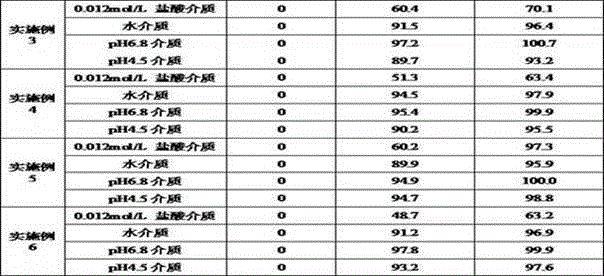
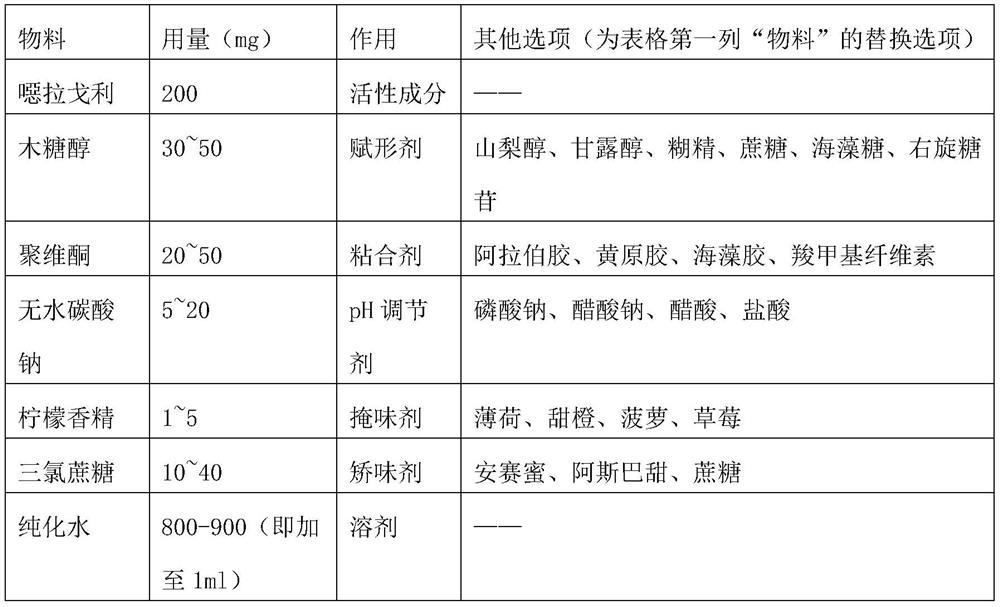
ActiveCN113876728AReduce pollutionPiece weight smallOrganic active ingredientsInorganic non-active ingredientsBULK ACTIVE INGREDIENTDigestion
The invention discloses elagolix freeze-dried tablets. The elagolix freeze-dried tablets comprise an active ingredient elagolix, an excipient, an adhesive, a pH regulator, a taste masking agent, a flavoring agent and a solvent, and comprises the following components in parts by weight: 200 parts of active component elagolix, 30-50 parts of excipient, 20-50 parts of adhesive, 5-20 parts of pH regulator, 1-5 parts of taste masking agent, 10-40 parts of flavoring agent and 800-900 parts of solvent. The invention further discloses a preparation method of the elagolix freeze-dried tablets. The elagolix freeze-dried tablets and the preparation method thereof have the following advantages: no dust is generated in the production process, and the environmental pollution is less; the prepared products are smaller in tablet weight and adopt fewer auxiliary materials; the freeze-dried tablets are low in water content and stable in quality, and impurities are hardly increased in the storage process; in the clinical using process, the elagolix freeze-dried tablets can be quickly dissolved in the mouth, the dissolution and release of the drug cannot be influenced by the digestion process of gastrointestinal tracts, and the clinical response is quicker.
