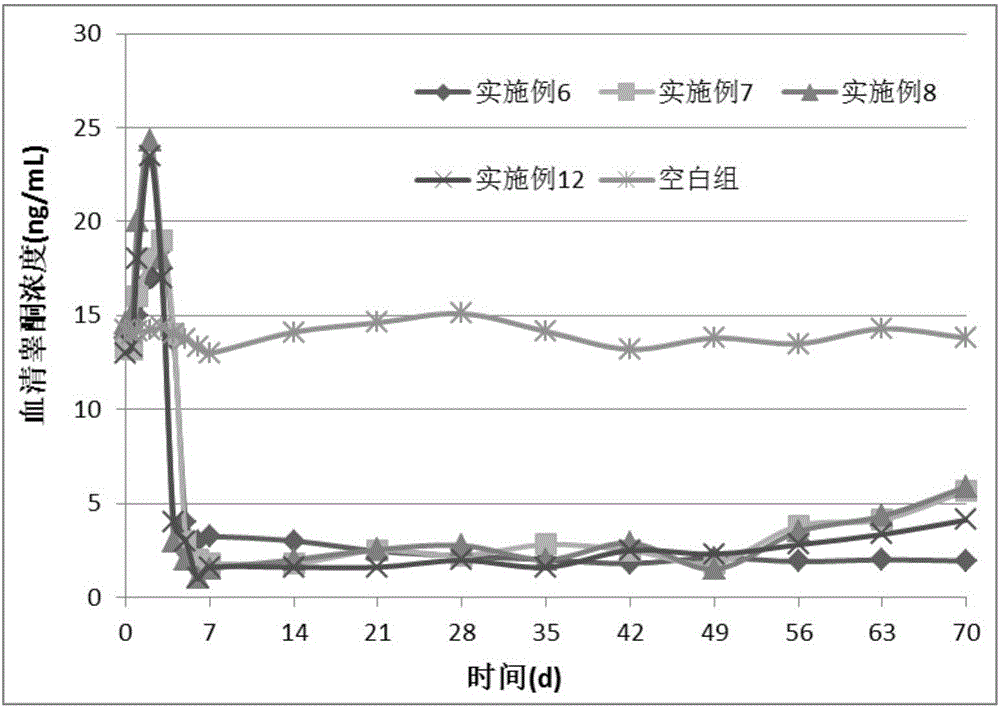
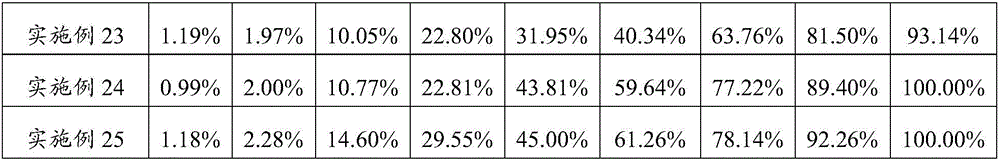
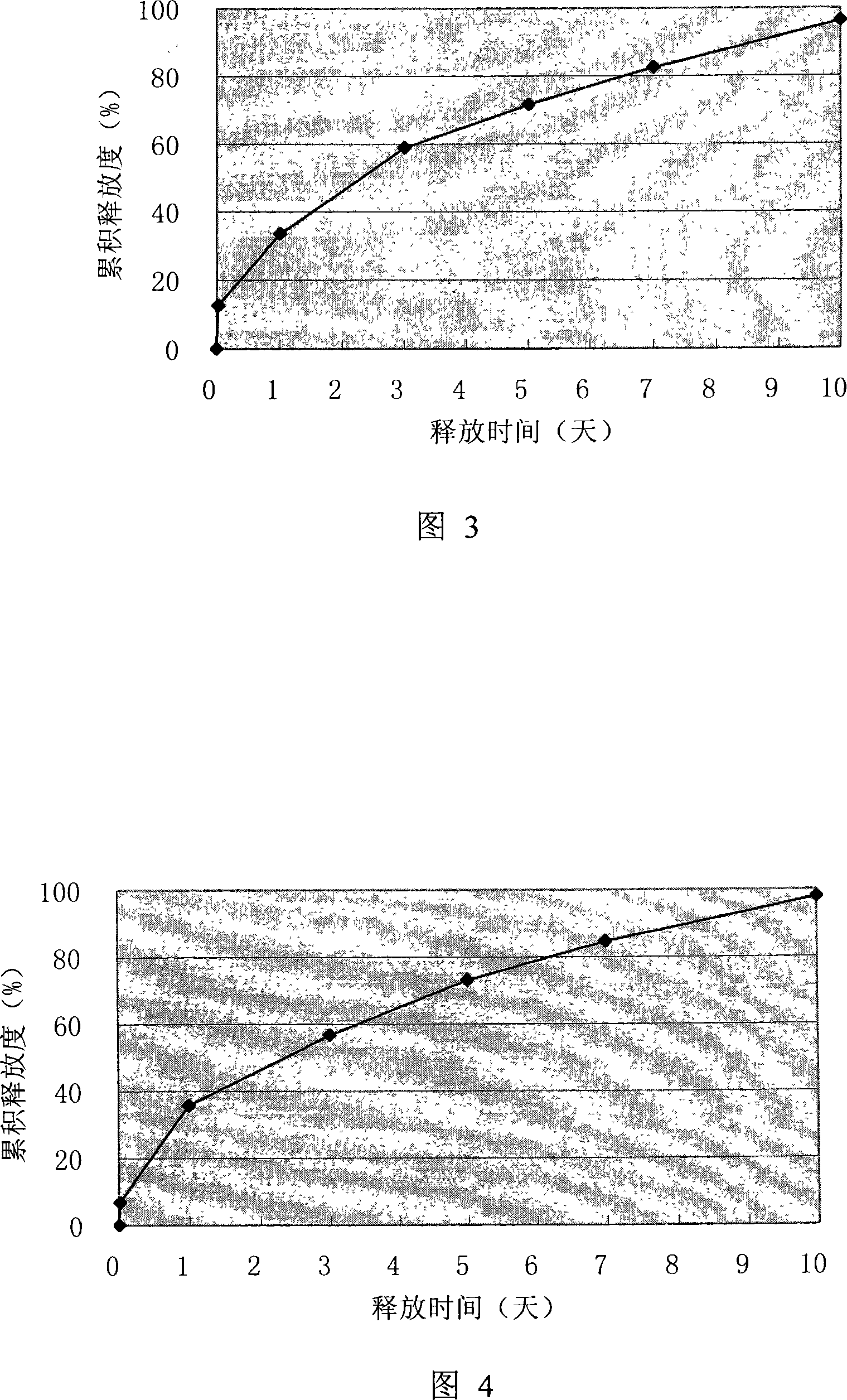
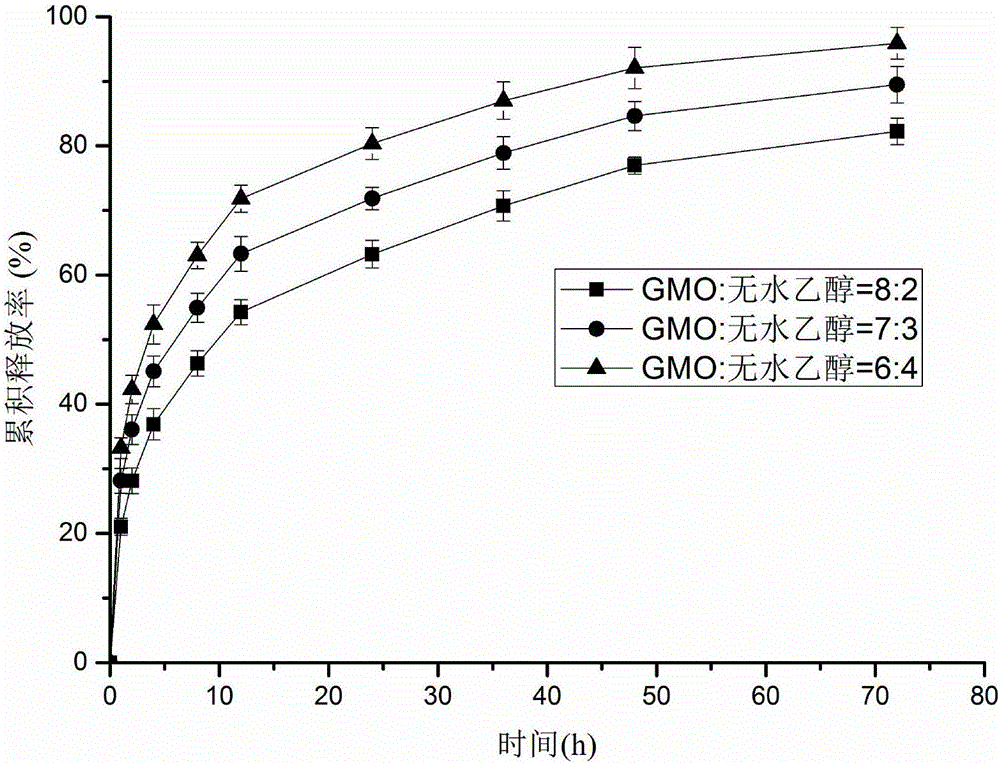
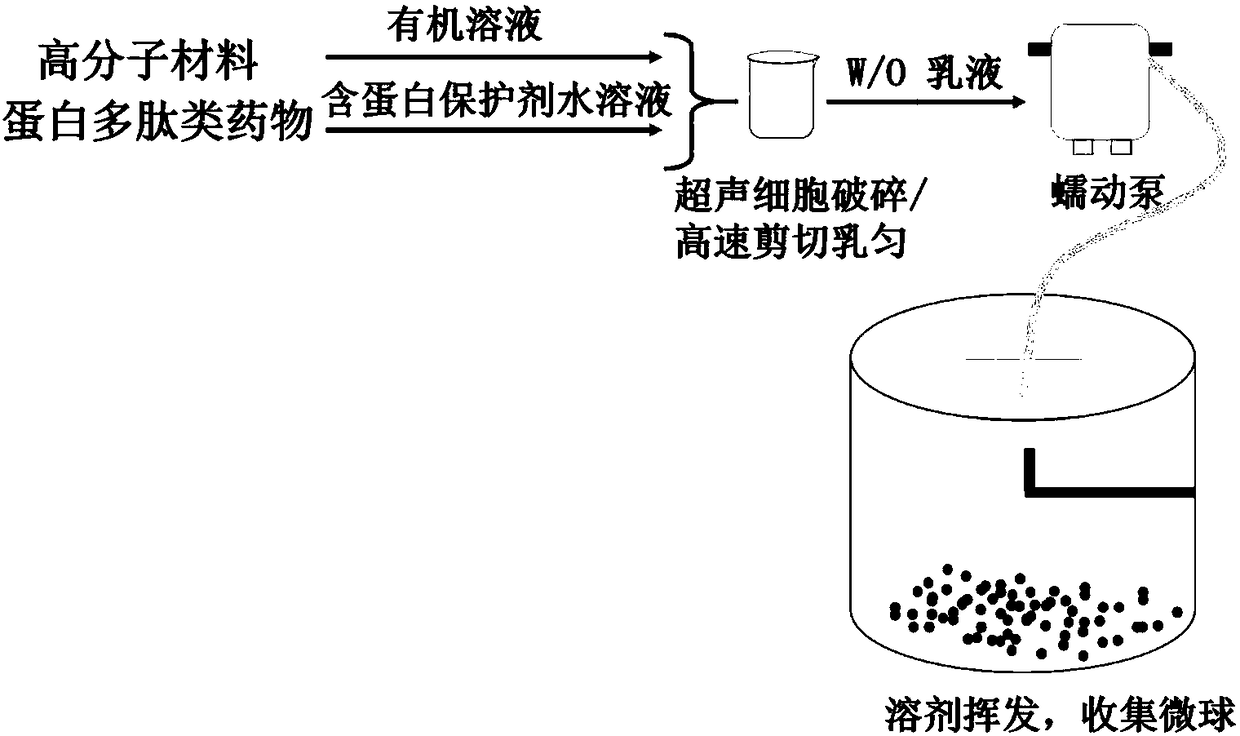
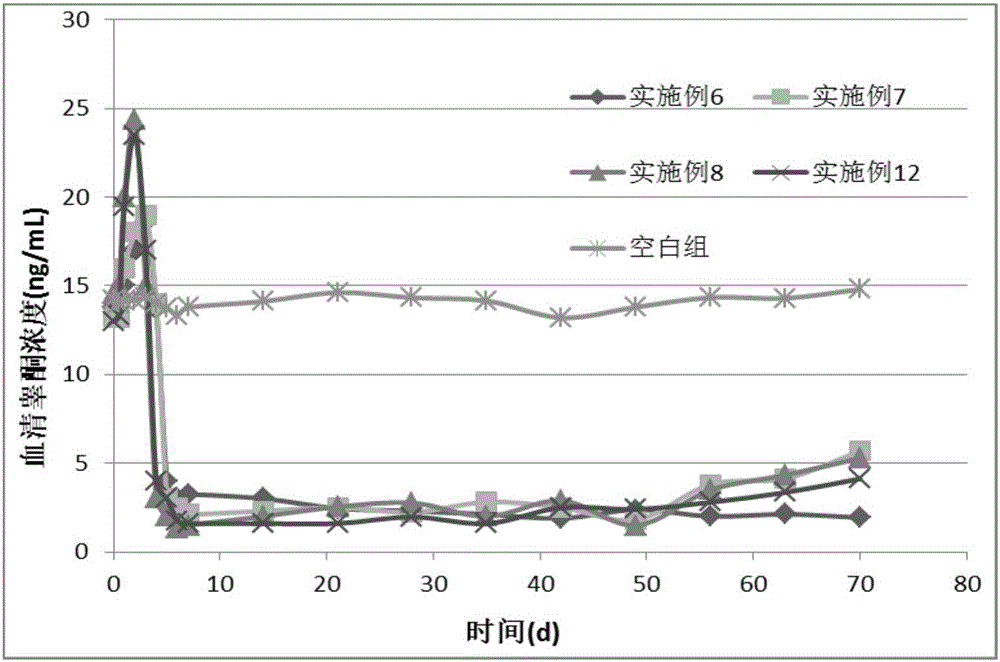
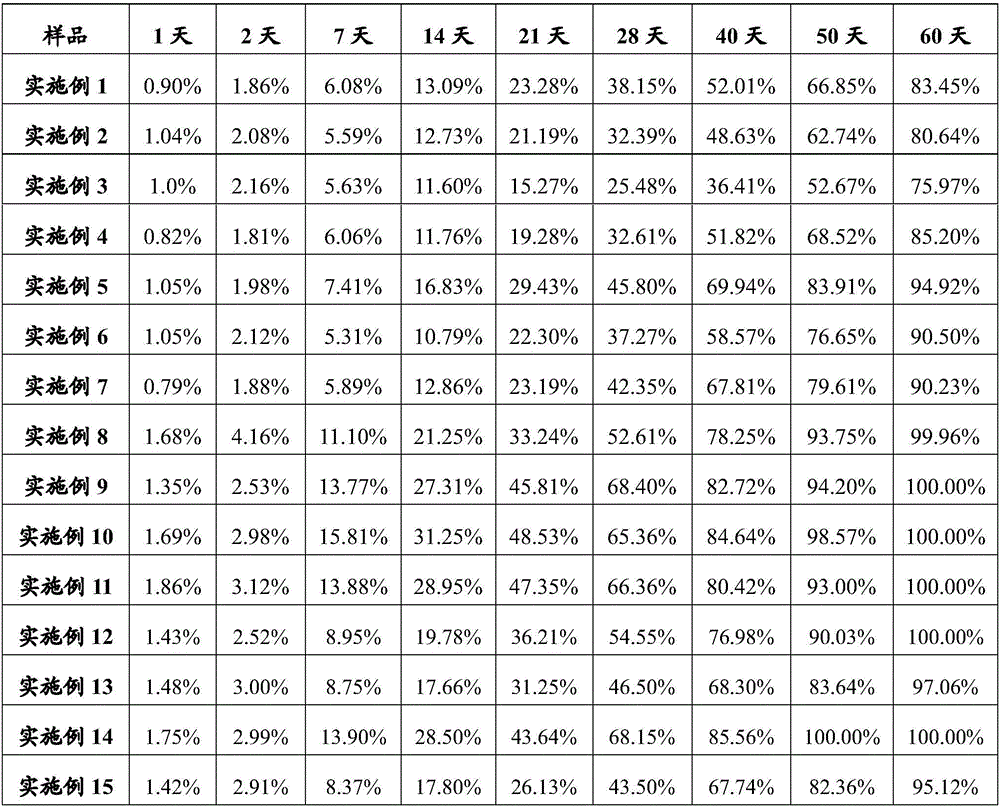
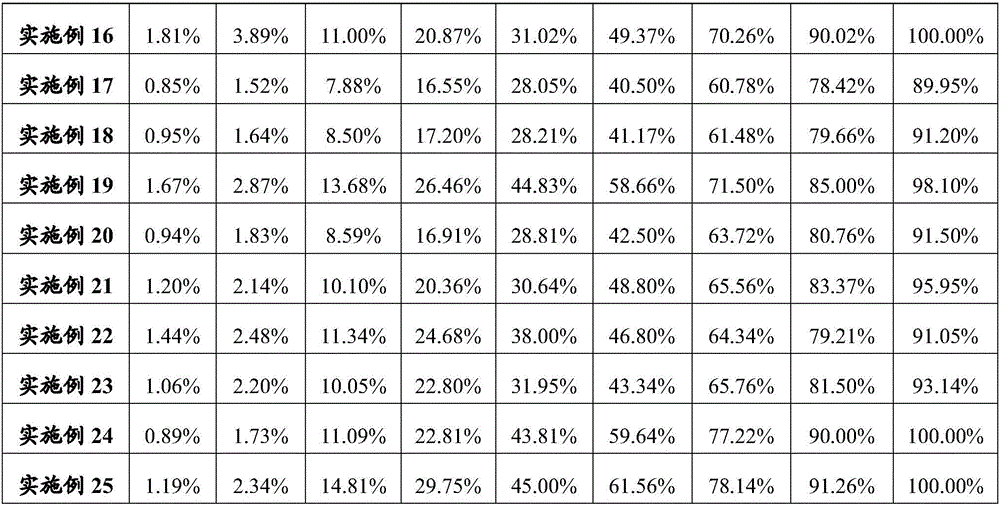
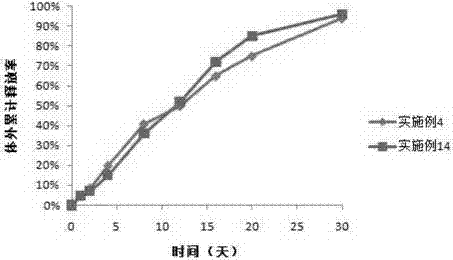
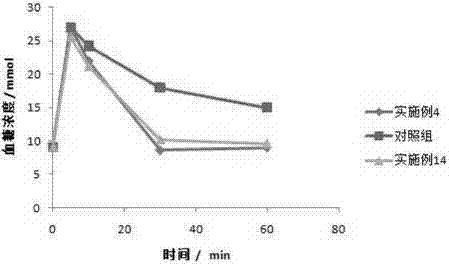
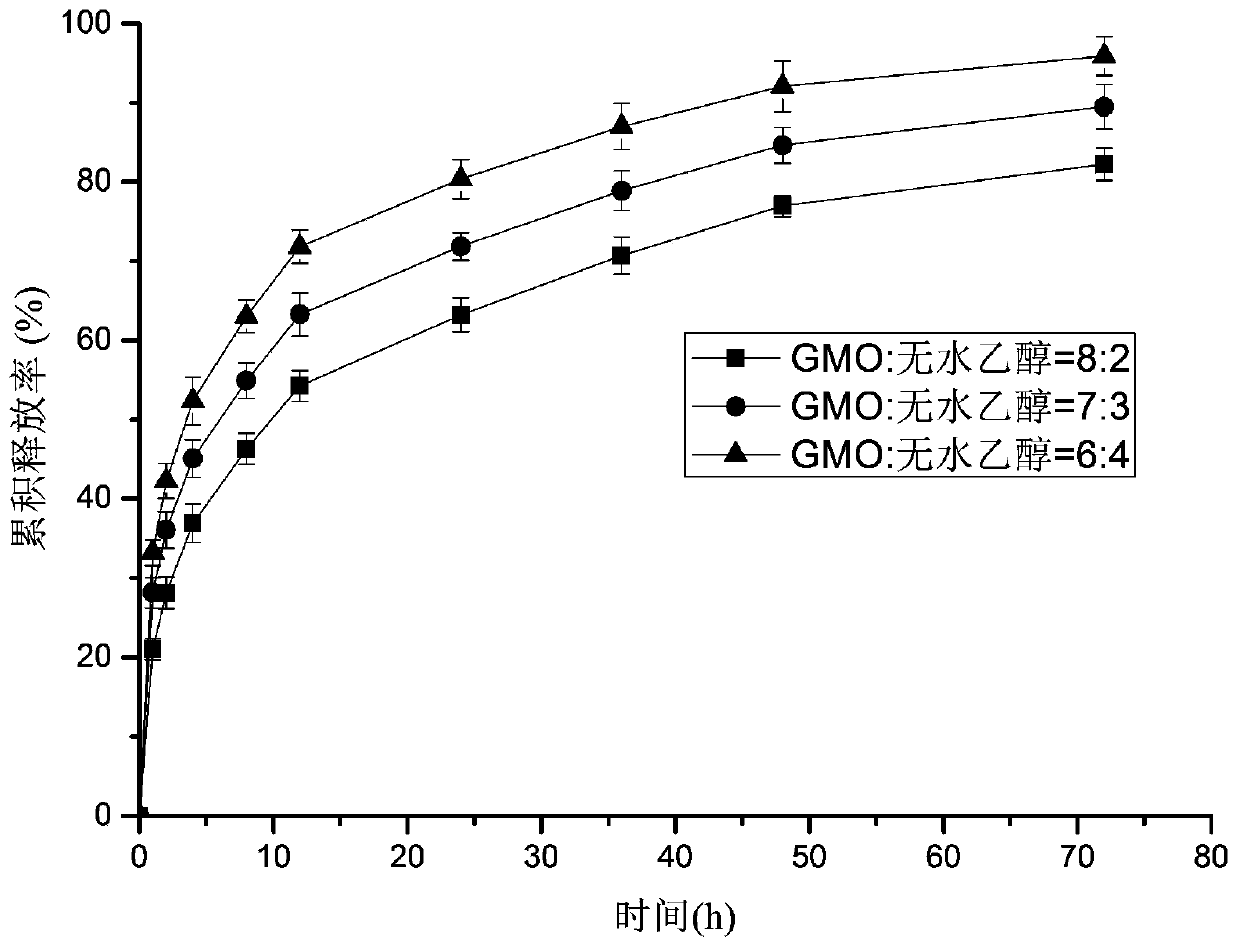
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
46results about How to "Low burst rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
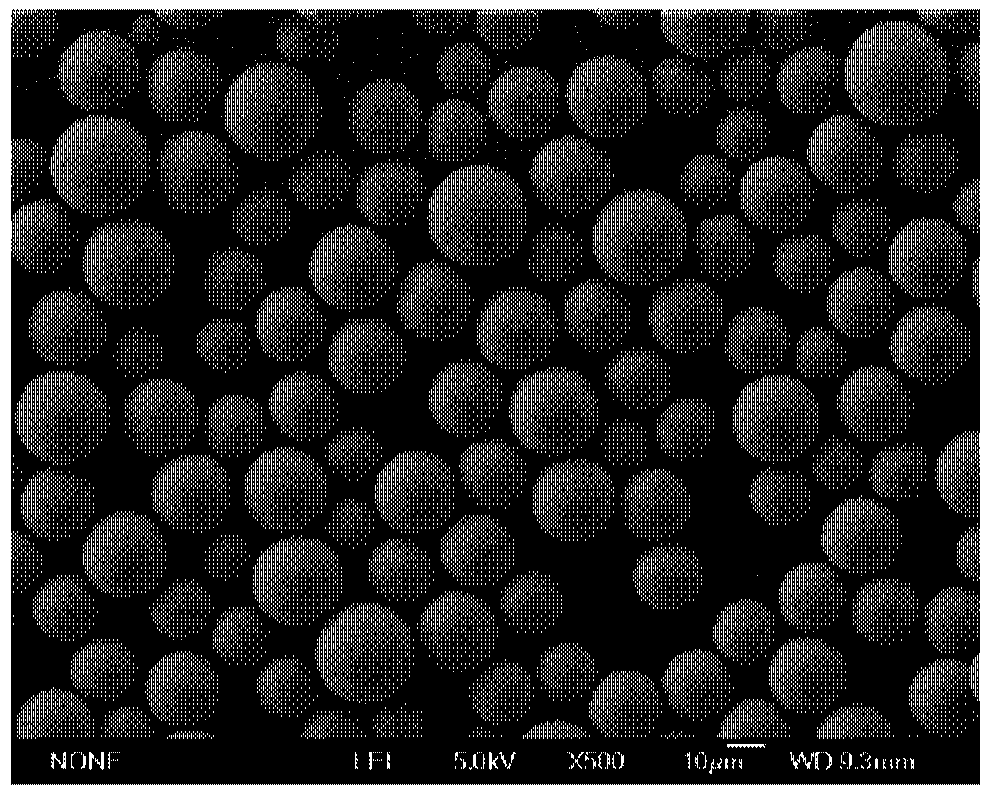
