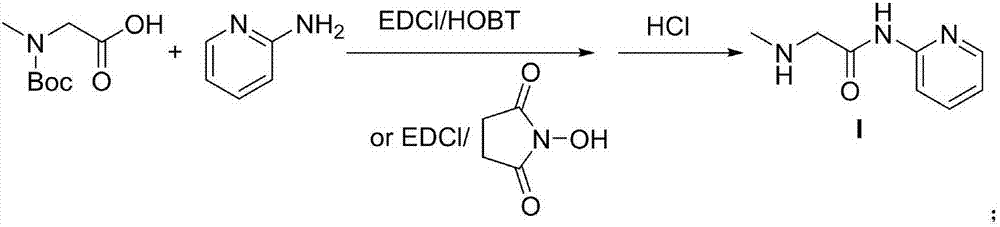
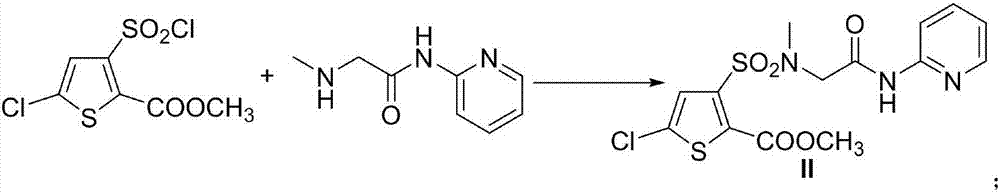
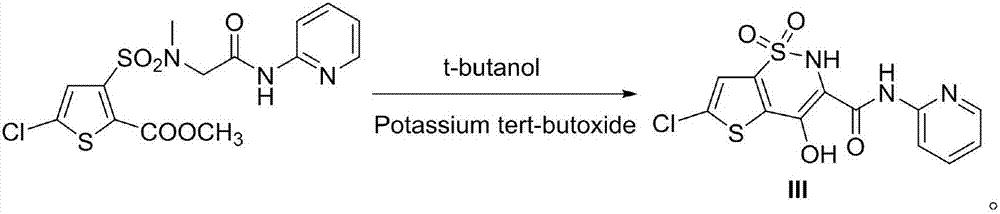
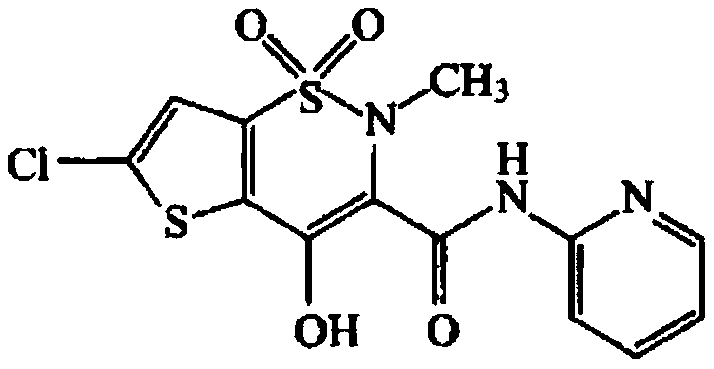
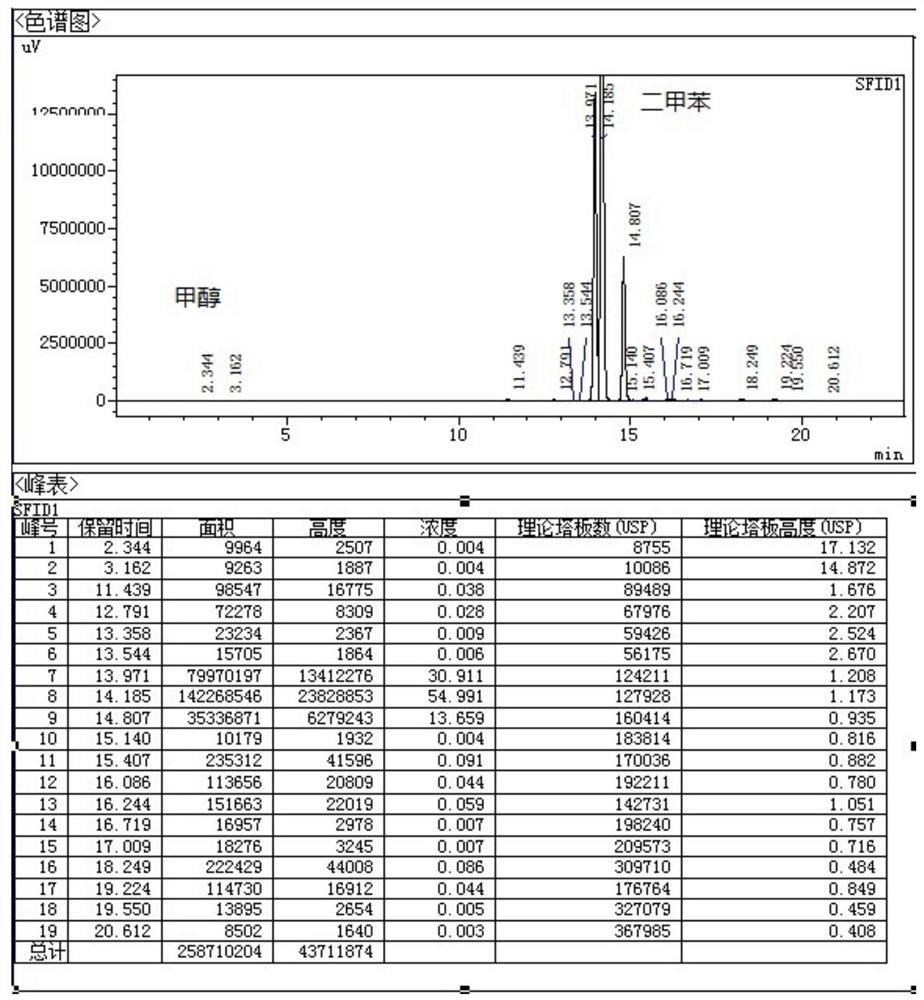
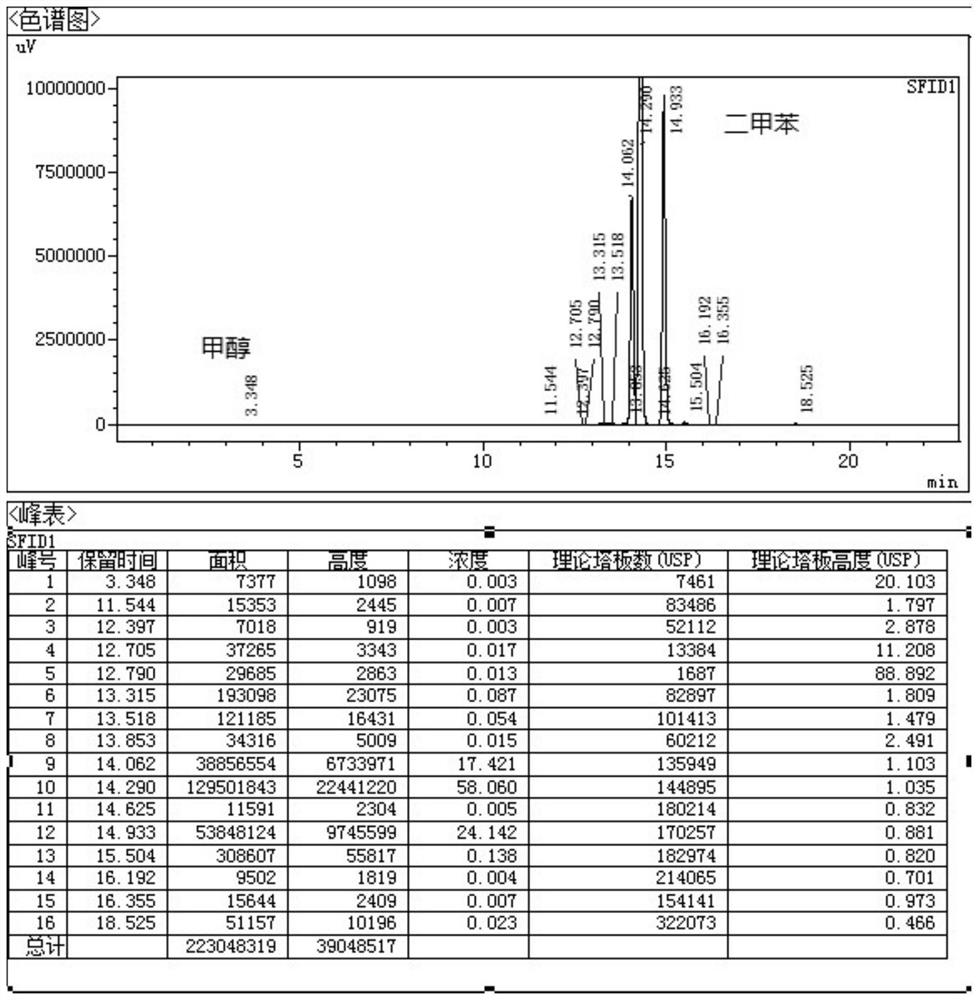
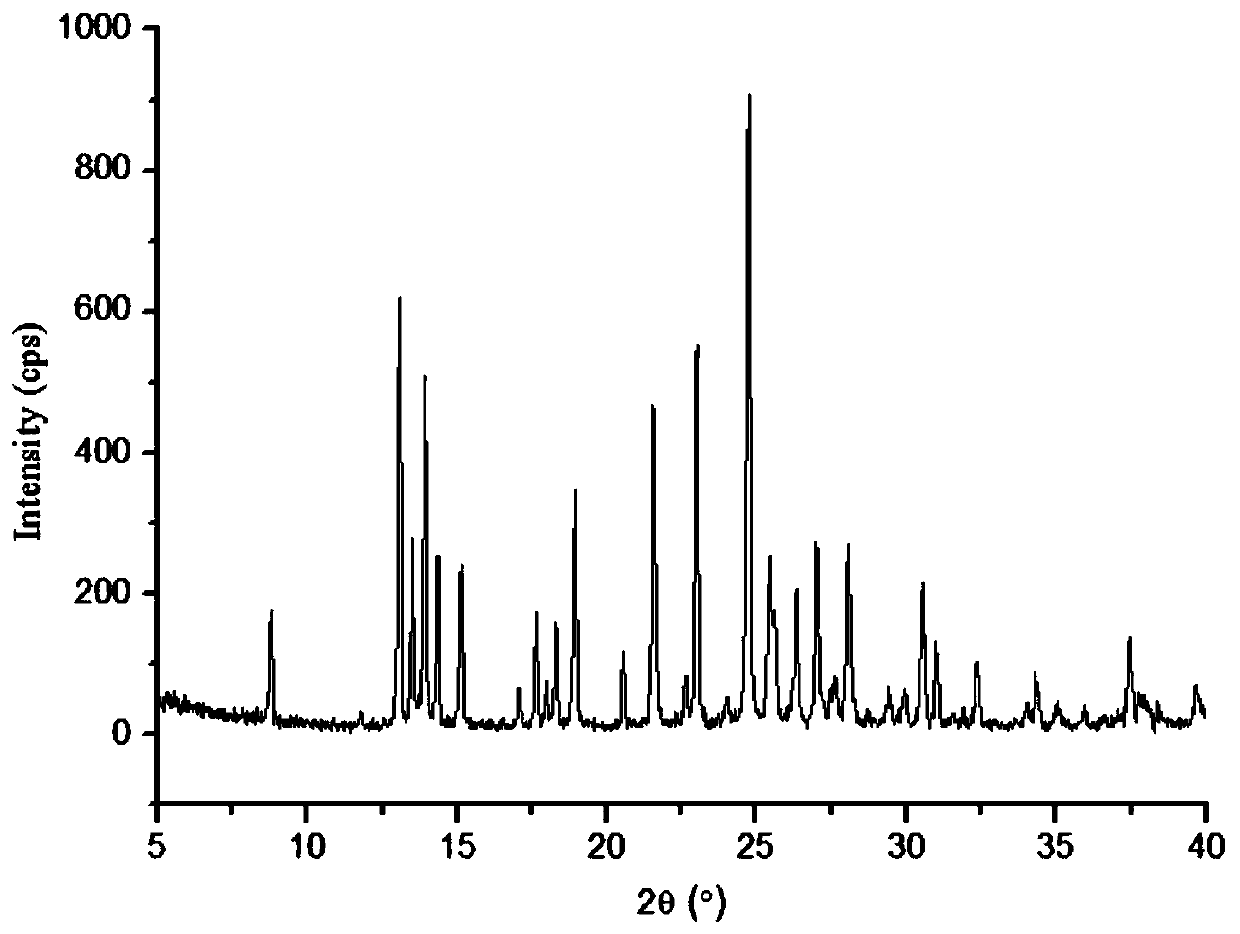
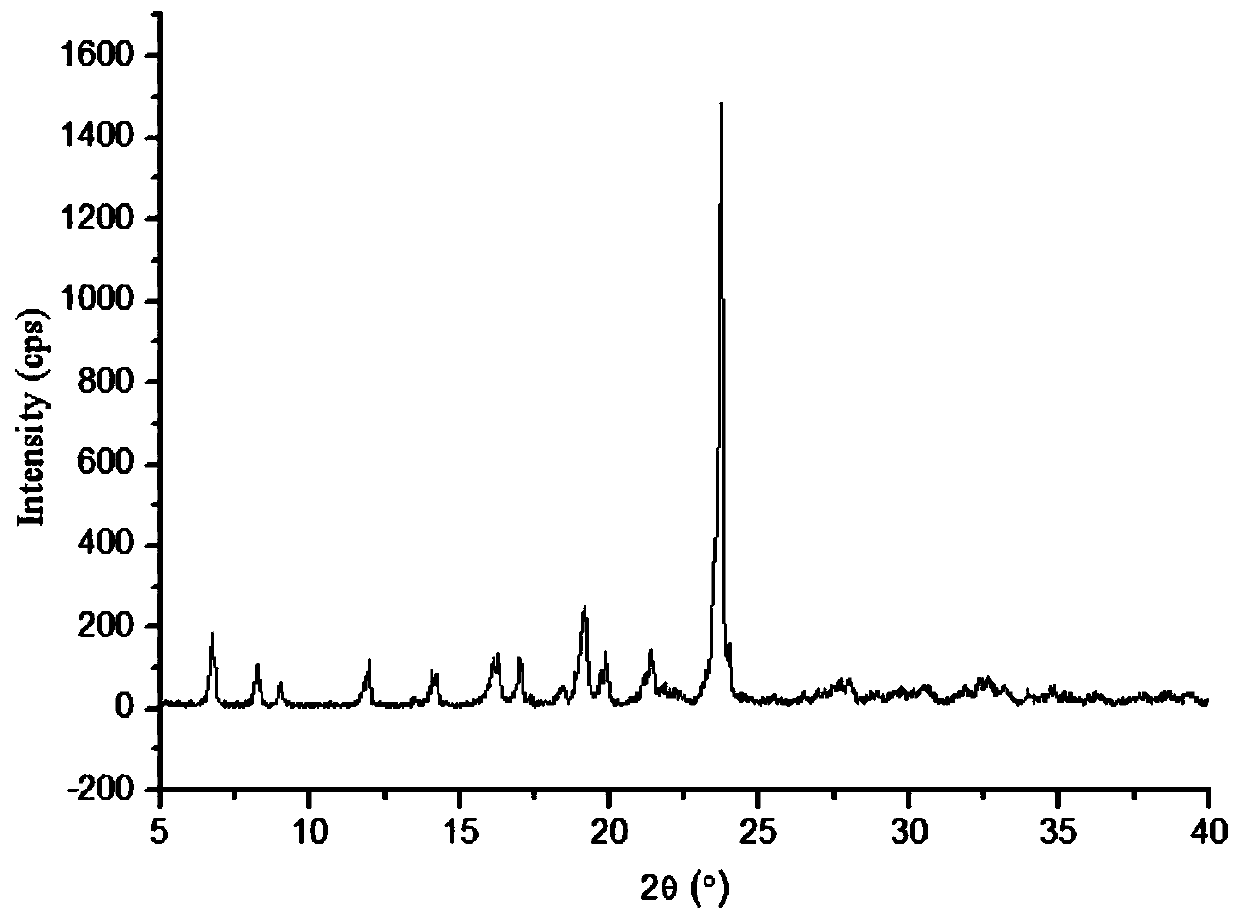
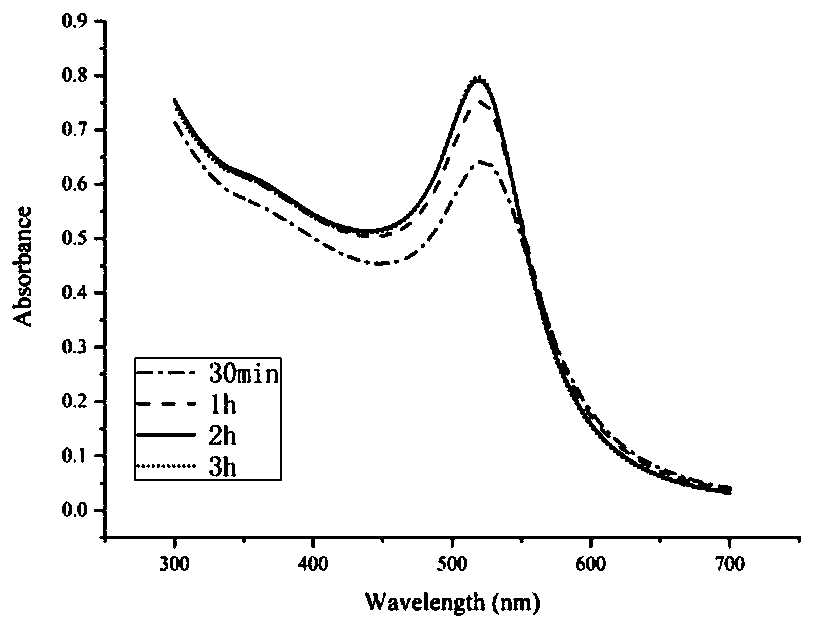
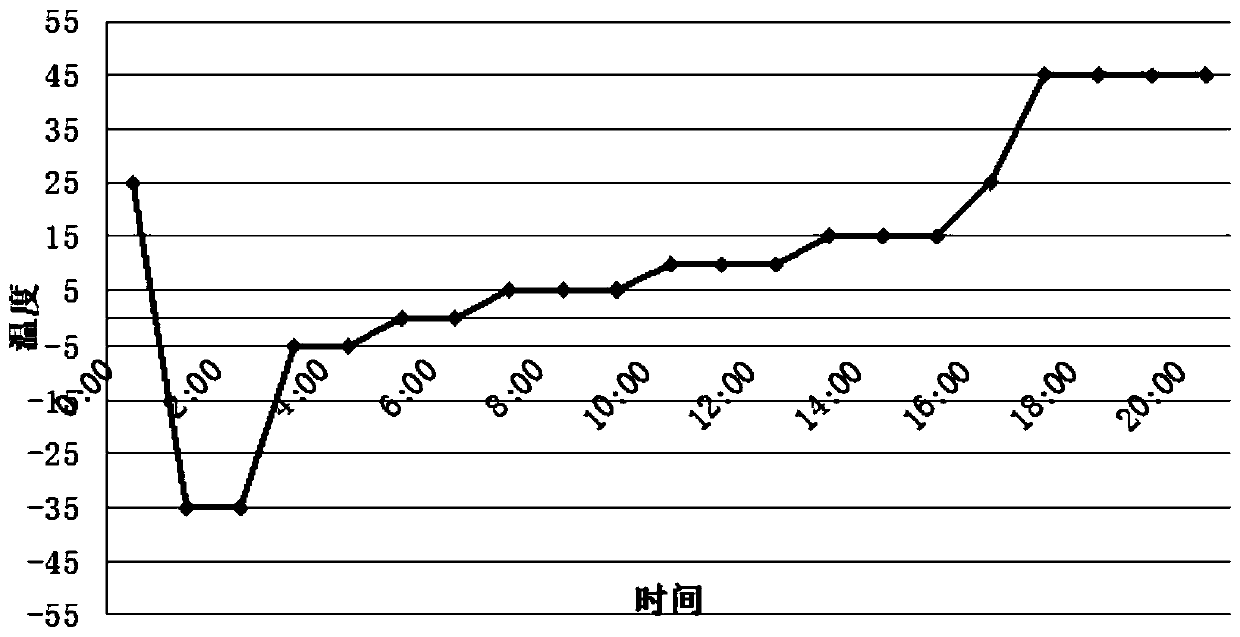
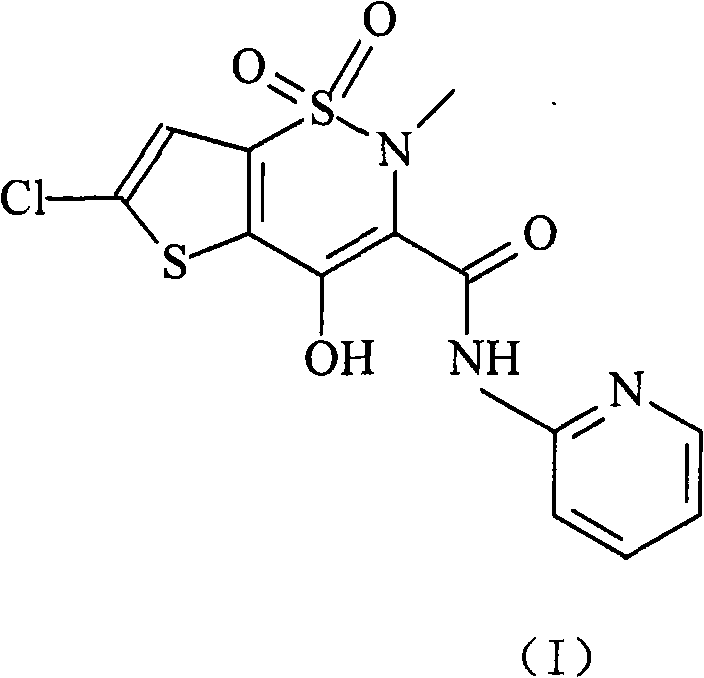
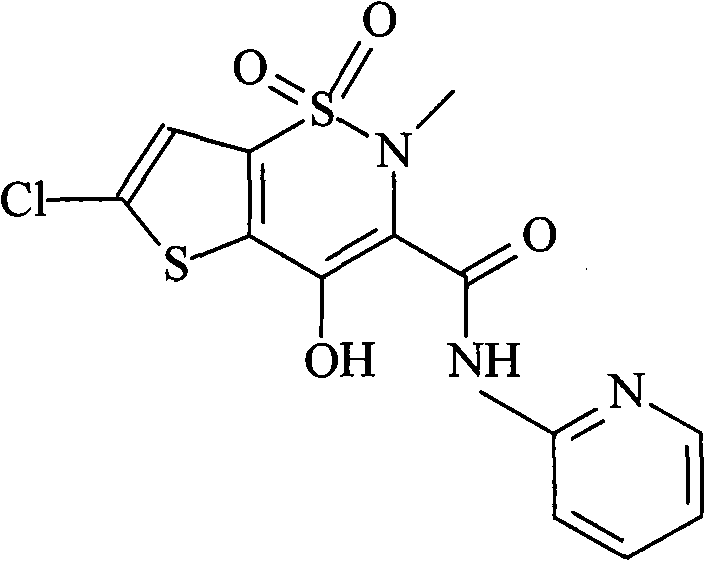
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
50 results about "Lornoxicam" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
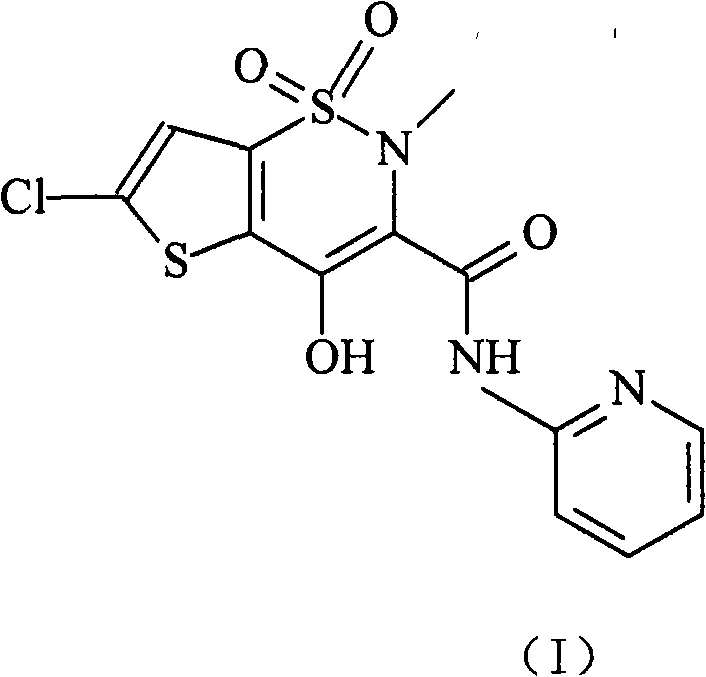
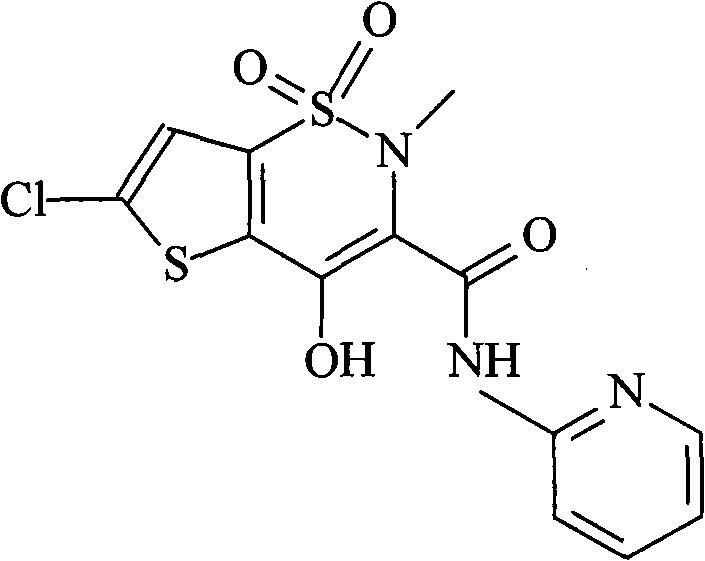
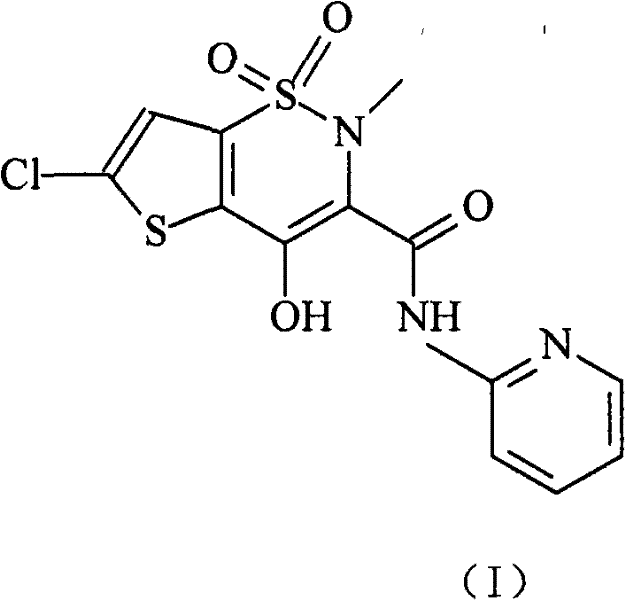
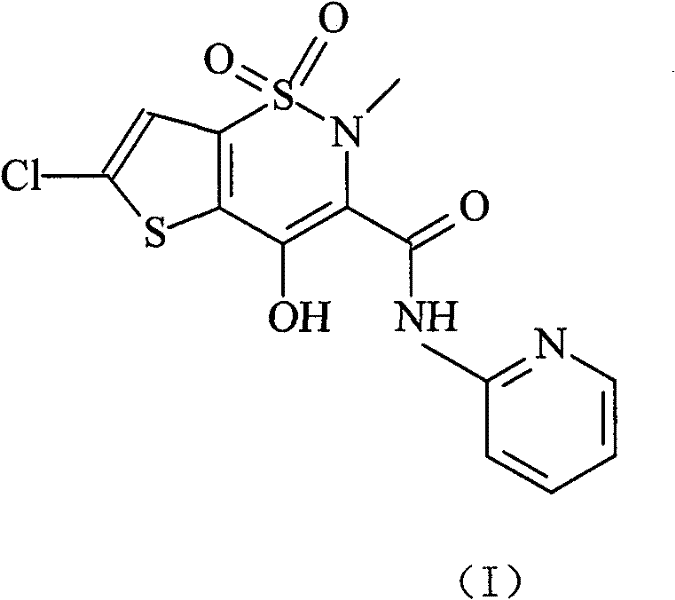
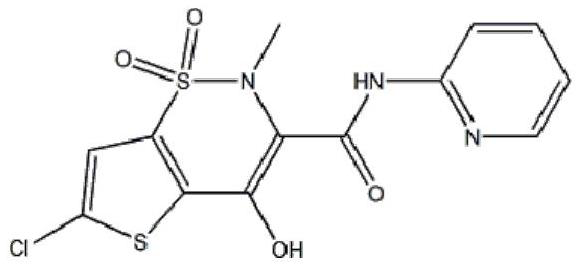
Lornoxicam, also known as chlortenoxicam, is a nonsteroidal anti-inflammatory drug (NSAID) of the oxicam class with analgesic (pain relieving), anti-inflammatory and antipyretic (fever reducing) properties. It is available in oral and parenteral formulations.