Owner:南京唯创远医药科技有限公司
Wind-dispelling and hair-restoring alopecia areata pellet
ActiveCN103565752AHigh fat contentPlay a supporting roleGranular deliveryDermatological disorderSalvia miltiorrhizaMedicine
The invention relates to a pellet preparation, and in particular relates to a wind-dispelling and hair-restoring alopecia areata pellet. The pellet comprises the following components in parts by weight: 148 parts of rehmannia, 148 parts of prepared rehmannia, 148 parts of prepared polygonum multiflorum, 98 parts of Chinese angelica, 98 parts of salvia miltiorrhiza, 98 parts of white peony root, 98 parts of schisandra chinensis, 50 parts of notopterygium root, 50 parts of pawpaw, and 10-1,000 parts of medical auxiliaries. The invention also relates to a preparation method of the alopecia areata pill (pellet). By adopting the method, alopecia areata pellet with diameter of 3.5-4mm is prepared from the traditional alopecia areata pill, so that the alopecia areata pellet is stable in quality and remarkable in drug effect, has extremely good fluidity and dissolution, and is suitable for clinical application.
Owner:ZHEJIANG WECOME MEDICINE IND
Novel preparation process for oral solid preparation of aliskiren and amlodipine/levamlodipine
InactiveCN103070863ACreate an advantageNovelty advantageOrganic active ingredientsCapsule deliveryLevamlodipineAmlodipine
The invention relates to a novel preparation process for a solid oral preparation which comprises the active components consisting of an oral active renin inhibitor (aliskiren) in an appropriate carrier medium or a medicinal salt thereof and a calcium channel blocker (CCB) amlodipine / levamlodipine or a medicinal salt thereof. Particularly, the invention provides a galenical preparation which comprises a composition of aliskiren hemifumarate and amlodipine or levamlodipine and a novel preparation process. The invention also relates to novel preparation methods for the above-mentioned preparations and application of the preparations as medicines. According to the invention, the method of dry granulation is employed, the problem that the method of wet granulation easily causes increase of relevant substances is overcome, and drug stability is improved; through optimization of the process, the usage amount of excipients is reduced, the purpose of reducing the weight of a tablet is achieved, cost is saved, and the preparations can be easily taken; through particle coating, unwanted reaction of two drugs after mixing is prevented.
Owner:河南省健康伟业生物医药研究股份有限公司 +1
Metronidazole effervescent tablet for vagina and preparation technology of metronidazole effervescent tablet
InactiveCN104666273AAdvantages and Significant AdvancementsFix stability issuesOrganic active ingredientsInorganic non-active ingredientsEffervescent tabletVagina
The invention discloses a metronidazole effervescent tablet for vagina and a preparation technology of the metronidazole effervescent tablet. The tablet is prepared from a metronidazole tablet core and an alkaline coating layer, wherein the metronidazole tablet core contains an acid component; and the metronidazole tablet core is wrapped with the alkaline coating layer. Compared with the prior art, an acid effervescing agent and an alkaline effervescing agent are effectively separated; the problem of the stability of the metronidazole effervescent tablet in preparation and storage processes is successfully solved; meanwhile, the amount of auxiliary materials is greatly reduced; the tablet weight is reduced; and medication of patients is facilitated.
Owner:尹克春
Trelagliptin succinate tablets
InactiveCN106309393AEasy to prepareEasy to operateOrganic active ingredientsMetabolism disorderMannitolChemistry
The invention provides trelagliptin succinate tablets and a preparation method thereof. The trelagliptin succinate tablets contain the following components in parts by weight: 64.0%-78.2% of trelagliptin succinate, 3.7%-5.0% of cross-linking sodium cellulose glycolate, 2.2%-5.0% of hydroxypropyl cellulose, 6.8%-25.1% of mannitol, 6.8%-15.2% of microcrystalline cellulose and 0.1%-5% of sodium stearoyl fumarate. The invention aims at providing the trelagliptin succinate tablets with even quality, high disintegration speed, good digestion degree, small tablet weight, simple production process, easy operation and suitability for industrial production.
