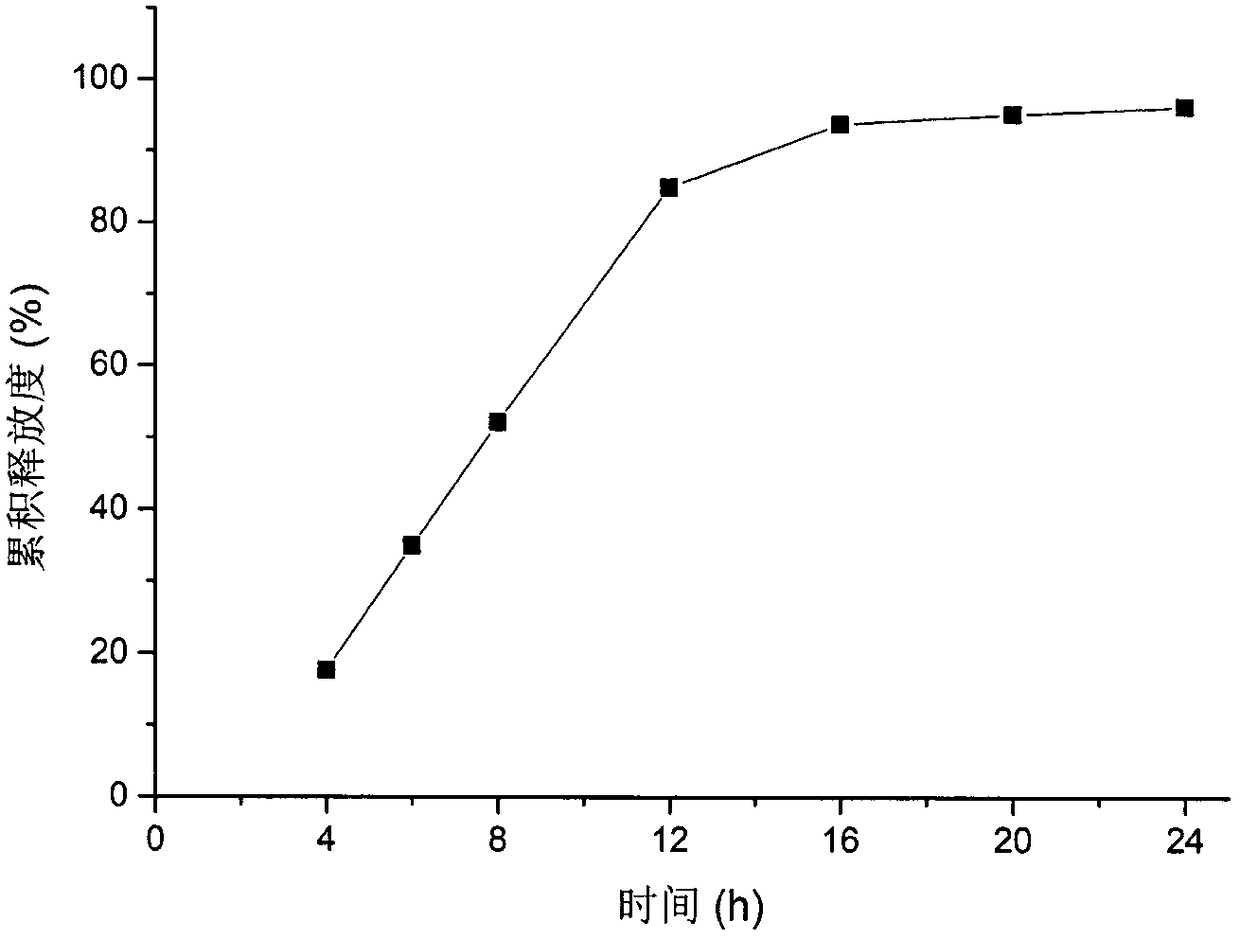
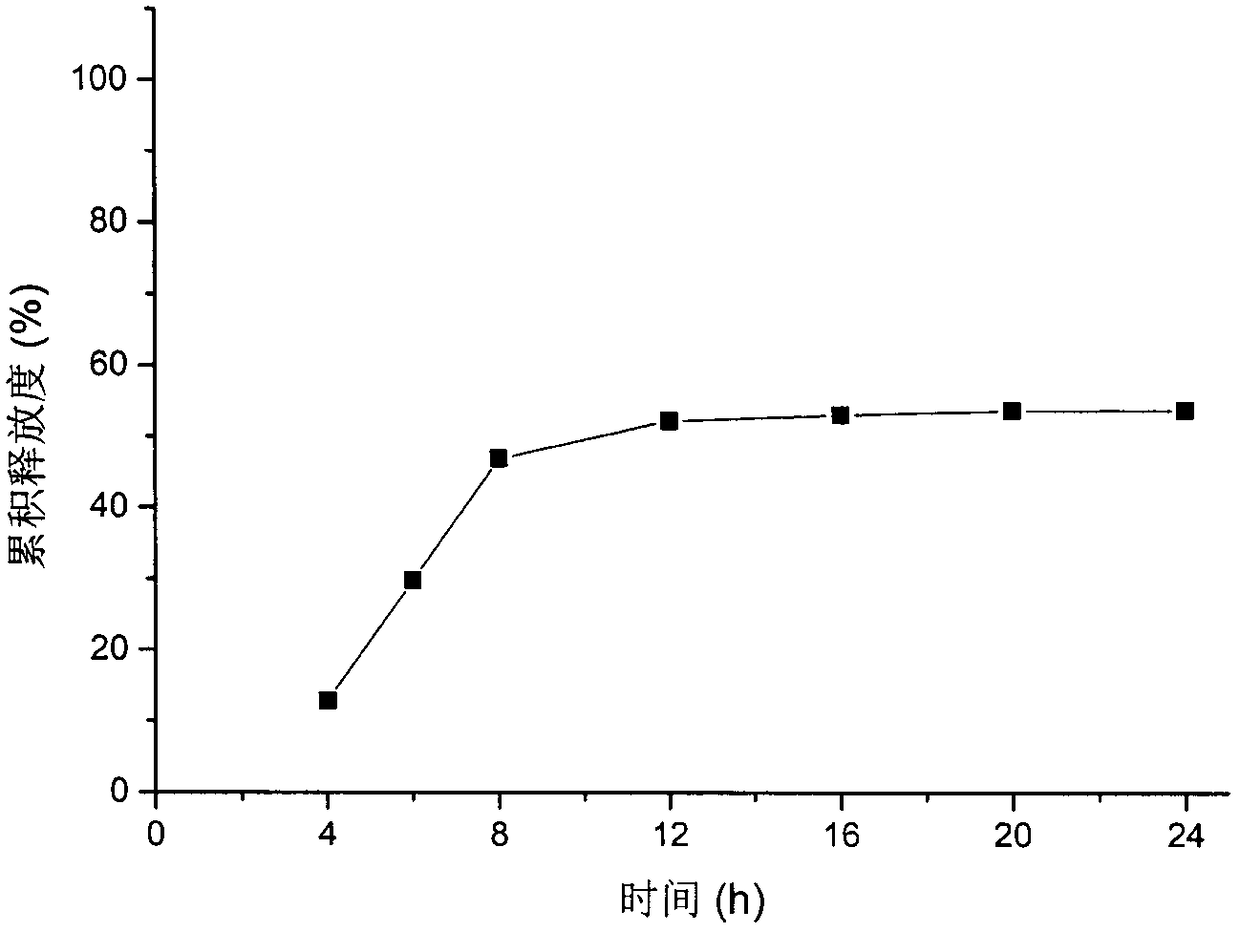
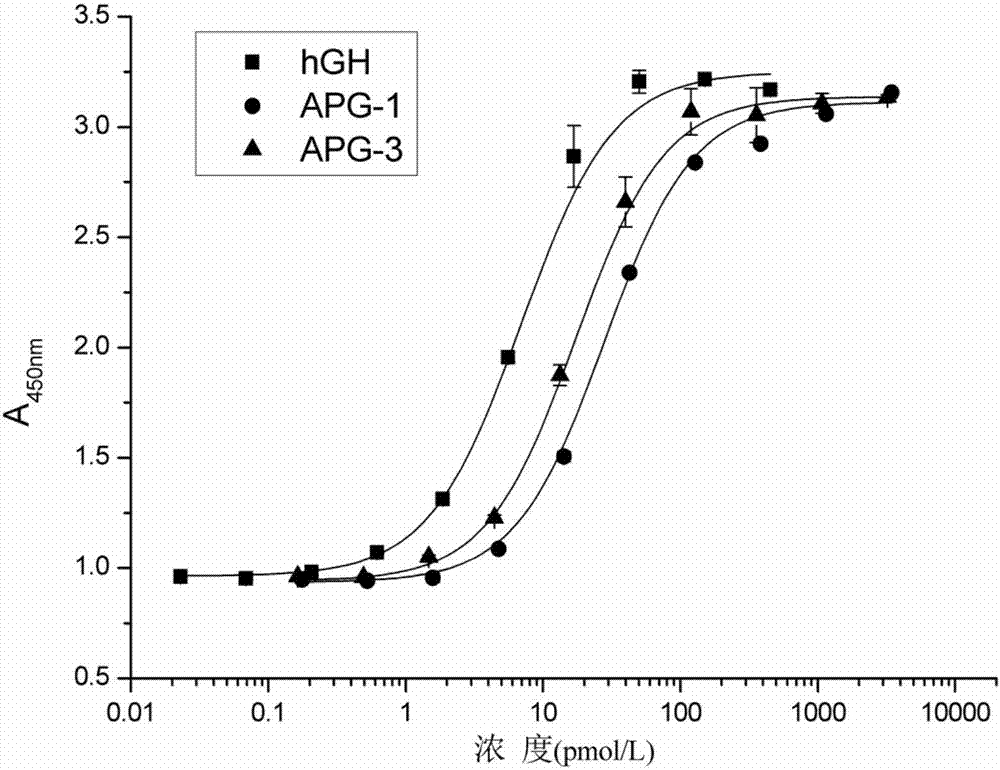
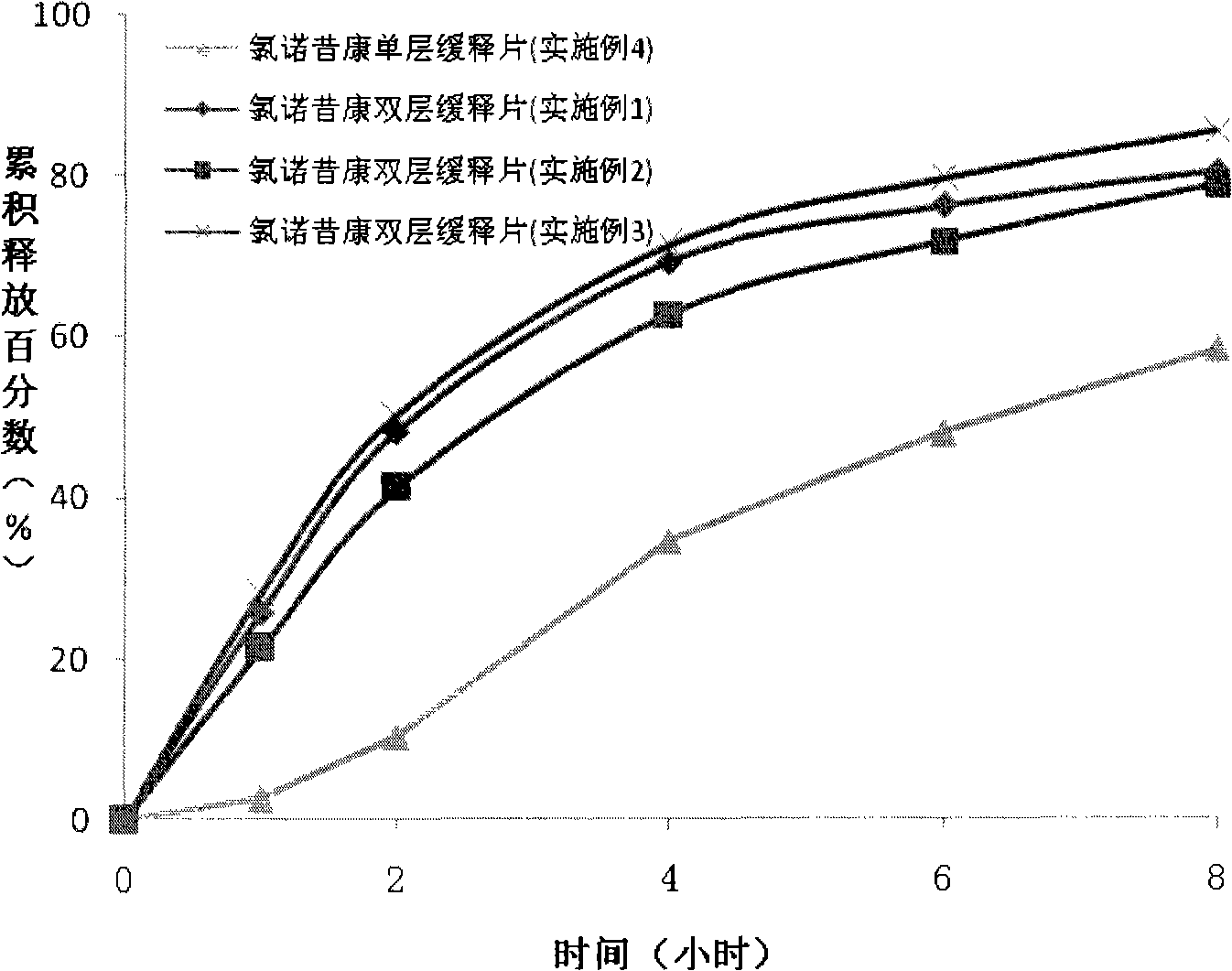
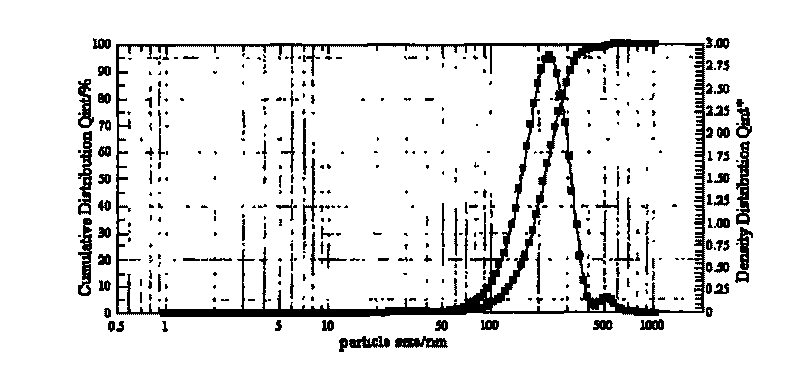
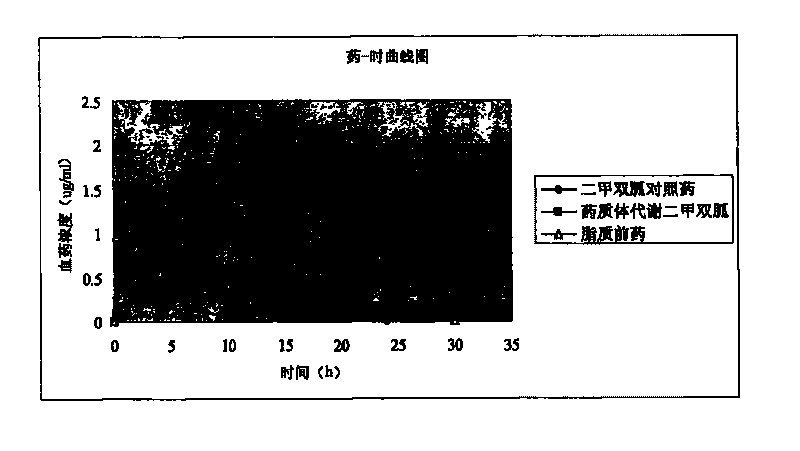
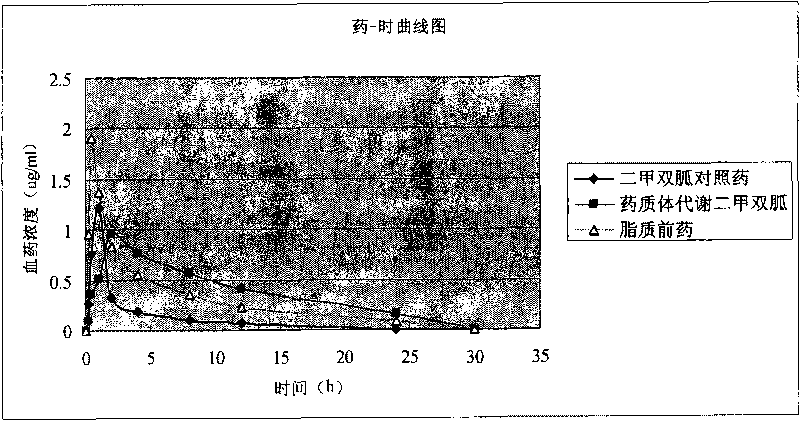
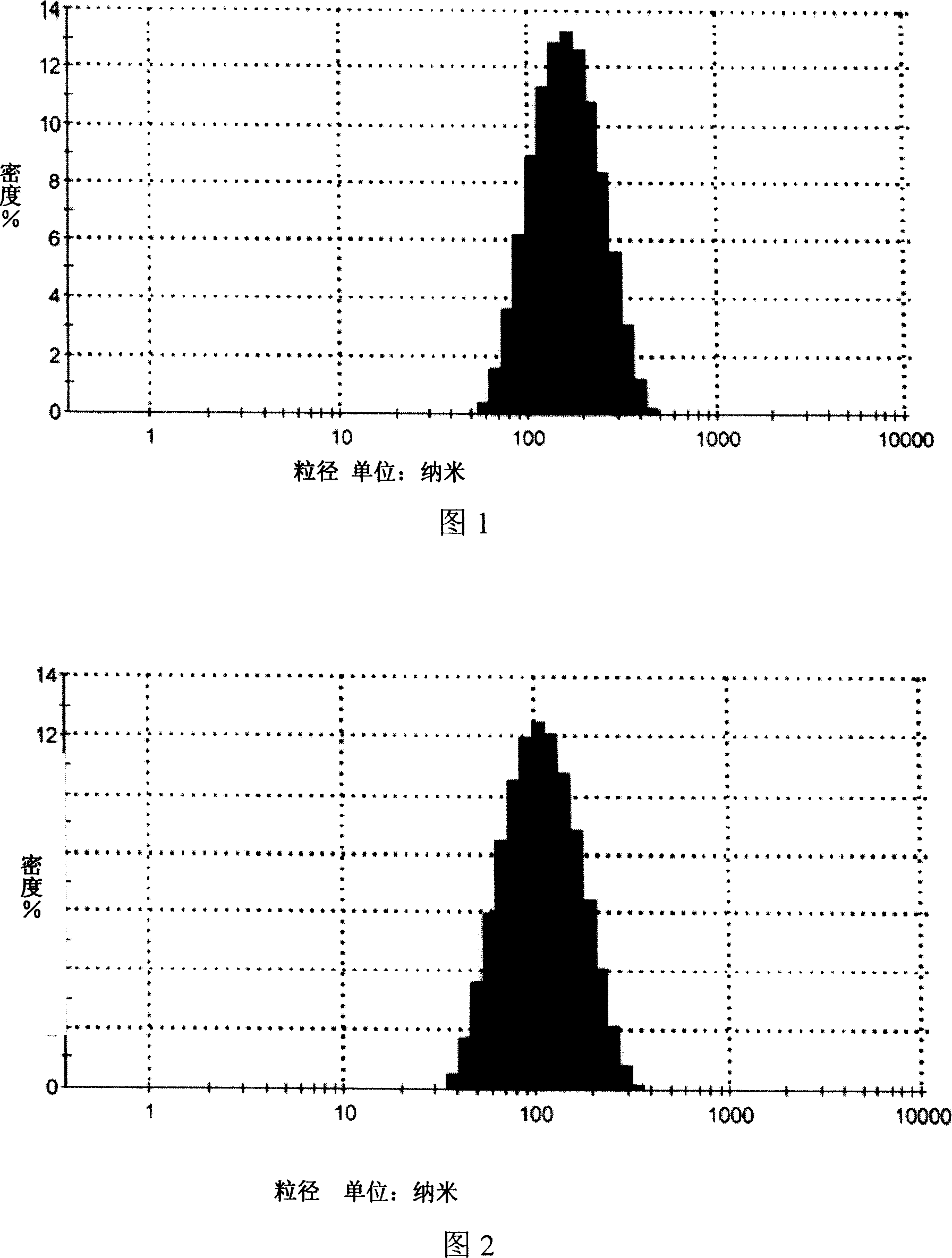
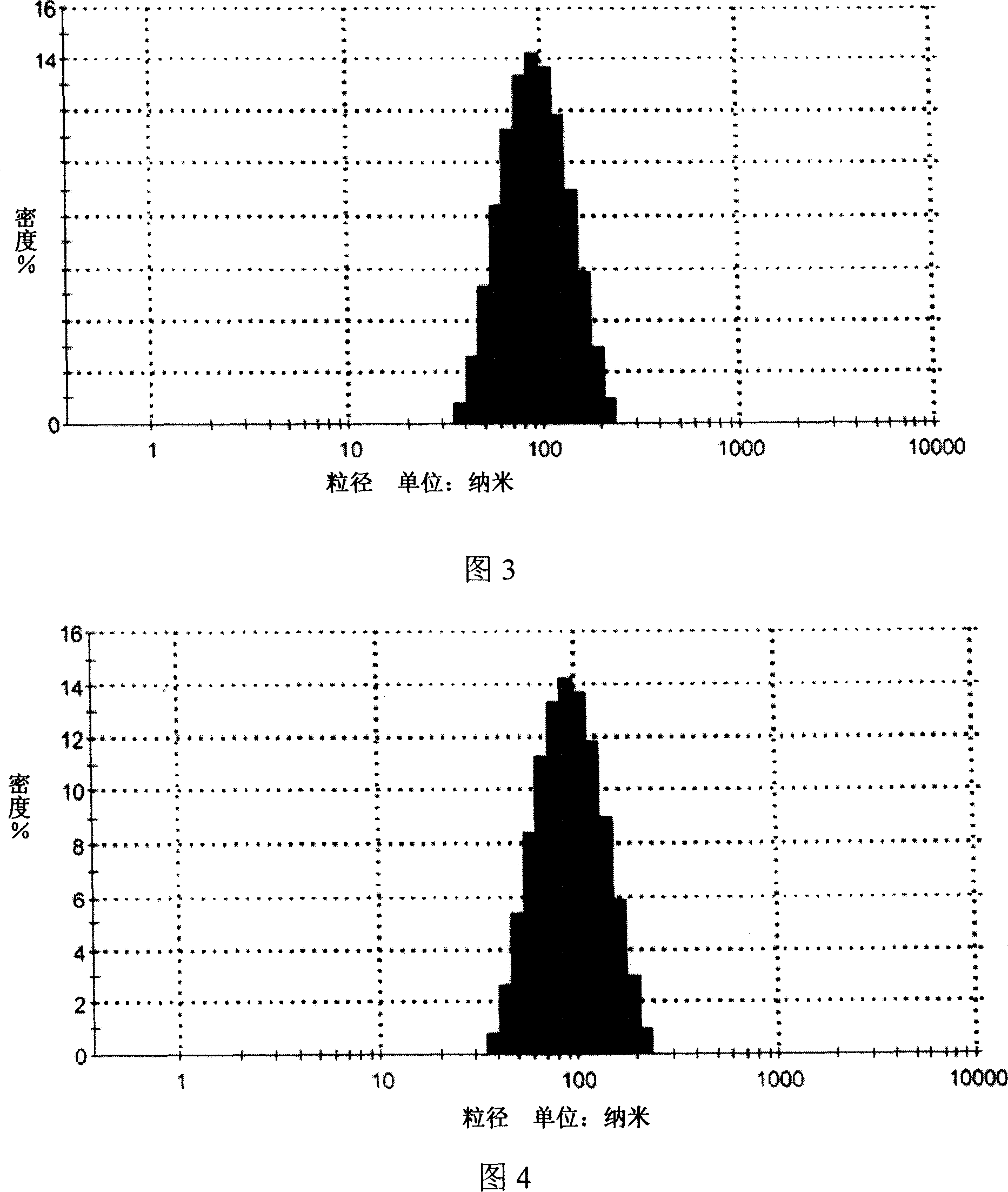
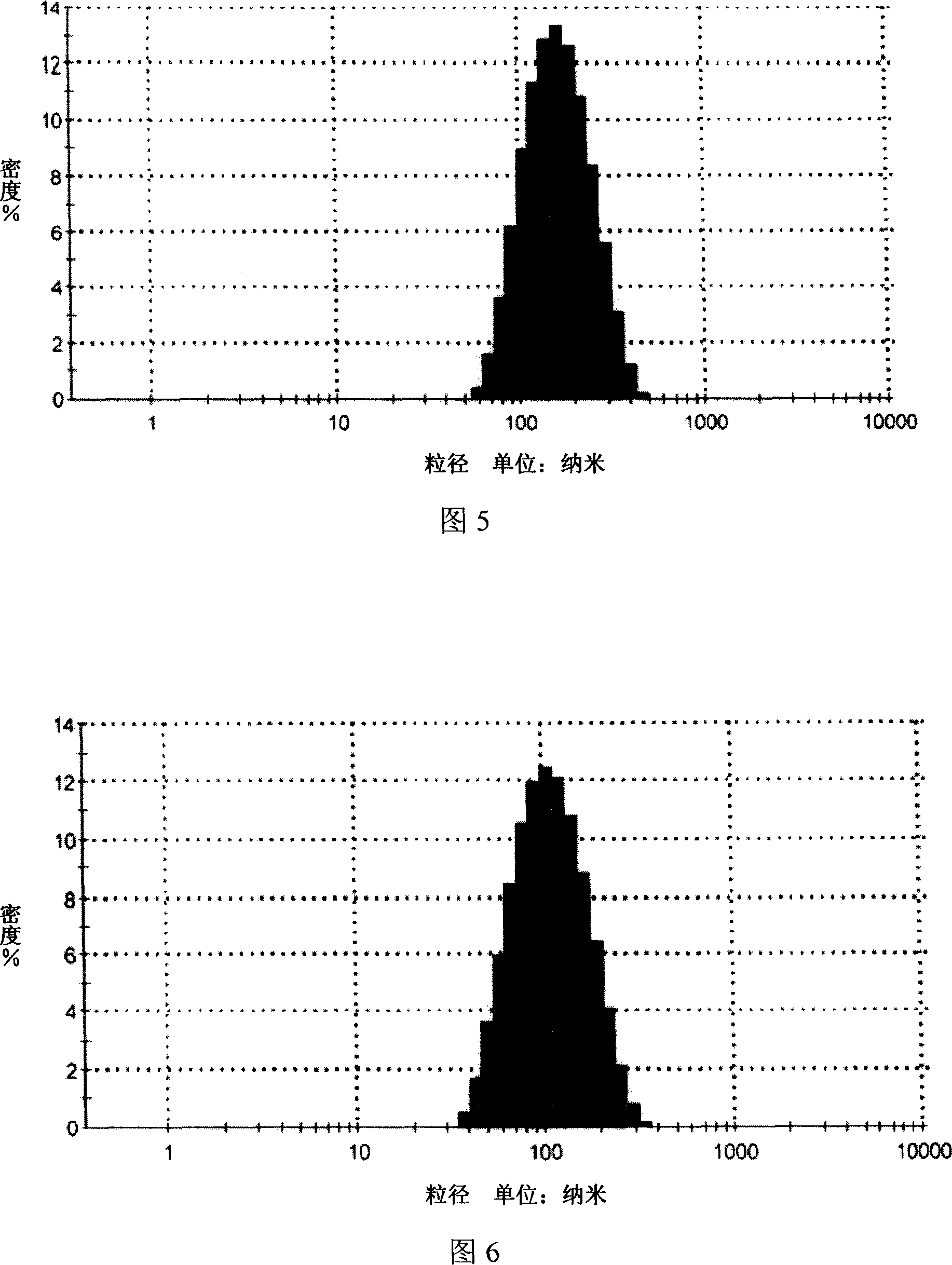
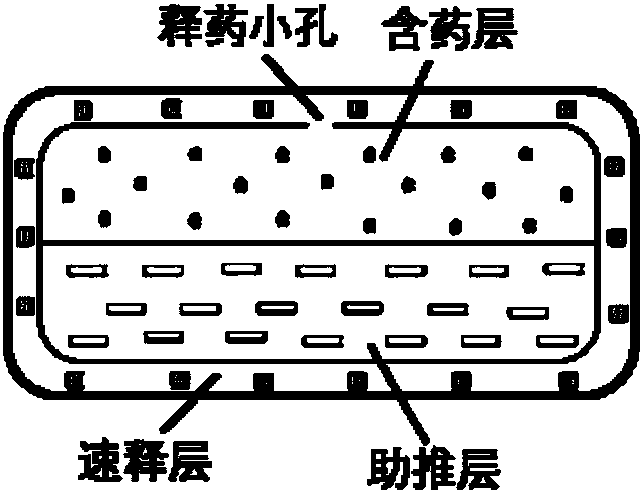
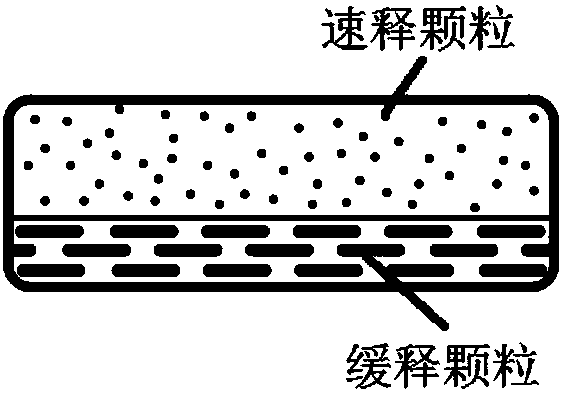
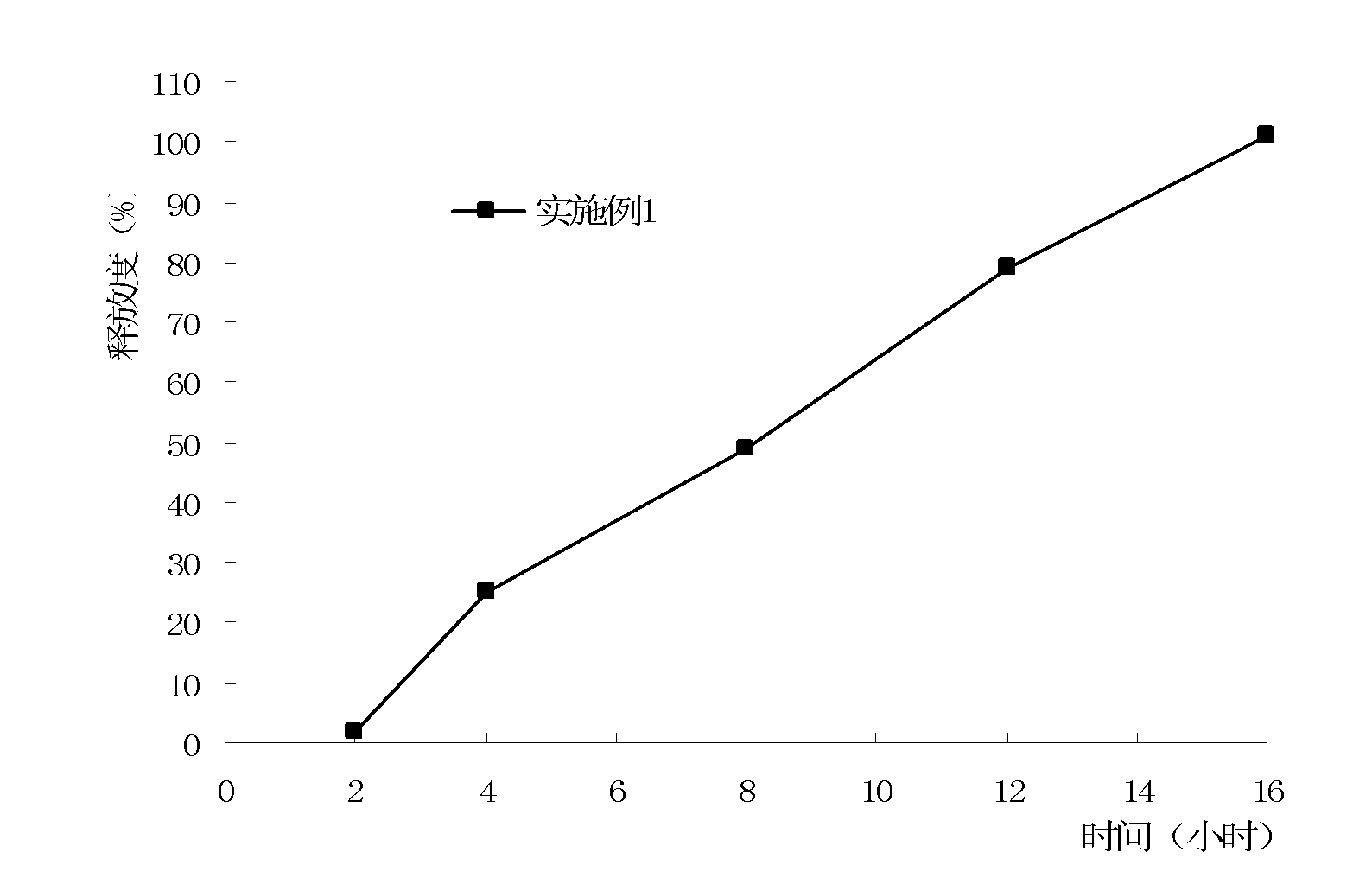
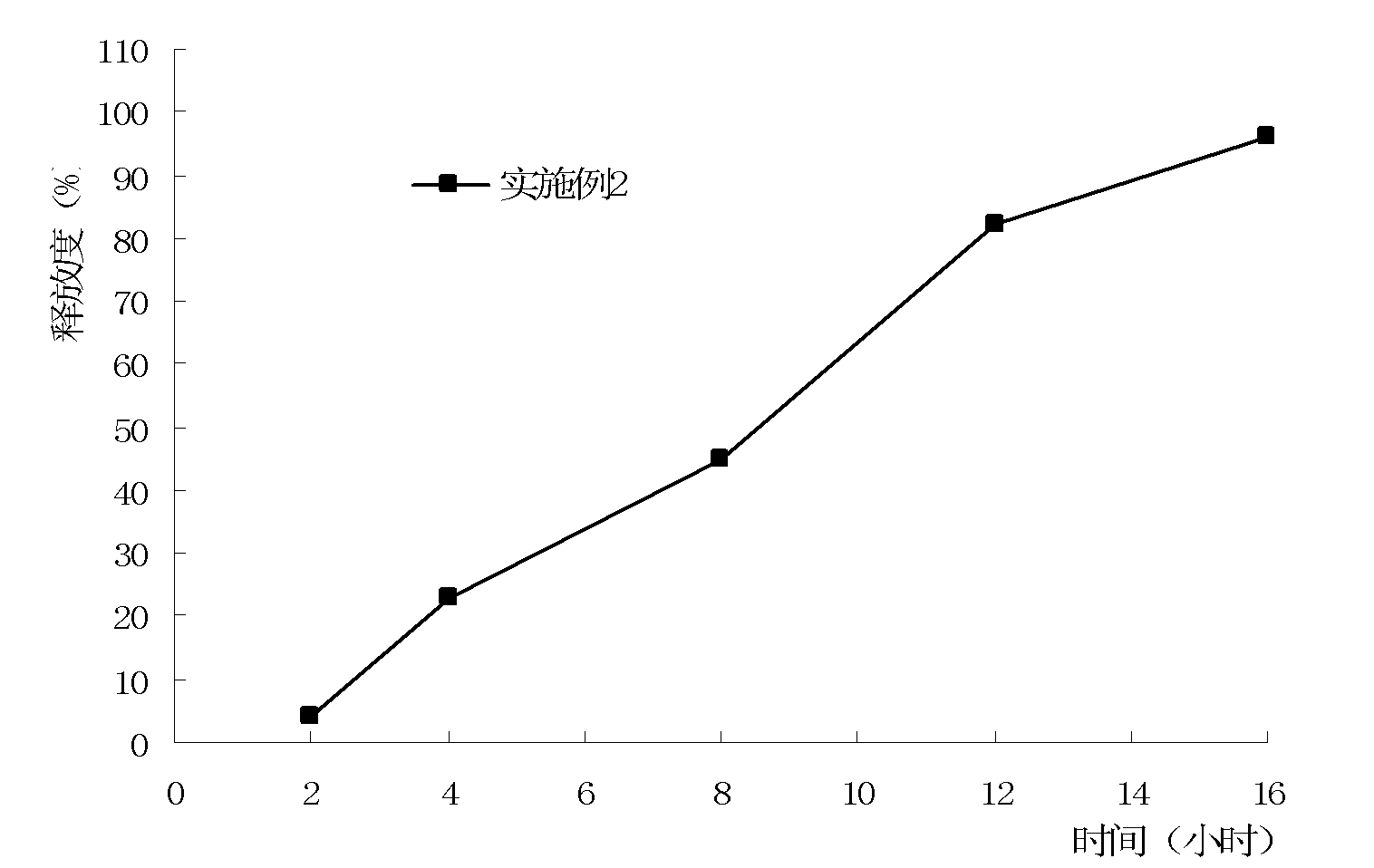
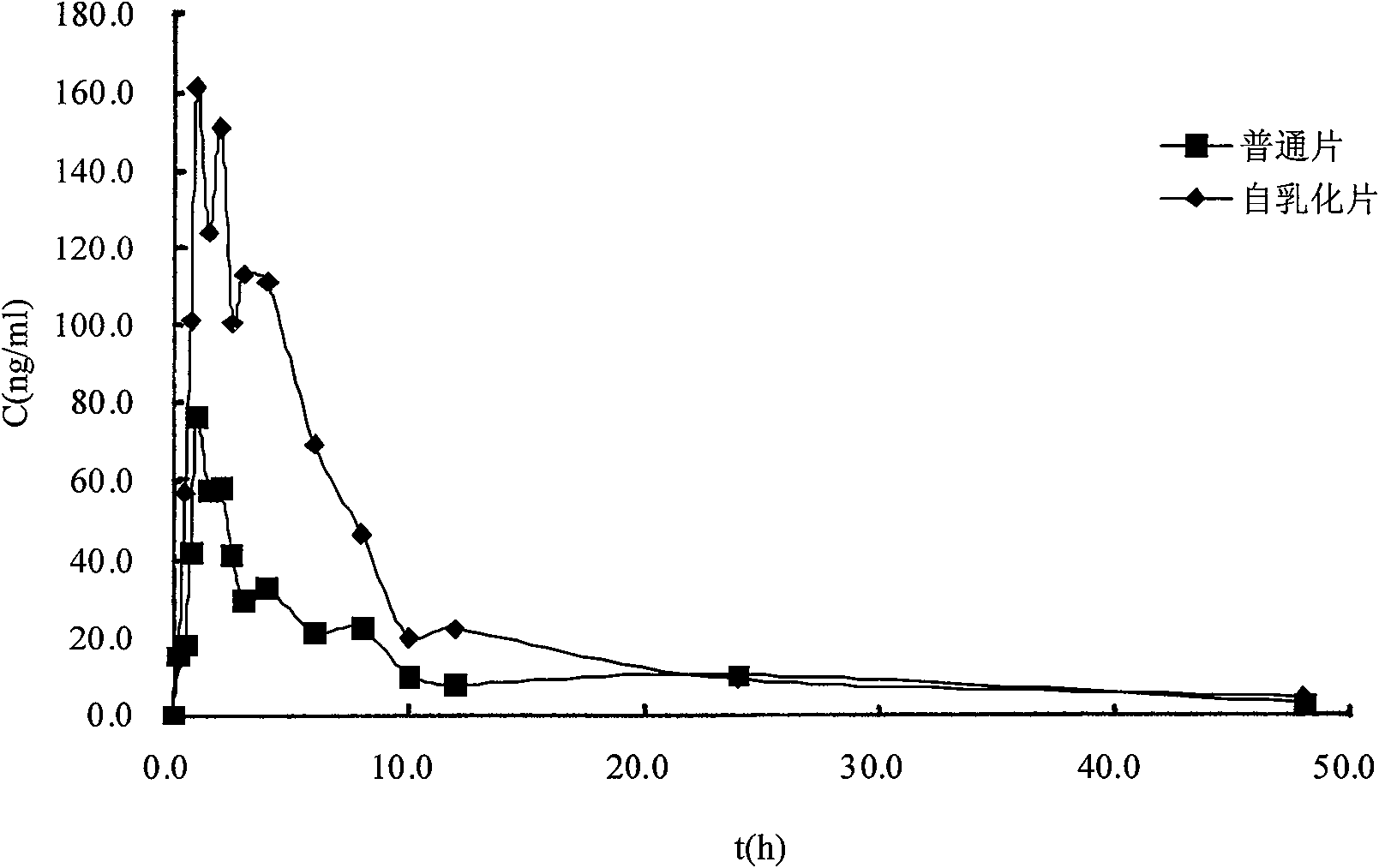
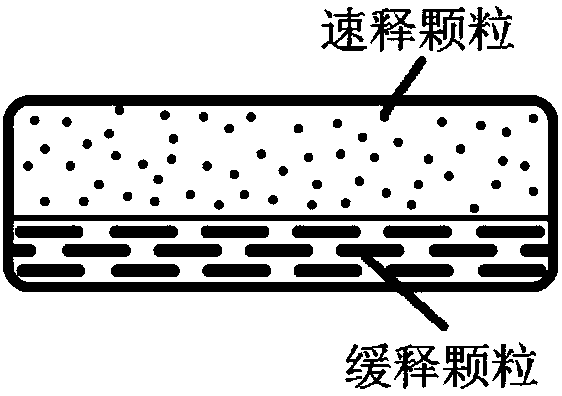
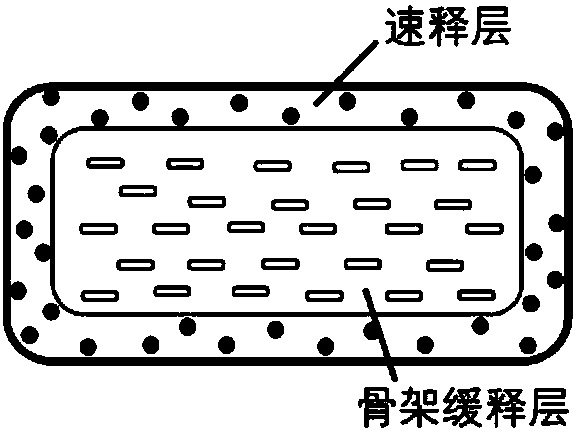
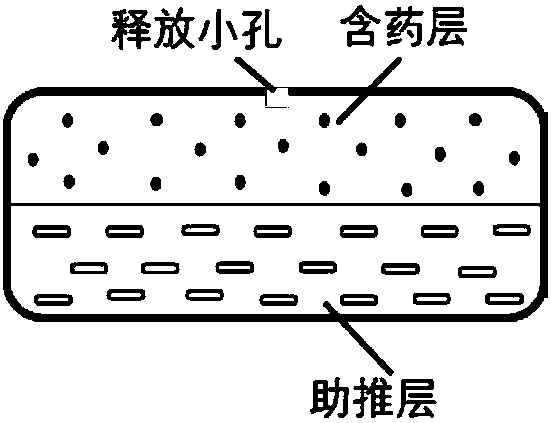
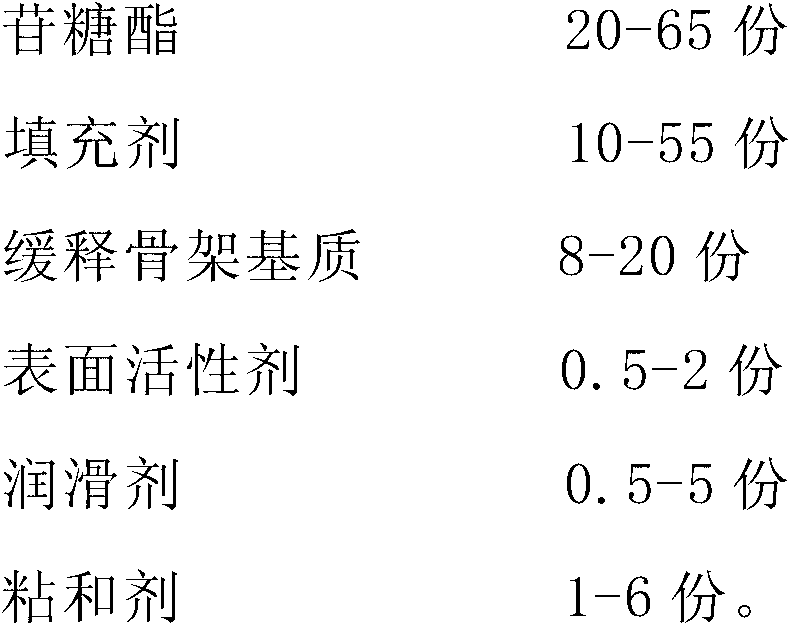
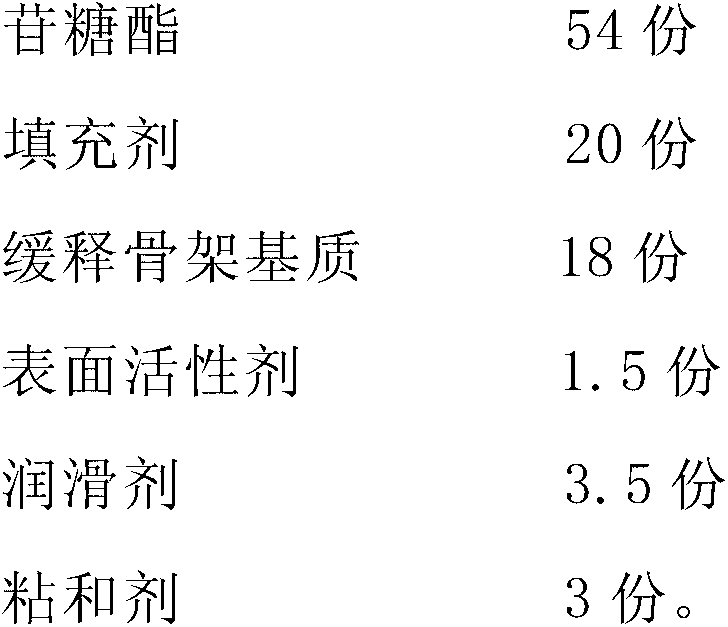
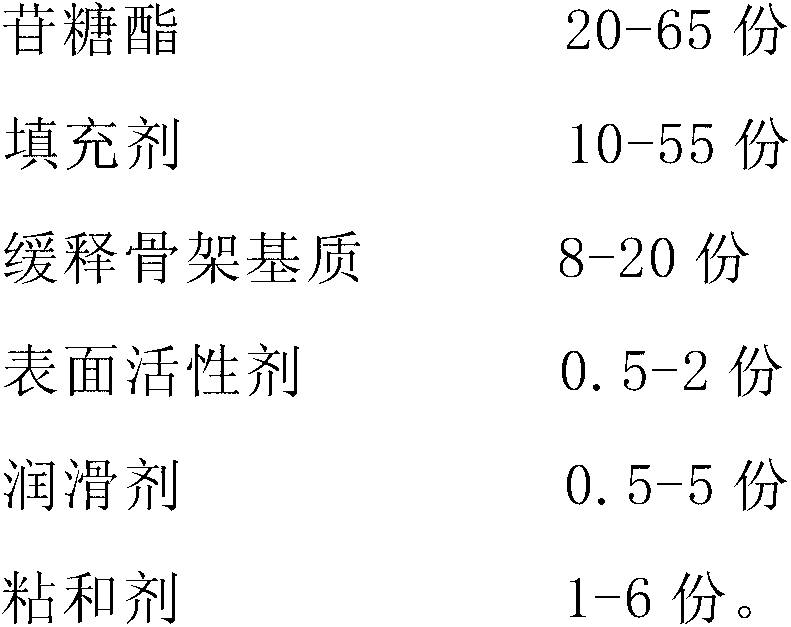
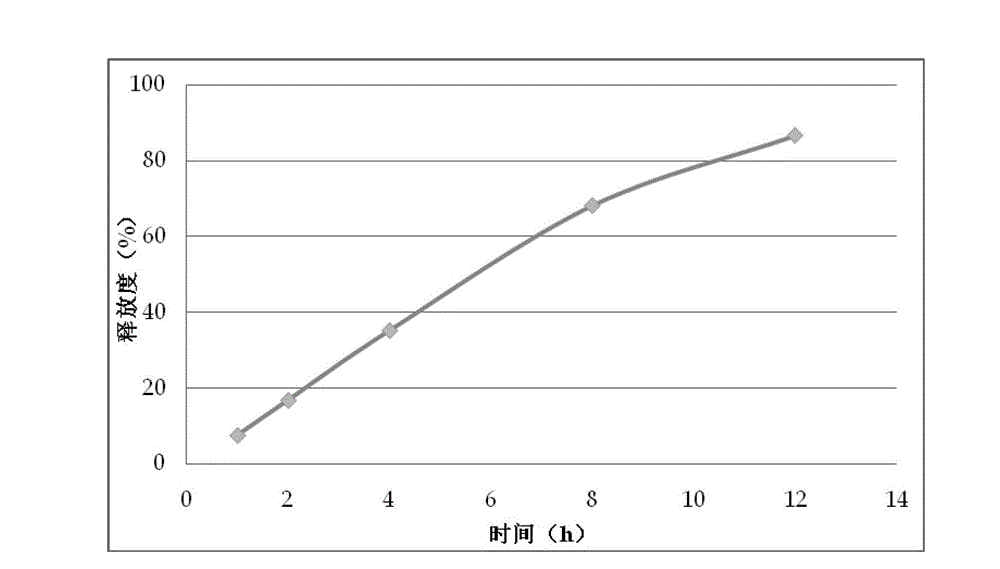
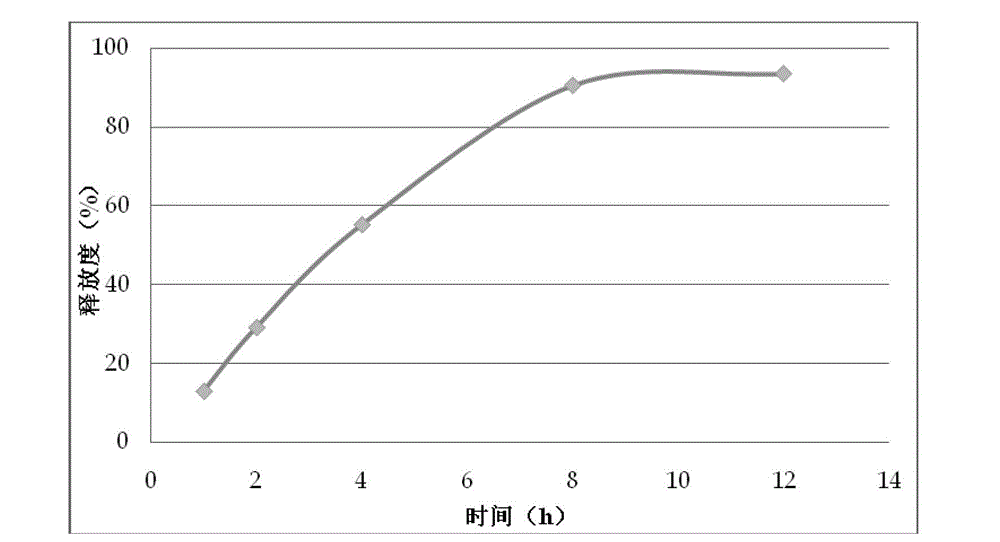
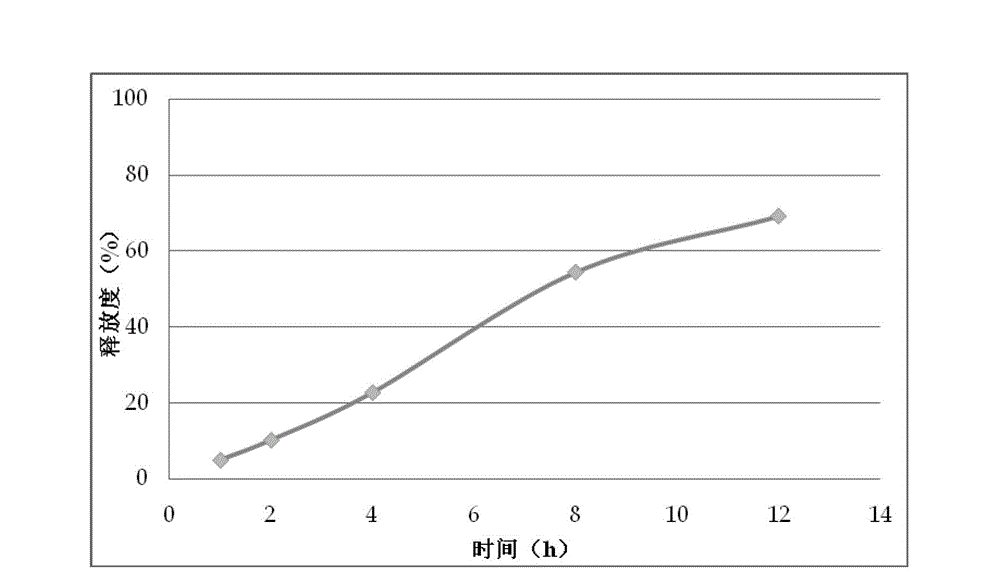
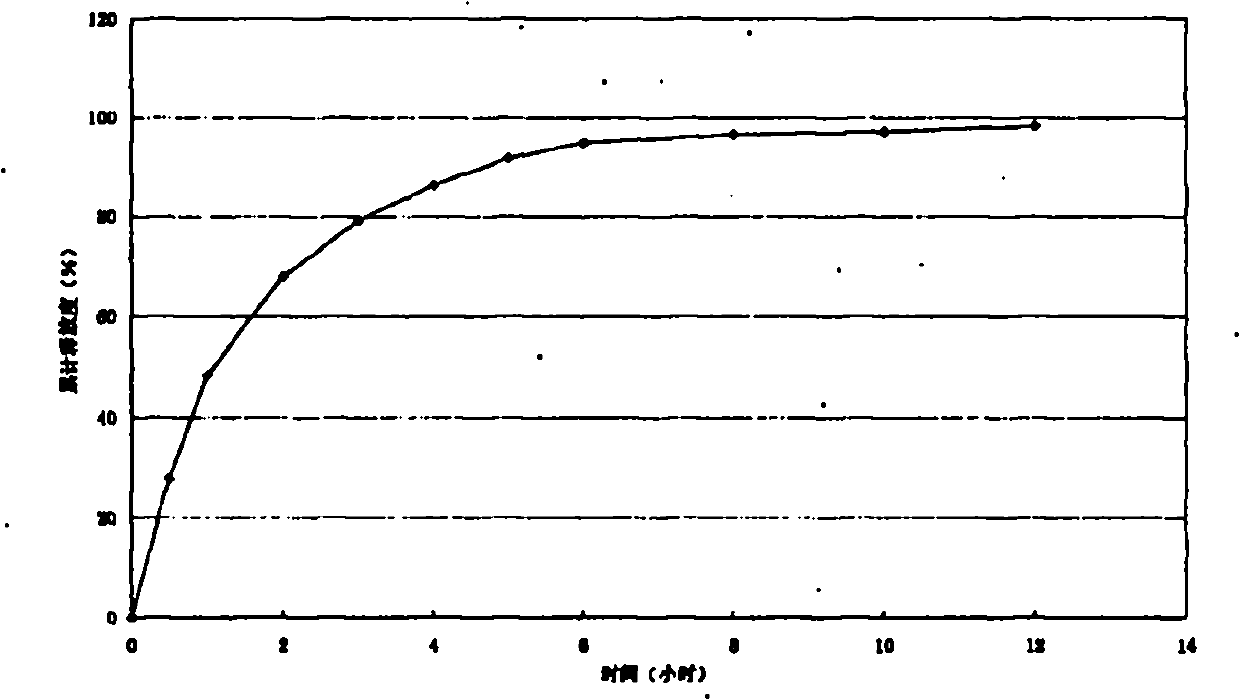
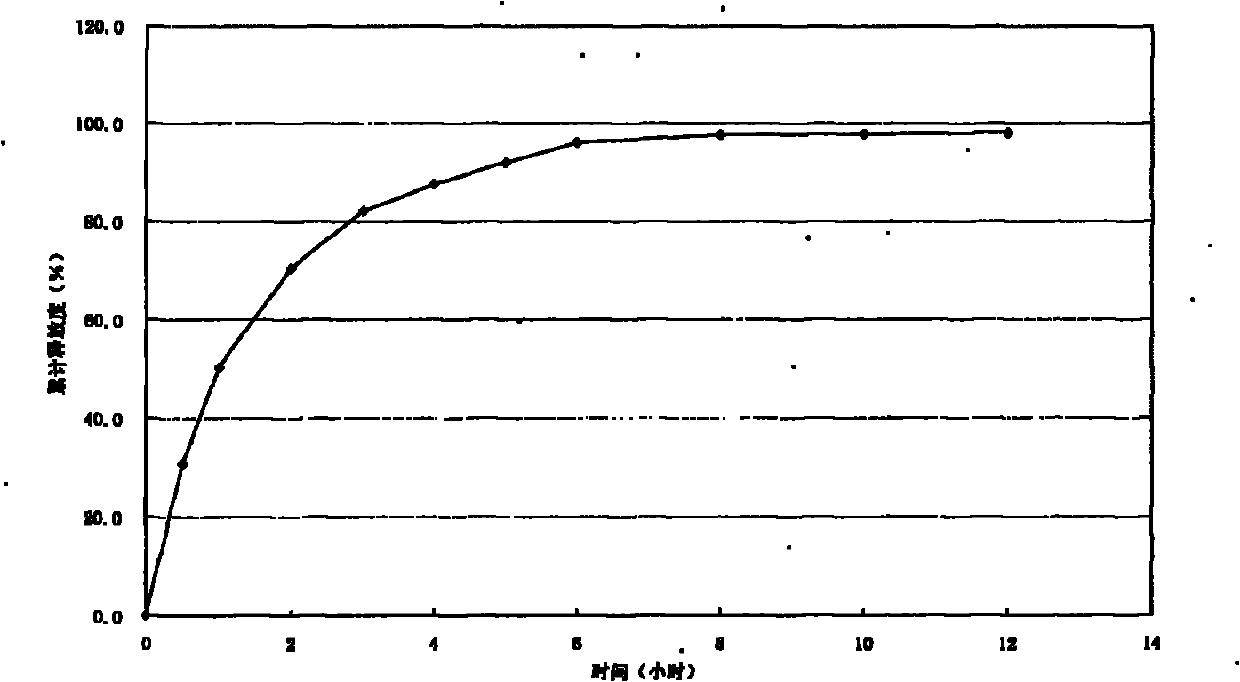
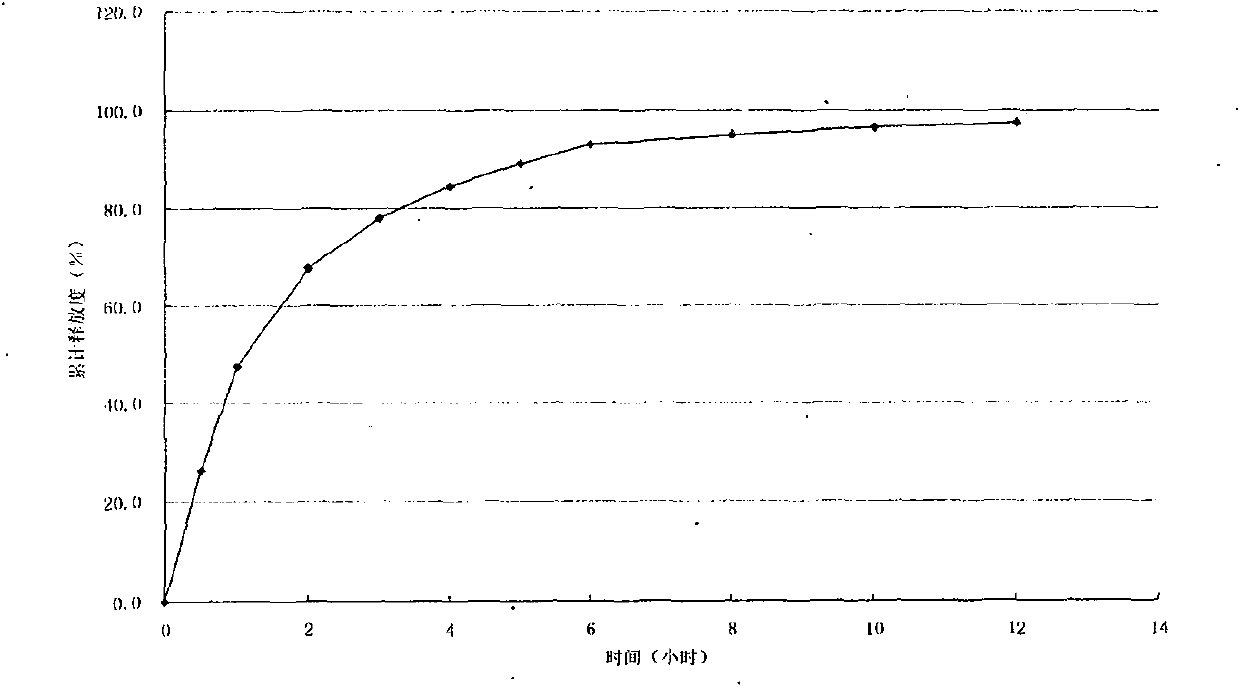
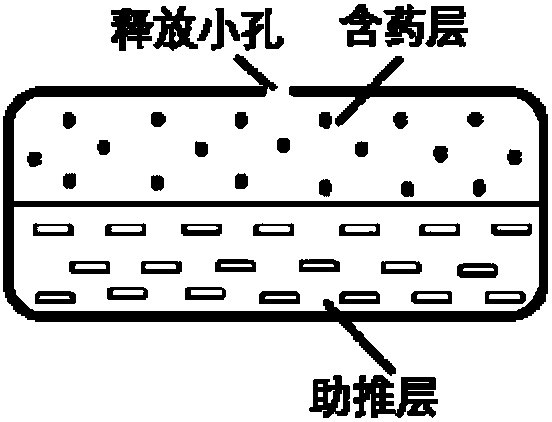
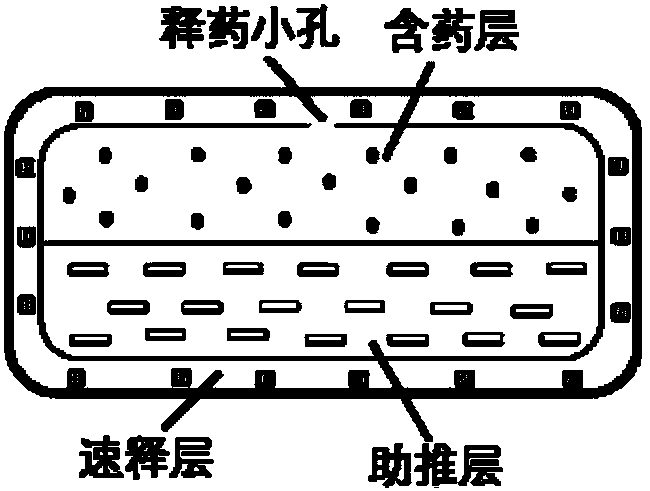
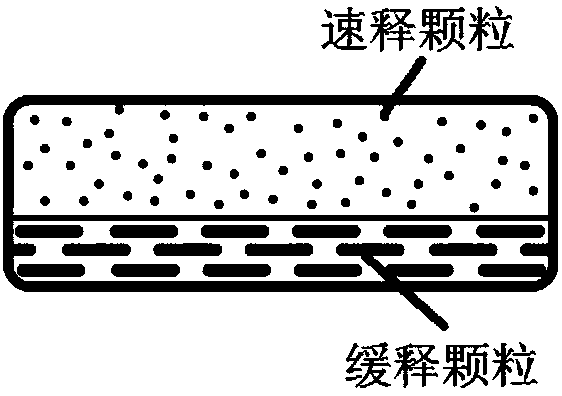
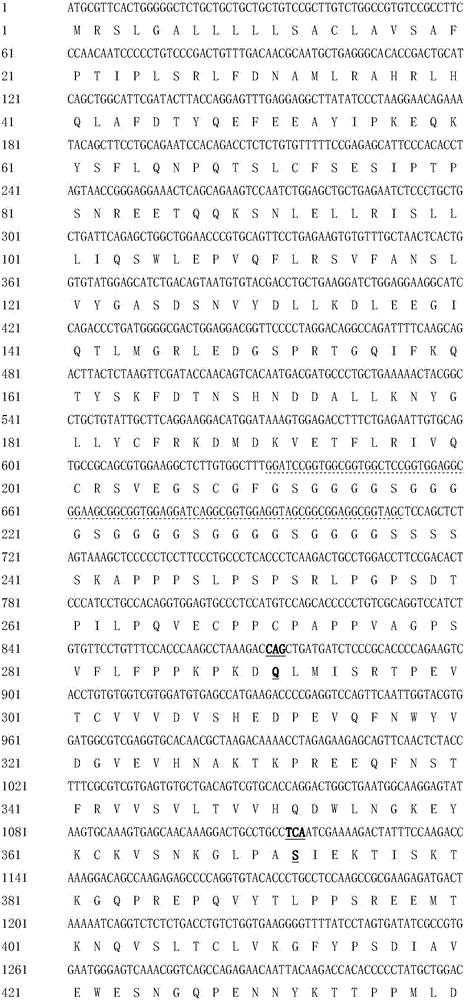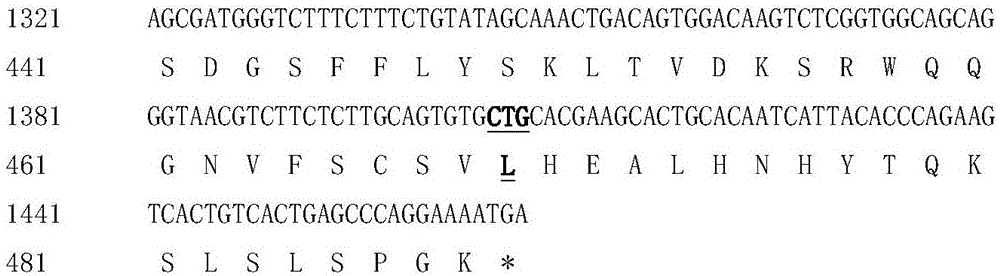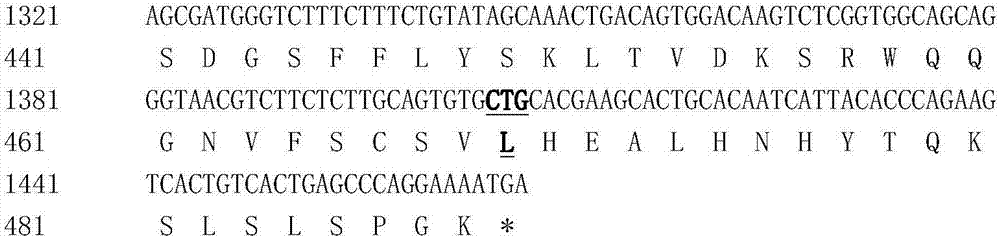Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