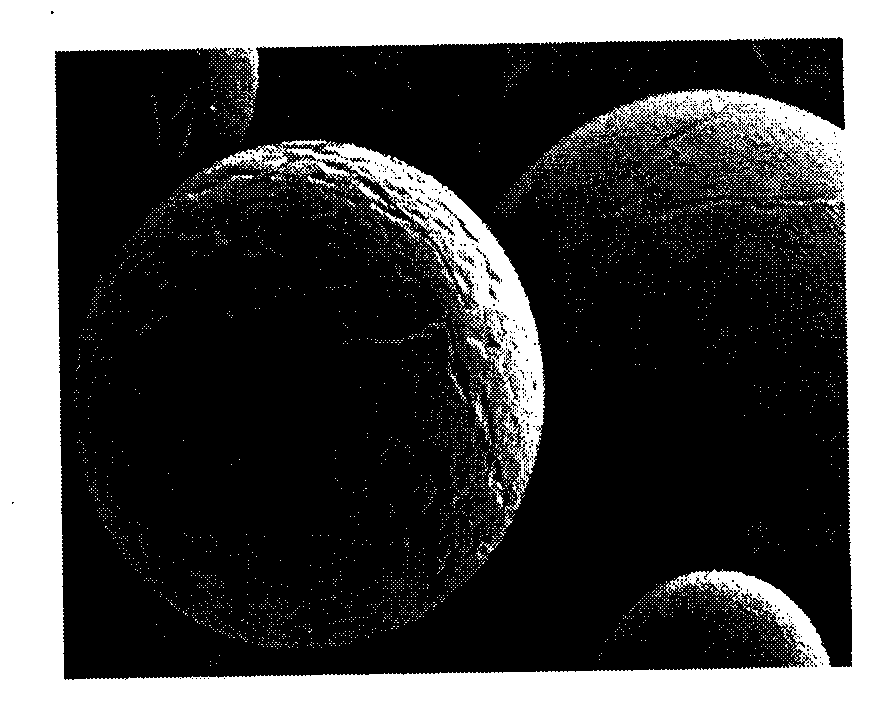
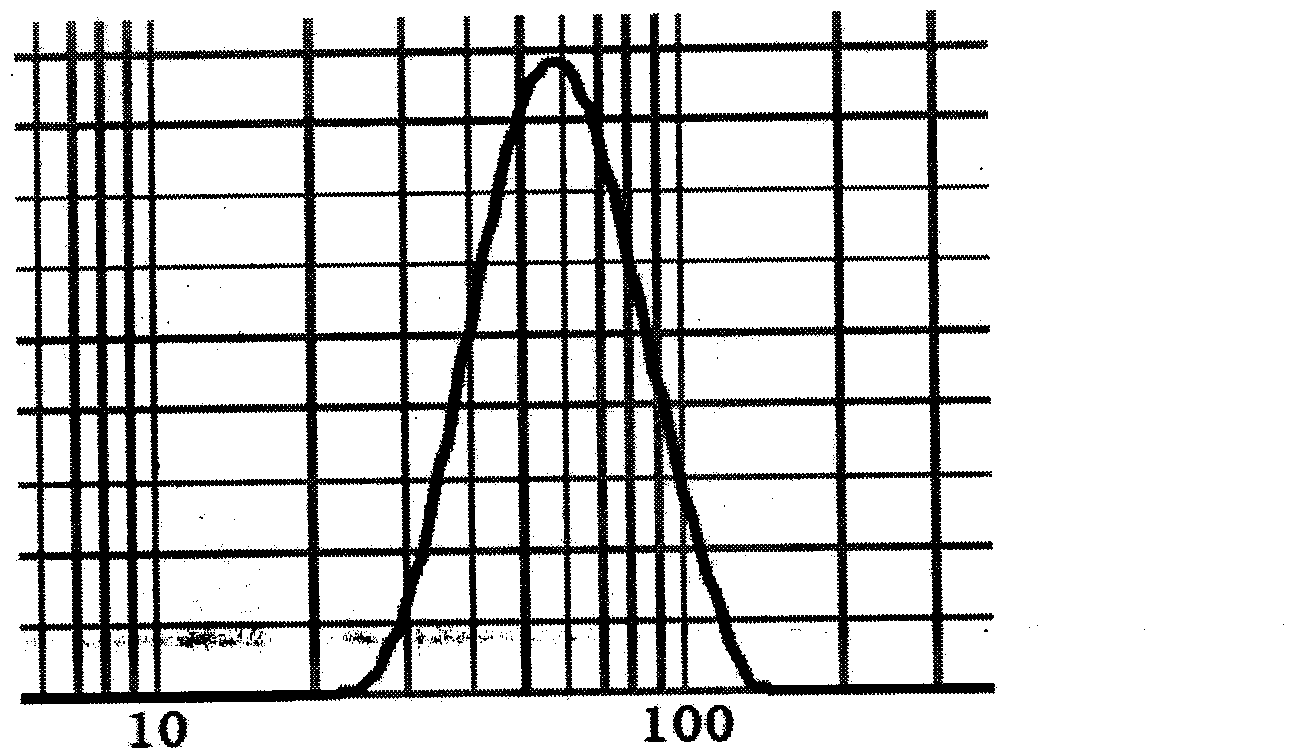
Polypeptide drug sustained-release microsphere preparation and preparation method thereof
ActiveCN102688198AUniform particle sizeNarrow particle sizePeptide/protein ingredientsMetabolism disorderFreeze-dryingMicrosphere
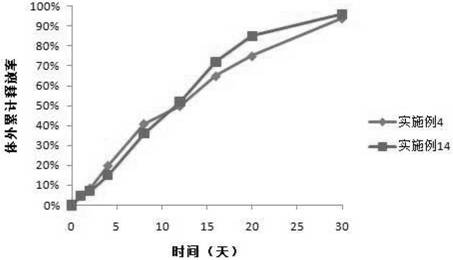
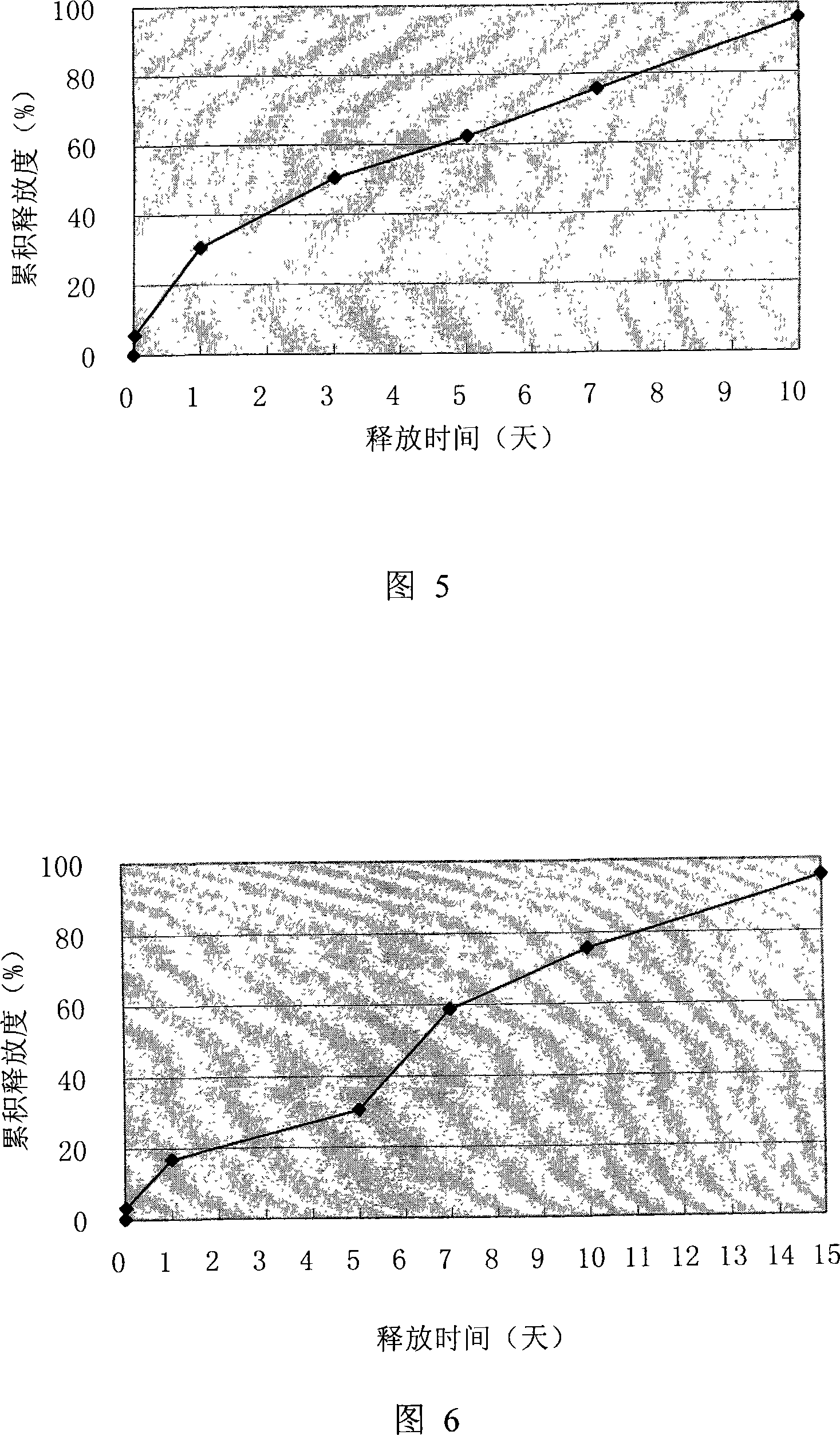
The invention discloses a polypeptide drug sustained-release microsphere preparation and a preparation method thereof. The method comprises the following steps of: dissolving the polylactic acid-glycollic acid