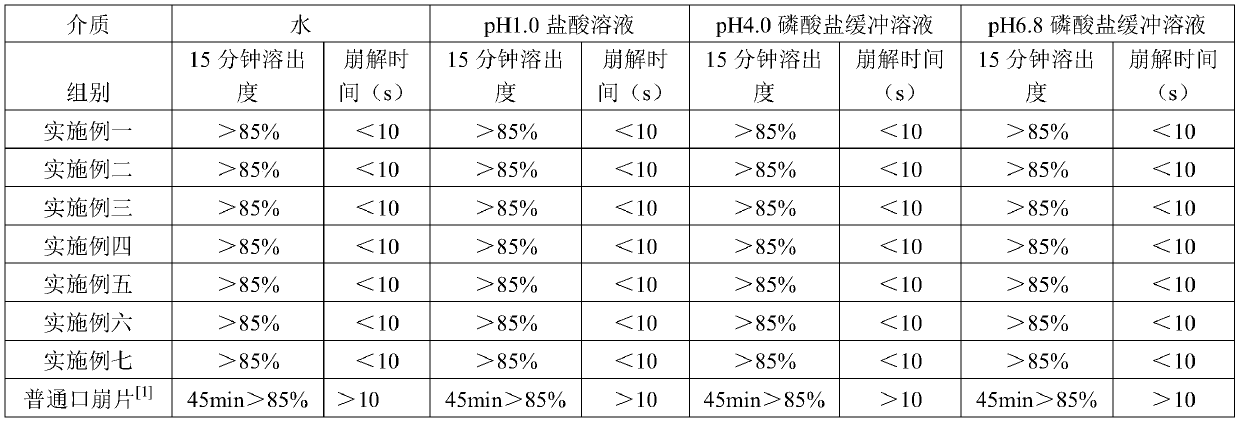
Lornoxicam freeze-dried injection and preparation method thereof
ActiveCN101327193AHigh clarityGood formabilityPowder deliveryOrganic active ingredientsMedicineFreeze-drying
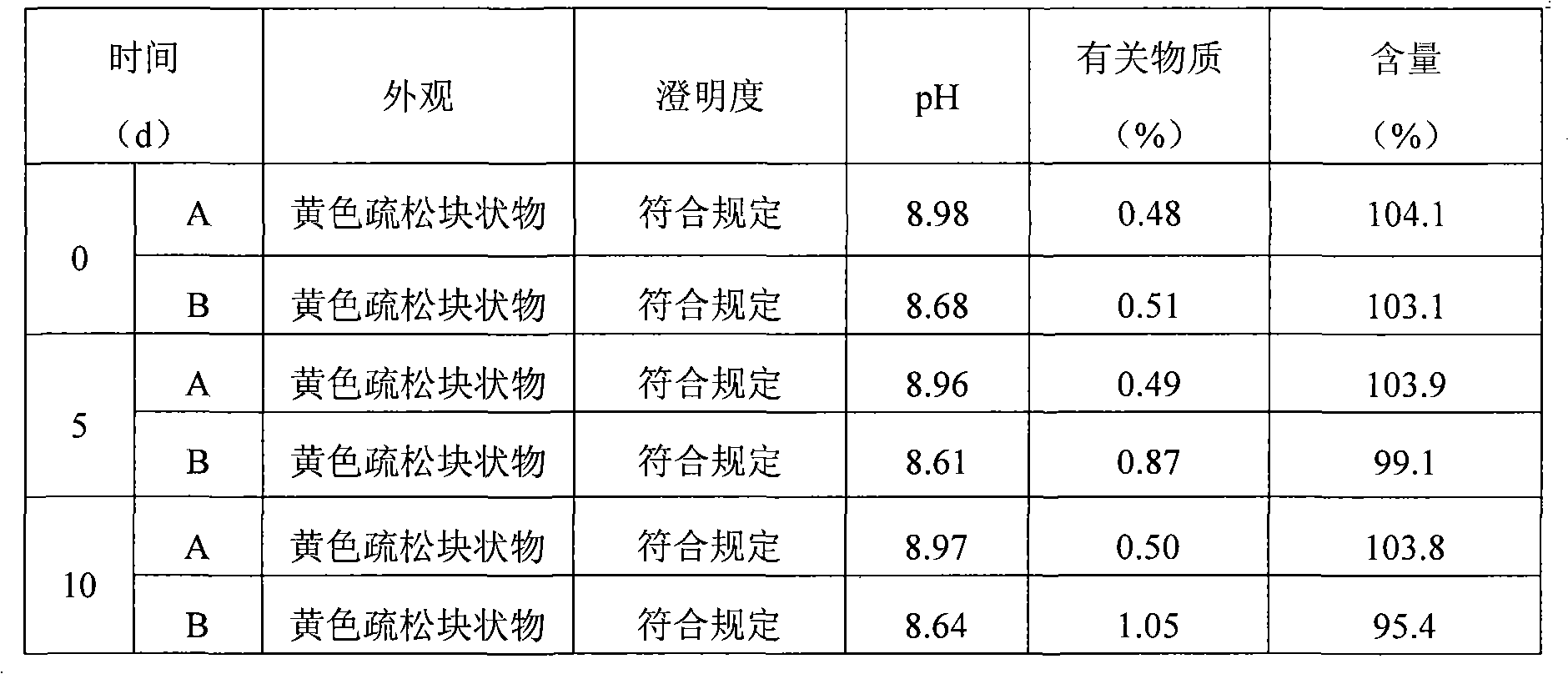
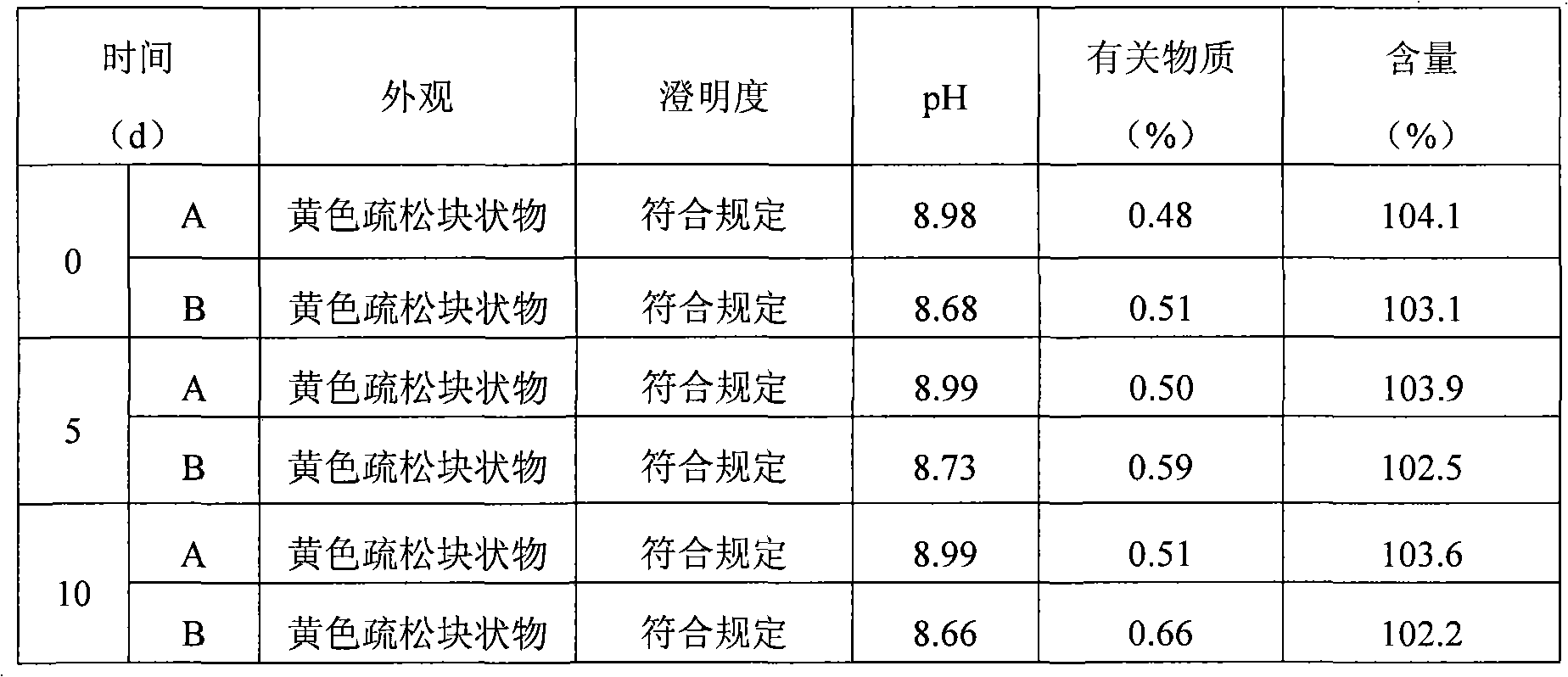
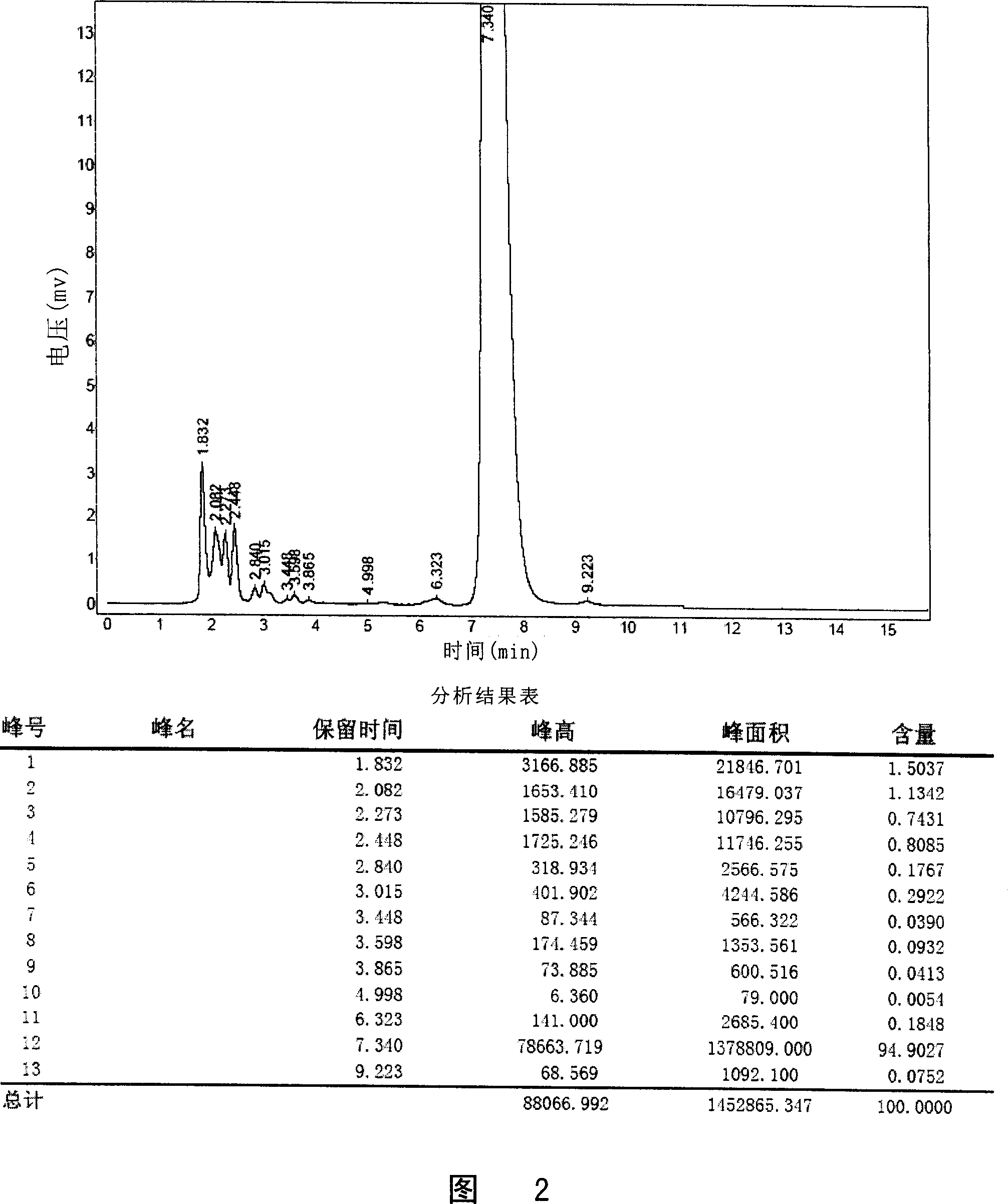
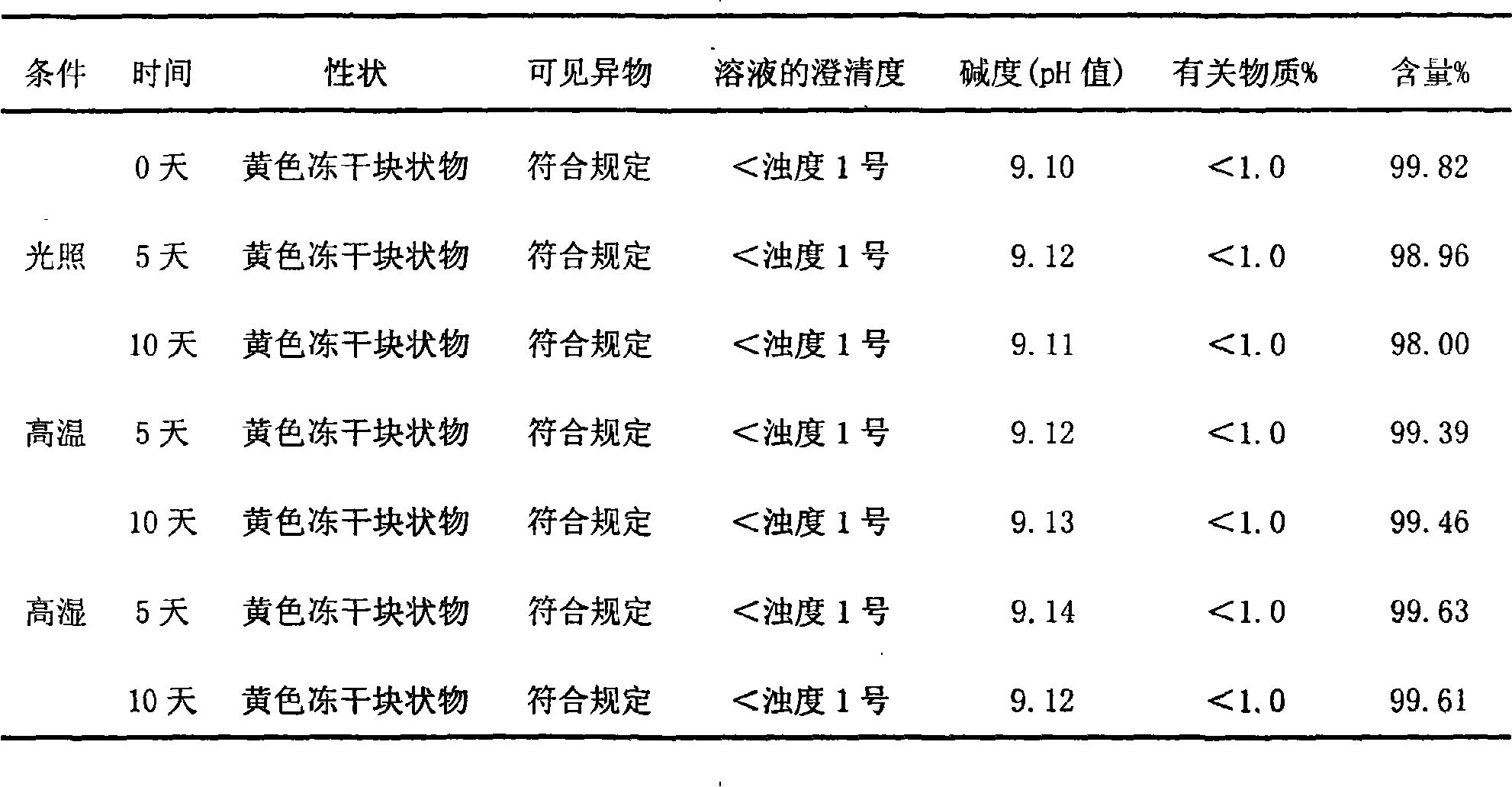
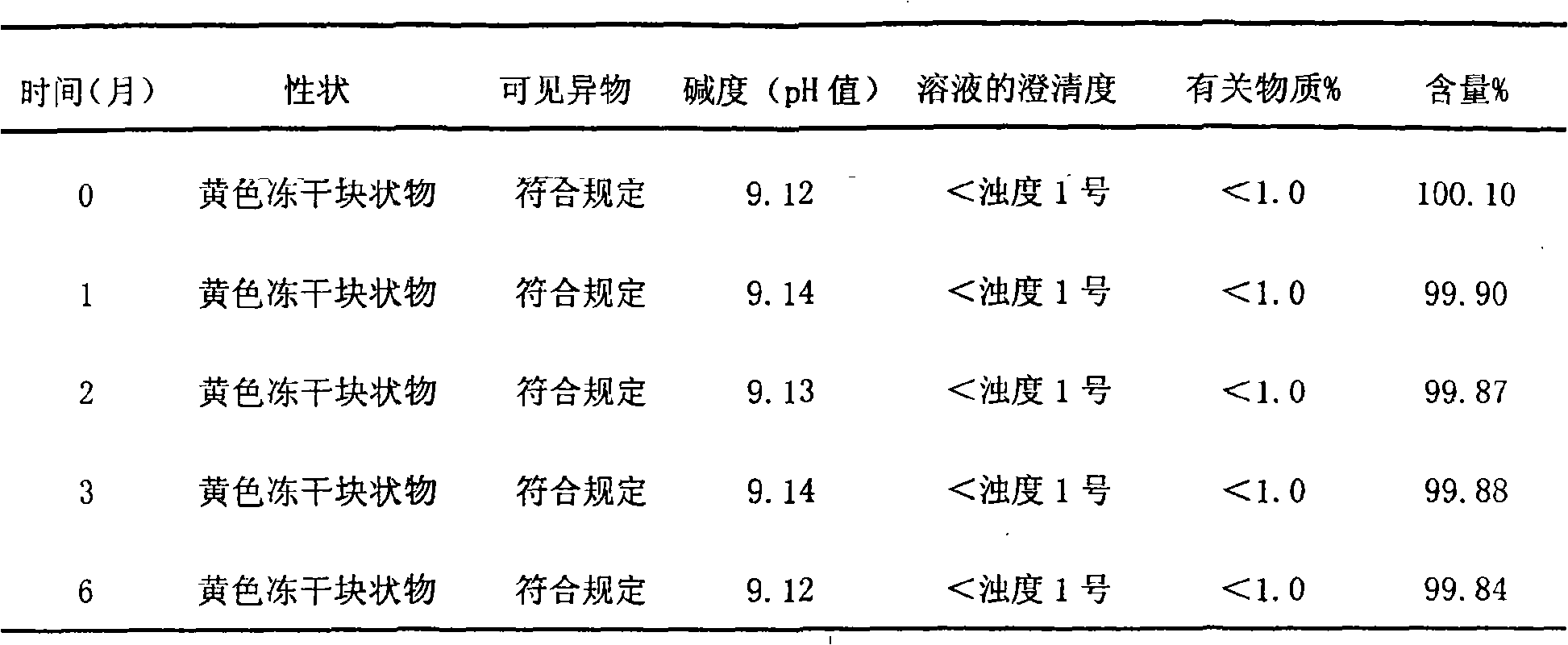
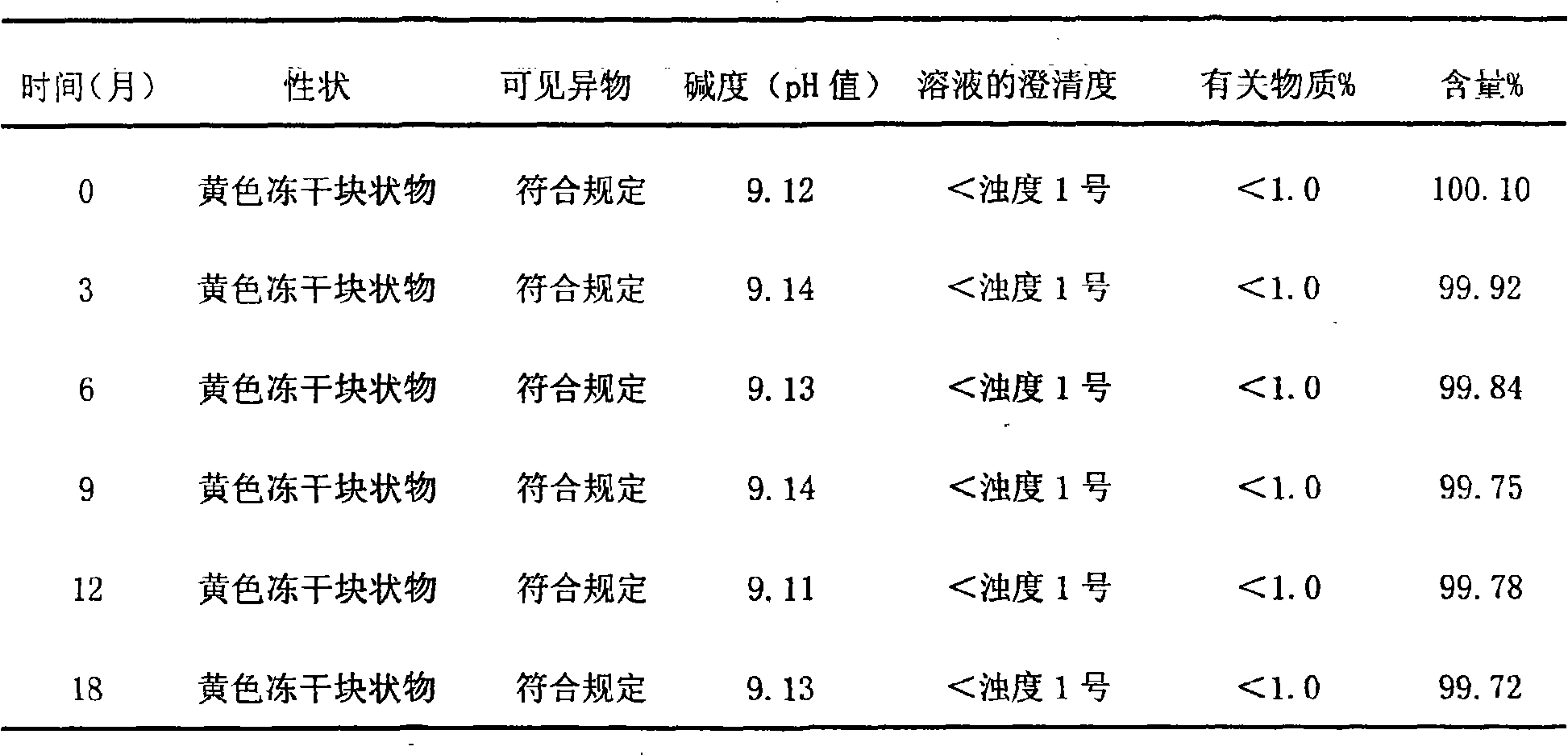
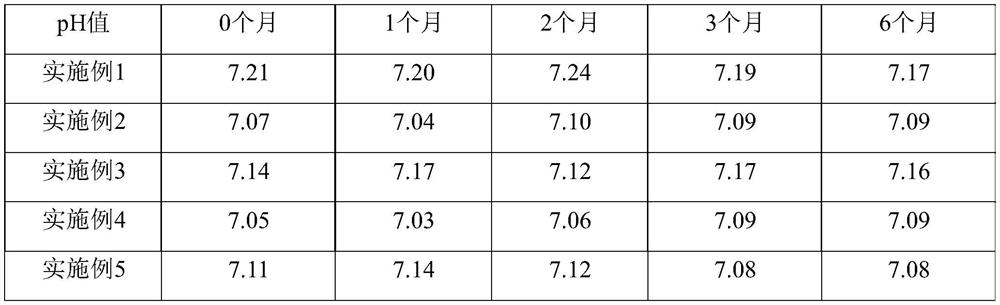
The invention relates to lomoxicam freeze-dried powder injection and a preparation method thereof. The freeze-dried powder injection comprises omoxicam, mannite, tromethamine, EDTA and pH regulator. The dosage of the mannite is 3.5 to 8.5 g of mannite per gram of lomoxicam, and the dosage of the EDTA is 0.015 to 0.025g of EDTA per gram of lomoxicam. The invention adopts EDTA to replace EDTA-disodium salt and selects the dosages of the mannite and EDTA so that the clarity of the reconstituted obtained freeze-dried powder is greatly improved, and the formability is good; in addition, the invention adopts two pH regulators, in the process, one pH regulator is used to regulate the solution to a pH range, then the other pH regulator is used to regulate the solution to another pH range, and the freeze drying technology is controlled strictly. The prepared freeze-dried powder injection has great improvement of stability and favorable resolubity.