87results about How to "Small fluctuations in blood concentration" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
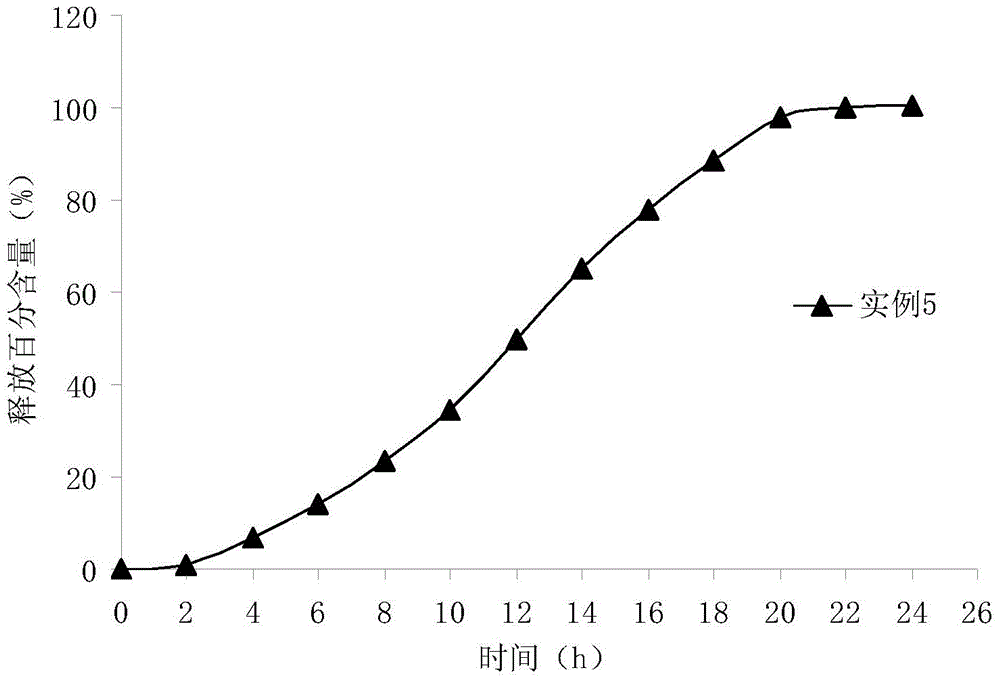
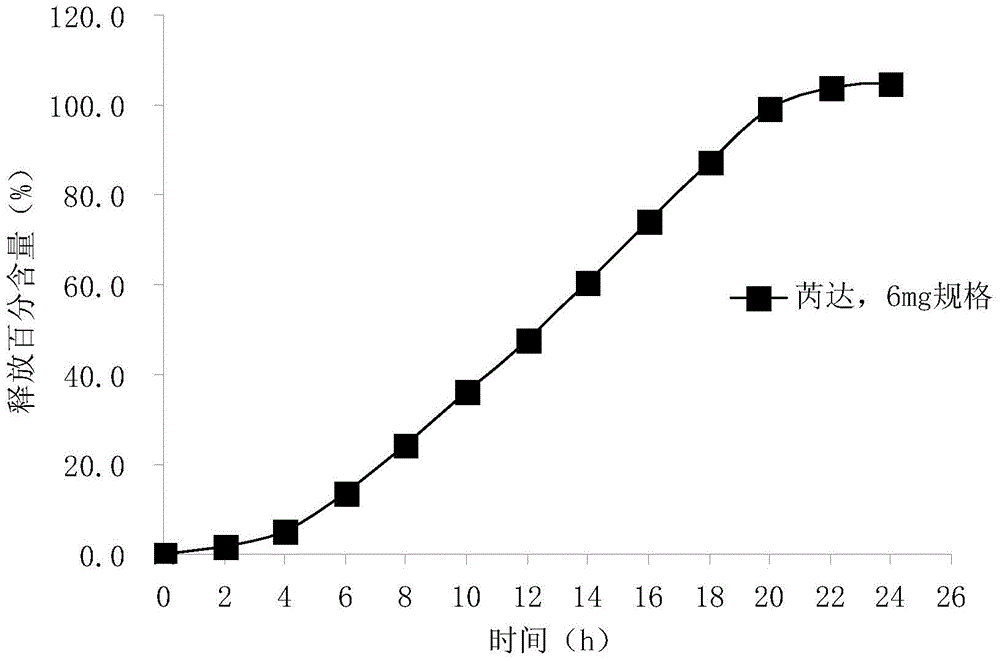
Memantine hydrochloride capsule sustained-release preparation and preparation method for same
InactiveCN102552218ALarge distribution areaImprove bioavailabilityNervous disorderPharmaceutical delivery mechanismControl layerSide effect
The invention discloses a memantine hydrochloride capsule sustained-release preparation, wherein the preparation is composed of two parts, namely, immediate-release grains and sustained-release grains; the sustained-release part is composed of a blank pellet core, a medicine layer and a release control layer; and the immediate-release part is composed of a blank pellet core and a medicine layer. The capsule is uniform in content, good in release effect, stable in blood concentration, good for reducing the toxic and side effects of medicines, and is capable of being used for treating moderate-to-severe Alzheimer-type dementia, and the medicine-taking times of the patient are reduced,.
Owner:无锡万全医药技术有限公司
Compound preparation of calcium antagonist and timishatan for reducing blood pressure and its use