copolymer or polylactic acid, a protective agent and a polypeptide drug in an organic solvent to form a completely uniform mixed solution; adding the mixed solution into an oil phase to form emulsion; removing the organic solvent; and performing centrifugal washing and freeze drying to obtain the polypeptide drug sustained-release microsphere. In the invention, an O / O method is adopted, the problem that the drug spreads toward the outer aqueous phase in the multiple-emulsion preparation method is solved, and the drug encapsulation efficiency is improved to 60-95%. The biological active polypeptide drug is degraded in the body and slowly released with the polymer material of the microsphere through the pores on the microsphere surface; the release time can be as long as several weeks and even several months; and the in-vitro release test indicates that the release conforms to similar zero-order release.
Owner:AC PHARMA CO LTD
Liraglutide sustained-release microsphere preparation and preparation method thereof
InactiveCN104382860AUniform particle sizeLow burst ratePeptide/protein ingredientsMetabolism disorderSucroseMicrosphere
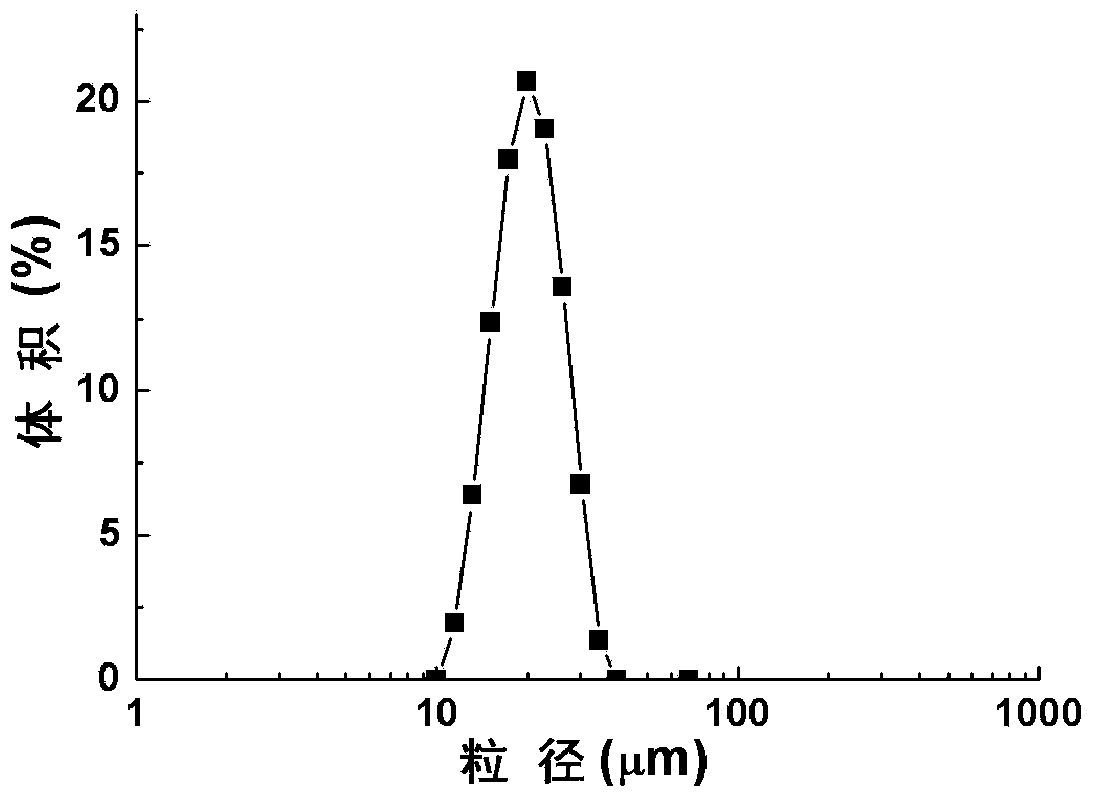
The invention relates to a liraglutide sustained-release micro sphere preparation and a preparation method thereof. The liraglutide sustained-release microsphere preparation comprises 5mg to 100mg of liraglutide, 0.5mg to 10mg of a protective agent and 50mg to 1000mg of a polylactic acid-glycolic acid copolymer, wherein the protective agent is one or a mixture of a plurality of sucrose, mycose, gelatin, mannitol, glycine, lysine and human serum albumin; the molecular weight of the polylactic acid-glycolic acid copolymer is 5000-20000 Daltons, and the ratio of polylactic acid to glycolic acid in the polylactic acid-glycolic acid copolymer is 1:3 to 3:1. According to the liraglutide sustained-release microsphere preparation disclosed by the invention, regular microspheres and medicines uniformly distributed in the microspheres can be obtained by just emulsifying and volatizing an organic solvent; processing procedures are simple; operation is simple; good repeatability is realized in preparation; the prepared liraglutide sustained-release microsphere preparations in batches have no remarkable difference; the obtained microspheres are uniform in grain size, narrow in distribution, controllable in grain size, round and orderly in surfaces and low in burst release rate.