Owner:HAINAN JINRUI PHARMA CO LTD
Lornoxicam hydrogel patch and preparation method thereof
ActiveCN101879147ALarge drug releaseLittle side effectsOrganic active ingredientsAntipyreticMedicineHalf-life
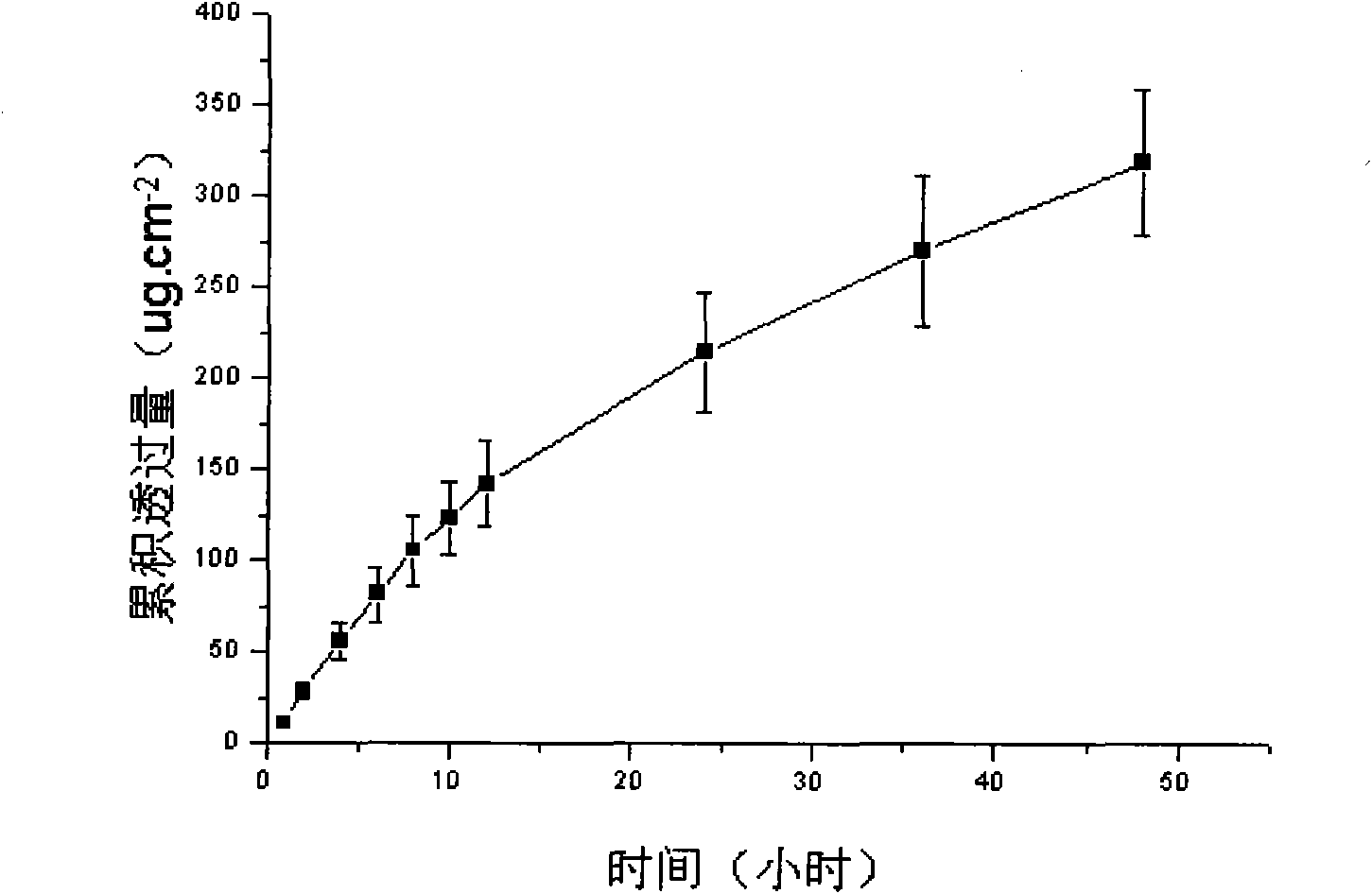
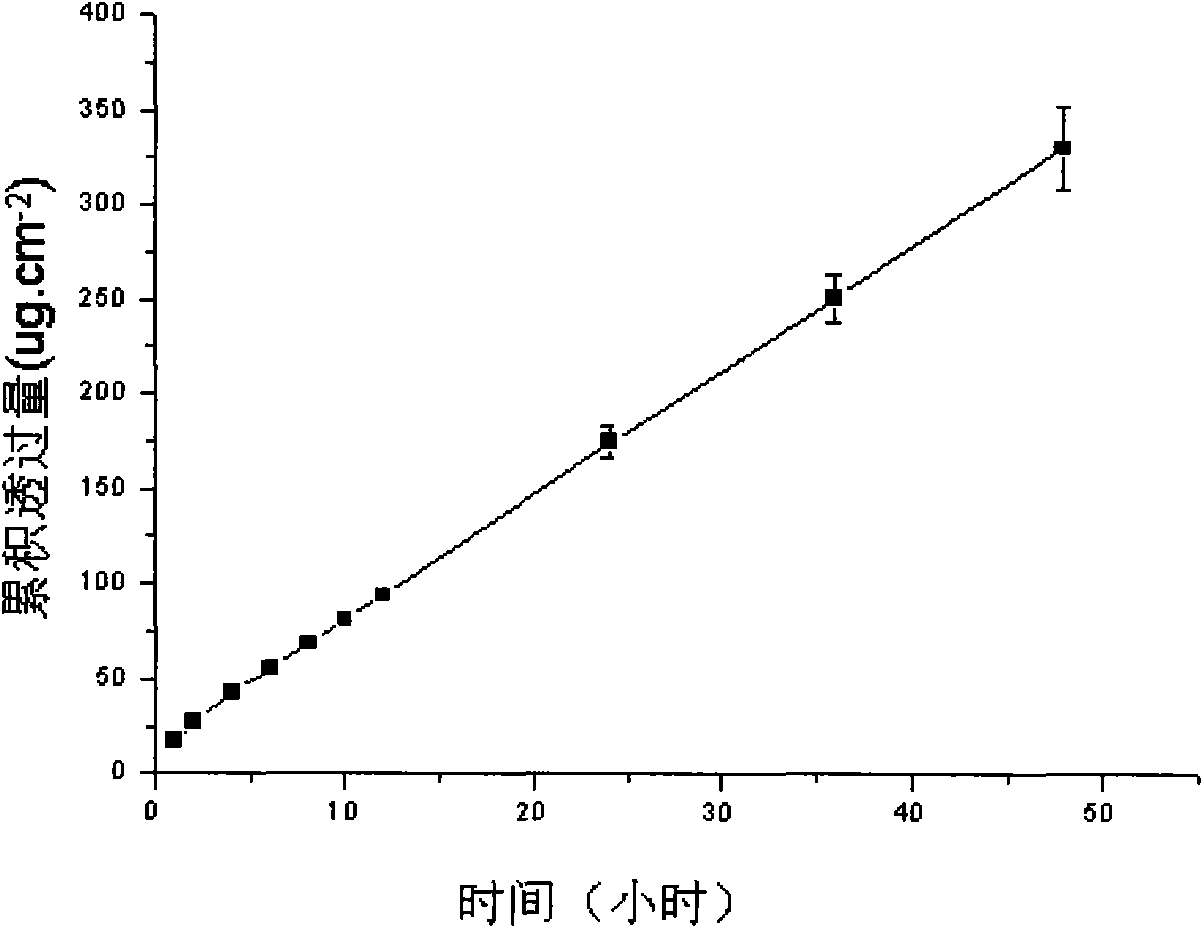
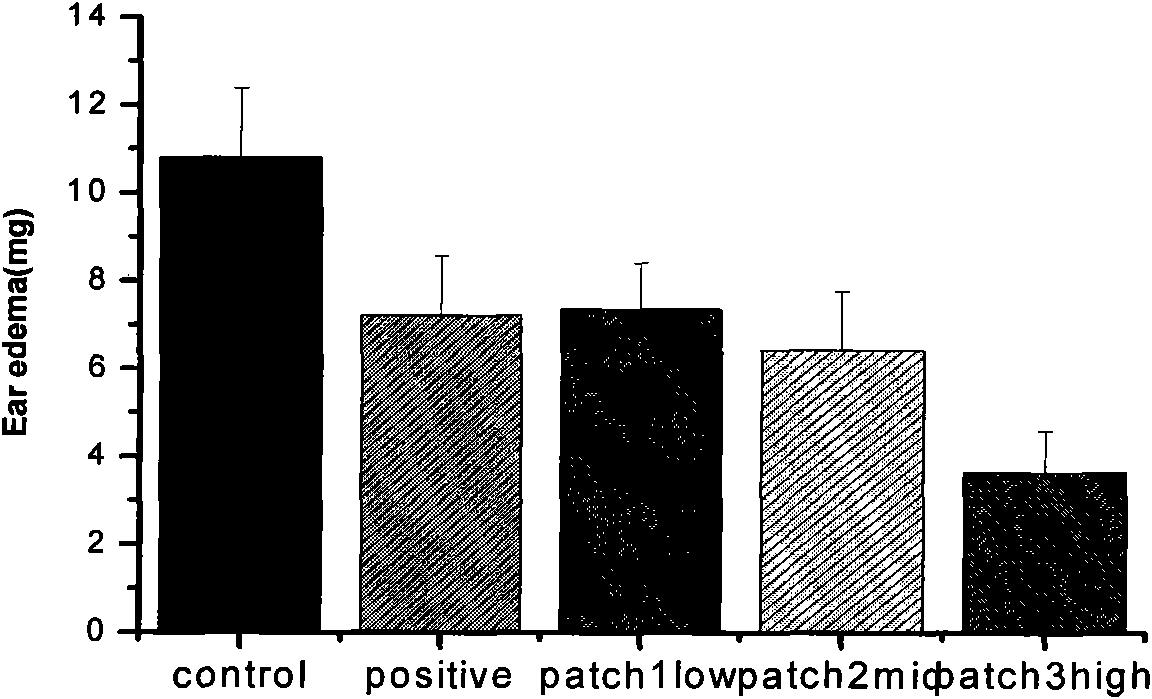
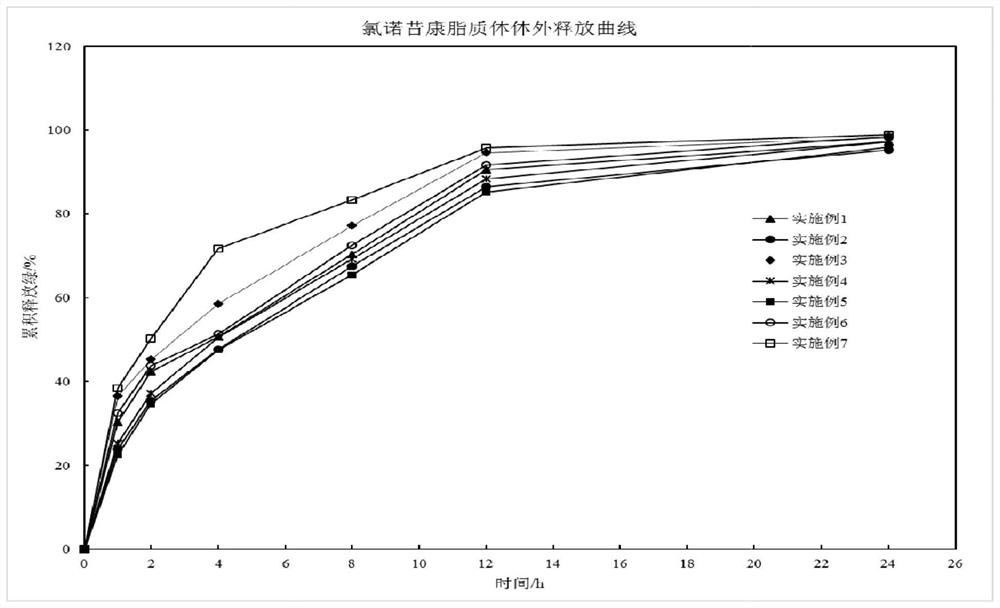
The invention discloses a lornoxicam hydrogel patch and a preparation method thereof. In the hydrogel patch, lornoxicam is used as a medicinal active ingredient, and the hydrogel patch consists of a medicament-containing matrix, an adhesive layer, a lining layer and a protective layer, wherein the medicament-containing matrix consists of the lornoxicam, a hydrogel matrix and a penetrating agent. The patch makes the medicament release stably to prolong half-life period, is remarkably superior to other conventional preparations of the lornoxicam, and has the 48-hour accumulated permeation quantity of over 300ug.cm<-2> through percutaneous experiments in vitro. Pharmacodynamic tests prove that the lornoxicam hydrogel patch has excellent effect of relieving pain and diminishing inflammation.
Owner:MICROBE EPIDEMIC DISEASE INST OF PLA MILITARY MEDICAL ACAD OF SCI
Orally quick disintegrating lornoxicam composition and its prepn process
ActiveCN1739523ANo grittinessGreat tasteOrganic active ingredientsAntipyreticLornoxicamTraditional medicine
The present invention relates to orally quick disintegrating lornoxicam composition and its preparation process. The orally quick disintegrating lornoxicam composition includes lornoxicam in 100 weight portions, pharmaceutically acceptable lipoid taste mask 25-100 weight portions, and pharmaceutically acceptable disintegrating agent 50-500 weight portions. The present invention also provides the preparation process of the oral disintegrating lornoxicam composition. The composition of the present invention can disintegrate inside oral cavity in good taste and act in stomach. The composition of the present invention is easy taken, especially for those people with dysphagia.
Owner:ZHEJIANG ZHENYUAN PHARMA CO LTD +1
Pharmaceutical composition for anti-inflammation ease-pain
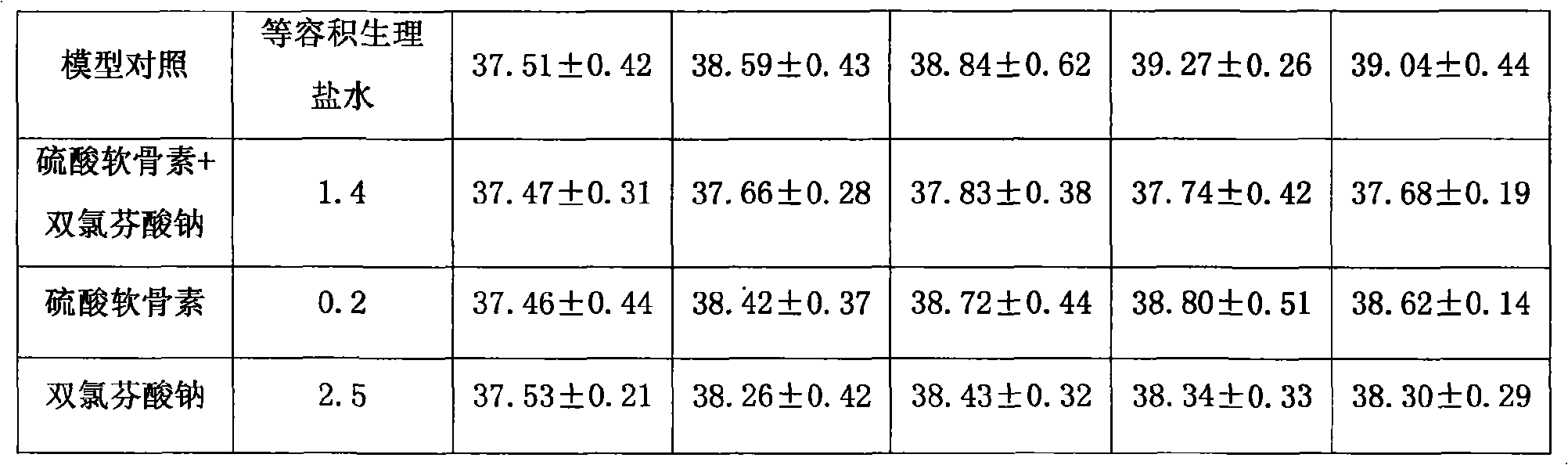
The invention provides an anti-inflammatory and pain-killing medical composition; chondroitin sulfate and a non-steroidal anti-inflammatory agent are used as active ingredients and mixed with a medical carrier to form the composition. Non-steroidal anti-inflammatory agent includes but not confines in diclofenac sodium (potassium), naprosyn and Lornoxicam; the composition exists in forms of squirt, freeze-dried powder and large-capacity transfusion for intravenous medication of a human body. The composition can be applied to symptomatic treatments of various febrile illnesses, various arthritises and inflammatory rheumatic diseases, soft tissue rheumatisms, neuralgia, arthralgia and pains, wounds relevant to degenerative diseases of spines, or postoperative pains.