ActiveCN1679954AUnique Pharmacological PropertiesPharmacological properties are slowOrganic active ingredientsCardiovascular disorderReflexREFLEX DECREASE
The invention discloses a compound preparation of calcium antagonist and timishatan for reducing blood pressure and its use. The inventive compound preparation is composed of following components by weight portion: 10-160 portion of timishatan, 0.5-50 protion of calcium antagonist. The calcium antagonist expands blood vessel to induce reflex sympathetic nerve excitement and the timishatan antagonizes neurohormone activity which is able to release from the sympathicus over-activation in the CCB blood pressure reduction, reduce the adverse effect induced by CCB treatment, provide all-around protection of the target organ such as heart, brain and kidney. The association of two acts synergistic effect to reduce blood pressure.
Owner:SUZHOU DAWNRAYS PHARM CO LTD
Stomach detention sustained and controlled release medicament releasing system and preparation method
ActiveCN101371822AEasy to swallowImprove complianceSurface/boundary effectCarbohydrate active ingredientsControlled releaseOrganic acid
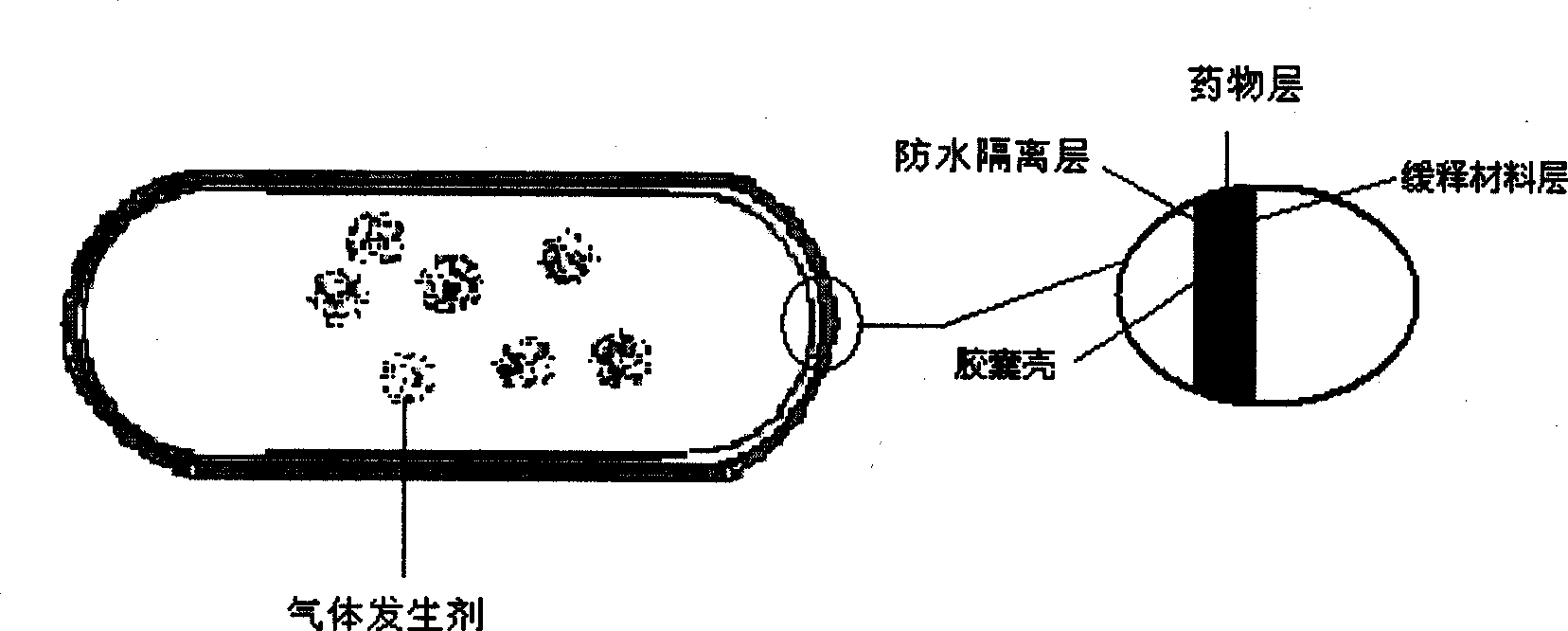
