Lomoxicam sustained release tablet and preparation method thereof
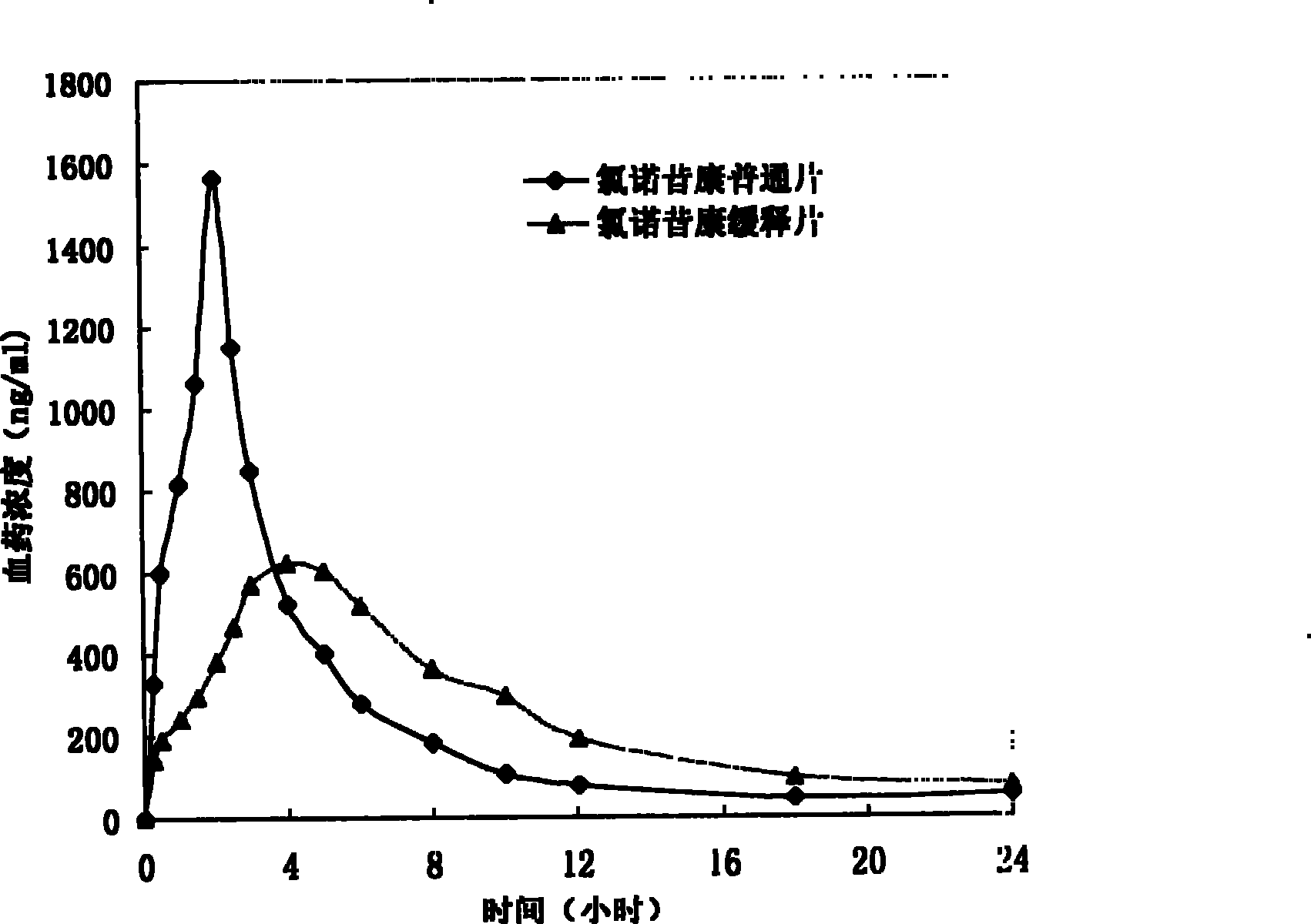
A technology for lornoxicam and sustained-release tablets, which is applied in the field of sustained-release preparations of non-steroidal anti-inflammatory drugs and its preparation, can solve the problem of large fluctuations in blood concentration of lornoxicam, inconvenience in clinical medication for patients, and easy occurrence of Adverse reactions and other problems, to achieve the effect of reducing the incidence of adverse reactions, reducing blood concentration fluctuations, and improving convenience
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0076] The preferred preparation method of lornoxicam sustained-release preparation of the present invention comprises the following steps:
[0077] (i) Mix 2.0 to 60.00 parts by weight of lornoxicam, 10.00 to 95.00 parts by weight of slow-release retarding materials and fillers, add binders, and make soft materials; wherein, the slow-release retarding materials include : Hypromellose or a mixture of hydroxypropylmethylcellulose and other celluloses;
[0078] (ii) granulating, drying, sizing, drying and tableting the soft material to obtain lornoxicam sustained-release tablets.
[0079] Any method known in the field of formulation can be used to carry out the steps of mixing, preparing the soft material, granulating, drying, sizing, drying or tableting the soft material.