Owner:FUKANGREN BIO PHARMA
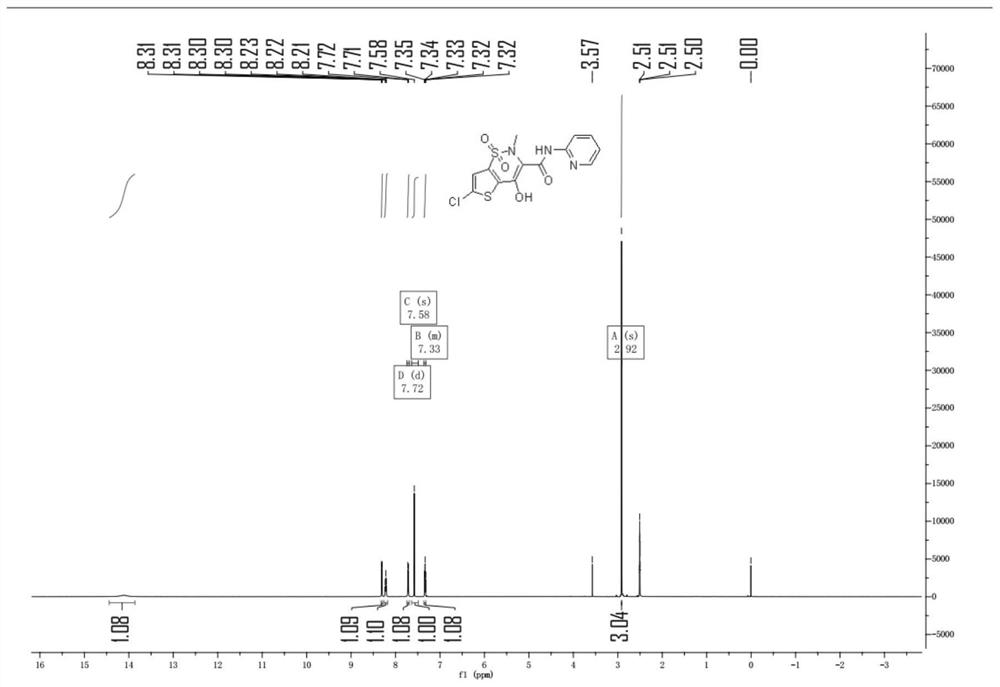
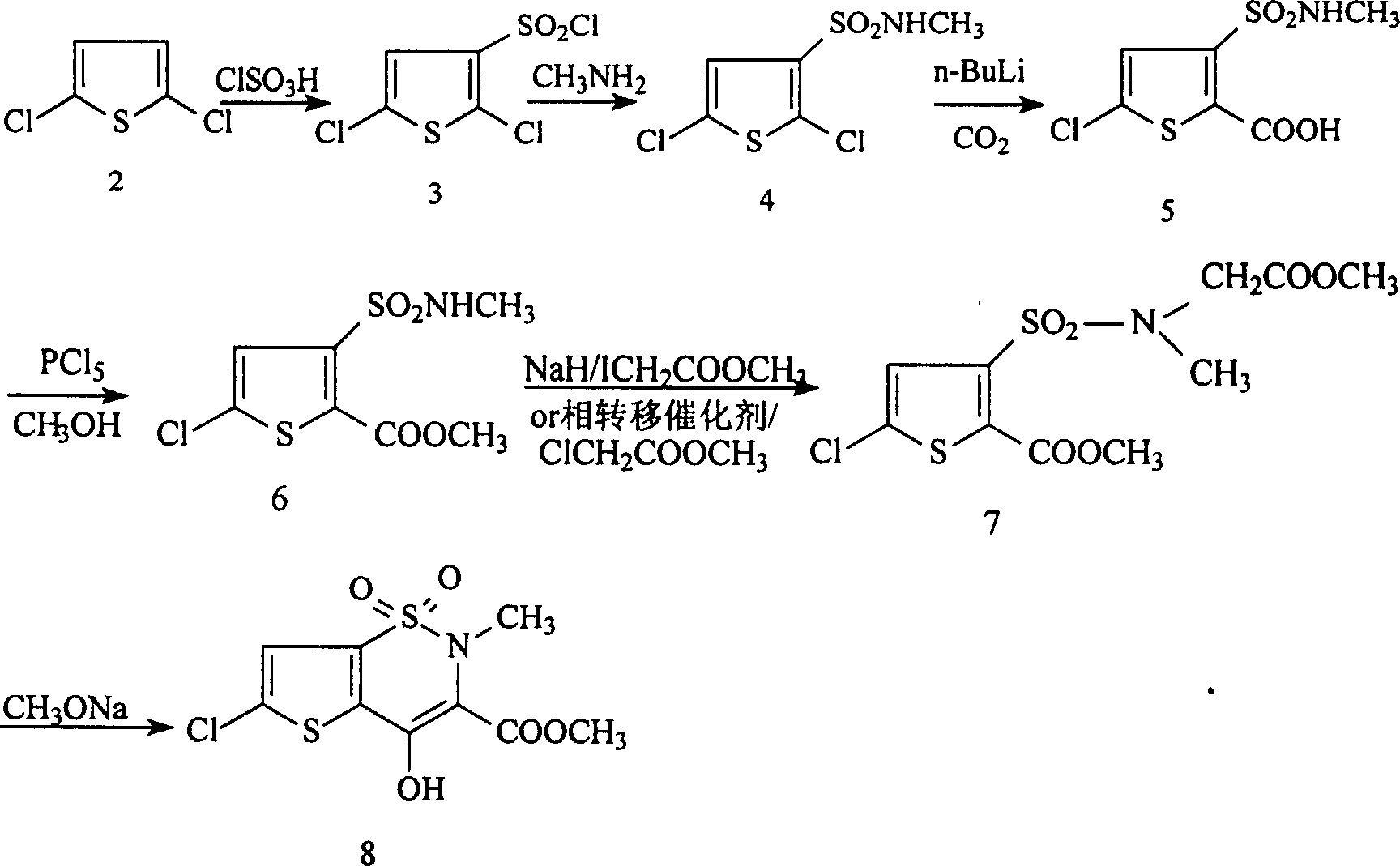
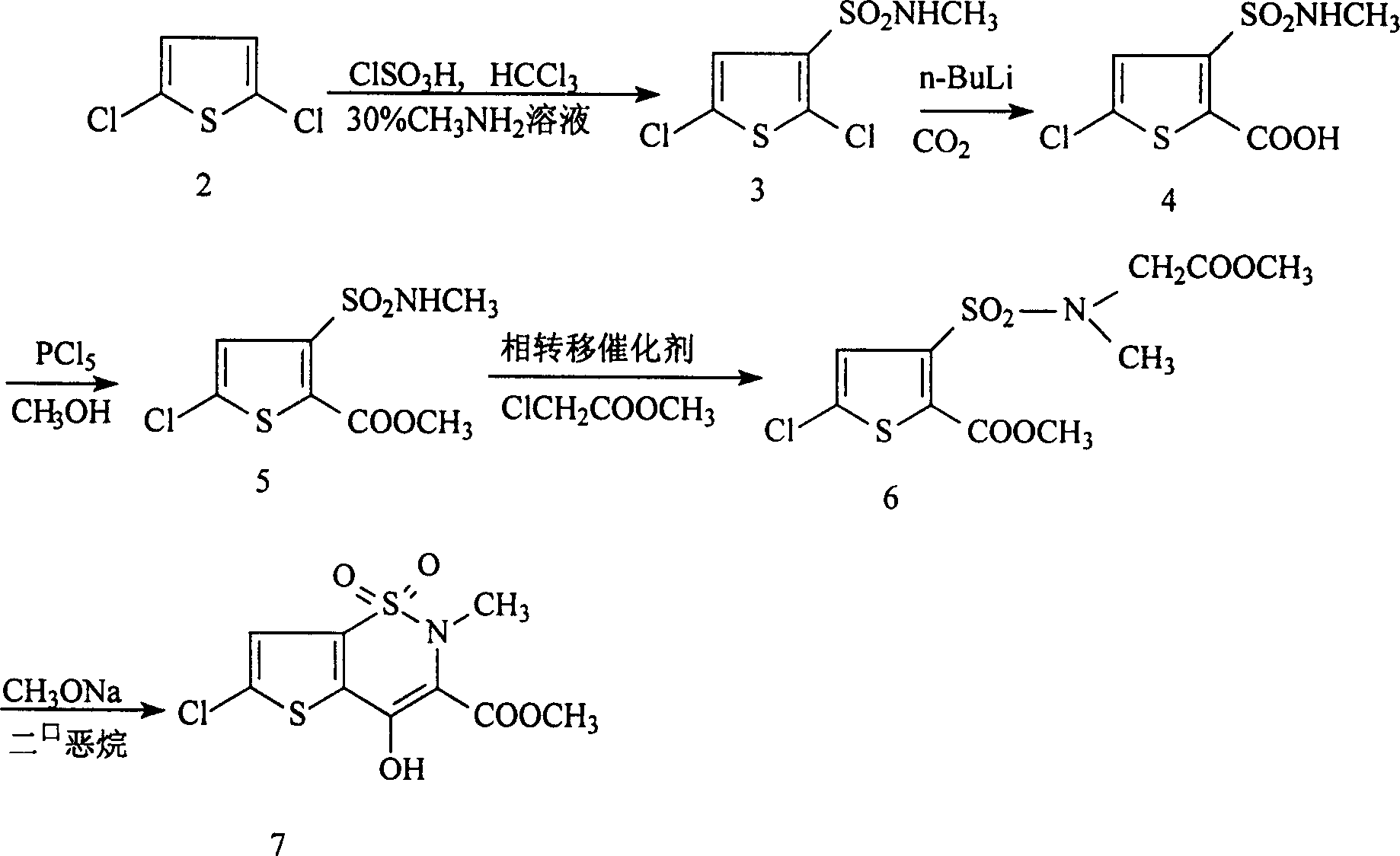
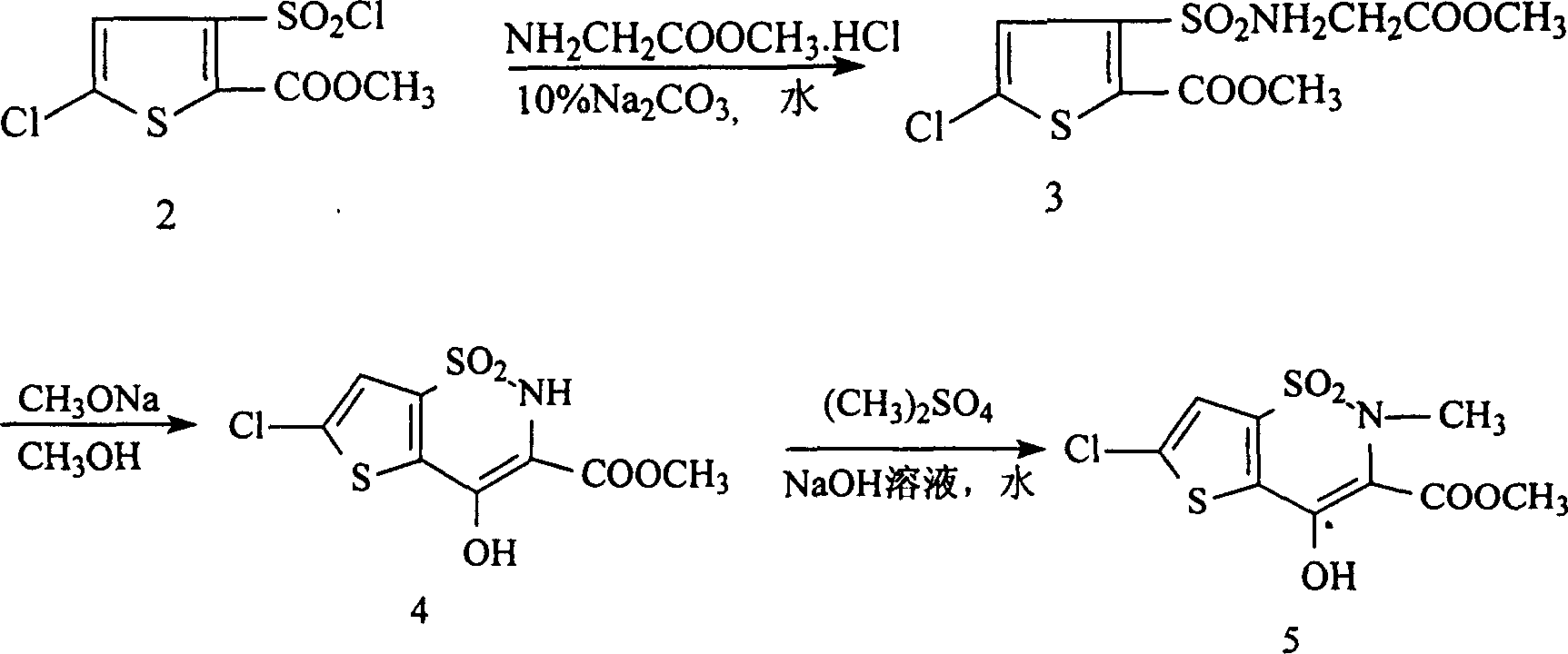
Synthesis method of lornoxicam
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Process for synthesizing lornoxicam intermediate against inflammation and pain
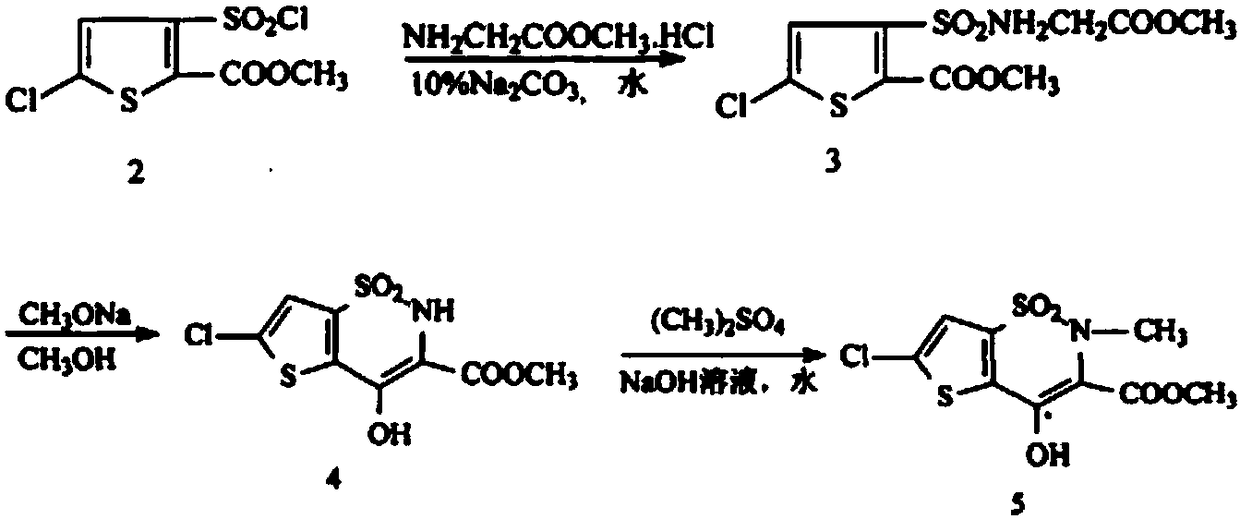
Disclosed is a process for synthesizing lornoxicam intermediate against inflammation and pain, which comprises using 5-chloro-3-thiophene sulfonyl chloride-2-carboxylate as raw material, charging 2-10% of sodium carbonate aqueous solution and 5-30% of glycine methylester hydrochloride simultaneously into water and methanol phases, thermal insulating 6-26 hours at 10-60 deg. C, then filtering and drying to obtain 6-chloro-3-sulfamoyl amido methyl acetate thiophene-2 methyl formate, reacting it with 5-27% of sodium methylate methanol solution 1-15 hours at 30-75 deg. C, filtering to obtain 6-chloro-4-hydroxyl-2H-thione[2,3-e]-1 and 2-thiazine-3-methyl formate-1,1-dioxide, then reacting the filtered substance with dimethyl sulfate in 1-10% aqueous solution of sodium-hydroxide.
Owner:JIANGSU POLYTECHNIC UNIVERSITY
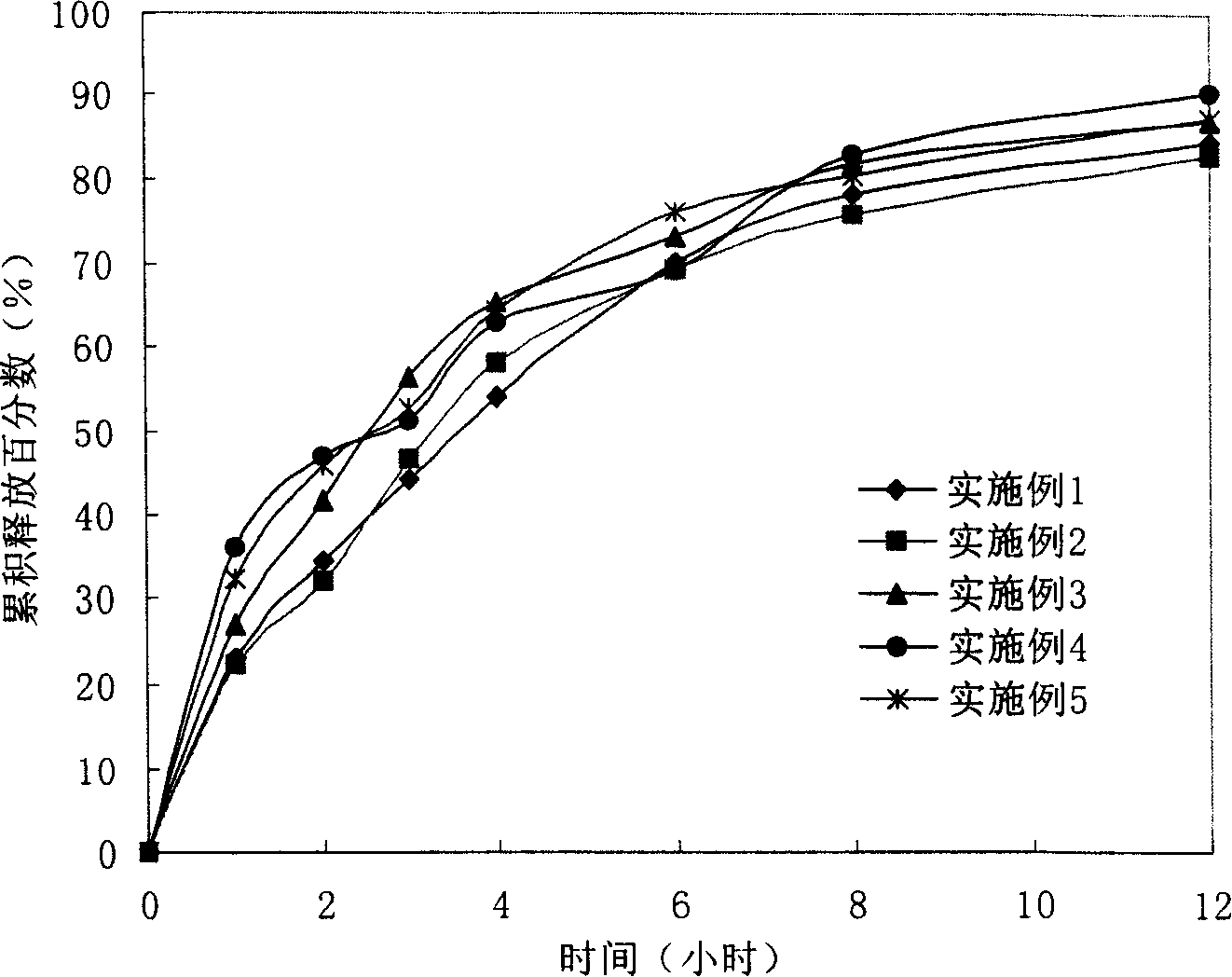
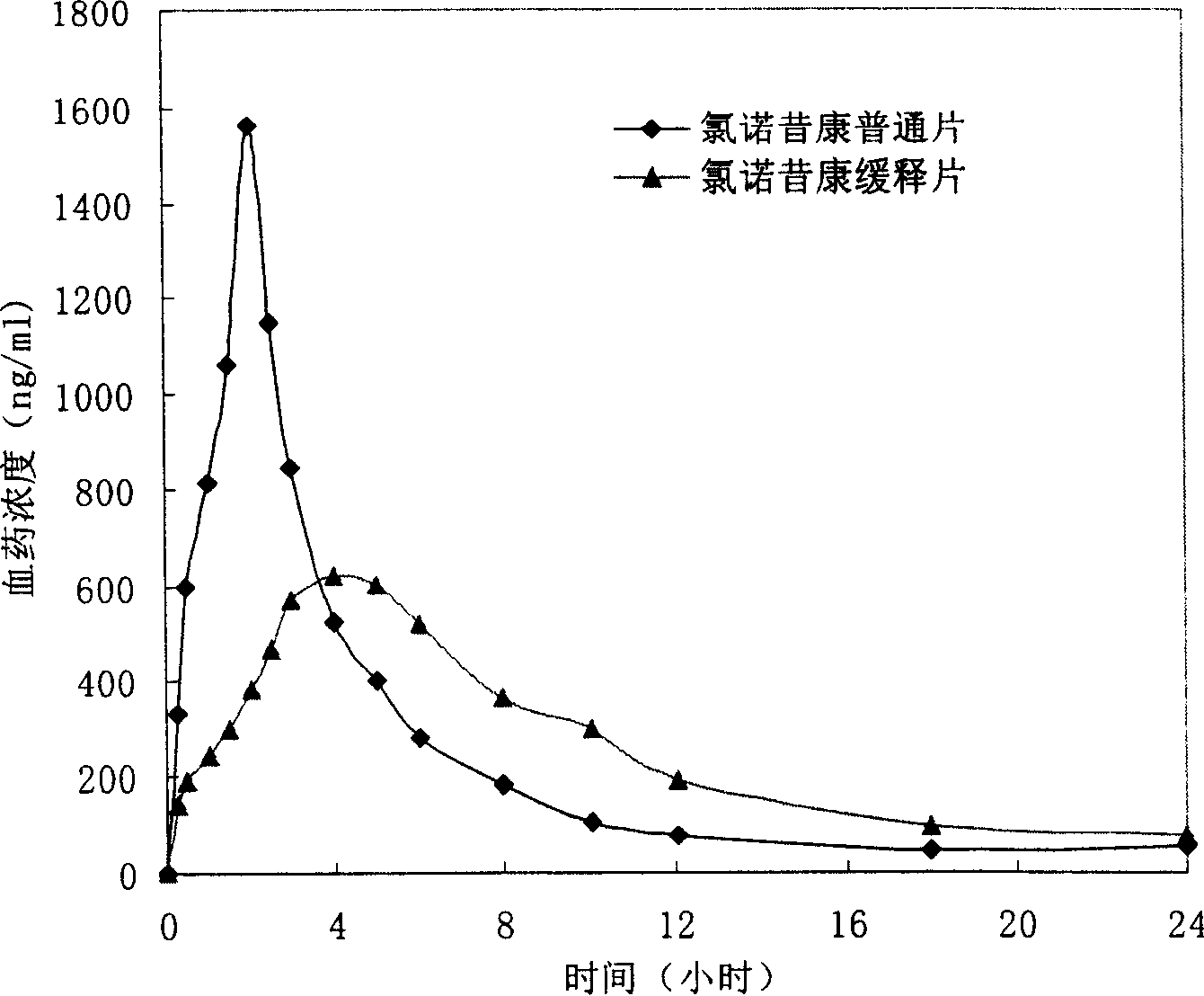
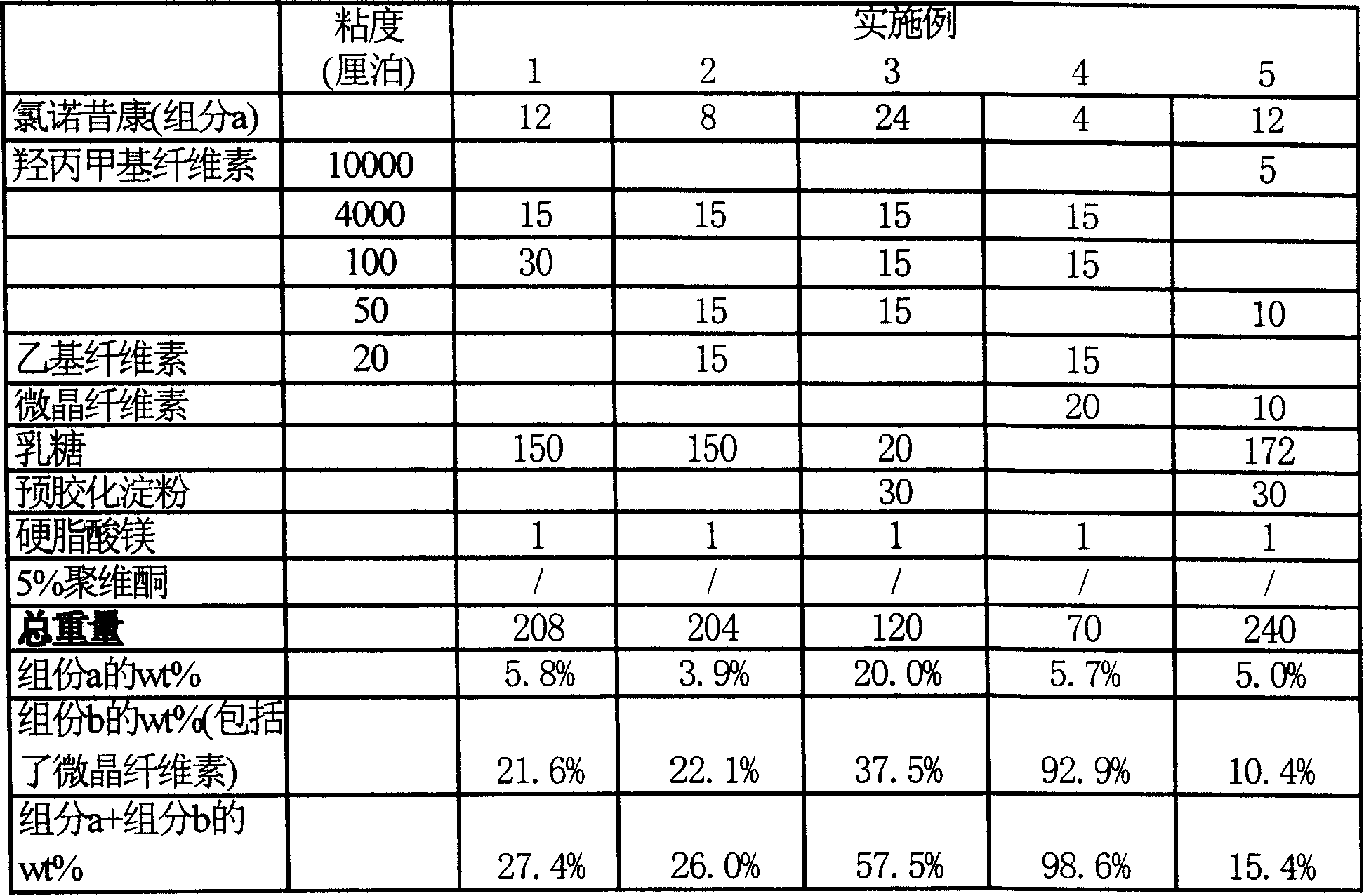
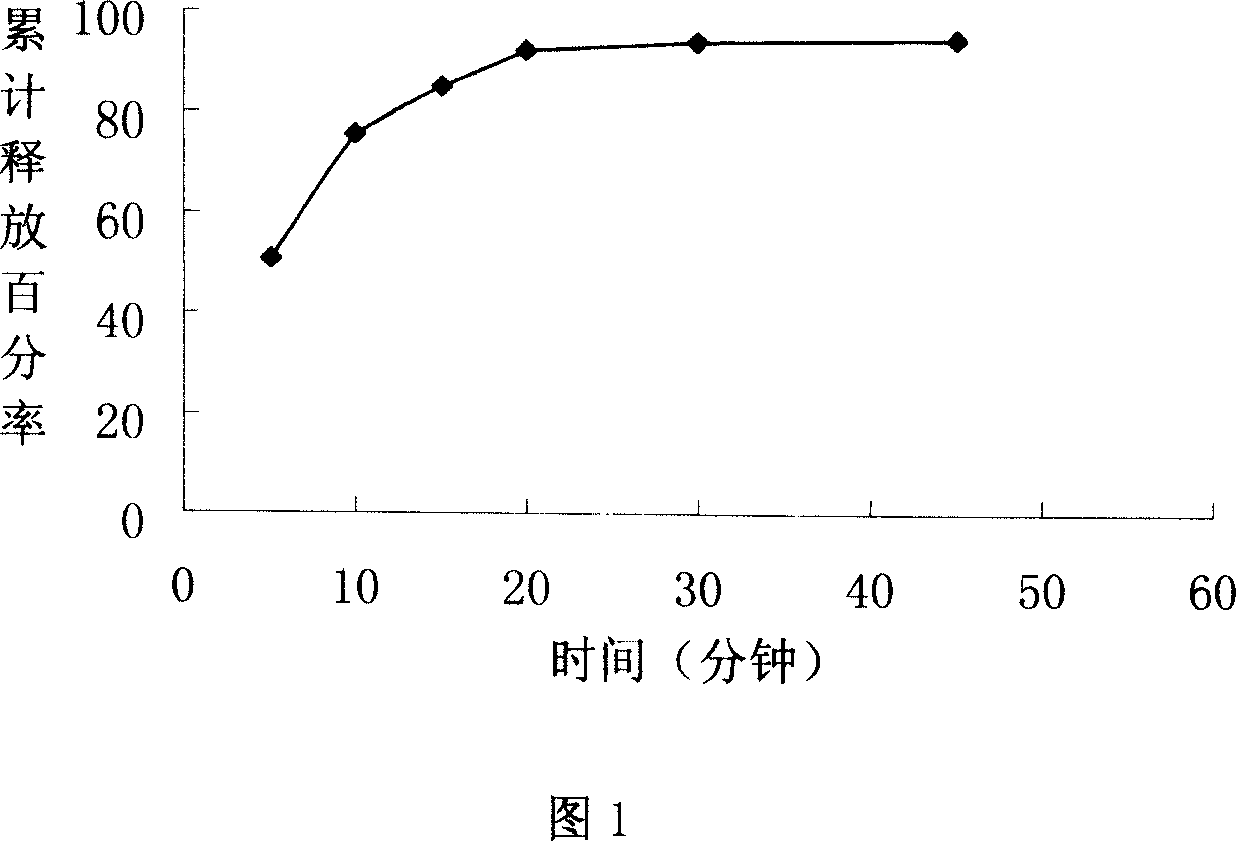
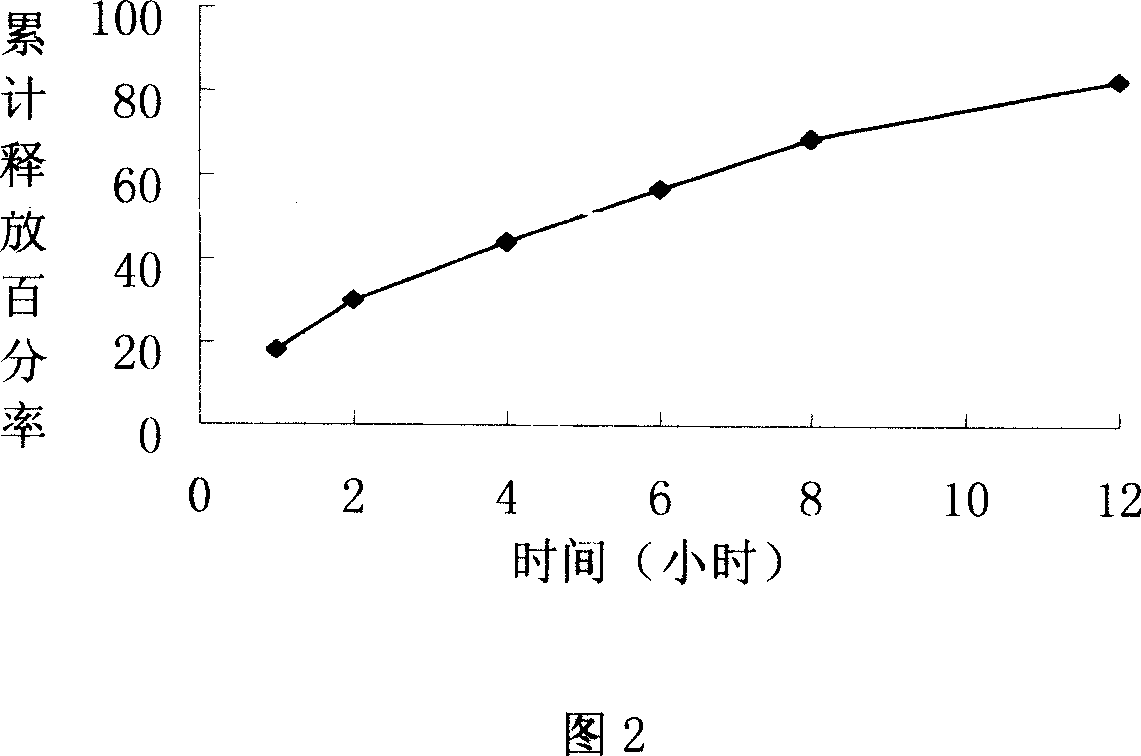
Lomoxicam sustained release tablet and preparation method thereof
ActiveCN101185640AReduce the frequency of takingReduce the fluctuation of blood drug concentration in the bodyOrganic active ingredientsAntipyreticCelluloseAdhesive
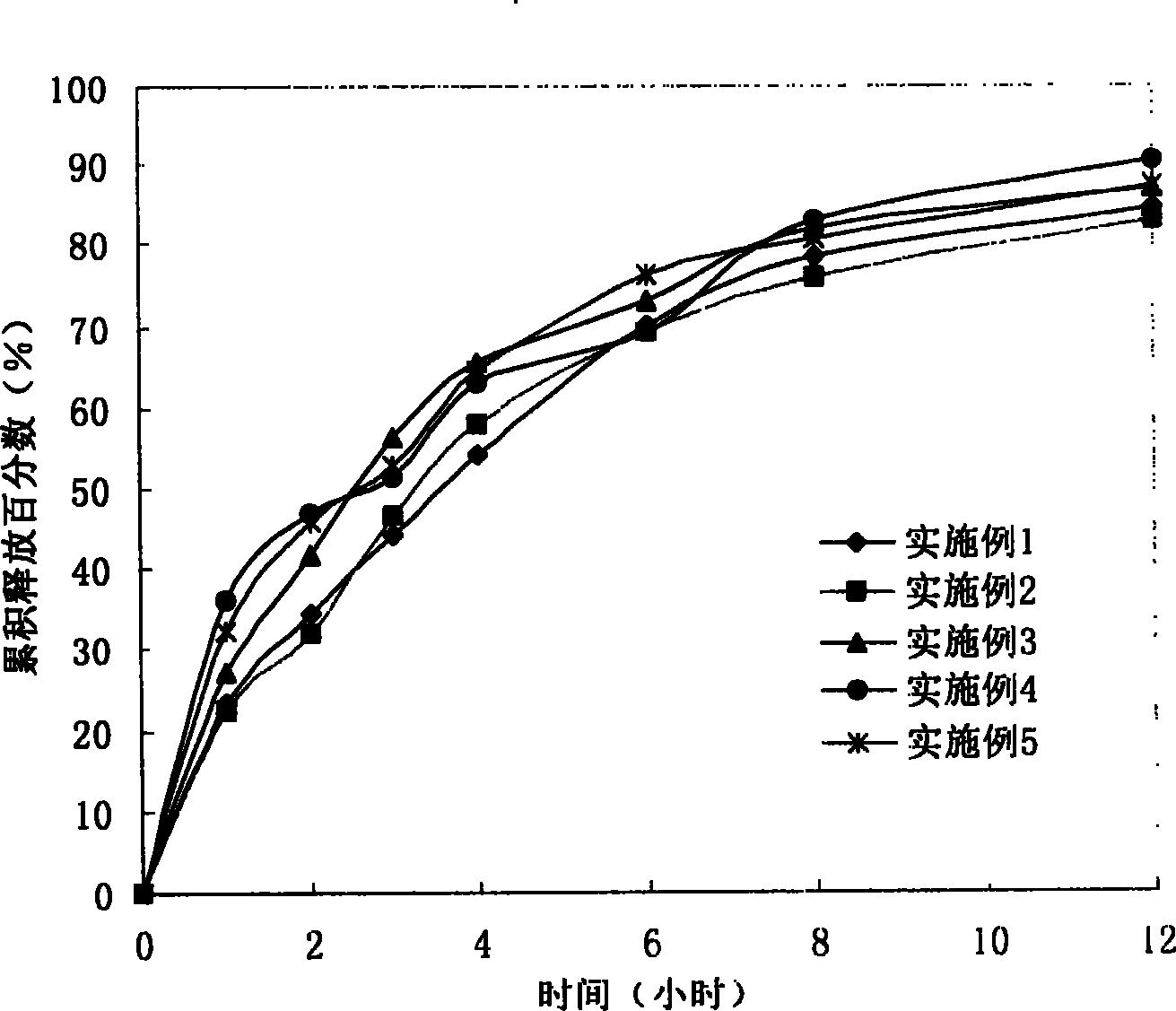
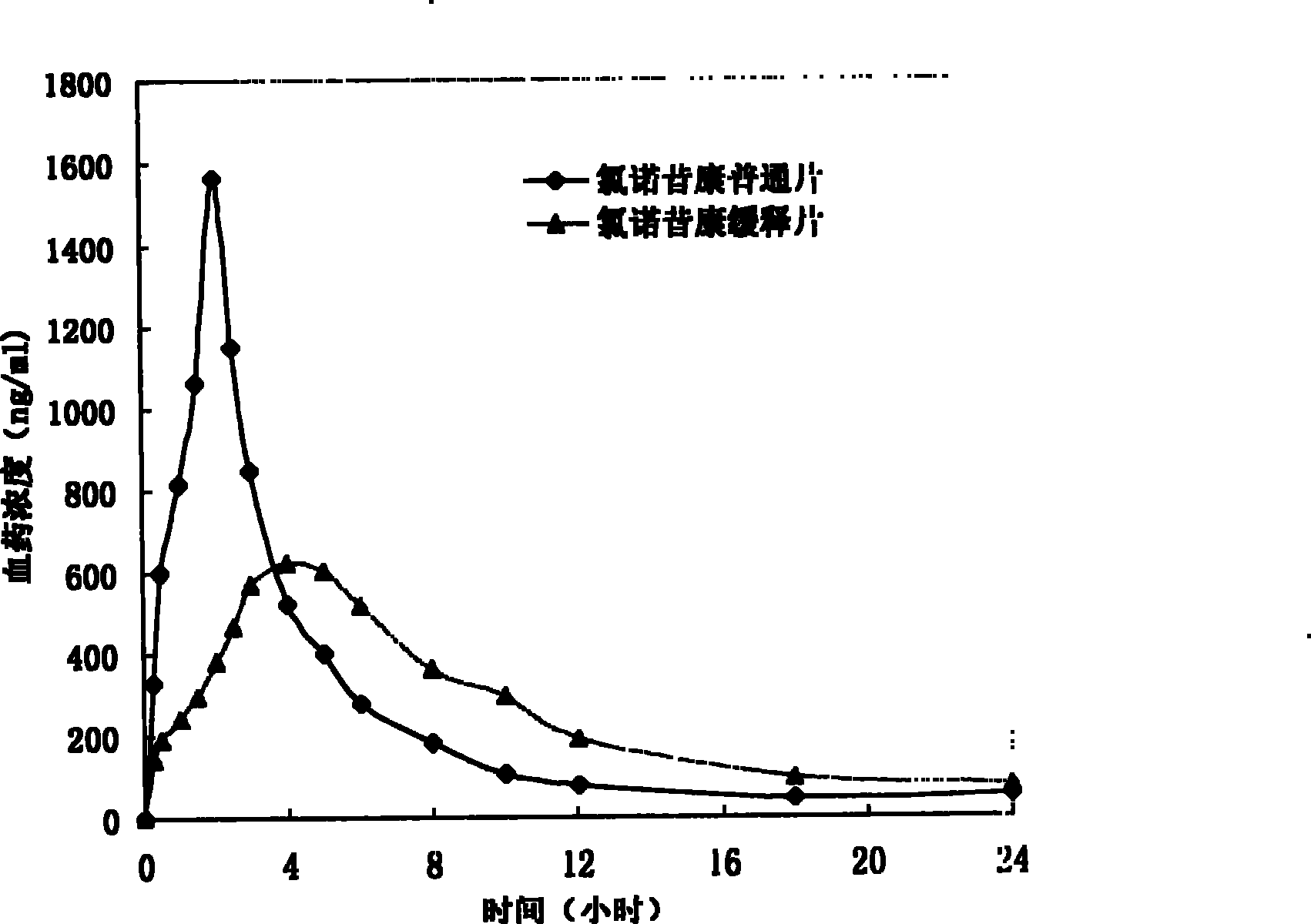
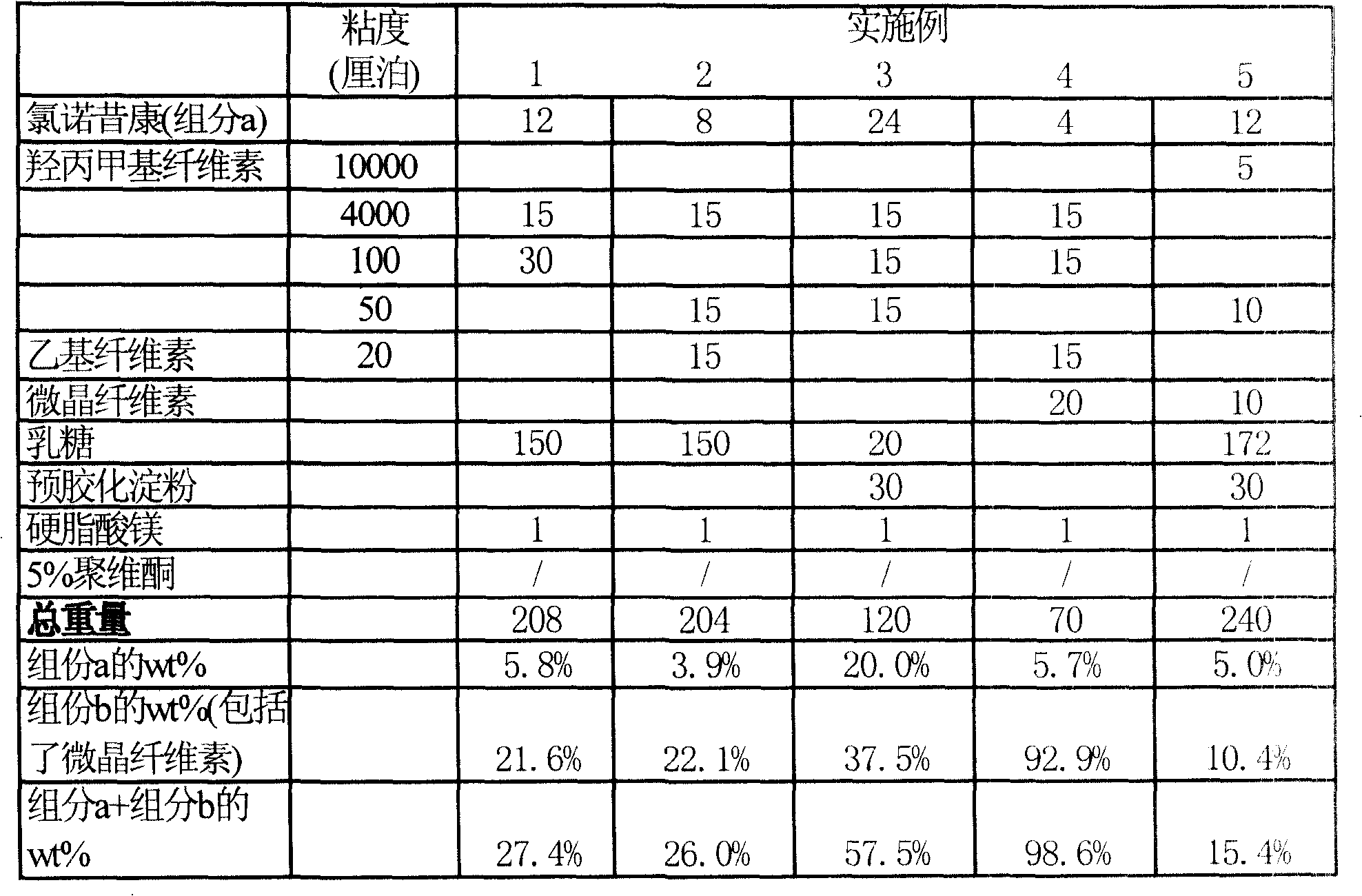
The invention provides a lornoxicam sustained release tablet and the preparation method thereof. The sustained release tablet includes 2.0-60.00 parts of lornoxicam by weight and 10.00-95.00 parts of sustained release block material by weight; wherein, the sustained release block material includes hydroxypropyl methyl cellulose or compound of hydroxypropyl methyl cellulose and other celluloses. The preparation method is that raw material prescription doses of lornoxicam, sustained release block material and filler are evenly mixed, then added with adhesive to be prepared into soft material; pelletization, drying and finishing granule are carried out according to the conventional technique of the tablet preparation, and the dry grain after finishing granule is added with lubricant, evenly mixed and pressed to make the preparation. Compared with conventional oral lornoxicam preparation, the lornoxicam sustained release tablet can maintain a longer effect after oral administration of the drug.
Owner:ZHEJIANG ZHENYUAN PHARMA CO LTD +1
Lornoxicam composition for injection and preparation process thereof
ActiveCN1927206AImprove stabilityWide range of clinical applicationsPowder deliveryOrganic active ingredientsSolubilityMedicine