The invention provides a new air sac type gastric retention controlled-release medicine release system, including (1) an air sac which is formed by wrapping high molecular film-forming materials and hydrophobic materials outside a hollow sac; (2) a medicine containing layer which is composed of medicines and pharmaceutically acceptable excipient; the medicine containing layer covers the outside of the air sac and includes a medicine controlled release layer or a medicine slow release layer. If necessary, a quick release layer, which is composed of medicines and excipient and covers the outside of the controlled release layer or the slow release layer, can also be included. The air sac can also be internally filled with a certain amount of gas generant which includes carbonate and pharmaceutically acceptable organic acid. The average density of the whole formulation can be generally controlled below 0.6 / cm<3> and is obviously superior to the present gastro-floating formulation on sale, and the floating time in the stomach is rather longer than a common gastro-floating formulation.
Owner:北京天衡药物研究院有限公司
Pregabalin stomach floating-type slow-release tablet and preparation method thereof
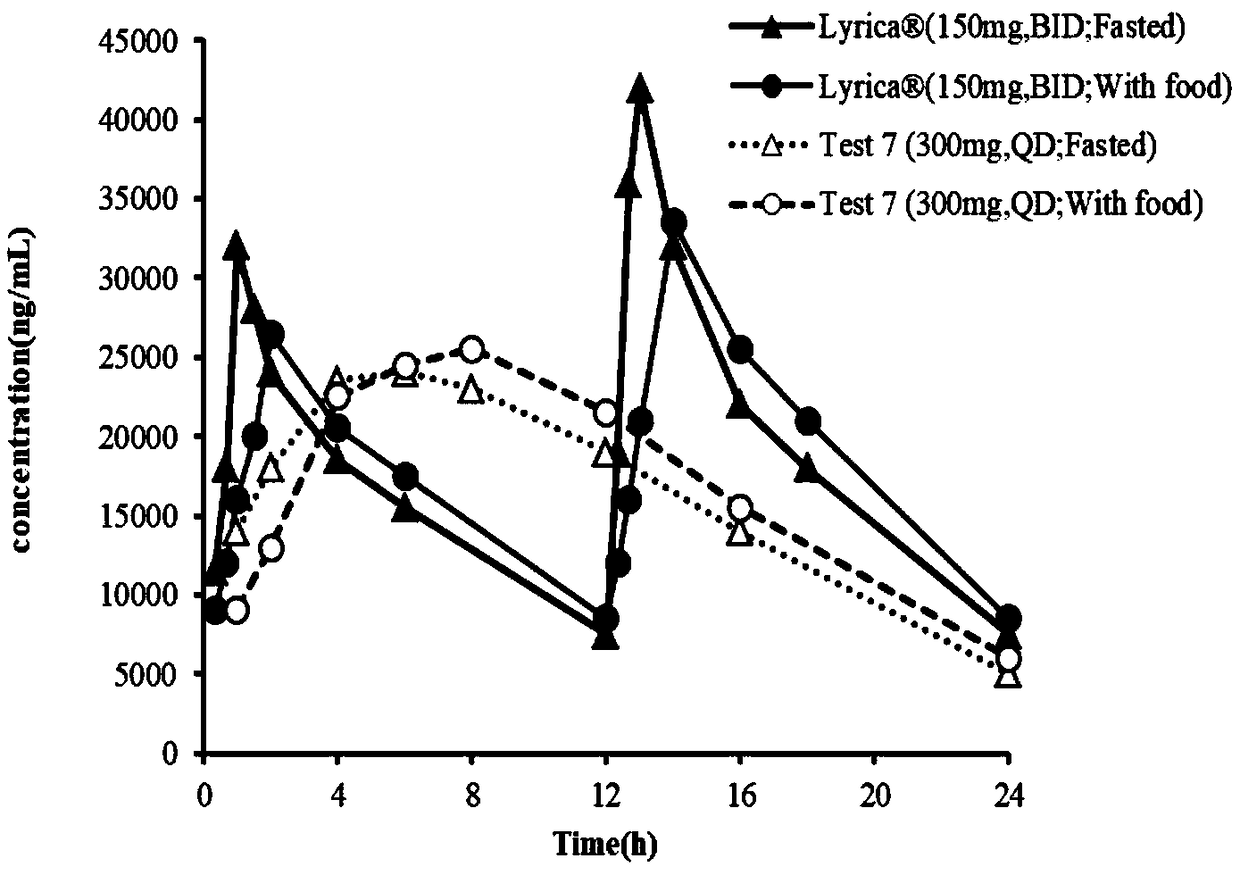
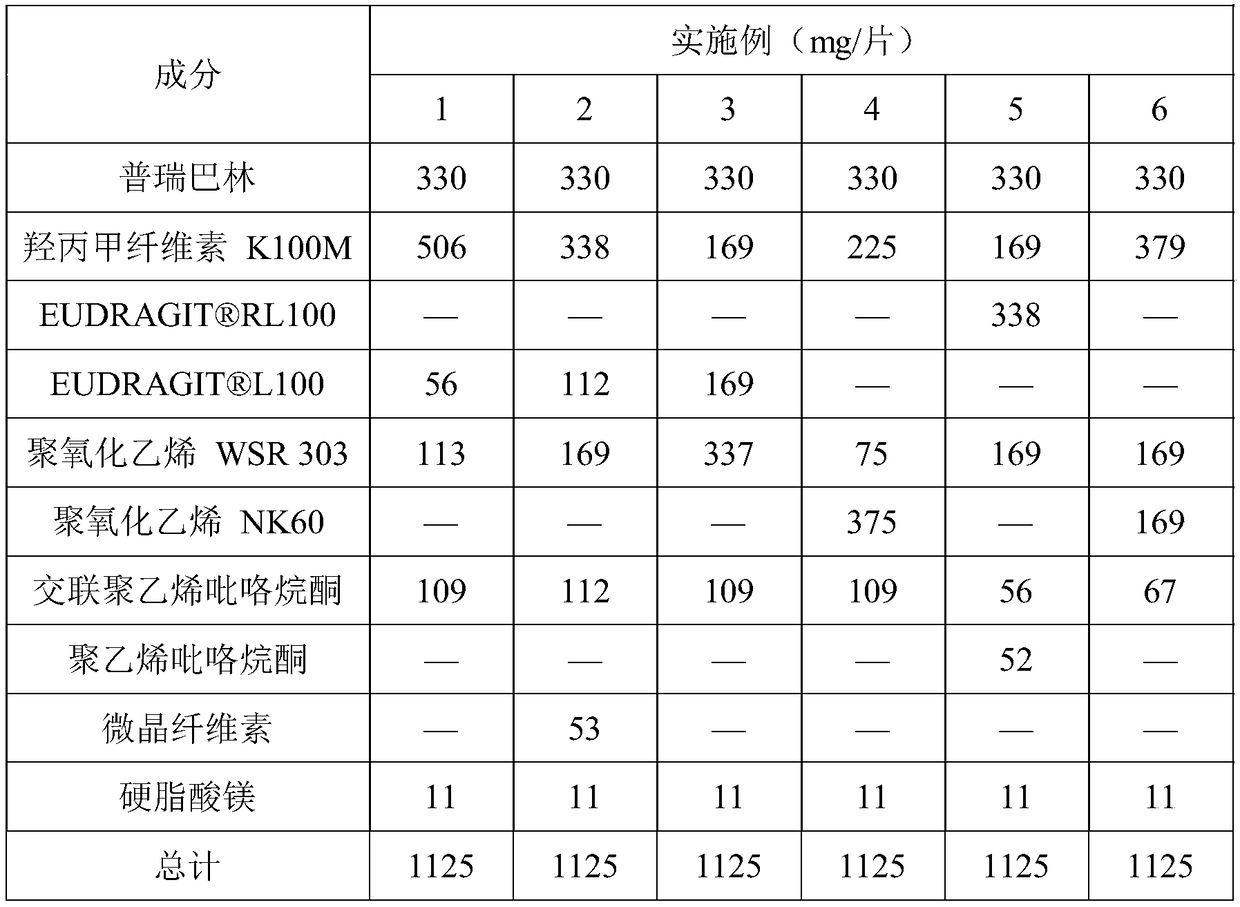
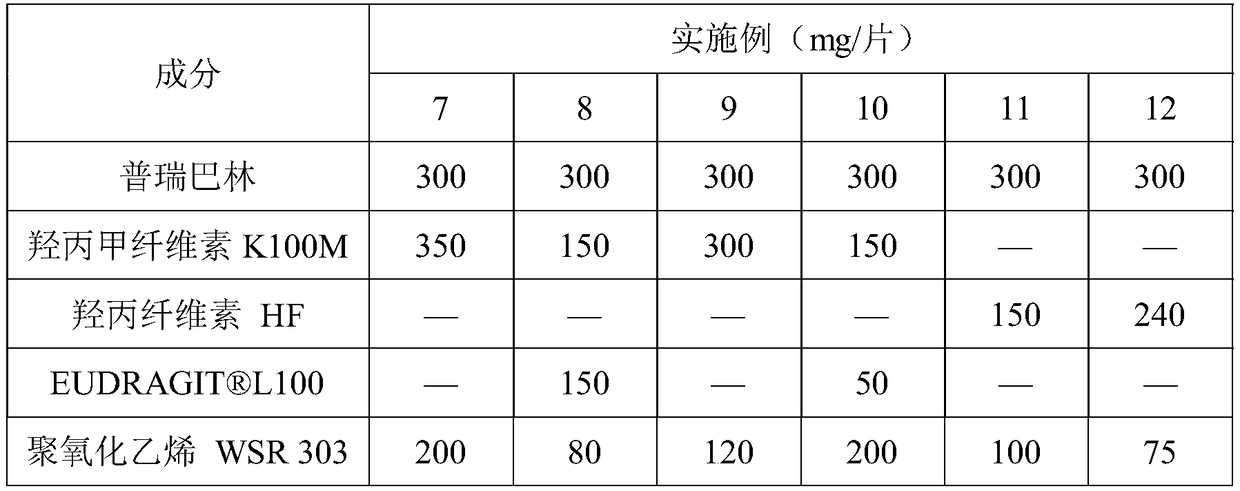
ActiveCN109044981AProlong gastric residence timePromote absorptionOrganic active ingredientsNervous disorderBlood concentrationAcrylic resin
The invention discloses a pregabalin stomach floating-type slow-release tablet. The pregabalin stomach floating-type slow-release tablet comprises an active ingredient, a framework material, a swelling agent and an excipient. The active ingredient is pregabalin and pharmacologically acceptable salt, a solvate, a hydrate or a complex thereof. The framework material is combination of any one or moreof hydroxypropyl methylcellulose, hydroxypropylcellulose, acrylic resin and derivatives thereof, wherein the active ingredient accounts for 7-33% of a total weight of the slow-release tablet, the framework material accounts for 5-50% of the total weight of the slow-release tablet, the swelling agent accounts for 5-55% of the total weight of the slow-release tablet, and the balance of the excipient. The pregabalin stomach floating-type slow-release tablet is capable of, through selecting the suitable combination of the framework material and the swelling agent, achieving the following purposes: 1) prolonging residence time of the slow-release tablet in a stomach, enabling a drug to be continuously released and absorbed, and reducing fluctuation of blood concentration; and 2) reducing the physiological condition effect of a patient, and enabling the efficacy of the slow-release tablet to have a smaller individual difference.