[0080] Other sustained-release tablet preparation methods known in the art can also be used to prepare the lornoxicam sustained-release preparation of the present invention. For example, dry granulation ta...
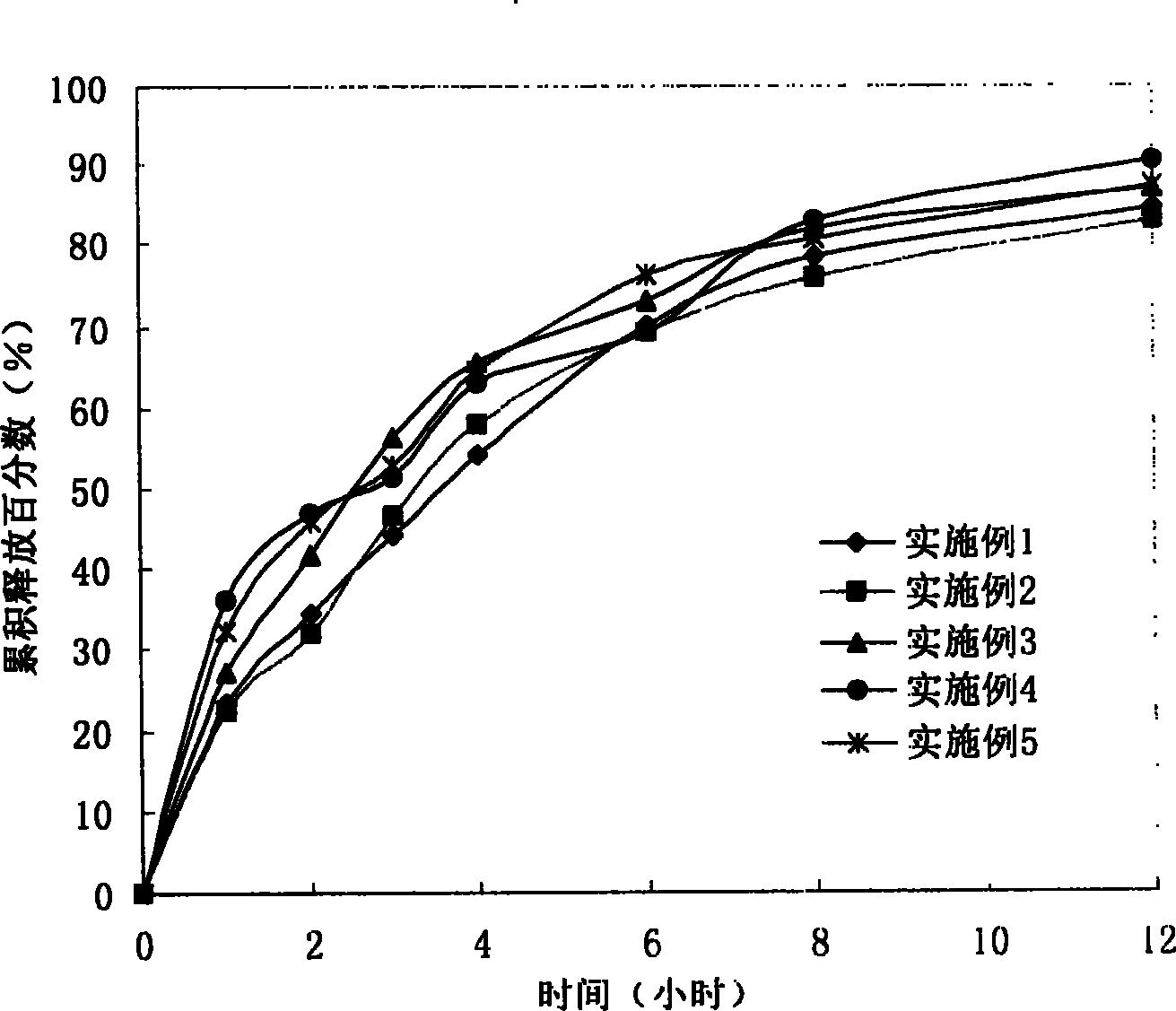
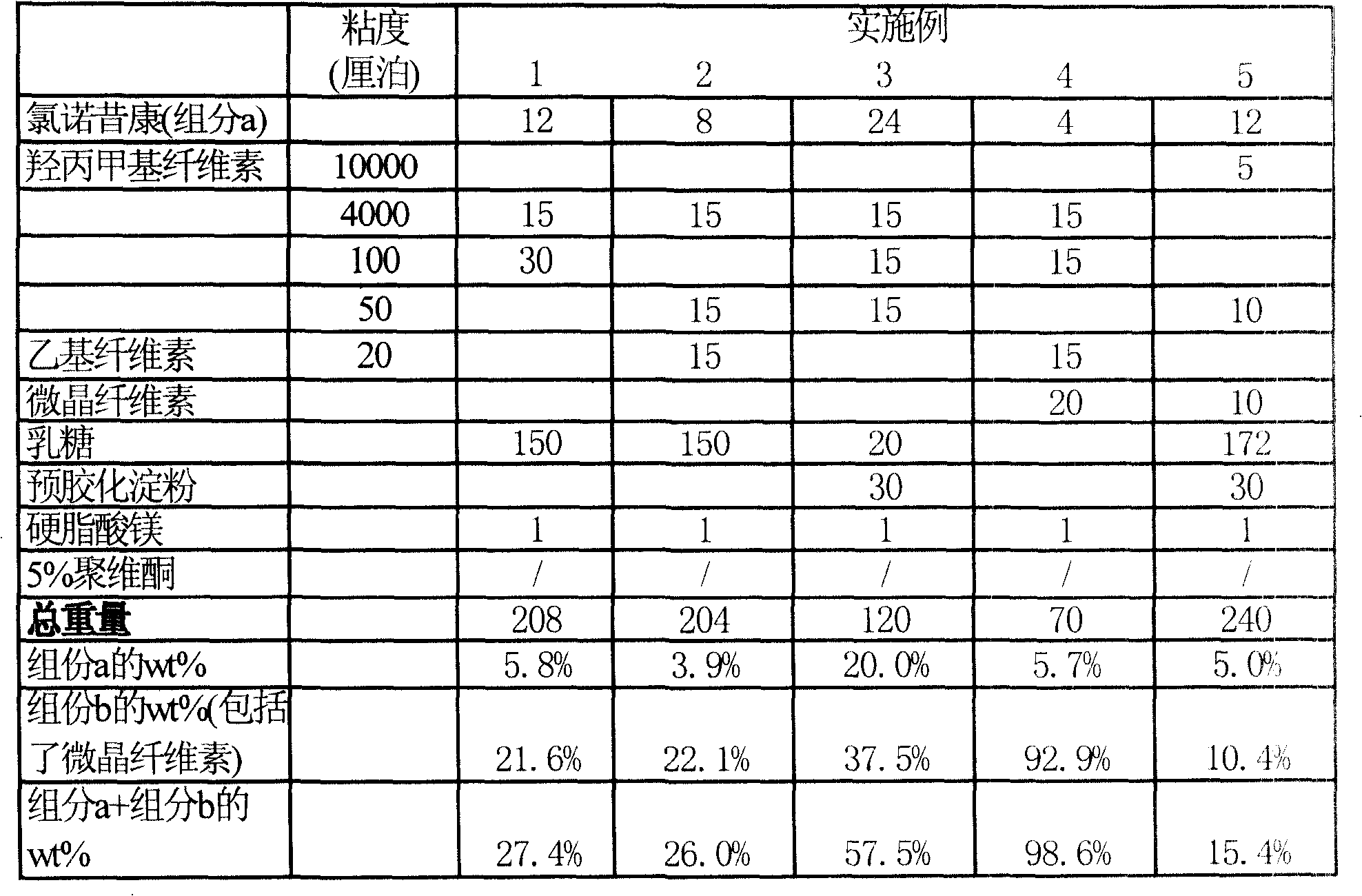
Embodiment 1
[0095] Preparation of Lornoxicam Sustained Release Tablet 1
[0096] 1000 pieces
[0097] Lornoxicam 12g
[0098] Hypromellose (viscosity: 4000 centipoise) 15g
[0099] Hypromellose (viscosity: 100 centipoise) 30g
[0100] Lactose 150g
[0102] Total weight 208g
[0103] Preparation:
[0104] Grind lornoxicam, hypromellose (viscosity: 4000 centipoise), hypromellose (viscosity: 100 centipoise) and lactose, pass through a 100-mesh sieve, and mix mechanically. Add 5% povidone aqueous solution (g / 100 ml, model: K30) to granulate during stirring, dry, and granulate. Then the dry granules after granulation are added with magnesium stearate dry powder, placed in a granulator, and mixed evenly. Punch the tablet with 8mm to get 1000 lornoxicam sustained-release tablets 1.
Embodiment 2
[0106] Preparation of Lornoxicam Sustained Release Tablet 2
[0107] 1000 pieces
[0108] Lornoxicam 8g
[0109] Hypromellose (viscosity: 4000 centipoise) 15g
[0110] Hypromellose (viscosity: 50 centipoise) 15g
[0111] Ethyl cellulose (viscosity: 20 centipoise) 15g
[0112] Lactose 150g
[0114] Total weight 204g
[0115] Preparation:
[0116] Grinding lornoxicam, hypromellose (viscosity: 4000 centipoise), hypromellose (viscosity: 50 centipoise), ethyl cellulose (viscosity: 20 centipoise), lactose, 100 mesh sieve, mechanically mixed. During stirring, add 5% povidone (type: K30) aqueous solution to granulate, dry, and granulate. Then the dry granules after granulation are added with magnesium stearate dry powder, placed in a granulator, and mixed evenly. Punch the tablet with 8mm to get 1000 lornoxicam sustained-release tablets 2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com