Owner:中山万远新药研发有限公司
Wind-dispelling and hair-restoring alopecia areata pellet
ActiveCN103565752BSmall disintegration effectHigh mechanical strengthGranular deliveryDermatological disorderSalvia miltiorrhizaMedicine
Owner:ZHEJIANG WECOME MEDICINE IND
Metformin hydrochloride controlled-release tablet and preparation method thereof
InactiveCN111840242ASmall fluctuations in blood concentrationLow incidence of adverse reactionsOrganic active ingredientsMetabolism disorderMetforminPlasticizer
The invention discloses a metformin hydrochloride controlled-release tablet and a preparation method thereof. The metformin hydrochloride controlled-release tablet comprises a tablet core, a coating aqueous dispersion forms a controlled-release coating film outside the tablet core, and the tablet core is prepared from, by weight, 80-120 parts of metformin hydrochloride, 10-14 parts of a tablet core controlled-release material, 2-4 parts of filler and 0.2-1.2 parts of an adhesive, and the aqueous dispersion is prepared from the following raw materials in parts by weight: 2 to 6 parts of coatingslow-release material, 1.2 to 1.6 parts of plasticizer, 1.2 to 1.6 parts of pore-foaming agent, 0.4 to 0.8 part of anti-sticking agent and 0.1 to 0.5 part of emulsifying agent. The controlled-releasetablet provided by the invention is coated by adopting the aqueous dispersion, so that the pollution of an organic solvent to the environment is avoided, and the potential safety hazard is reduced. According to the invention, a double controlled release technology of tablet core slow release and membrane controlled coating is adopted, the release degree in the second hour is 10%-35%, the releasedegree in the sixth hour is 40%-70% and the release degree in the twelfth hour is more than 80% in an in-vitro release degree test, constant-speed release is realized, swallowing is easy, and the compliance of a patient is improved.
Owner:CHONGQING CONQUER PHARML
Clopidogrel sulfate tablet and preparation process thereof
ActiveCN104173309AAdvantages and Significant AdvancementsFix stability issuesOrganic active ingredientsPharmaceutical non-active ingredientsHydrogen SulfateCombinatorial chemistry
The invention discloses a clopidogrel sulfate tablet and a preparation process thereof. The tablet contains compound of clopidogrel sulfate and polyvinylpolypyrrolidone, wherein the compound is prepared by the steps of dissolving clopidogrel sulfate in an alcohol solvent, adding polyvinylpolypyrrolidone, uniformly mixing and drying by spray. The clopidogrel sulfate and polyvinylpolypyrrolidone compound prepared by the process is capable of avoiding adhesion in the producing process and successfully solving the stability problem in clopidogrel sulfate preparing and storing processes, is simple in process, and is easy for industrial large-scale production.
Owner:南京康川济医药科技有限公司
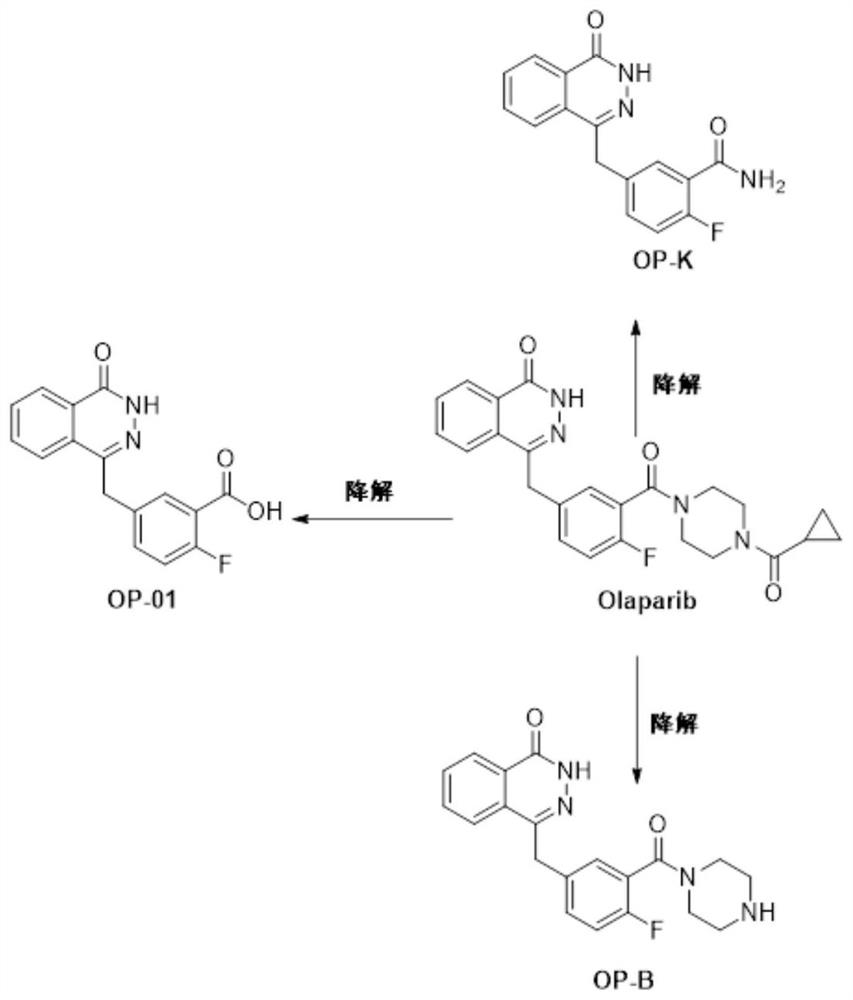
Olaparib release related pharmaceutical composition
ActiveCN112438954AImprove solubilityImprove dissolution rateOrganic active ingredientsOther chemical processesCombinatorial chemistryPharmaceutical Substances