Owner:AC PHARMA CO LTD
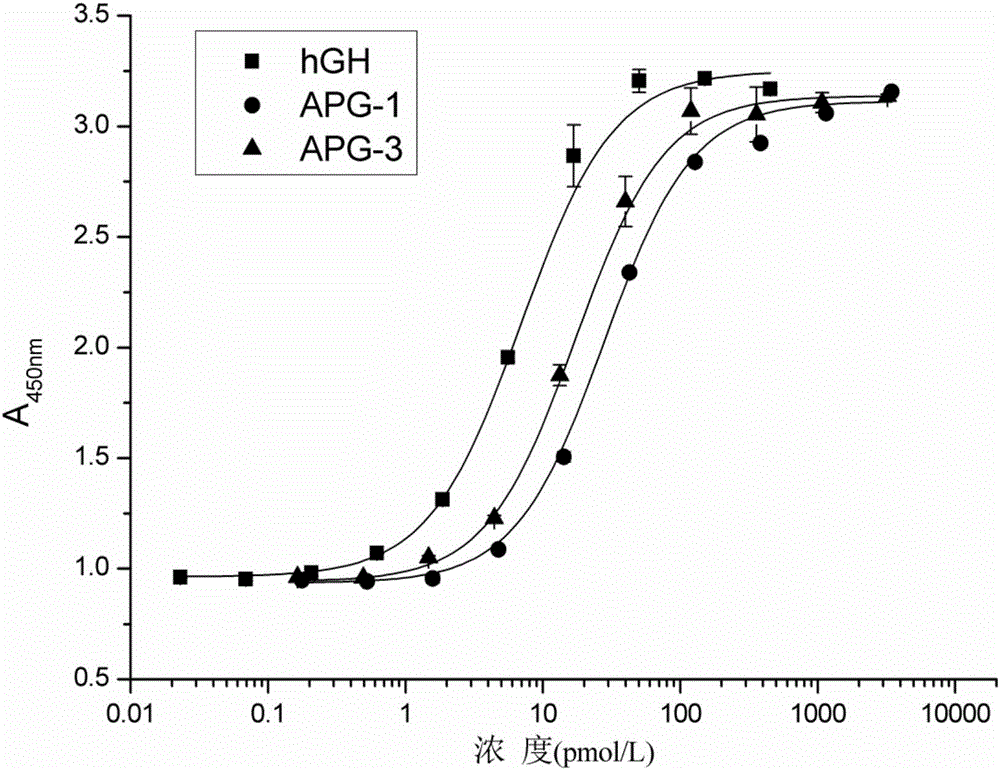
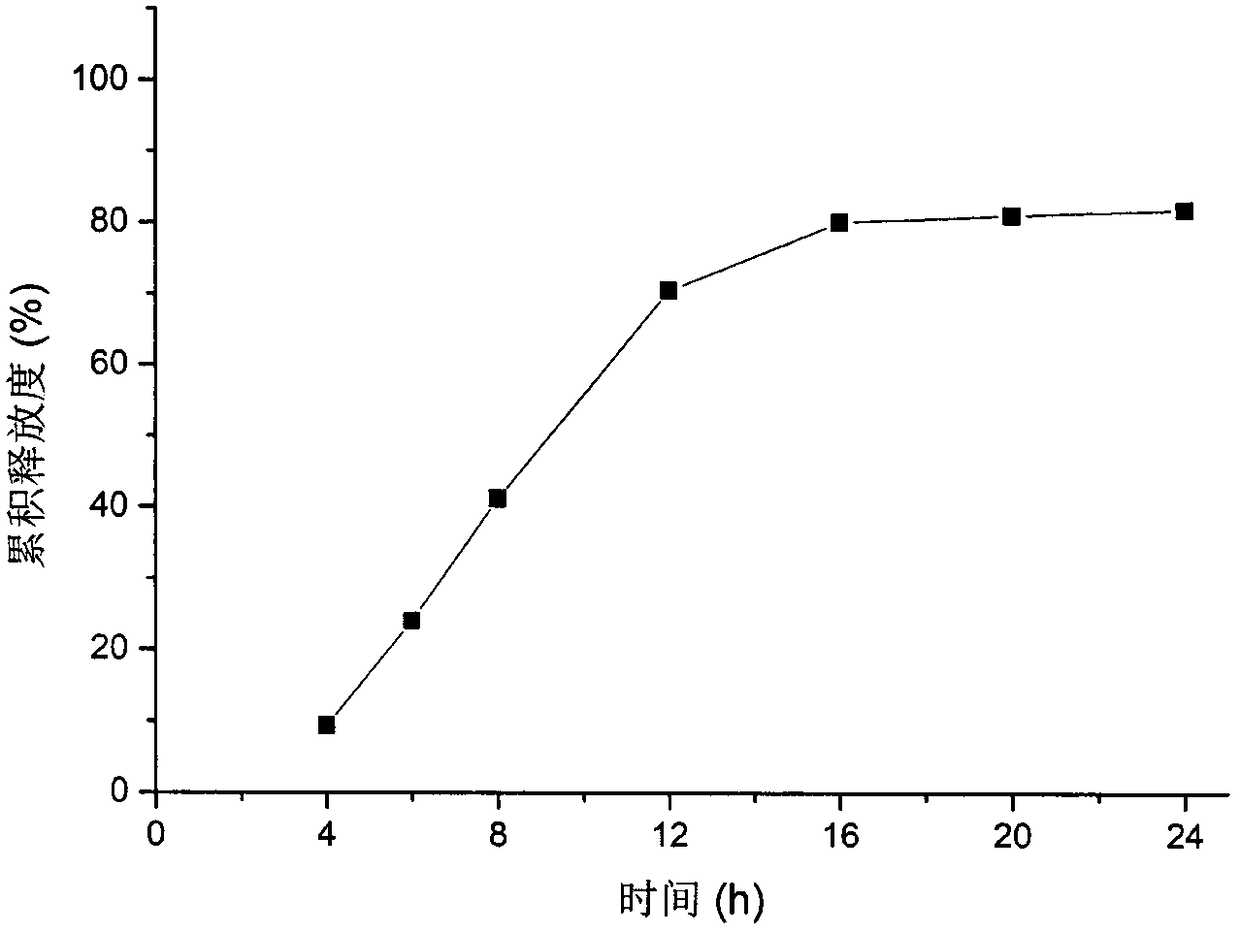
Hyperglycosylated human growth hormone fusion protein and preparation method and application thereof
InactiveCN106256835AImprove stabilityLow immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsHalf-lifeImmunoglobulin Fc Fragments
The invention discloses hyperglycosylated human growth hormone fusion protein. The human growth hormone fusion protein sequentially contains a human growth hormone (hGH), a flexible peptide joint (L), human chorionic gonadotropin beta-carboxyl terminal rigid peptide (CTP) and a human immunoglobulin Fc fragment from the N terminal to the C terminal. The invention further discloses a method for efficiently preparing the fusion protein. Compared with a recombinant hGH, the built fusion protein has more excellent in-vivo drug efficacy, the in-vivo circulation half-life period is prolonged, the administration frequency is greatly decreased, and the bioavailability is improved; meanwhile, the production process is simpler and more efficient.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Nifedipine double-layer osmotic pump tablet and preparation method thereof
PendingCN108338976AFacilitated releaseReduce the number of dosesOrganic active ingredientsPharmaceutical non-active ingredientsNifedipineMedicine
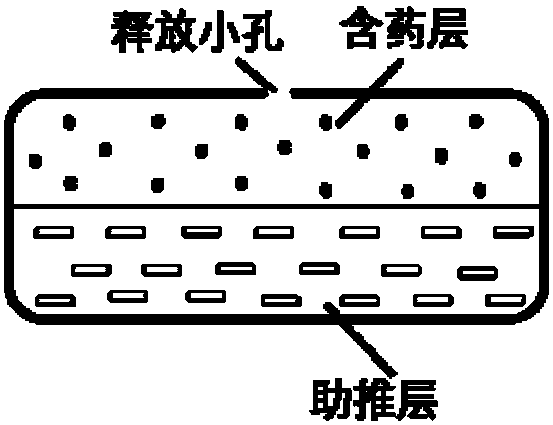
The invention provides a nifedipine double-layer osmotic pump tablet. The nifedipine double-layer osmotic pump tablet includes a medicated-layer tablet core, a boosting-layer tablet core, a semipermeable membrane and single drug-releasing holes arranged in the surface of the semipermeable membrane at one side of a medicated layer. The nifedipine double-layer osmotic pump tablet is simple in preparation process and low in cost and has stable drug-releasing rate; zero-level release is basically realized in 416 hours, and the the drug release is basically complete; the purpose of administration of preparation once per day is achieved, and the compliance of a patient is improved. The invention provides a preparation method of a nifedipine controlled-release tablet.
Owner:CHINA PHARM UNIV
Oral-applied slow-releasing or release-controllable solid clozapine
InactiveCN1395931ASmall fluctuations in blood concentrationStable blood concentrationOrganic active ingredientsNervous disorderMedicineCurative effect
An oral-applied slow-releasing or release-controlalble solid clozapine for treating psychosis is prepared from clozepine, skeleton assistant material and filler (starch, hydroxypropyl cellulose, ethylcellulose, etc.). Its advantages are high curative effect, and stable concentration of medicine in blood.
Owner:DAYA PHARMA HUIZHOU
High-glycosylation human growth hormone fusion protein and preparation method and purpose thereof
ActiveCN107286248AReduced effector functionImprove stabilityPeptide/protein ingredientsAntibody mimetics/scaffoldsHuman Chorionic Gonadotropin Beta SubunitHalf-life
The invention discloses high-glycosylation human growth hormone fusion protein. The human growth hormone fusion protein provided by the invention is characterized in that human growth hormone (hGH), flexible peptide joint (L), at least one human chorionic gonadotropin beta-subunit carboxyl terminated rigid peptide (CTP) and human immunoglobulin Fc fragments are sequentially contained from the end N to the end C. The invention also discloses a method for effectively preparing the fusion protein. The built fusion protein has more excellent in vivo medicine effect than recombination hGH; the in vivo circulation half-life period is prolonged; the medication administration frequency is greatly reduced; in addition, the bioavailability is improved; meanwhile, the production process is more simple and efficient.
Owner:AMPSOURCE BIOPHARMA (SHANGHAI) INC
Lornoxicam double-layer sustained release tablets
ActiveCN101342177AReduce the number of dosesBlood concentration is effectiveOrganic active ingredientsAntipyreticPharmaceutical preservativesSustained-Release Preparations
The invention relates to a double-layer sustained release lornoxicam tablet comprising (a) a quick release layer and (b) a sustained release layer, wherein, the quick release layer comprises (a1) the lornoxicam, (a2) alkaline matter and (a3) an other optional carrier or excipient acceptable to pharmacy and the sustained release layer comprises the (b1) lornoxicam, (b2) sustained release substance and (b3) the other optional carrier or the excipient acceptable to the pharmacy. The weight proportion of (a1) and (b1) is 1:50 to 50:1. The invention also provides a preparation method of the double-layer sustained release lornoxicam tablet. The double-layer sustained release preparation of the invention has the advantages of the quick effect of the quick release preparation and the sustained effect of sustained release preparation. In addition, the double-layer sustained release preparation can maintain the effect of effective blood concentration continuously and stably after the effective blood concentration is reached rapidly.
Owner:CHINA PHARM UNIV +1
Lipid prodrug of drug containing guanidino and pharmacosome thereof
InactiveCN101723857ASolve the problem of low bioavailability due to difficulty in crossing biofilmsSolve the problem of low bioavailabilityOrganic active ingredientsOrganic chemistryLong chain fatty acidLipid formation
The invention relates to a lipid prodrug of a drug containing guanidino and pharmacosome thereof. The lipid prodrug is a medicinal compound which is in covalent union with long-chain fatty acid with a single chain or double chains and contains guanidino. The pharmacosome is formed in such a way that the lipid prodrug disperses freely in a proper medium, is orderly arranged into a single-layer or double-layer structure, and is automatically assembled finally. The pharmacosome has biological targeting property, can effectively enhance the bioavailability of the drug and opens up a novel concept for solving the problems of poor transmembrane capability, low bioavailability and the like of a water soluble drug.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Microemulsion containing matrine
InactiveCN100998592AImprove stabilityEasy to prepareAntibacterial agentsOrganic active ingredientsMatrineOil phase
A microemulsion of matrine with high biologic utilization rate and stability is proportionally prepared from matrine, emulsifier, emulsifying aid, oil phase and water phase. Its preparing process is also disclosed.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Olaparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
InactiveCN108201536AAchieve absorptionAccurate blood drug concentration in vivoOrganic active ingredientsPill deliveryControl releaseEnzyme inhibition
The present invention relates to an olaparib oral controlled-release and sustained-release pharmaceutical composition, which contains dissolution form improved olaparib and a matrix polymer for release rate adjustment. According to the present invention, the in vivo absorption behavior, the blood drug level and the PARP enzyme inhibition level of the pharmaceutical composition can be controlled, the pharmaceutical composition has the improved olaparib loading and / or oral absorption and / or bioavailability and / or blood drug concentration control and / or enzyme inhibition level control, and can beused as the sole preparation or can be combined with other treatment methods in the treatment of cancer.