The invention provides a lornoxicam injection combination, including injection and freeze-dried powder. The combinations contain 0.86-4.3 weight portion of lornoxicam and pharmaceutically acceptable basic amino acids, and the weight rate of lornoxicam and basic amino acids is 1:0.5-20, the present invention also provides corresponding preparation methods. The said combination has good solubility in solution, high stability and it improves the convenience and safety of clinical use.
Owner:ZHEJIANG ZHENYUAN PHARMA CO LTD +1
Stable lornoxicam solution agent, preparation method and application thereof
InactiveCN101278906AImprove solubilityOrganic active ingredientsAntipyreticSolubilityIntramuscular injection
The invention relates to stable lornoxicam solution and a preparation method and an application thereof; the solution contains the lornoxicam or the pharmaceutically-acceptable salt of the lornoxicam, meglumine, potassium phosphate salt and / or sodium phosphate salt, and has the advantages of good solubility, good stability and good security. The solution of the invention is suitable for parenteral administration such as intramuscular injection, intravenous injection or ophthalmic purpose; the solution which is suitable for the invention is selected from injection preparation, lyophilization formulation or spraying desiccant, preferably the lyophilization formulation.
Owner:南京易亨制药有限公司
Preparation method of non-steroidal anti-inflammatory analgesic lornoxicam
InactiveCN106892932ACheap and easy to getHigh purityOrganic chemistryPotassium tert-butoxideMethyl carbonate
The invention discloses a preparation method of non-steroidal anti-inflammatory analgesic lornoxicam. The preparation method includes the steps of enabling 5-chloro-3-chlorosulfonyl-2-methyl carbonate as a starting material to react with 2-N-methyl-2-N-Boc-acetyl(2-pyridyl)amine, and enabling a reactant to react with tertiary butanol and potassium tert-butoxide to obtain the lornoxicam. The preparation method has the advantages of safety, short synthesis route, high yield and environmental protection, and is suitable for industrialized production.
Owner:IANGSU COLLEGE OF ENG & TECH
Lornoxicam freeze-dried orally disintegrating tablet and preparation method thereof
InactiveCN110652500AEasy to takeDisintegrates quicklyOrganic active ingredientsAntipyreticOrally disintegrating tabletLornoxicam
The invention provides a lornoxicam freeze-dried orally disintegrating tablet and a preparation method thereof. The lornoxicam freeze-dried orally disintegrating tablet consists of lornoxicam and a matrix, wherein the single dosage effective component lornoxicam is 4-10mg; and the matrix comprises 0.8-10mg of an adhesive, 10-200mg of a framework support agent, 0-10mg of a flavoring agent and 0-10mg of an essence. The preparation method of the lornoxicam freeze-dried orally disintegrating tablet provided by the invention comprises steps of dissolution, mold injection, quick freezing, freeze-drying and product packaging. The lornoxicam freeze-dried orally disintegrating tablet provided by the invention is simple and convenient to take and rapid to disintegrate, can be dissolved immediately after being orally taken, can be directly taken without water and is rapid to absorb, and a first pass effect can be avoided.