The invention relates to pharmaceutical compositions, in particular to an olaparib release related pharmaceutical composition. The invention discloses an olaparib composition containing impurities with relatively low levels and having relatively high release level, and a method for maintaining the stability of such pharmaceutical compositions.
Owner:BEIJING KANG LISHENG PHARMA TECH DEV
Metformin hydrochloride dual sustained and controlled release composition as well as preparation method and application thereof
ActiveCN112999182APiece weight smallEasy to swallowOrganic active ingredientsMetabolism disorderControl releaseFoaming agent
The invention belongs to the field of pharmaceutical preparations, and discloses a metformin hydrochloride dual controlled release composition, which is prepared from a controlled release coating film coated outside a tablet core of metformin hydrochloride containing a controlled release material, the metformin hydrochloride tablet core containing the sustained-release material is composed of metformin hydrochloride, the sustained-release material, a filling agent and an adhesive, the coating layer is composed of a coating sustained-release material, a plasticizer and a pore-foaming agent, the coating sustained-release material is cellulose acetate, the tablet core sustained-release material is a mixture of octadecanol and polyoxyethylene, and the coating sustained-release material is cellulose acetate. According to the technical scheme, compared with a traditional film-coated controlled release tablet, the film-controlled metformin hydrochloride dual controlled release composition has the advantages that the time for keeping the stable release degree is longer, the storage stability is better, the process is simple, expensive production equipment is not needed, the cost is low, and industrialization is easy.
Owner:CHONGQING CONQUER PHARML
Metformin hydrochloride controlled release tablets and preparation method thereof
InactiveCN111870585ATo achieve the effect of double controlled releaseLow incidence of adverse reactionsOrganic active ingredientsMetabolism disorderMetformin HydrochloridePharmaceutical drug
The invention provides metformin hydrochloride controlled-release tablets and a preparation method thereof. The metformin hydrochloride controlled-release tablets contain a main drug namely metforminhydrochloride, an auxiliary material namely a filler, an auxiliary material namely a sustained-release material, and auxiliary materials namely an adhesive and a sustained-release coating, and is characterized in that the metformin hydrochloride, the filler and the sustained-release material are pressed into a tablet core; the sustained-release material in the tablet core and the sustained-releasecoating film outside the tablet core form a double controlled-release system of a controlled-release drug, the sustained-release material is a mixture of octadecanol and polyoxyethylene, the ratio ofthe octadecanol to the polyoxyethylene in parts by weight is 3: 1, and the molecular weight of the polyoxyethylene is 400,000-900,000. The metformin hydrochloride controlled release tablets providedby the invention have a release rate of 7%-10% per hour, can be released at a constant speed for about 10 hours, is clinically verified to have a low adverse reaction rate after being taken by a patient, and obviously improves the tolerance of the patient.