Owner:SHANGHAI INST OF MATERIA MEDICA CHINESE ACAD OF SCI
Sinlmenine hydrochloride plaster and its preparation method
InactiveCN1557310AModerate adhesionIncreased matrix strengthOrganic active ingredientsSheet deliveryWater soluble polymersIrritation
The coculine hydrochloride cataplasma with raised patient compliance includes coculine hydrochloride as medicine component; matrix comprising water soluble polymer skeleton, stuffing, cross-linking agent, cross-linking regulator, humectant and transdermal promoter; and non-woven fabric as lining layer. The preparation process includes dispersing water soluble polymer in glycerin to obtain phase A; mixing cross-linking agent, cross-linking regulator and small amount of deionized water to obtain phase B; adding transdermal promoter, medicine and sufficient deionized water to obtain phase C; mixing phase an and phase B and adding gradually phase C to obtain mixture; painting the mixture onto the lining layer and covering with anti-sticking layer. The present invention has good skin hydrolyzing effect, proper skin affinity, no pain produced during taking off, no irritation on skin and obvious slowly releasing effect.
Owner:SHENYANG PHARMA UNIVERSITY
Preparation method of melbinum osmotic pump controlled release tablets
ActiveCN102133204AHigh dissolution rateIntrinsic quality is stableOrganic active ingredientsMetabolism disorderIrritationControlled Release Tablet
The invention relates to a preparation method of melbinum osmotic pump controlled release tablets for treating type 2 diabetes. The preparation method comprises the steps of preparing tablet cores, coating semi-permeable membrane layers, perforating, drying and coating damp-proof layers, characterized in that when the tablet cores are prepared, a medicinal salt of melbinum and a nonionic surfactant are added, and the nonionic surfactant accounts for 2-20wt.% of the medicinal salt of melbinum. The medicament disclosed by the invention has stable release, no irritation on intestinal tracts and few times of administration, is acceptable by patients, and greatly improves the compliance of patients.
Owner:SHANDONG XINHUA PHARMA CO LTD
Aceclofenac in extended-released tablets and method of manufacturing the same
ActiveCN101108170AReduce stimulationSmall fluctuations in blood concentrationOrganic active ingredientsAntipyreticSustained Release TabletAdditive ingredient
The invention relates to a drug sustained-release preparation and its preparation method, in particular to an aceclofenac sustained-release tablet and its preparation method, which comprises the following ingredients according to the weight percentage: aceclofenac of 60 to 95 per cent, skelecton retarder of 3 to 30 per cent, adhesive of 1 to 10 per cent and lubricant of 0.5 to 15 per cent. The hydroxypropylmethyl cellulose and carboxyvinyl polymer are adopted as optimized skelecton retarder. With such a technical proposal in the invention, the one aceclofenac sustained-release tablet can be taken a day to effectively reduce the fluctuation of blood drug level, prolong the maintenance duration of effective blood drug level and lower down the incitement to gastrointestinal tract. Besides, the invention also provides the preparation method of the aceclofenac sustained-release tablet.
Owner:ZHEJIANG JIANFENG PHARM CO LTD
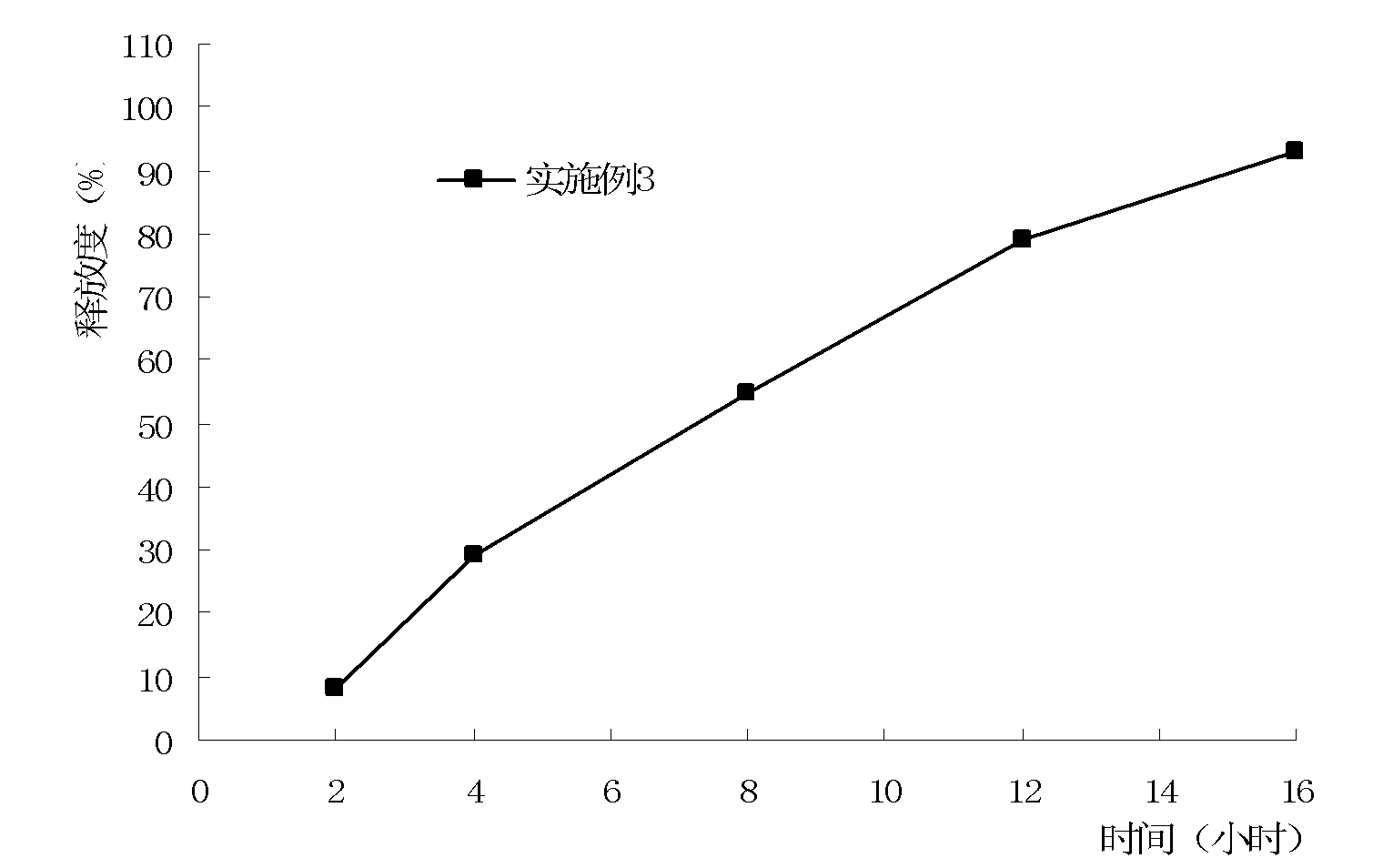
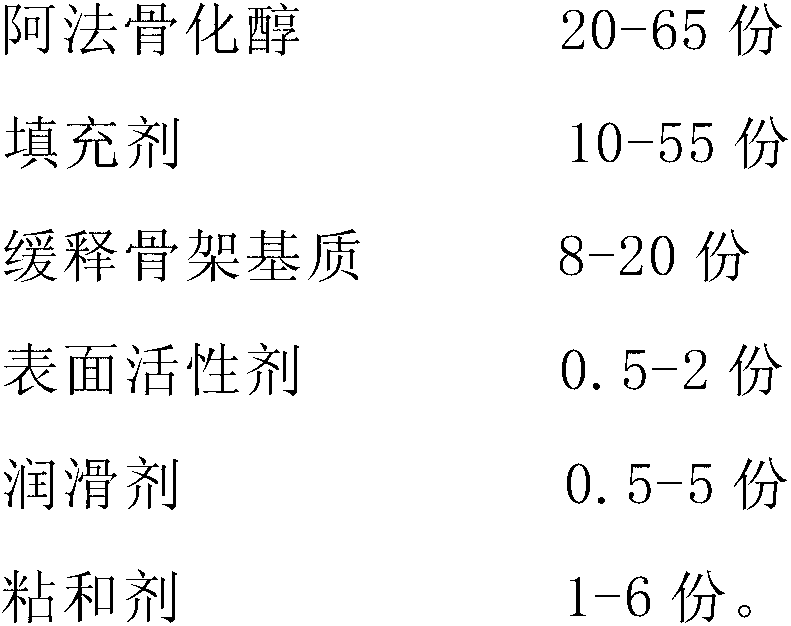
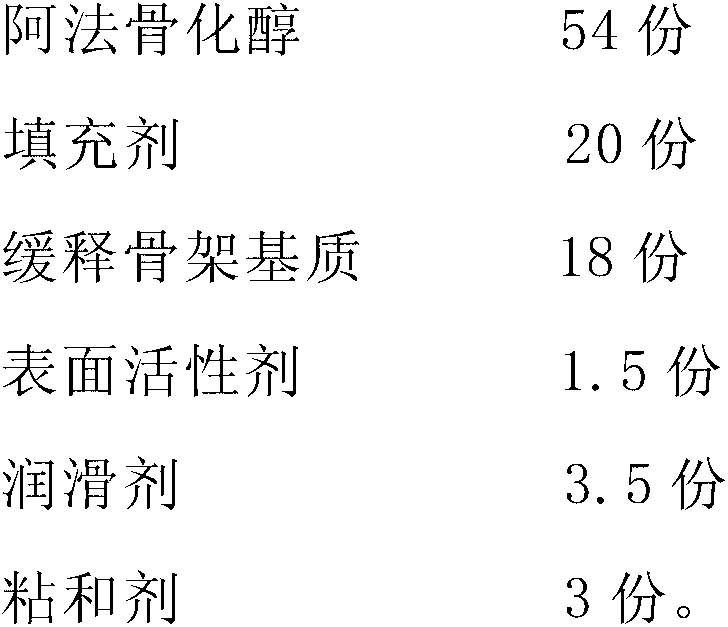
Alfacalcidol sustained-release preparation and preparation method thereof
ActiveCN103127020ASmooth releaseSmall fluctuations in blood concentrationOrganic active ingredientsMetabolism disorderSurface-active agentsAlfacalcidol
The invention discloses an alfacalcidol sustained-release preparation and a preparation method thereof. The alfacalcidol sustained release preparation comprises alfacalcidol, a sustained-release skeleton matrix, a filling agent, a surface active agent, a lubricating agent and an adhesive. The alfacalcidol sustained-release preparation can achieve long-acting release, and can stably release effective ingredients in a balanced mode within 24 hours.
Owner:CP PHARMA QINGDAO CO LTD
Bezafibrate sustained-release composition
InactiveCN101120931AReduce releaseSmall fluctuations in blood concentrationPeptide/protein ingredientsMetabolism disorderSustained release pelletsSide effect
A slow-release compound of benzafibrate is a slow-release capsule, the main part of which are the slow-release pellets. The slow-release pellets comprise the benzafibrate served as the active component and the slow-release layer with slow-release property covering outer layer of the medical pellet. The other excipient comprises the excipient material with pharmaceutical approval after mixing with benzafibrate and the slow-release material with slow-release property. The ratio among the benzafibrate, the excipient material and the slow-release material (weight ratio) is 1 to 0.01 till 1 to 0.01 till 1. The compound can prolong residence time of the medicine in the stomach. The compound is also capable of controlling the drug release, reducing the administration times, reducing toxic and side effects and improving the treatment effect.
Owner:珠海天翼医药技术开发有限公司
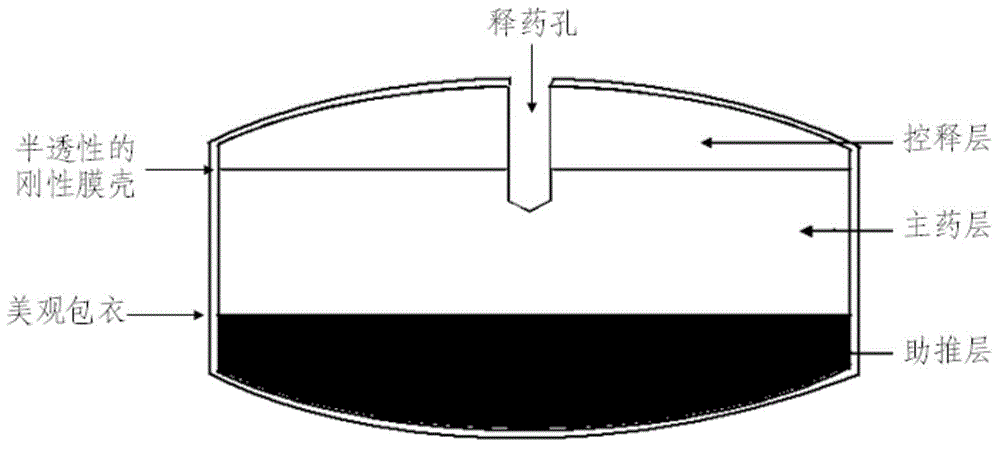
Paliperidone release rate progressive increasing preparation and preparation method thereof
ActiveCN106265583AControlled release rateThe blood concentration curve is stableOrganic active ingredientsNervous disorderPaliperidoneOsmotic pump
The invention discloses a paliperidone release rate progressive increasing preparation and a preparation method thereof. The paliperidone release rate progressive increasing preparation provided by the invention, from outside to inside, comprises a non-essential damp-proof beautiful coating, a semipermeable rigid film shell which plays a vital role, and an osmotic pump tablet core provided with one or more holes. The osmotic pump tablet core, from top to bottom, is composed of a controlled-release layer, a drug-containing layer and a push promoting layer; and penetration enhancers in the push promoting layer, in accordance with different coating weight increasing degrees, include a coating penetration enhancer I, a coating penetration enhancer II and a coating penetration enhancer III. The various coating penetration enhancers, which are different in coating weight increasing degree, differ in osmotic pressure in water. With the application of the paliperidone release rate progressive increasing preparation disclosed by the invention, when water enters the tablet core along with an osmotic pressure difference, the push promoting layer absorbs water to be swelled so as to push the outward release of the drug-containing layer and the controlled-release layer, and as time varies, the isolating coating penetration enhancers are exposed in the water, so that the osmotic pressure is continuously increased, a water-absorbing rate is increased and a drug-release rate is increased as well, thus progressive increasing drug release is achieved; therefore, the drug-release rate is effectively controlled, a blood drug concentration curve is kept stable and the fluctuation of blood drug concentration is relieved.