Owner:HANGZHOU BIO SINCERITY PHARMA TECH CO LTD
Slow-release, controlled-release prepn. contg. chlortenozicam
InactiveCN1947706AGood curative effectAvoid peaks and valleysOrganic active ingredientsNervous disorderControlled releaseCurative effect
A slow-release or release-controllable lornoxicam with durable antalgic and anti-inflammation effect is disclosed. The povidone, polyethanediol and poloxamer are used to prepare the dispersing substance of lornoxicam or the beta-cyclodextrin or its derivative is used for preparing its inclusion substance. The slow-releasing material is used for controlling its release.
Owner:何岩
Lornoxicam dropping pill and its preparing method
The present invention discloses chlortenozicam dripping pills and preparation method. Said dripping pill preparation belongs to a medicine for resisting inflammation and stopping pain, and is formed from 1.5%-20% of medicine and 80-99.5% of auxiliary material, in which the medicine is chlortenozicam and the auxiliary material is polyethylene glycol. Besides, said invention also provides the concrete steps of its preparation method, and said chlortenozicam dripping pills can be used for curing various acute pains, arthralgia, inflammation pain, acute lumber vertebrae pain and sciatica, etc.
Owner:贵阳高新瑞得科技开发有限公司
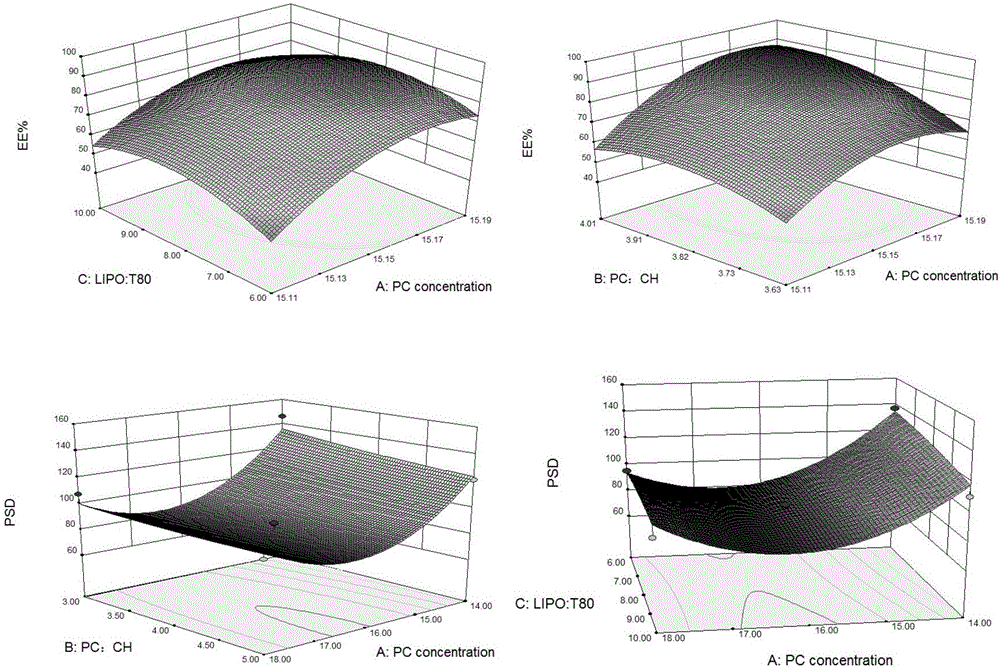
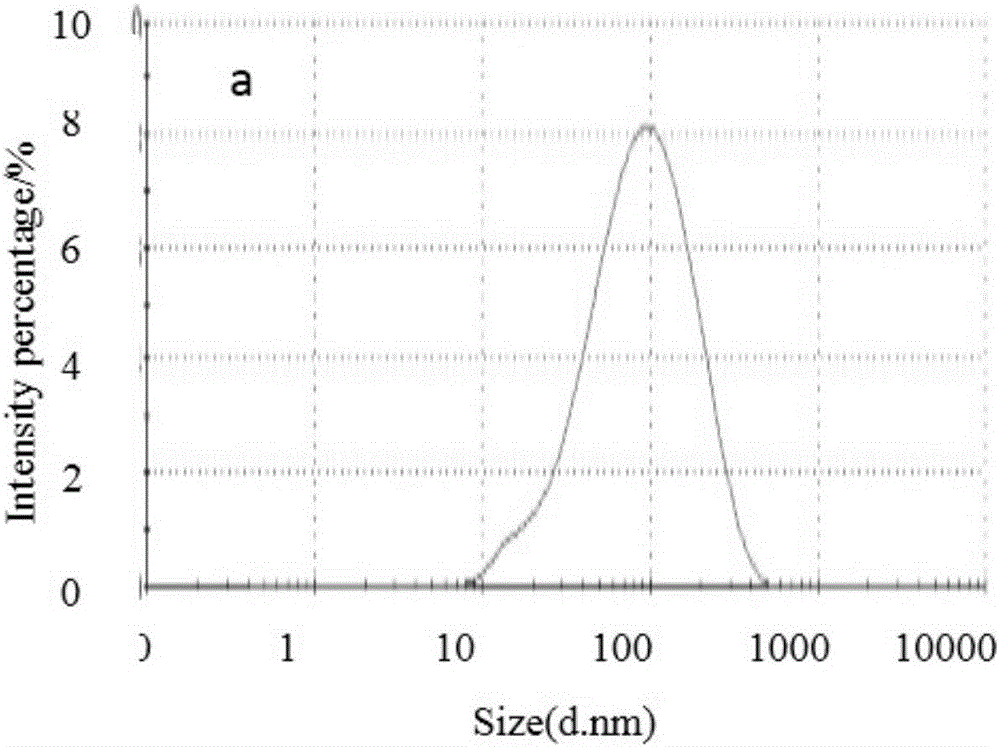
Method for optimally preparing lornoxicam flexible liposome with Box-Behnken response surface method
InactiveCN106474066ASimple preparation processHigh encapsulation efficiencyOrganic active ingredientsAntipyreticLipid formationMass ratio
The invention aims at providing a method for preparing an optimal preparation of lornoxicam flexible liposome in a screening mode with the Box-Behnken response surface method. The response surface method has the advantages that experiment design is comprehensive, scientific and accurate, and the interaction of multiple factors can be studied. According to the method, four factors including the phospholipid concentration, the phospholipid-cholesterol mass ratio, the total lipid-Tween 80 mass ratio and the total lipid-medicine mass ratio which influence the flexible liposome preparing process greatly are investigated separately, Box-Behnken response surface experiment design is conducted with the encapsulation rate and the particle size as the optimization indexes, and the optimal preparation of the lornoxicam flexible liposome is obtained through optimization. The lornoxicam flexible liposome obtained through optimization is high in encapsulation rate and uniform in size distribution and has the characteristic of slow release, which provides a good experimental basis for the process of preparing the lornoxicam flexible liposome into hydrophilic gel at the later stage.
Owner:沈阳医学院附属中心医院
Method for synthesizing lornoxicam intermediate by one-pot method
The invention belongs to the technical field of drug synthesis, and in particular relates to a method for synthesizing a lornoxicam intermediate by a one-pot method. The method is as follows: using 5-chloro-3-methylsulfonamidethiophene-2-carboxylic acid methyl ester as a raw material, and adding methyl bromoacetate and sodium methoxide for alkylation reaction; adding again the sodium methoxide forcyclization reaction to obtain 6-chloro-4-hydroxy-2-methyl-2H-thieno [2,3-e]-1,2-thiazine-3-carboxylic acid methyl ester-1,1-dioxide. The lornoxicam intermediate is synthesized by the one-pot methodby alkylation and cyclization two-step reaction, use of dangerous reagents such as dimethyl sulfate and sodium hydride can be avoided, no dangerous gas is produced during the reaction, side reactionsare reduced, production environment is improved, production safety is ensured, operation is simplified, and product yield is improved.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Lornoxicam freeze-dried injection and preparation method thereof
ActiveCN100560061CHigh clarityGood formabilityPowder deliveryOrganic active ingredientsMedicineLornoxicam
Owner:HAINAN JINRUI PHARMA CO LTD
Synthesis method of high-purity lornoxicam
ActiveCN113480561AAchieve adsorptionReduce usageIon-exchange process apparatusOrganic chemistryXylylenePtru catalyst
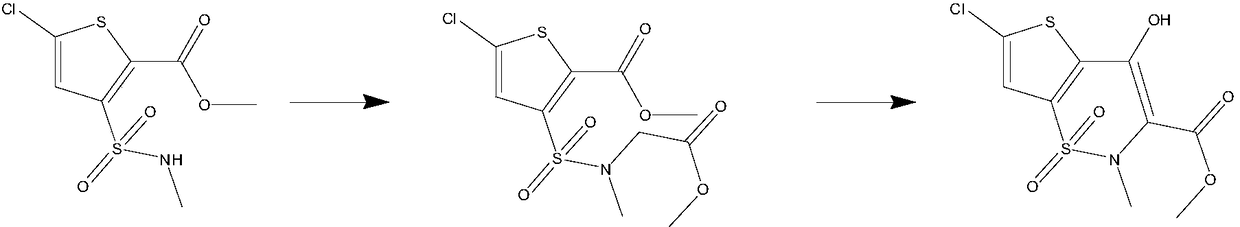
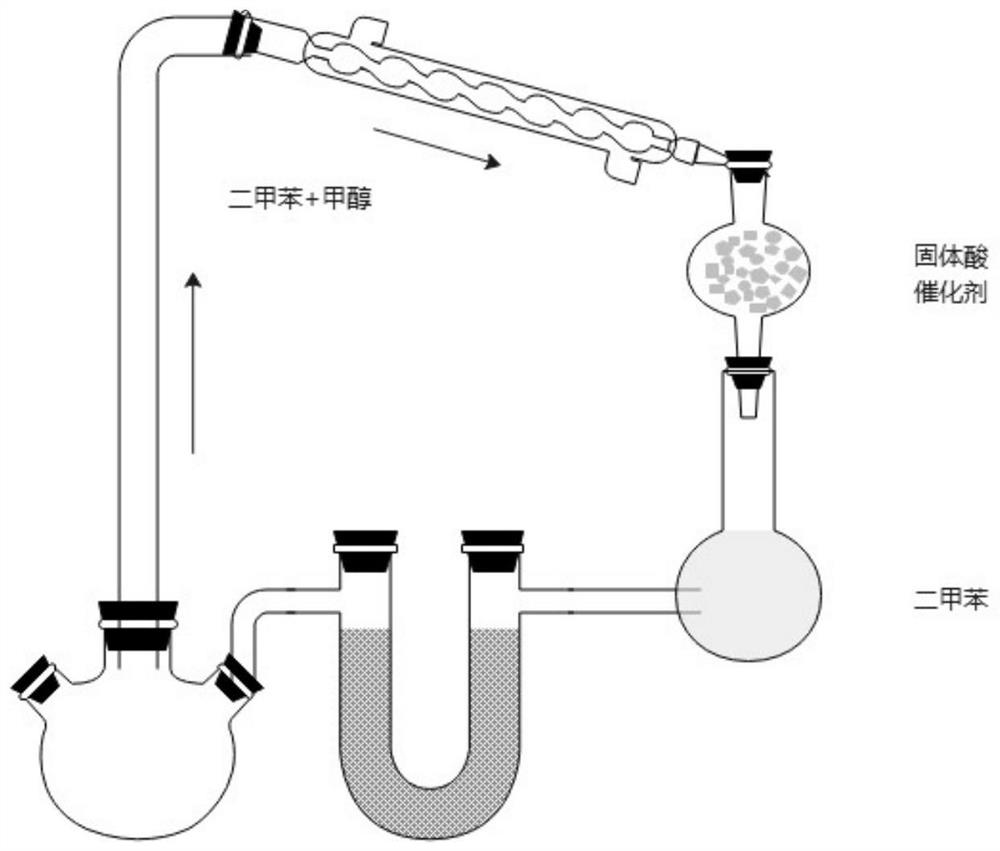
The invention belongs to the technical field of medicine synthesis, and particularly relates to a synthesis method of high-purity lornoxicam. The method comprises the following steps: by taking 6-chloro-4-hydroxy-2-methyl-2-H-thieno [2, 3-e]-1, 2-thiazine carboxylic acid methyl ester-1, 1-dioxide and 2-aminopyridine as raw materials and xylene as a solvent, carrying out distillation reaction, condensing mixed gas obtained by the distillation reaction to obtain condensate, adsorbing methanol in the condensate by adopting a solid acid catalyst, and recycling the adsorbed condensate. According to the invention, the methanol generated by the reaction is distilled out so as to promote the reaction to proceed forwards, and then the methanol is absorbed under the catalysis of H2SO4 / MxOy superacid solid acid, so that the xylene returned to the reaction system does not contain methanol, and the coking of the reaction is reduced so as to improve the product quality and yield; and the purity of the prepared lornoxicam is high and can reach 99.9% or above, the solvent amount is reduced, and the method is suitable for industrial production.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
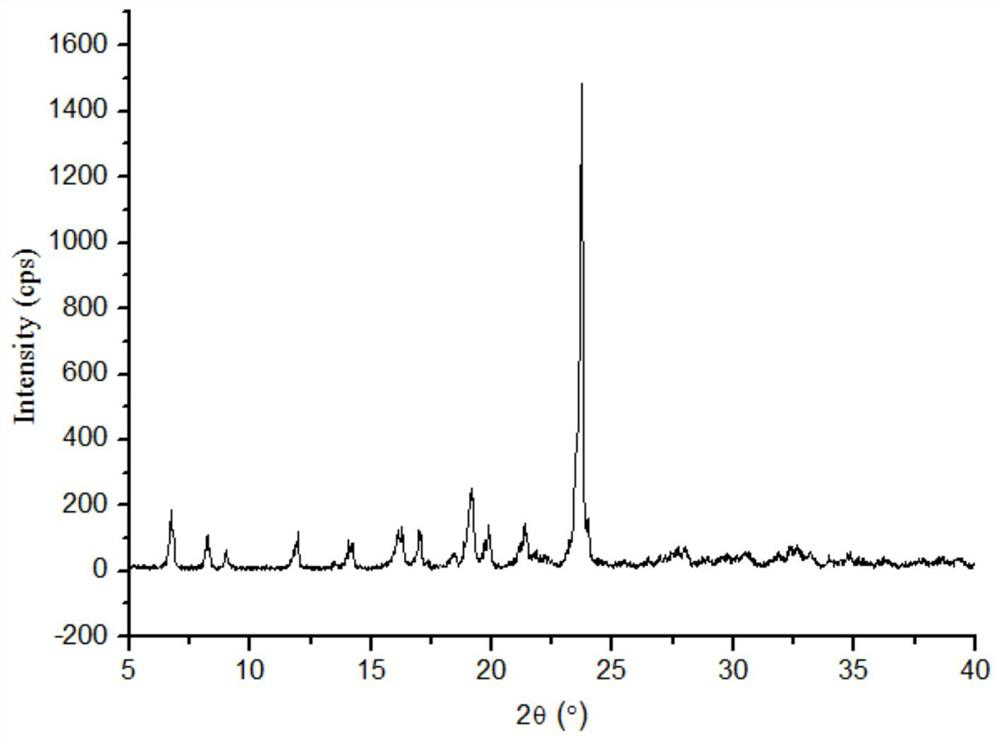
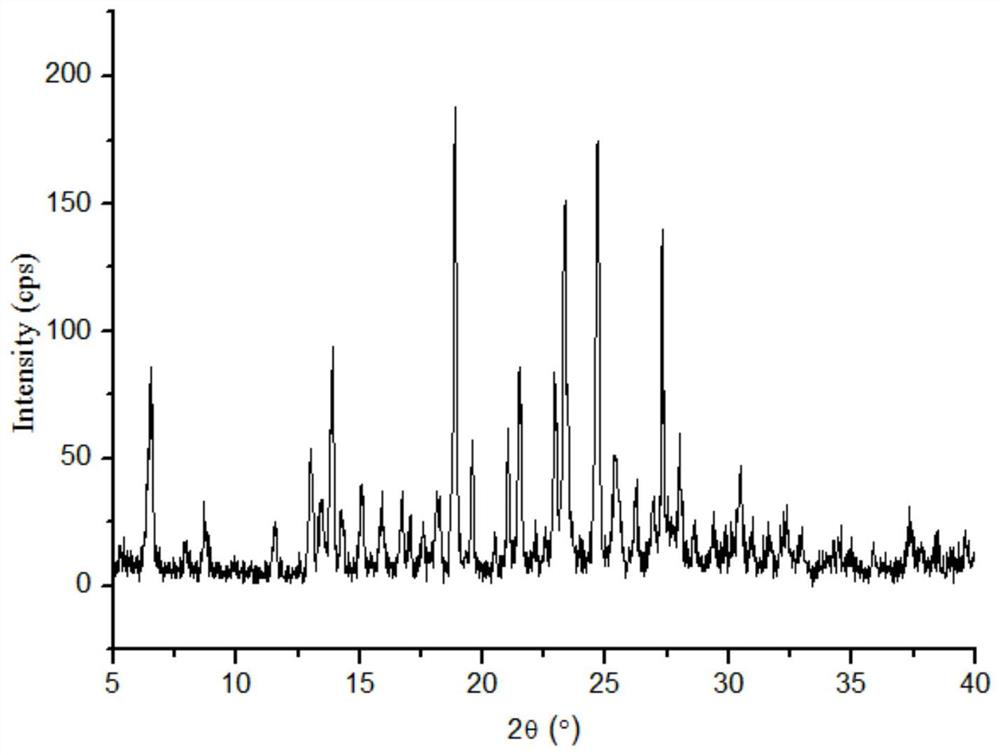
Lornoxicam and puerarin eutectic crystal and preparation method thereof
ActiveCN111004256AGood compressibilityImprove solubilityOrganic chemistry methodsPhysical chemistryLornoxicam
The invention discloses a lornoxicam and puerarin eutectic crystal, which is formed by combining lornoxicam and puerarin according to a molar ratio of 1: 1, and has a powder X-ray diffraction patternexpressed by a 2theta angle value, and an infrared spectrogram. The method comprises the following steps: dissolving the lornoxicam and the puerarin into an organic solvent according to a certain molar ratio, carrying out reduced pressure rotary evaporation on the solvent, carrying out vacuum drying, and putting into a resistance furnace to remove the solvent, thereby obtaining the lornoxicam andpuerarin eutectic crystal. The eutectic crystal is different from a lornoxicam monomer, a puerarin monomer and a physical mixture of the lornoxicam monomer and the puerarin monomer in powder X-ray diffraction and infrared ray, the crystal form of the eutectic crystal is a new crystal form different from the monomers and the physical mixture, and the compressibility of lornoxicam and the water solubility of the lornoxicam and the puerarin are improved.