Owner:CHONGQING CONQUER PHARML
Improved Zhenyuan tablet and preparation method thereof
InactiveCN110721171ALong release timeGood sustained release effectNervous disorderAntinoxious agentsLACTOSE MONOHYDRATEMagnesium stearate
The invention relates to an improved Zhenyuan tablet and a preparation method thereof in the technical field of pharmaceutical preparations. The improved Zhenyuan tablet includes a tablet core and a coating, wherein the tablet core includes ginseng fruit saponins and a sustained-release material, and the coating includes a sustained-release coating material. By adding the sustained-release material in the tablet core and adopting the coating containing the sustained-release coating material, the slow release time of the Zhenyuan tablet is prolonged, and the bioavailability of ginseng fruit saponins in the Zhenyuan tablet is improved. The Zhenyuan tablet has a good slow-release effect. Each 1000 Zhenyuan tablets preferably include the following ingredients: 25 g of ginseng fruit saponins, 28.5 g of starch, 60 g of lactose monohydrate, 1.52 g of povidone, 2 g of silica, 0.5 g of magnesium stearate and 2.01 g of gastric soluble opadry. The povidone is used as both a sustained-release material and a binder to reduce the tablet weight.
Owner:HONG KONG JOWA & HUAYUAN GRP CHUZHOU PHARMA CO LTD
Metformin hydrochloride sustained-release tablet and method for preparing the same
InactiveCN1209101CReduce dosageEasy to swallowOrganic active ingredientsMetabolism disorderCelluloseMetformin hcl
The invention provides a metformin hydrochloride sustained-release tablet and a preparation method thereof. The sustained-release tablet is composed of 46.5-70% of metformin hydrochloride, 13.5-33.0% of hydroxypropyl methylcellulose, and 10.0-14.0% of micronized ethyl cellulose , filler 1.3~9.4%, lubricant 1.2~1.5%, and its preparation method is that metformin hydrochloride, hypromellose and filler are granulated according to the conventional process of tablets, dried, and processed Granules, dry granules after granulation, add micropowder ethyl cellulose and lubricant, mix well, and then compress into tablets.
Owner:GUANGZHOU PHARMACEUTICAL INDUSTRIAL RESEARCH INSTITUTE +1
Metformin hydrochloride tablet and preparation method thereof
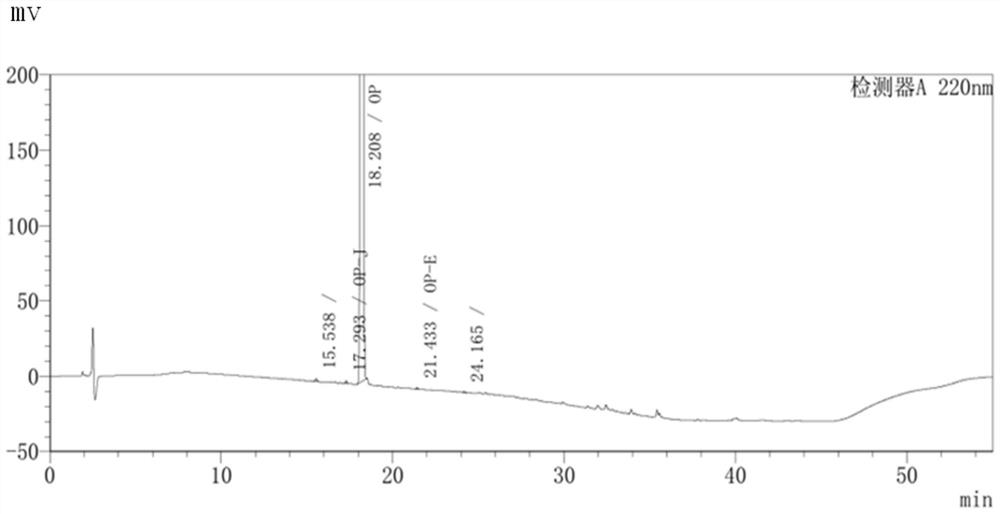
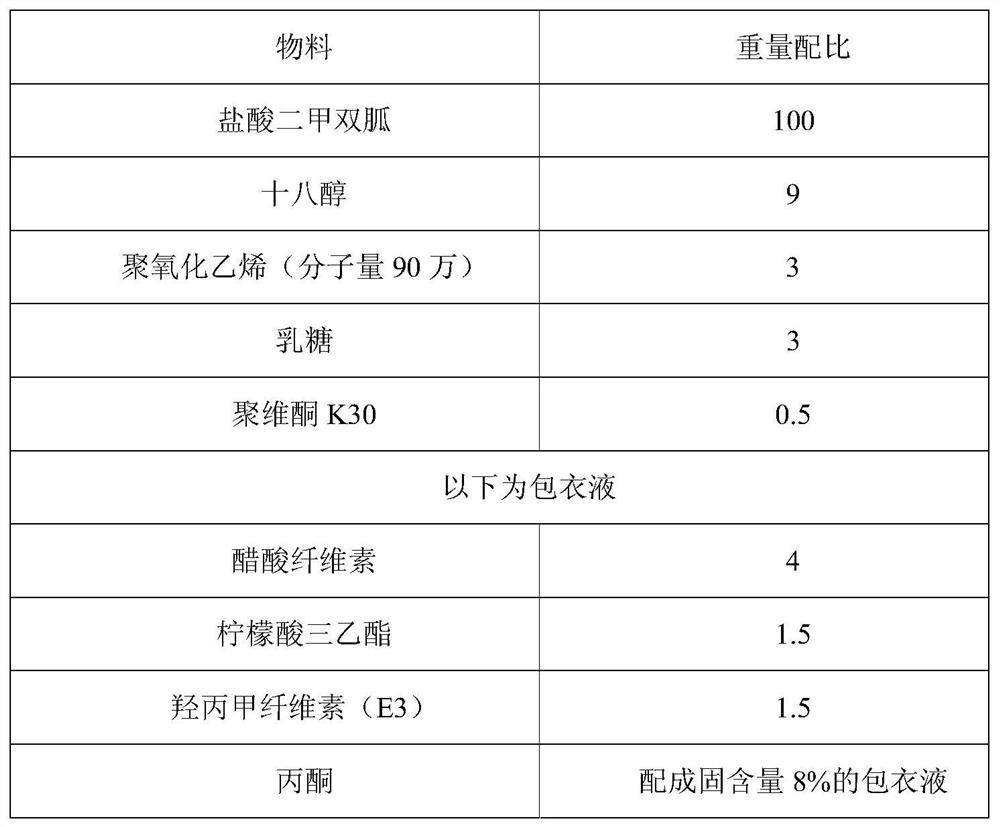