Owner:ZHEJIANG JINGXIN PHARMA
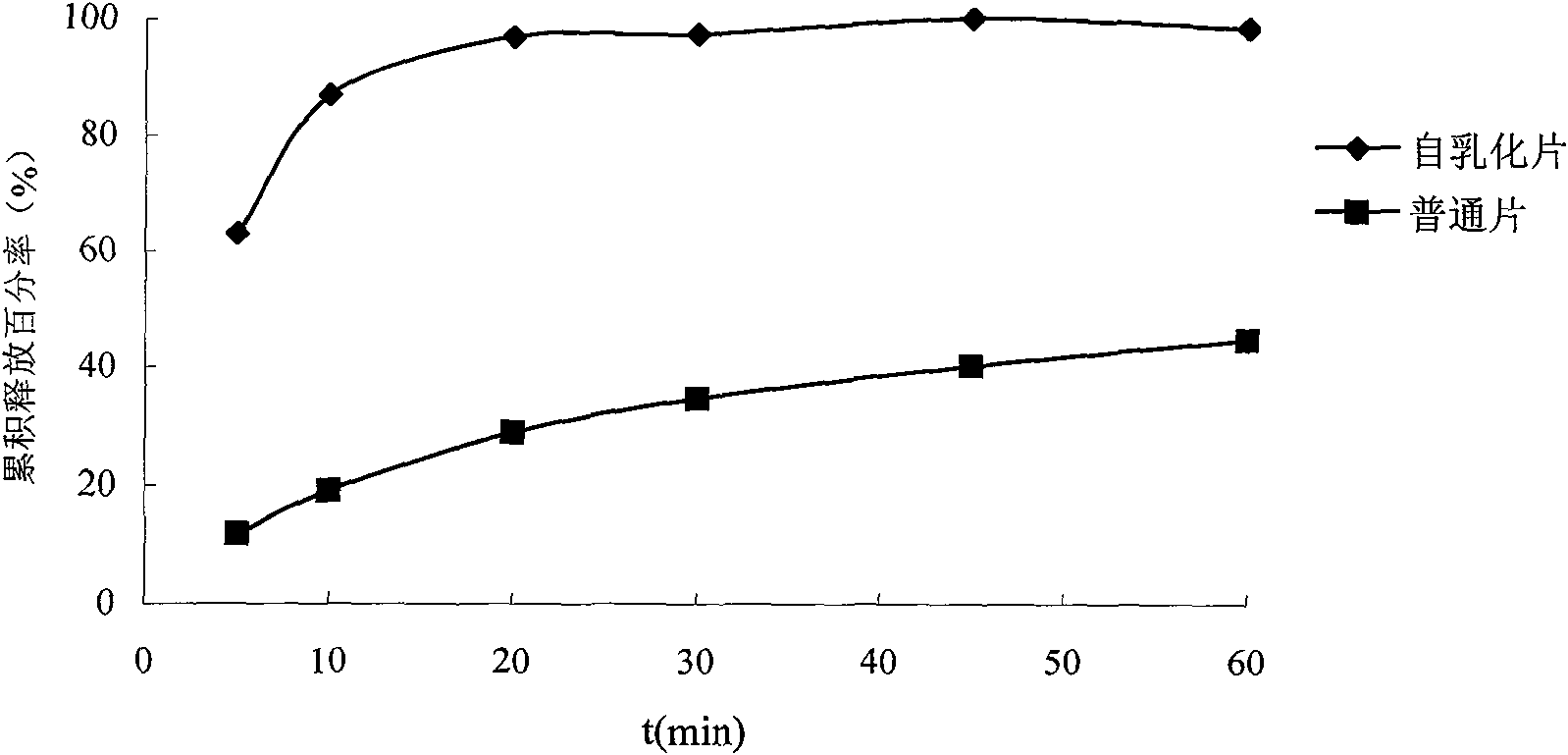
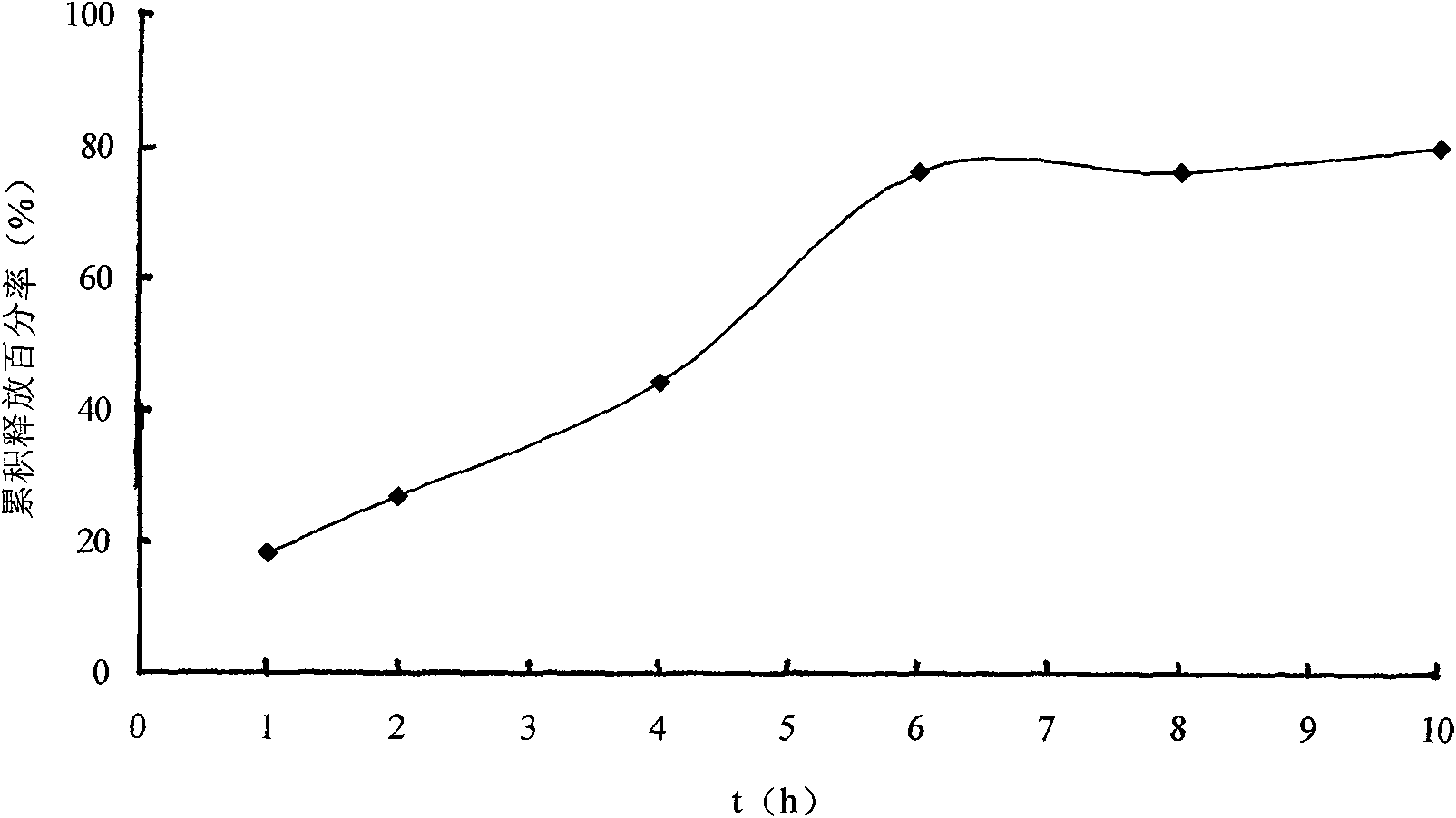
Solid self-emulsifying oral administration system of dihydropyridine calcium ion antagonist and preparation method thereof
InactiveCN101618020AEasy to manufacturePrevent precipitationOrganic active ingredientsPill deliverySorbentOil phase
The invention relates to a solid self-emulsifying oral administration system of a fat-soluble dihydropyridine calcium ion antagonist and a preparation method thereof. The solid self-emulsifying oral administration system comprises a fat-soluble dihydropyridine calcium ion antagonist and a solid medicine transmission system. The preparation method comprises the following steps: taking an oil phase, a surfactant and a cosurfactant according to the quantity of a recipe, evenly mixing, adding a medicine according to the quantity of the recipe, stirring by magnetic force at 50 DEG C, completely dissolving and evenly mixing the medicine, and forming a uniform medicine-containing self-emulsifying system. If the system is in a solid state at room temperature, the system can be prepared into a self-emulsifying semisolid skeleton capsule after being heated and melted; the system also can be prepared into various oral solid preparation forms after the medicine-containing self-emulsifying system is solidified by adopting an adsorbent; and the system also can be prepared into corresponding slow-release and controlled-release solid preparations by selecting an appropriate skeleton material and a coating technique. The preparation method is simple and convenient, has low cost and is convenient for industrialized production, and the obtained solid preparation has diversified preparation forms, convenient taking, high bioavailability, stable blood concentration, good stability and convenient storage and transportation.
Owner:SHENYANG PHARMA UNIVERSITY
Zinc gluconate pellets and preparation method thereof
InactiveCN101703478AGood dispersionQuick effectOrganic active ingredientsMetabolism disorderGluconic acidDissolution
The invention provides a zinc gluconate pellet preparation, which is prepared from zinc gluconate and pharmaceutic adjuvant, and is characterized in that: the pharmaceutic adjuvant is an excipient and an adhesive, wherein in the pellet preparation, the weight percent of the zinc gluconate is 10 to 80 percent, the weight percent of the excipient is 12 to 89 percent and the weight percent of the adhesive is 1 to 8 percent. The pellet preparation can be prepared into a slow release preparation or an enteric-coated preparation. The zinc gluconate pellet preparation has high dissolution rate, and high bioavailability; and the method is simple, convenient, and easy to operate.
Owner:JF PHARMALAND TECH DEV
Rucaparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
InactiveCN108201534AAchieve absorptionAccurate blood drug concentration in vivoPharmaceutical non-active ingredientsPill deliveryEnzyme inhibitionPolymer
The present invention relates to a rucaparib oral controlled-release and sustained-release pharmaceutical composition, which contains dissolution form improved rucaparib and a matrix polymer for regulating release rate. According to the present invention, the in vivo absorption behavior, the blood drug level and the PARP enzyme inhibition level of the pharmaceutical composition can be controlled,the pharmaceutical composition has the improved rucaparib loading and / or oral absorption and / or bioavailability and / or blood drug concentration control and / or enzyme inhibition level control, and canbe used as the sole preparation or can be combined with other treatment methods in the treatment of cancer.
Owner:SCI RAINBOW BIOPHARMA CO LTD
Tripterygium wilfordii hook extract cataplasm and preparation method thereof
ActiveCN102008536AImprove securityIncrease drug concentrationAntipyreticAnalgesicsBlood concentrationSide effect
The invention discloses a tripterygium wilfordii hook extract cataplasm and a preparation method thereof. The tripterygium wilfordii hook extract cataplasm provided by the invention contains tripterygium wilfordii hook extract and a substrate, wherein the weight ratio of the tripterygium wilfordii hook extract to the substrate is 1:3-12. The tripterygium wilfordii hook extract cataplasm provided by the invention improves the clinical medication security of the tripterygium wilfordii hook, increases the medicament concentration of the tripterygium wilfordii hook at inflammatory joints, reduces adverse reaction and toxic or side effect caused by medicaments, and has enhanced anti-inflammatory action and reduced blood concentration fluctuation.
Owner:苏州迪星生物医药科技有限公司
Dexketoprofen coating sustained-release micro-encapsulated capsule
ActiveCN101756939AReduce the frequency of takingSmall fluctuations in blood concentrationOrganic active ingredientsAntipyreticUse medicationTherapeutic effect
The invention provides a dexketoprofen coating sustained-release micro-encapsulated capsule and a preparation method thereof, which belong to the technical filed of chemico-pharmaceutical preparations. The coating sustained-release micro-encapsulated capsule consists of coating sustained-release particles and a capsule shell. The coating sustained-release particles are made of core particles containing dexketoprofen basic remedy through a coating technique. The invention can reduce the stimulation of the dexketoprofen on gastrointestinal tract and taking times, consequently, the medication compliance of patients is improved, the fluctuation of dexketoprofen blood concentration is reduced, bioavailability and treatment effect are improved and adverse reactions are reduced.
Owner:NEW FOUNDER HLDG DEV LLC +2
Chondroitin sulfate pellet and preparation method thereof
InactiveCN101703475AGood dispersionQuick effectOrganic active ingredientsAntipyreticDissolutionBioavailability
The invention provides a chondroitin sulfate pellet preparation, which is prepared from chondroitin sulfate and pharmaceutic adjuvant. The pellet preparation is characterized in that the pharmaceutic adjuvant is excipient and bonding agent, wherein the pellet preparation comprises the following components in percentage by weight: 10 to 90 percent of the chondroitin sulfate, 3 to 89 percent of the excipient, and 1 to 7 percent of the bonding agent. The pellet preparation can be prepared into a slow-release preparation or an enteric-coated preparation as requested. The chondroitin sulfate pellet preparation has high dissolution rate and high bioavailability, and the method is simple and convenient and is easy to operate.
Owner:JF PHARMALAND TECH DEV
Propylene glycol marinate sulfate-containing sustained-release preparation and preparation method thereof
ActiveCN103099796ASmooth releaseSmall fluctuations in blood concentrationOrganic active ingredientsMetabolism disorderPropylene glycolIsopropylene glycol
The invention discloses a propylene glycol marinate sulfate sustained-release preparation and a preparation method thereof. The propylene glycol marinate sulfate sustained-release preparation comprises: propylene glycol marinate sulfate, a sustained-release skeleton matrix, a filler, a surfactant, a lubricant and a binder. The propylene glycol marinate sulfate sustained-release preparation disclosed in the invention can achieve long-acting release, and can release drug ingredients stably and evenly within 24h.
Owner:CP PHARMA QINGDAO CO LTD
Daphnetin slow-release composition and preparation method thereof
InactiveCN103181909AStable and effective blood drug concentrationTo achieve the effect of sustained releasePharmaceutical delivery mechanismOil/fats/waxes non-active ingredientsMedicineHydrophilic matrix
The invention relates to a daphnetin slow-release composition and a preparation method thereof. The slow-release composition is a matrix tablet. The matrix tablet comprises daphnetin, filler, a matrix material, a bonding agent and a lubricating agent. The matrix material is a high-viscosity hydrophilic matrix material or a soluble matrix material. The daphnetin slow-release tablet prepared with the method has a good slow-release effect and is long in action time, low in dosage, low in frequency of taking and high in bioavailability in comparison with a capsule on the market. The process is simple in operation, low in cost and easy to control, and is suitable for industrial mass production.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Trimetazidine sustained release capsule and preparation method thereof
InactiveCN102552217APromote absorptionImprove complianceOrganic active ingredientsSenses disorderControl releaseSustained Release Capsule
The invention relates to a trimetazidine or salt sustained release capsule and a preparation method thereof. The trimetazidine sustained release capsule comprises the following components in percentage by weight: 5-50 percent of trimetazidine, 5-30 percent of auxiliary materials playing a role in sustained release and 45-85 percent if other auxiliary materials. The trimetazidine sustained release capsule and the preparation method thereof are characterized in that (1), a capsule content is a pellet; (2), the medicated pellet is firstly prepared, and a sustained release preparation is then prepared; and (3), the release of a drug is controlled through a controlled release membrane enwrapped by the pellet.
Owner:TIANJIN KAIWEN BIO TECH
Niraparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof
InactiveCN108201537AIncrease doseImprove efficacyOrganic active ingredientsPill deliveryBlood concentrationEnzyme inhibition
The invention discloses a niraparib oral controlled-release and sustained-release pharmaceutical composition and uses thereof. According to the presebt invention, by regulating the niraparib release behavior, the in vivo absorption rate and the absorption time of niraparib can be controlled, and the blood concentration and the blood concentration fluctuation range of niraparib in vivo can be regulated, such that the in vivo PARP enzyme inhibition activity of niraparib can be efficiently and permanently provided so as to provide the antitumor treatment effect in the high-efficiency and low-toxicity manner; a purpose of the present invention is to control the in vivo blood concentration and the efficacy providing of niraparib through the controlled-release and sustained-release preparation so as to improve the treatment effect of the drug and reduce the toxic-side effect of the drug; and the efficient and long-acting PARP enzyme activity inhibition pharmaceutical composition is providedfor patients, wherein the antitumor effect is improved, the acting time is long, the compliance is good, and the toxic-side effect is low.
Owner:SCI RAINBOW BIOPHARMA CO LTD
Preparation method of pharmaceutical composition for treating type II diabetes
ActiveCN101984974BImprove complianceReduce volumeMetabolism disorderSulfonylurea active ingredientsSide effectPatient compliance
The invention relates to a preparation method of a pharmaceutical composition for treating type II diabetes, belonging to the medical preparations containing organic ingredients, particularly relating to a preparation method of a pharmaceutical composition of metformin hydrochloride and glimepiride. In the preparation method, the metformin hydrochloride and the glimepiride are taken as active ingredients of the pharmaceutical composition, the metformin hydrochloride is firstly prepared into pill cores by adopting an osmotic pump technology and then the metformin hydrochloride pill cores are coated with the glimepiride by using a coating technology. The preparation method comprises the following steps: 1), preparing the metformin hydrochloride and pharmaceutically acceptable auxiliary materials into pills which are coated with semipermeable membrane layers, punching by laser and producing the pill cores; and 2), preparing the glimepiride and the pharmaceutically acceptable auxiliary materials into a coating liquid dissolved in stomach and carrying out coating on the pill cores in step 1. The invention provides the preparation method of the pharmaceutical composition for treating the type II diabetes, wherein the pharmaceutical composition steady and slowly releases drugs, has reduced administration times, good patients compliance, small side effect and small tablet volume, is less influenced by pH values of different segments of a gastrointestinal tract, and is convenient for administration for the patients.
Owner:SHANDONG XINHUA PHARMA CO LTD
Red ginseng extract pellet and preparation method thereof
InactiveCN102462715AEffectiveSmall fluctuations in blood concentrationNervous disorderImmunological disordersPharmaceutical preservativesGINSENG EXTRACT
The invention provides a red ginseng extract pellet preparation, which is prepared from a red ginseng extract and pharmaceutical adjuvants, and is characterized in that: an excipient and a bonding agent are taken as the pharmaceutical adjuvants; and the pellet preparation comprises the following components in percentage by weight: 2-80 percent of a red ginseng extract, 15-97 percent of an excipient and 1-5 percent of a bonding agent. The pellet preparation can be prepared into a slow-release or enteric preparation as required. As proved by a pharmacological experiment, the pellet has the effects of reinforcing vital energy, restoring pulses, preventing exhaustion, tonifying qi and controlling blood.
Owner:JF PHARMALAND TECH DEV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com