Owner:CHINA PHARM UNIV
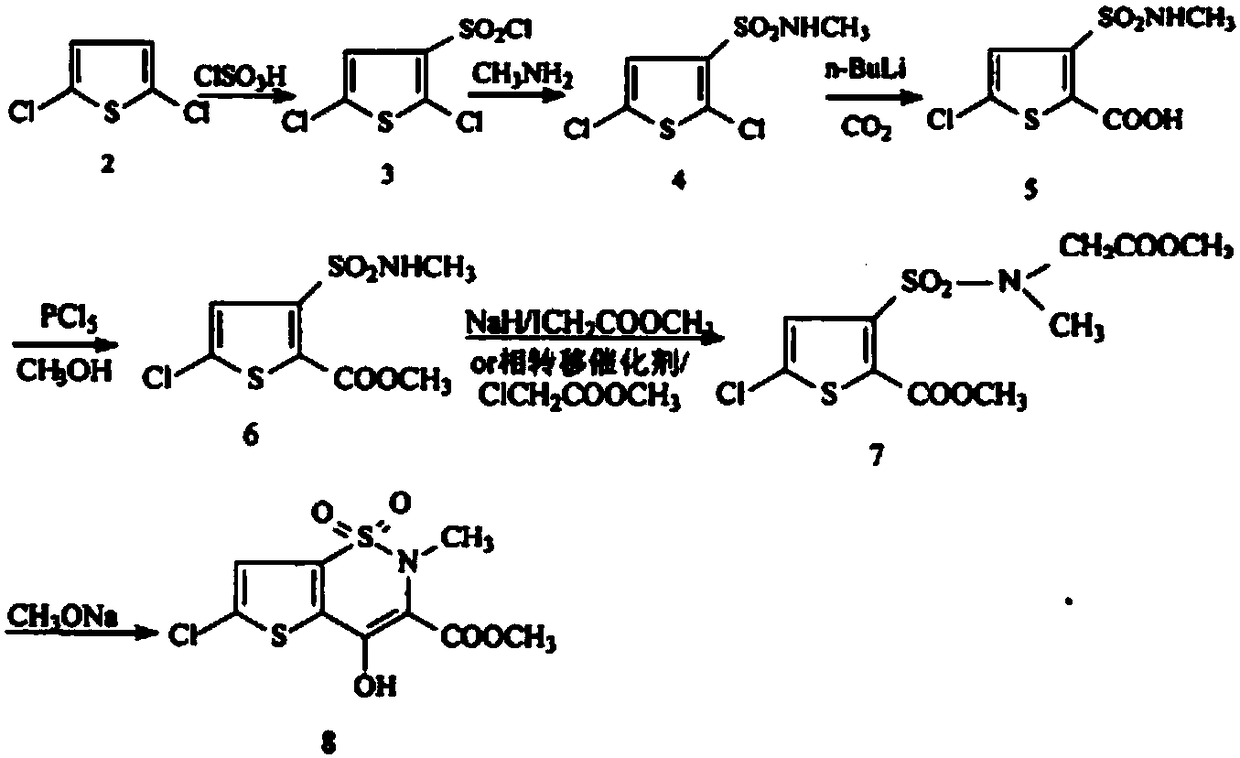
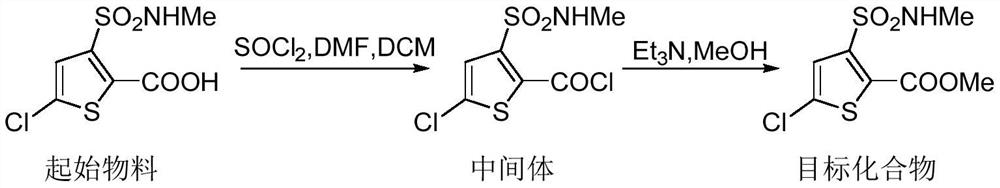
Synthesis method of lornoxicam intermediate 5-chloro-3-methylsulfonamide thiophene-2-carboxylic acid methyl ester
The invention belongs to the technical field of medicine synthesis, and particularly relates to a synthetic method of a lornoxicam intermediate 5-chloro-3-methyl sulfonamide thiophene-2-carboxylic acid methyl ester. The method comprises the following steps: by taking 5-chloro-3-methyl sulfonamide thiophene-2-carboxylic acid as an initial raw material, dichloromethane as a solvent, N,N-dimethylformamide as a catalyst and thionyl chloride as a chlorination reagent, reacting to generate an acyl chloride active intermediate, then adding triethylamine and methanol, and reacting to generate the 5-chloro-3-methyl sulfonamide thiophene-2-carboxylic acid methyl ester. According to the method, thionyl chloride is used as the chlorination reagent, so that the use of phosphorus pentachloride which is a phosphorus-containing reagent in the traditional process is avoided, and the process is more environment-friendly; meanwhile, dichloromethane is used as a solvent instead of high-toxicity chloroform, so that the pollution of the process to the environment is further reduced.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Lornoxicam tromethamine eutectic crystal
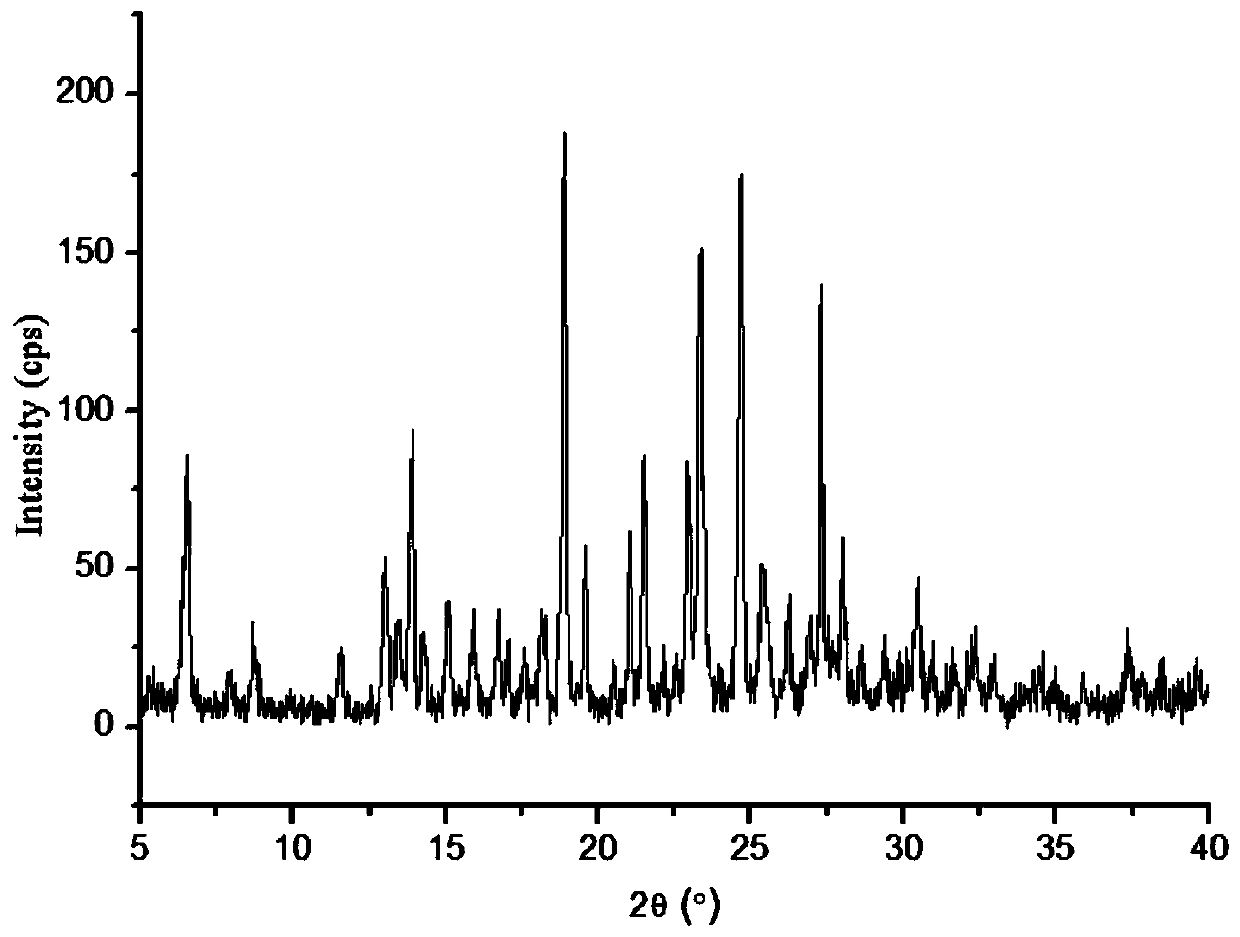
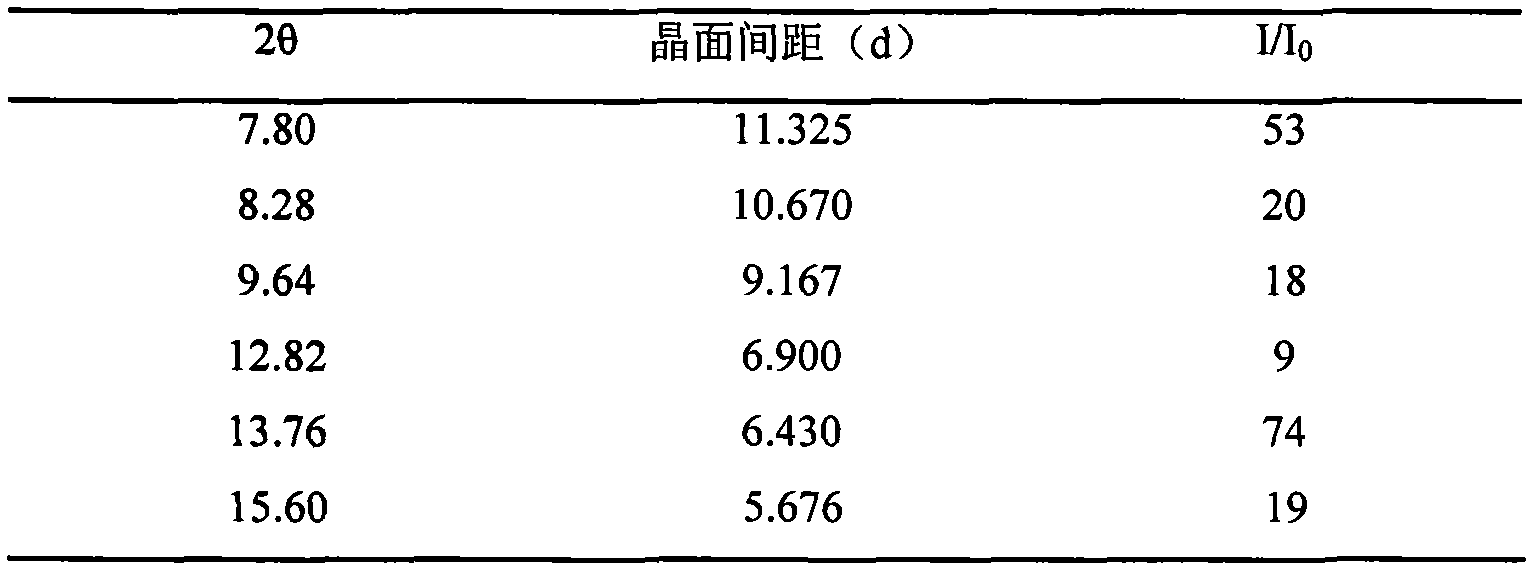
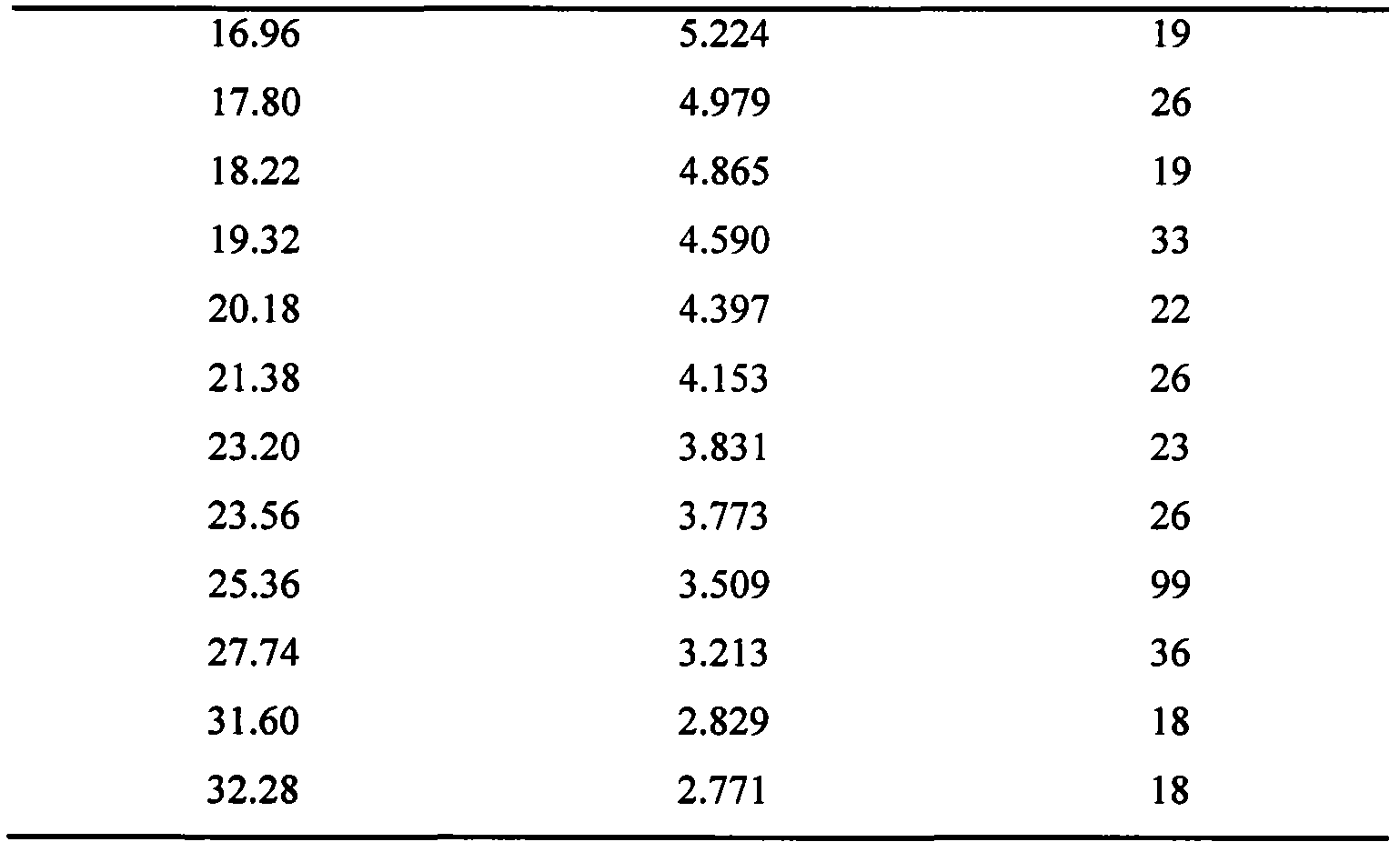
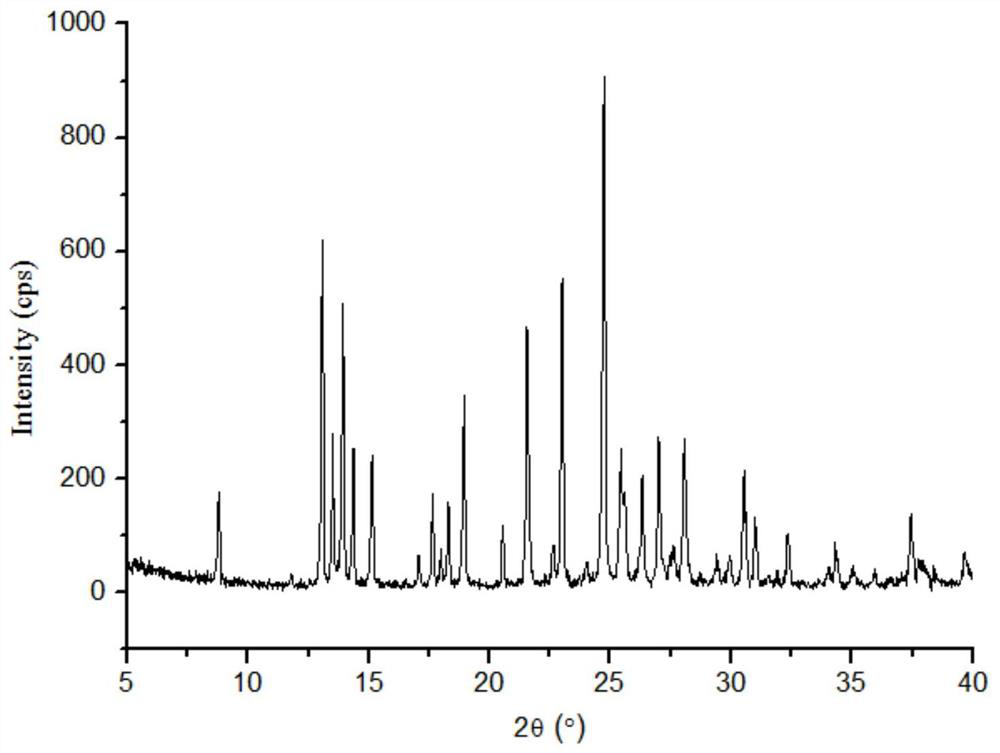
InactiveCN102093394AOrganic compound preparationAmino-hyroxy compound preparationLornoxicamLength wave
The invention relates to a lornoxicam tromethamine eutectic crystal formed by combining lornoxicam and tromethamine. By Cu-K alpha radiation, characteristic peaks are presented at the positions of 7.80, 8.28, 9.64, 12.82, 13.76, 15.60, 16.96, 17.80, 18.22, 19.32, 20.18, 21.38, 23.20, 23.56, 25.36, 27.74, 31.60 and 32.28 of X-ray powder diffraction spectrum which is represented in a degree of 2 theta; absorption peaks are presented at the positions of about 3352, 3294, 3103, 2937, 2887, 1627, 1573, 1533, 1496, 1473, 1433, 1392, 1332, 1236, 1188, 1155, 1045, 887, 821, 777, 634, 588, 551,522 and 462 cm<-1> wavelengths of infrared absorption spectrum obtained by KBr tebletting; and heat absorption transformation of differential scanning caborimetry (DSC) is mainly generated at the temperature of about 55.9 DEG C. The lornoxicam tromethamine eutectic crystal disclosed by the invention is different from the existing commercial lornoxicam powder in the aspects of X-ray diffraction, DSC, infrared spectrum and melting point, thus the crystal form of the lornoxicam tromethamine eutectic crystal is a crystal form which is completely different from the lornoxicam prepared by the prior art. Compared with the lornoxicam physical mixture of equivalent tromethamine, the digestion speed and degree of the lornoxicam tromethamine eutectic crystal are obviously improved.
Owner:CHINA PHARM UNIV
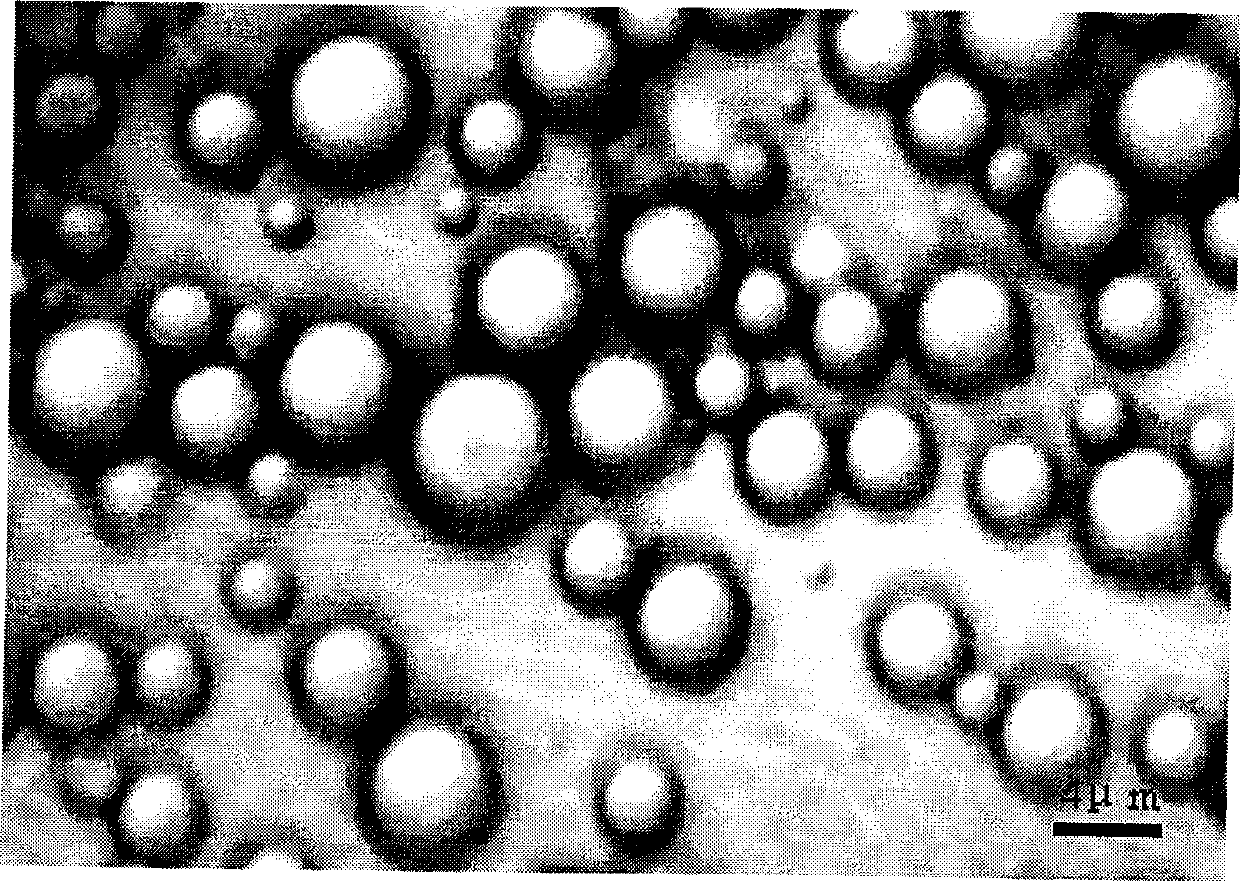
Lornoxicam microsphere and preparation method thereof
InactiveCN101371842AGood curative effectIncrease drug concentrationPowder deliveryOrganic active ingredientsSide effectMicrosphere
The invention discloses Lornoxicam microspheres, in which polylactic acid-glycolic acid copolymer is used as a carrier and Lornoxicam as a sustained-released medicament; the average diameter of the microspheres is 1-15mum, the span thereof is 1-2, and Lornoxicam accounts to 1-15 percent of the whole microsphere mass. The preparation method of the Lornoxicam microspheres is that: polylactic acid-glycolic acid copolymer is dissolved in dichloromethane to form an organic phase; the sustained-released medicament is mixed and suspended in distilled water to form an internal aqueous phase, and then the internal aqueous phase is added into the organic phase in a dropwise way, thus a primary emulsion is obtained. Next, the primary emulsion is injected by an injector into an external aqueous phase, and a W / O / W multiple emulsion is obtained, and then stirred to fully evaporate dichloromethane, the multiple emulsion is centrifugated to remove the supernatant liquid, cleaned by distilled water; and then the microspheres are obtained, and the microspheres are dried in vacuum, thus the dried powder of Lornoxicam microsphere is obtained. The microspheres enhances the curative effect of the medicament, reduces the toxicity and side effects, and avoids the shortcomings of long term administration, which has quite good effect on the treatment of arthritis.
Owner:SHANDONG UNIV
AuNPs/miR-140 compound as well as preparation method and application thereof
ActiveCN111529713AImprove drug activityImprove stabilityOrganic active ingredientsAutomatic syringesCartilage cellsDisease
The invention provides an AuNPs / miR-140 compound as well as a preparation method and application thereof. The AuNPs / miR-140 compound is obtained by compounding gold nanoparticles AuNPs and miR-140 through electrostatic adsorption, wherein the mass ratio of the AuNPs to the miR-140 is (5-28.8):1. The AuNPs / miR-140 compound can efficiently deliver the miR-140 into cartilage cells, the cell transfection rate is close to 100%, the AuNPs / miR-140 compound has no obvious toxicity to the cartilage cells, the expression of the miR-140 in the cartilage cells can be up-regulated, the expression level ofCOL2A mRNA in the cartilage cells can be increased, and the AuNPs / miR-140 compound has a repairing effect and a certain anti-inflammatory capability on the cartilage cells. A pharmaceutical composition of the AuNPs / miR-140 compound and lornoxicam has remarkable effects in the aspects of protecting cartilage, promoting cartilage repair and preventing and treating arthritis diseases.
Owner:SHANDONG UNIV
A kind of freeze-drying process of lornoxicam for injection
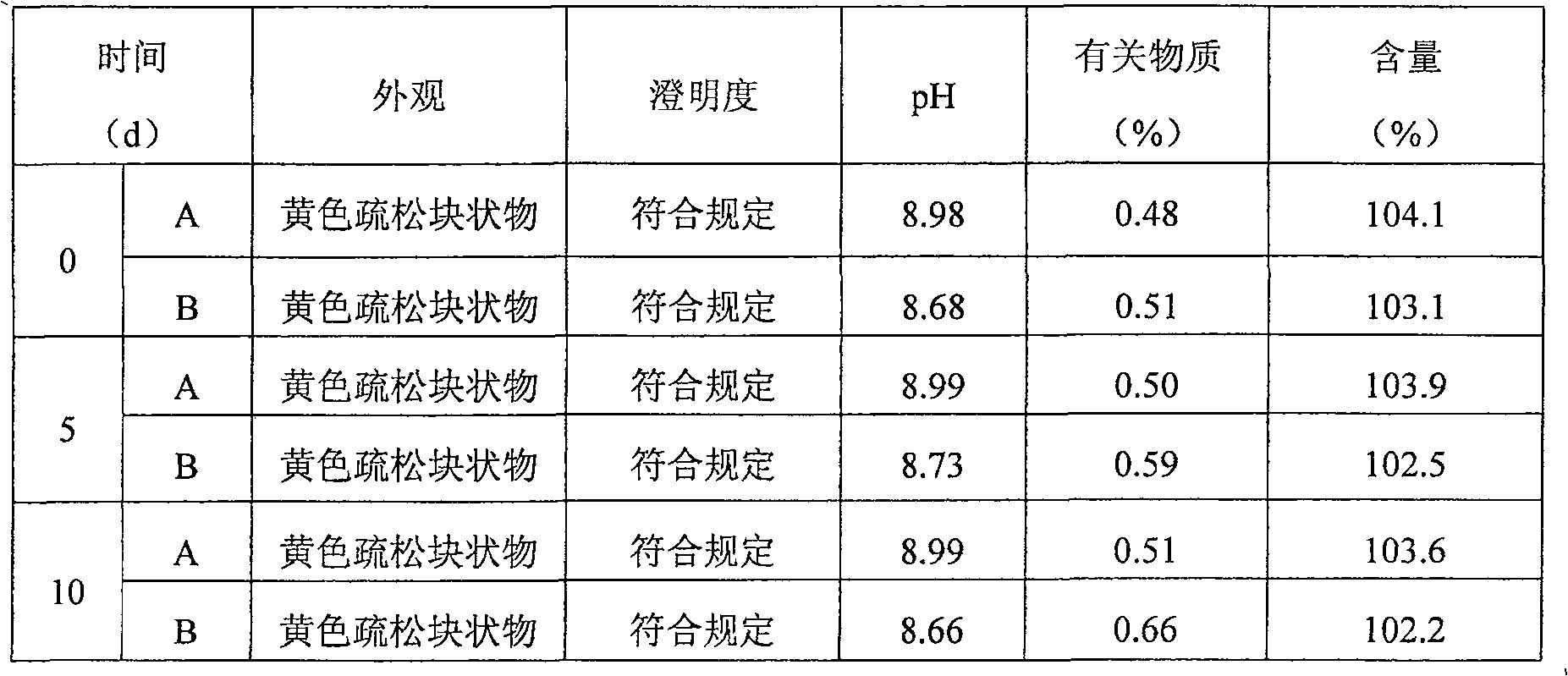
ActiveCN106727364BSufficient process capabilityGood resolubilityOrganic active ingredientsPowder deliveryLornoxicamEngineering
The invention discloses a freeze-drying technology for injection lornoxicam. The freeze-drying technology is characterized by sequentially comprising the following steps: (1) conducting pre-freezing; (2) conducting sublimation drying in a mode of six-sectional stepped temperature raising as follows: a, heating up a coolant to a minus (6-4) DEG C, preserving heat for 0.5-1.5h, conducting automatic pulsation to promote air permeation when a vacuum degree is 12-18Pa and stopping the air permeation when the vacuum degree is 22-28Pa; b, heating up the coolant to a temperature ranging from minus 1 DEG C to 1 DEG C, preserving heat for 1-1.5h and conducting pulsation to promote air permeation to 22-28Pa; c, heating up the coolant to 4-6 DEG C, preserving heat for 2-2.5h and conducting pulsation to promote air permeation to 22-28Pa; d, heating up the coolant to 9-11 DEG C, preserving heat for 2-2.5h and conducting pulsation to promote air permeation to 22-28Pa; e, heating up the coolant to 14-16 DEG C, preserving heat for 2-2.5h and conducting pulsation to promote air permeation to 22-28Pa; and f, heating up the coolant to 24-26 DEG C, preserving heat for 1-1.5h and conducting pulsation to promote air permeation to 30-36Pa; and (3) conducting secondary sublimation drying. With the application of the freeze-drying technology provided by the invention, moisture content and impurity limit of the product (the injection lornoxicam) can conform to requirements prescribed within, and meanwhile, the time of an entire freeze-drying process can be also shortened.