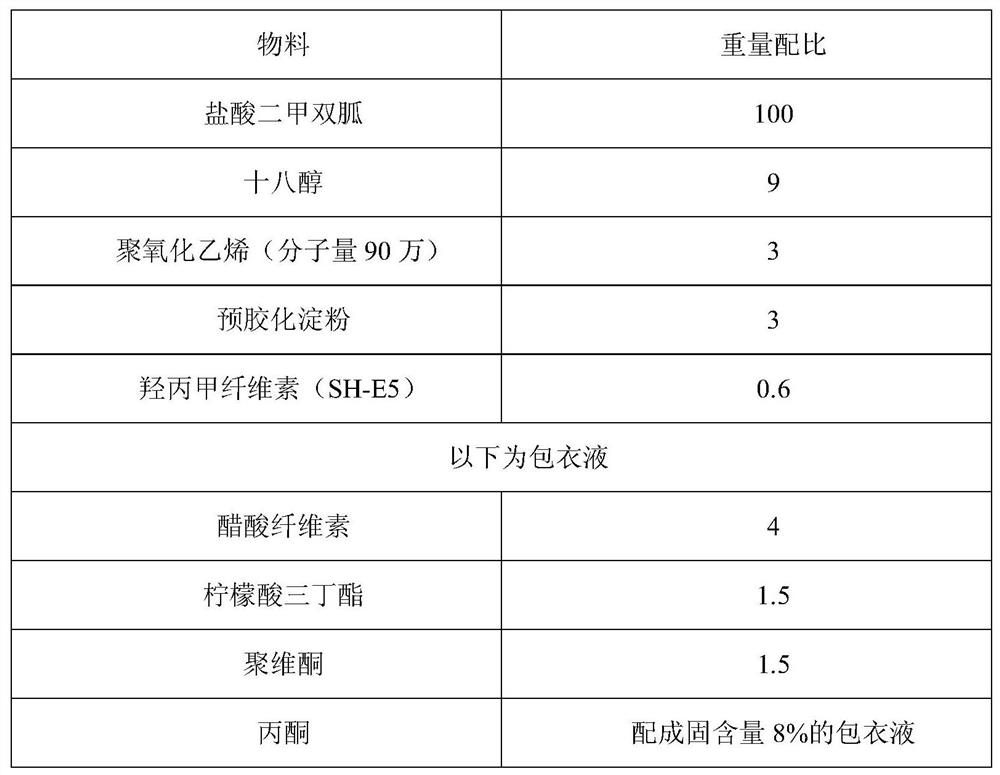
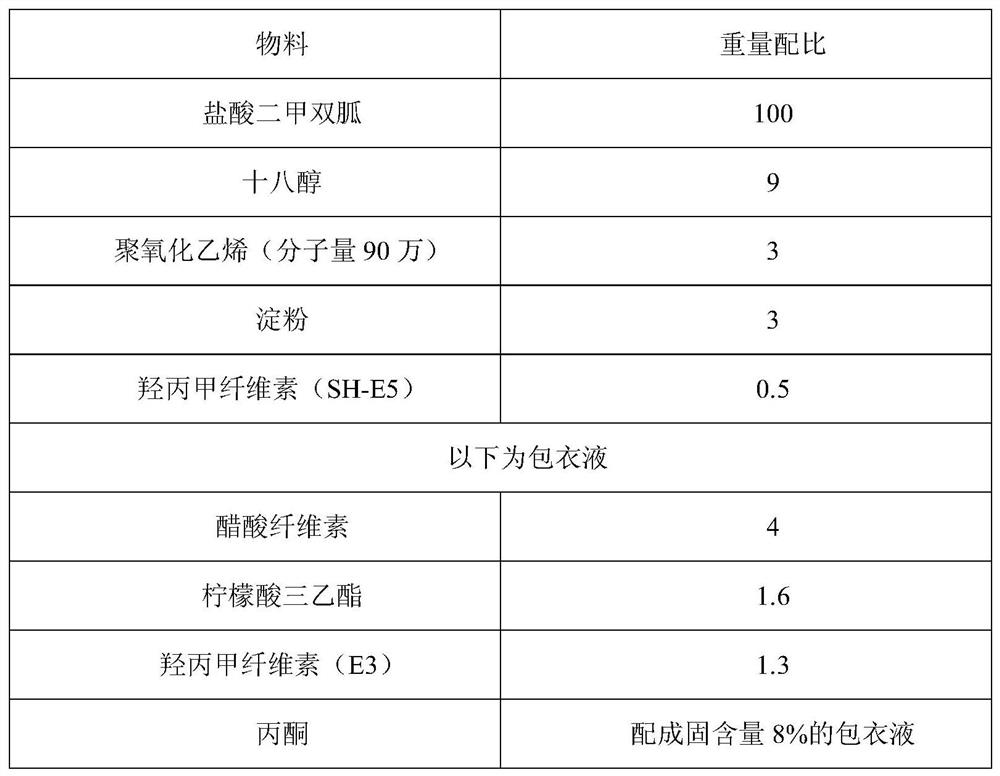
ActiveCN110215437AOvercome Irritation SymptomsPiece weight smallOrganic active ingredientsMetabolism disorderSodium stearateMetformin Hydrochloride
The invention relates to the technical field of pharmaceutical preparations, in particular to a metformin hydrochloride tablet and a preparation method thereof. The components of the metformin hydrochloride tablet comprise metformin hydrochloride, a binder, a framework film material and a lubricant, wherein the framework film material is selected from at least one of sodium stearate, magnesium stearate, stearic acid and the like. The preparation method of the tablet is a one-step granulation method. The in-vitro dissolution condition of the provided metformin hydrochloride tablet is highly similar to that of an original triturate, the tablet has excellent inter-batch stability, and the in-batch and inter-batch dissolution uniformity and inter-batch reproducibility of the tablet are superior to those of the original triturate.
Owner:BEIJING ZHONGHUI PHARM CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com