Owner:苏州天马医药集团天吉生物制药有限公司
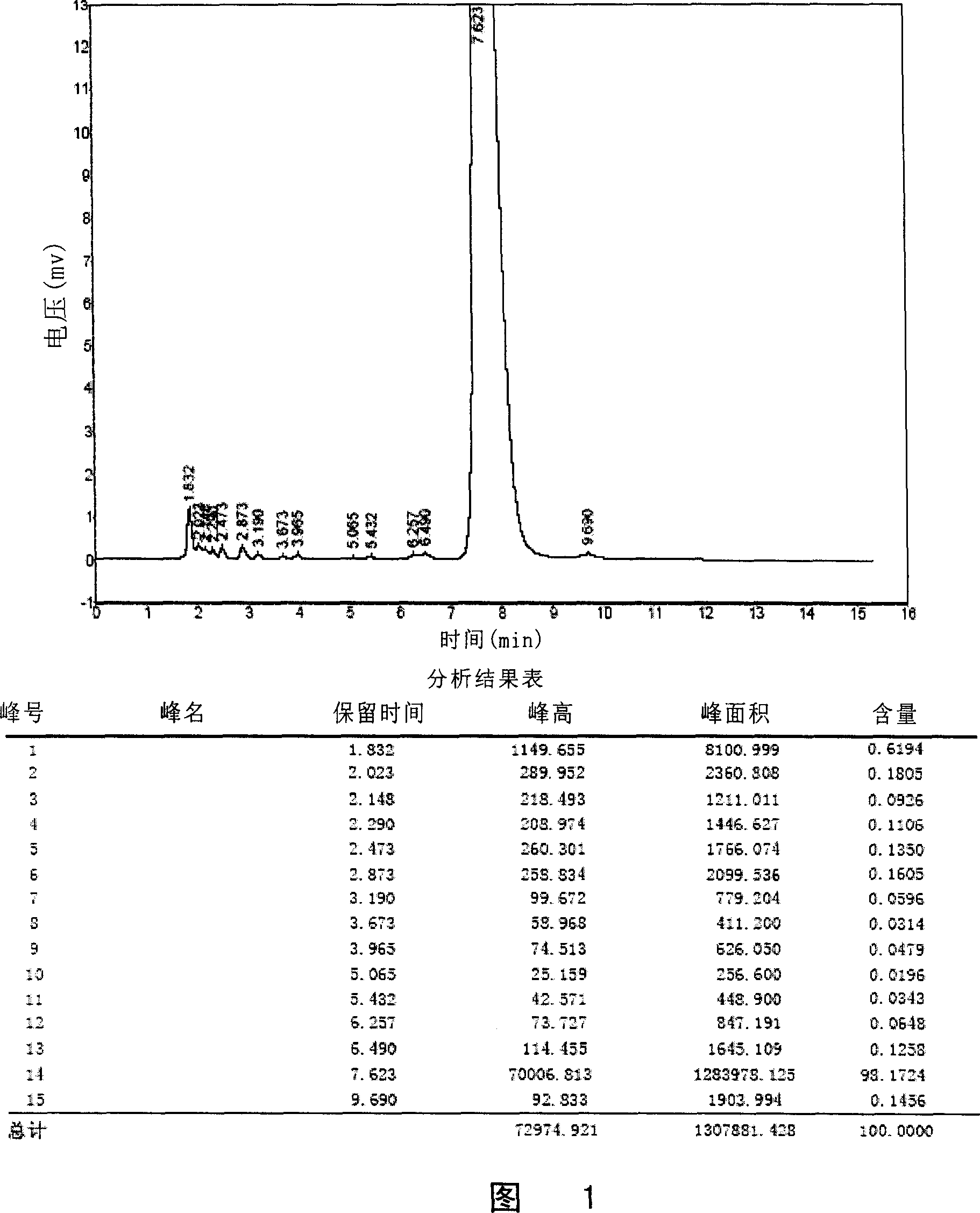
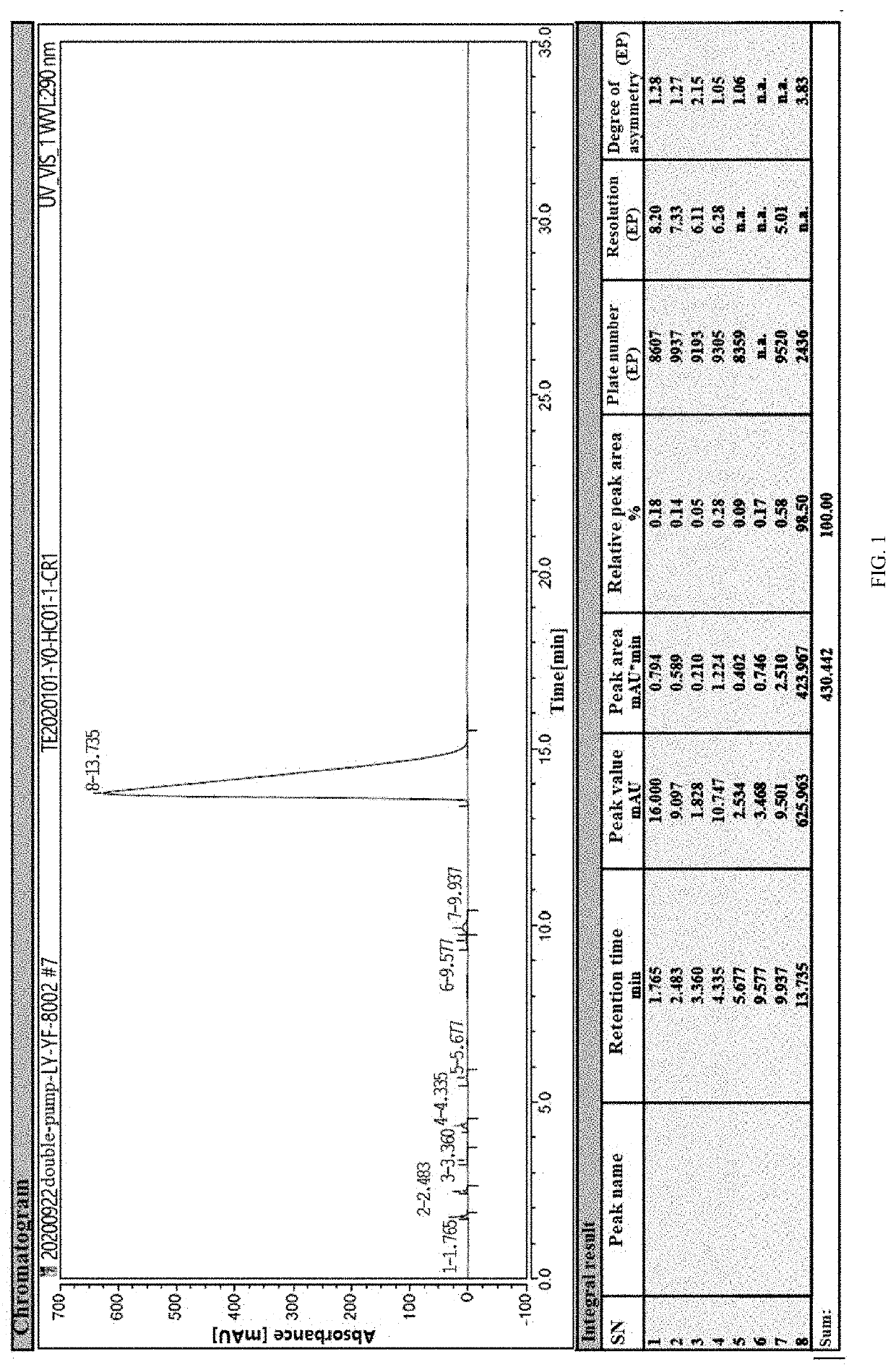
Lornoxicam compound and purifying method thereof
InactiveCN101633667AIon-exchange process apparatusOrganic chemistryStationary phasePurification methods
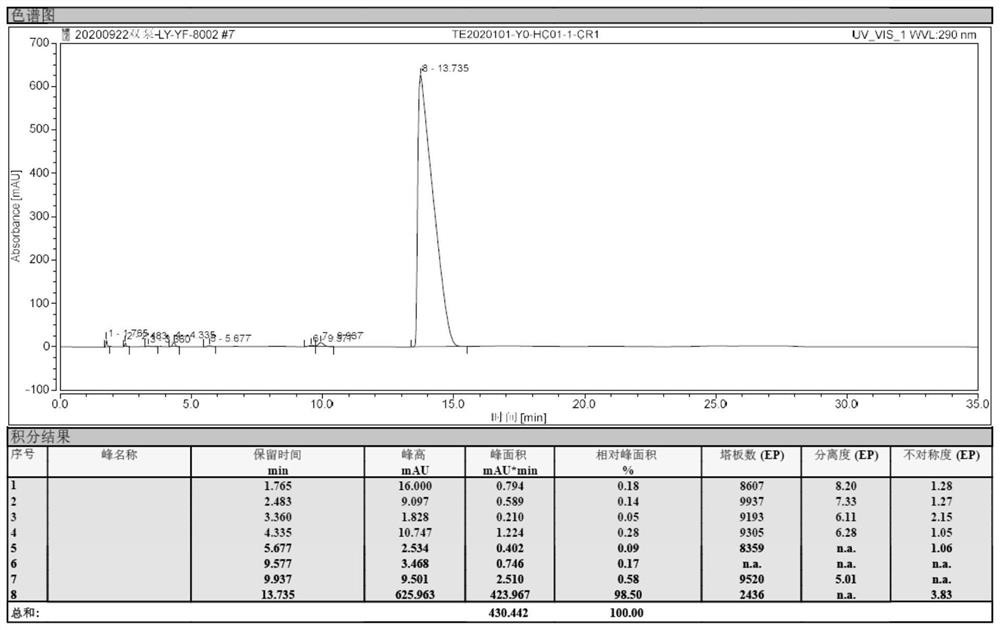
The invention discloses a lornoxicam compound and a purifying method thereof. In the method, a simple silicagel column chromatography method is used, and a proper stationary phase and a proper mobile phase are used, i.e. silicagel is used as the stationary phase and a mixed solvent of isopropanol and acetone with a certain proportion is used as the mobile phase, the temperature of the column is kept to be higher than the room temperature, and the lornoxicam can be efficiently purified. The method can achieve high yield and high purity, thereby being an effective method for obtaining the lornoxicam with high purity.
Owner:HAINAN YONGTIAN PHARMA INST
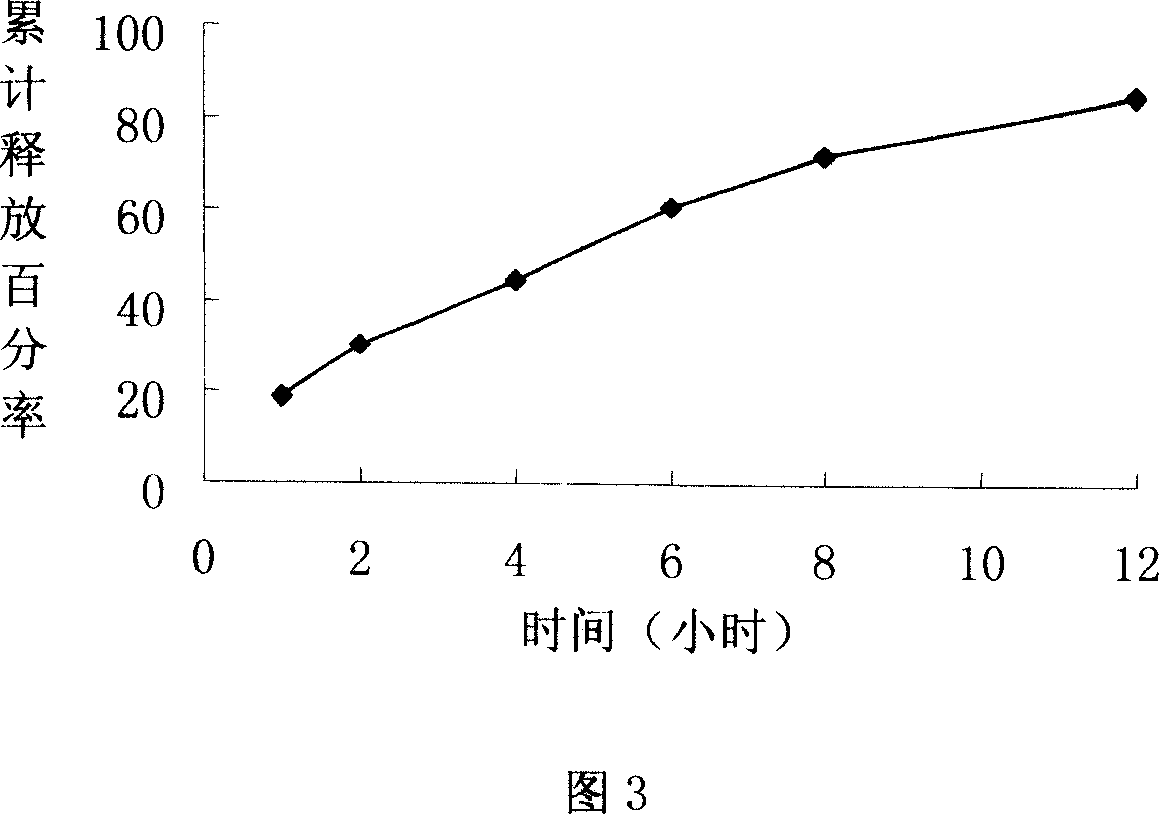
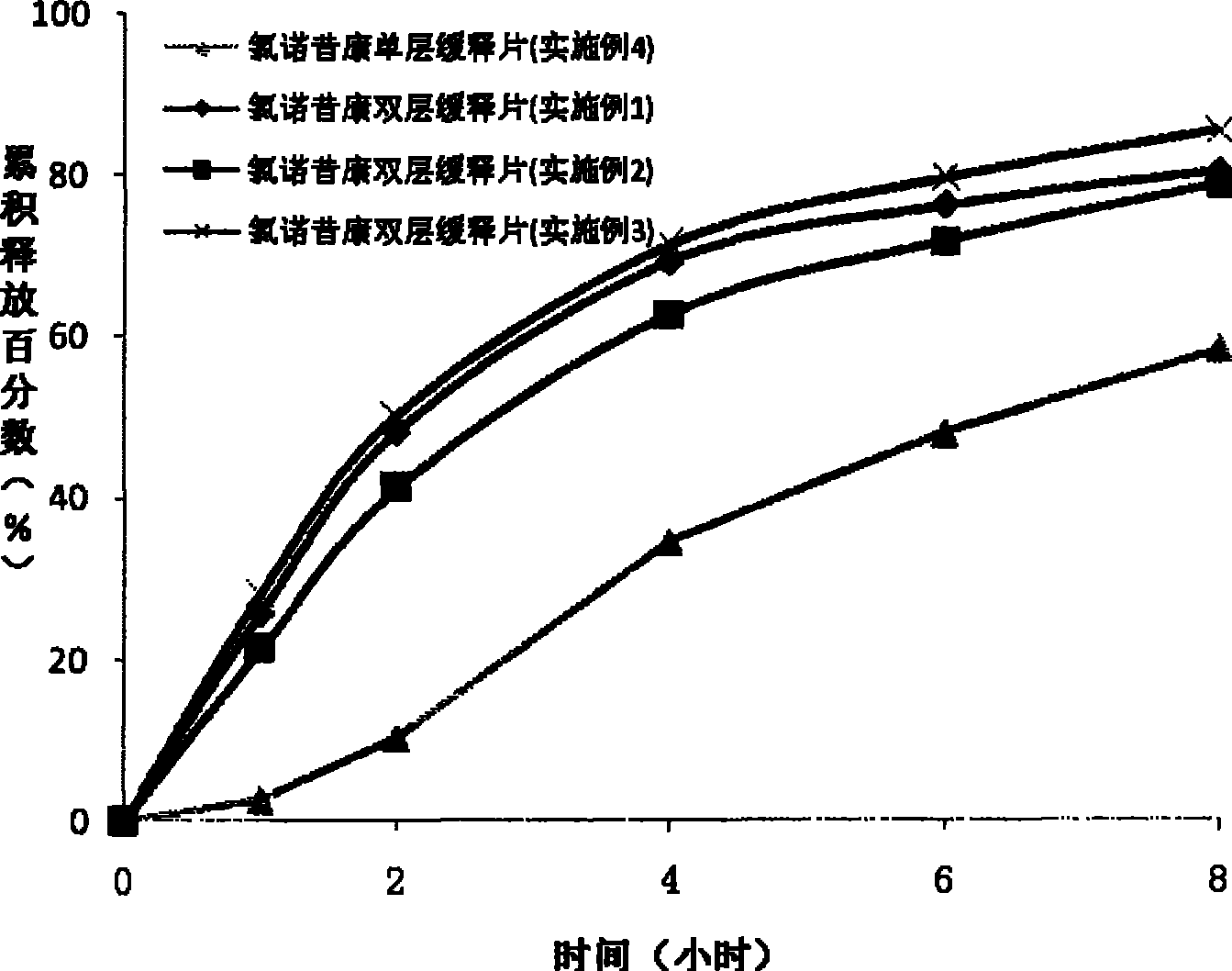
Lornoxicam double-layer sustained release tablets
ActiveCN101342177BReduce the number of dosesBlood concentration is effectiveOrganic active ingredientsAntipyreticBlood concentrationLornoxicam
The invention relates to a double-layer sustained release lornoxicam tablet which comprises (a) a quick release layer and (b) a sustained release layer, wherein, the quick release layer comprises (a1) the lornoxicam, (a2) alkaline matter and (a3) an other optional carrier or pharmaceutically acceptable excipient and the sustained release layer comprises the (b1) lornoxicam, (b2) sustained releasesubstance and (b3) the other optional carrier or the pharmaceutically acceptable excipient. The weight proportion of (a1) and (b1) is 1:50 to 50:1. The invention also provides a preparation method ofthe double-layer sustained release lornoxicam tablet. The double-layer sustained release preparation of the invention has the advantages of the quick effect of the quick release preparation and the sustained effect of sustained release preparation. In addition, the double-layer sustained release preparation can maintain the effect of effective blood concentration continuously and stably after theeffective blood concentration is reached rapidly.
Owner:CHINA PHARM UNIV +1
Lornoxicam compound and purifying method thereof
InactiveCN101633667BIon-exchange process apparatusOrganic chemistryStationary phasePurification methods
The invention discloses a lornoxicam compound and a purifying method thereof. In the method, a simple silicagel column chromatography method is used, and a proper stationary phase and a proper mobile phase are used, i.e. silicagel is used as the stationary phase and a mixed solvent of isopropanol and acetone with a certain proportion is used as the mobile phase, the temperature of the column is kept to be higher than the room temperature, and the lornoxicam can be efficiently purified. The method can achieve high yield and high purity, thereby being an effective method for obtaining the lornoxicam with high purity.
Owner:HAINAN YONGTIAN PHARMA INST
Lornoxicam liposome for injection
PendingCN114732786AReduce the number of dosesSustained analgesic effectOrganic active ingredientsAntipyreticCholesterolActive agent
The invention belongs to the technical field of chemical and biological medicines, and particularly relates to lornoxicam liposome for injection. The lornoxicam liposome for injection is prepared from the following components in parts by weight: 1 to 10 parts of lornoxicam, 2 to 20 parts of phospholipid, 1 to 8 parts of cholesterol, 0.1 to 5 parts of a surfactant, 1 to 10 parts of a buffering agent, 1 to 10 parts of a freeze-drying excipient and 1 to 10 parts of a freeze-drying protective additive. According to the lornoxicam lipidosome for injection disclosed by the invention, various components such as specific lornoxicam, phospholipid, cholesterol, a surfactant, a buffer agent, freeze-drying auxiliary materials (a protective agent and an excipient) and the like are matched with one another, so that the product has a milder pH value, a slow release property and a longer half-life period. When the lornoxicam is used, the administration frequency of the lornoxicam can be reduced, and the analgesic effect of the lornoxicam can be quickly and continuously exerted.
Owner:MAXENMED GUANGZHOU
Puerarin co-crystal of lornoxicam and preparation method thereof
ActiveCN111004256BGood compressibilityImprove solubilityOrganic chemistry methodsOrganic solventLornoxicam
The invention discloses a co-crystal of lornoxicam and puerarin, the co-crystal is formed by combining lornoxicam and puerarin at a molar ratio of 1:1, and has a powder X-ray diffraction pattern represented by 2θ angle value , and infrared spectra. The preparation method comprises the steps of: dissolving lornoxicam and puerarin in an organic solvent according to a certain molar ratio, rotating the solvent under reduced pressure, drying in vacuum, and placing it in a resistance furnace to remove the solvent, thereby obtaining lornoxicam puerarin eutectic. The eutectic of the present invention is different from the powder X-ray diffraction and infrared of lornoxicam monomer and puerarin monomer and their physical mixtures, and the crystal form of the eutectic is a new one different from monomers and physical mixtures. Crystal form, improve the compressibility of lornoxicam, and the water solubility of lornoxicam and puerarin.
Owner:CHINA PHARM UNIV
Method for preparing lornoxicam
The present disclosure relates to the technical field of drug synthesis, in particular to a method for preparing lornoxicam. The method includes the following steps: using 6-chloro-4-hydroxy-2-methyl-2-H-thieno[2,3-e]-1,2-thiazine methyl carboxylate-1,1-dioxide and 2-aminopyridine as raw materials, and xylene as a solvent; mixing the raw material and solvent, and adding a stabilizer, to obtain a mixture; subjecting the mixture to an ammonolysis; cooling the resulting reactant; conducting a vacuum concentration to remove the solvent; adding an organic solvent and slurrying, and filtering, to obtain a crude lornoxicam; and refining the crude lornoxicam to obtain the lornoxicam. In the present disclosure, p-toluenesulfonic acid is used as a stabilizer to reduce the reaction temperature and promote the reaction to proceed forward, thereby improving the quality and yield of the product.
Owner:BEIJING JINCHENG TAIER PHARMA CO LTD
Lomoxicam sustained release tablet and preparation method thereof
ActiveCN101185640BSmooth releaseSimple processOrganic active ingredientsAntipyreticCelluloseAdhesive
The invention provides a lornoxicam sustained release tablet and the preparation method thereof. The sustained release tablet includes 2.0-60.00 parts of lornoxicam by weight and 10.00-95.00 parts of sustained release block material by weight; wherein, the sustained release block material includes hydroxypropyl methyl cellulose or compound of hydroxypropyl methyl cellulose and other celluloses. The preparation method is that raw material prescription doses of lornoxicam, sustained release block material and filler are evenly mixed, then added with adhesive to be prepared into soft material; pelletization, drying and finishing granule are carried out according to the conventional technique of the tablet preparation, and the dry grain after finishing granule is added with lubricant, evenly mixed and pressed to make the preparation. Compared with conventional oral lornoxicam preparation, the lornoxicam sustained release tablet can maintain a longer effect after oral administration of thedrug.
Owner:ZHEJIANG ZHENYUAN PHARMA CO LTD +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com