Owner:浙江美华鼎昌医药科技有限公司 +1
Small molecule hydrophilic drug-embedded sustained-release capsule and preparation method thereof
ActiveCN104224753AReduce spreadHigh embedding ratePharmaceutical delivery mechanismMacromolecular non-active ingredientsSolventOil phase
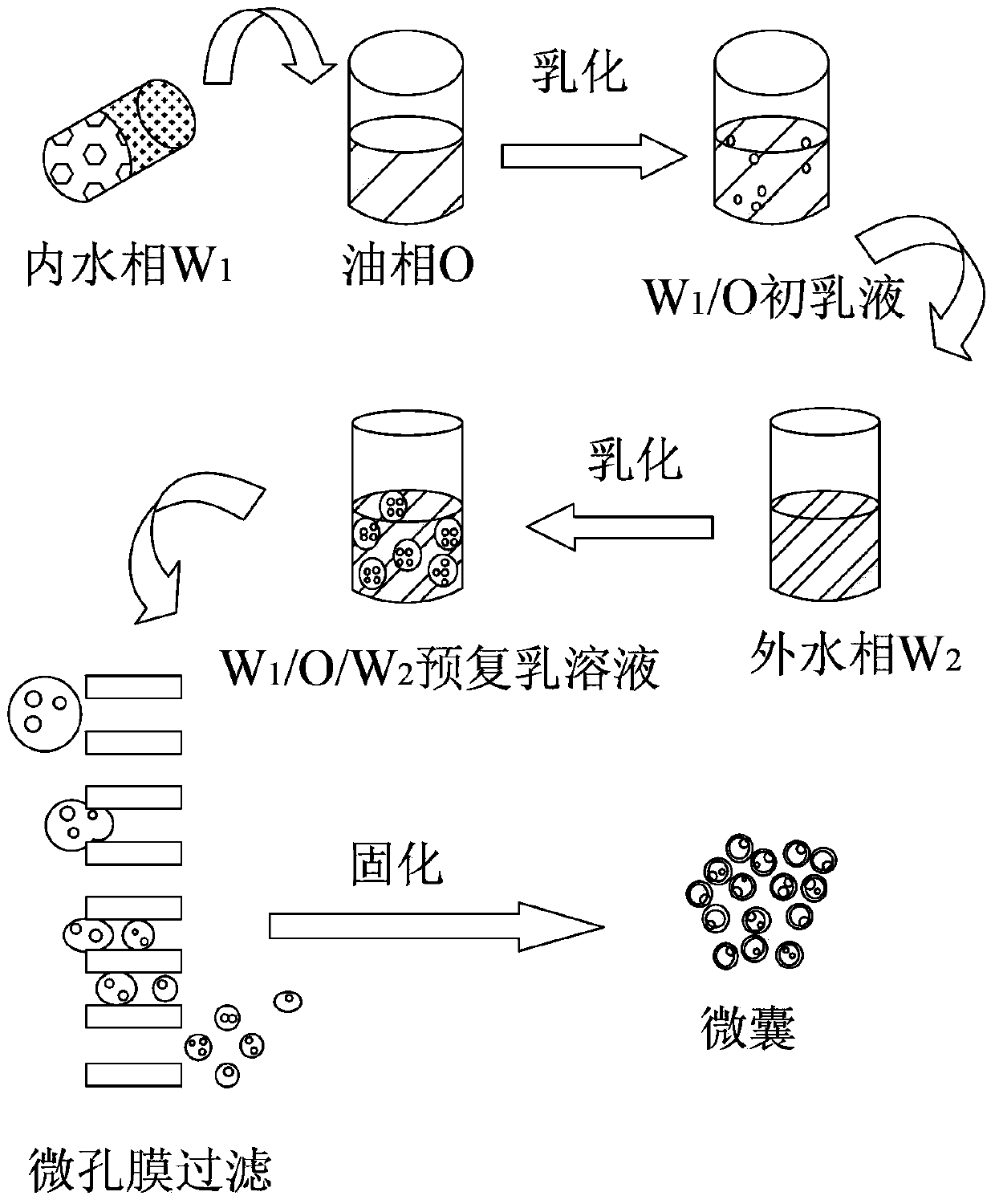
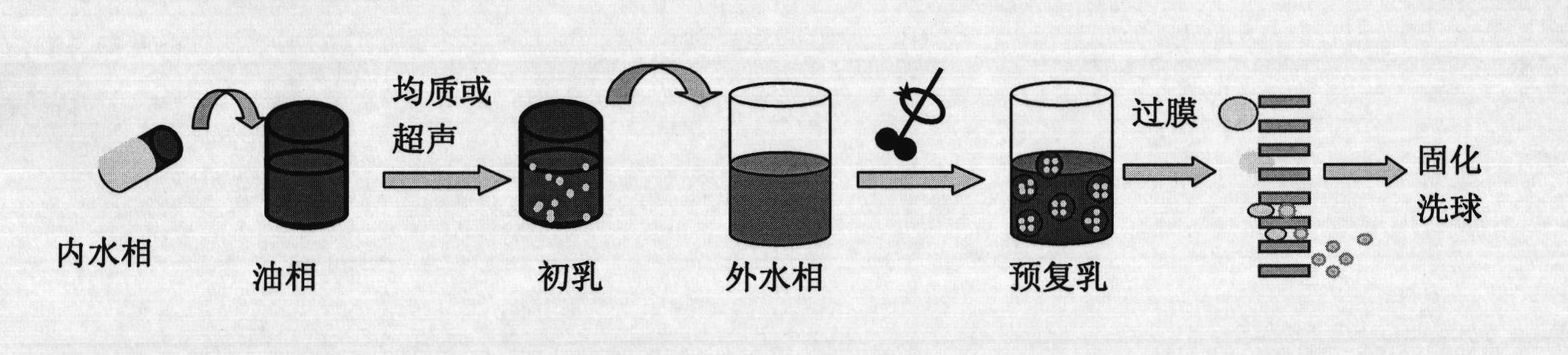
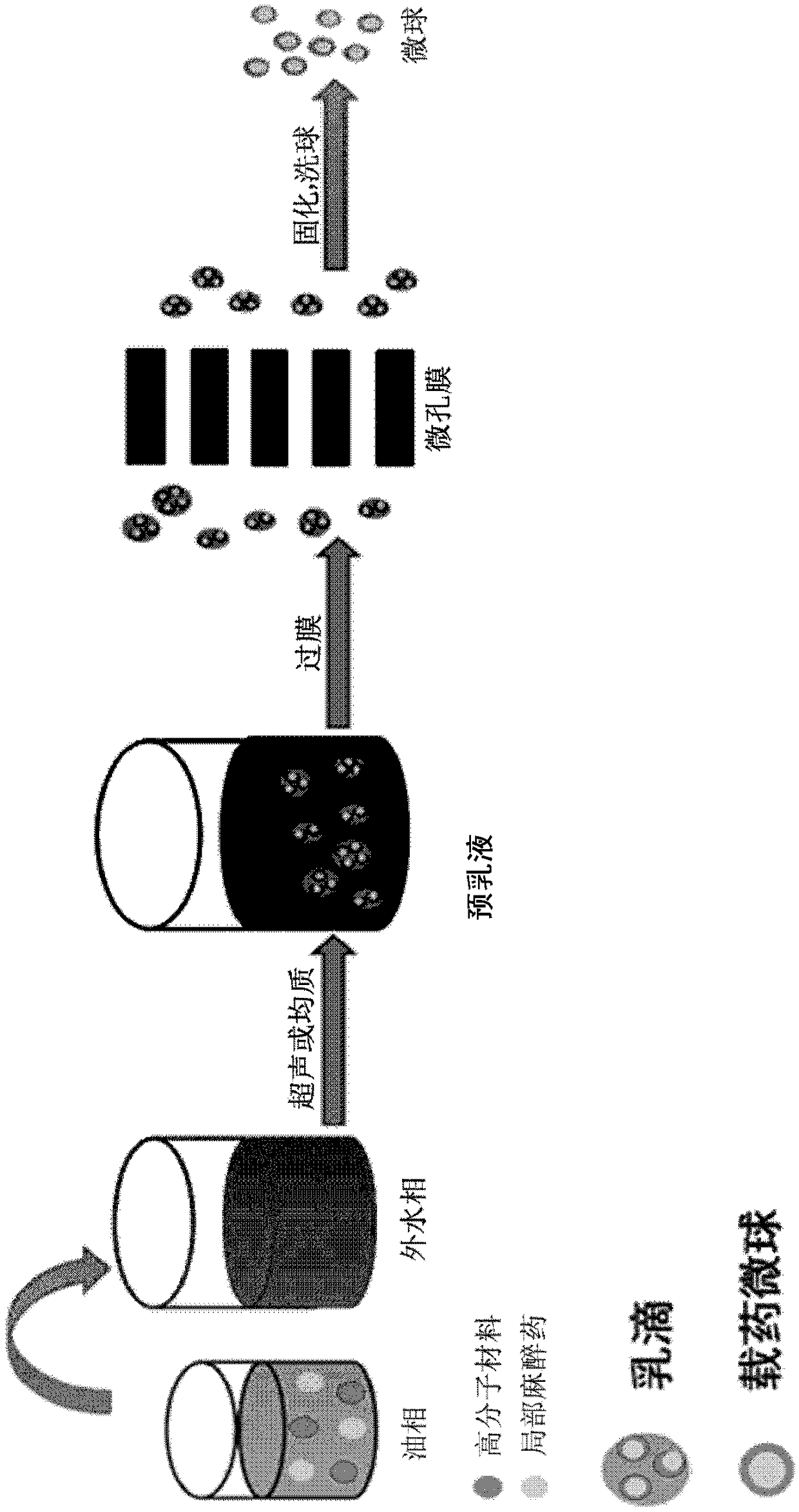
The invention relates to a method for preparing a small molecule hydrophilic drug-embedded sustained-release capsule. The method comprises the following steps: filtering a prepared'internal aqueous phase / oil phase / external aqueous phase' pre-multi-emulsion solution through a microporous membrane; and then removing a solvent, washing and drying to obtain the small molecule hydrophilic drug-embedded sustained-release capsule, wherein the internal aqueous phase comprises a small molecule hydrophilic drug and a thickening agent. The small molecule hydrophilic drug-embedded sustained-release capsule provided by the invention has the advantages of uniform grain diameter, high embedding rate, low burst-release rate, stable drug release and long action.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
Recombinant human growth hormone (rhGH) long-acting sustained-release microcapsule and preparation method thereof
ActiveCN102370630ALow burst rateSmall particle sizePeptide/protein ingredientsMetabolism disorderOrganic solventFreeze-drying
The invention relates to the field of medicine, and specifically, relates to a recombinant human growth hormone (rhGH) long-acting sustained-release microcapsule and a preparation method thereof. The preparation method of the rhGH long-acting sustained-release microcapsule comprises the following steps of 1, dissolving a diblock amphiphilic polymeric material in an organic solvent to obtain an oil phase O, 2, adding an rhGH-containing aqueous solution W1 or rhGH-containing particles S into the oil phase O obtained by the step 1, and carrying out emulsification preparation to obtain W1 / O or S / O primary emulsion, wherein the rhGH-containing aqueous solution W1 is utilized as an inner water phase, 3, adding the W1 / O or S / O primary emulsion into a stabilizer-containing outer water phase W2 to obtain W1 / O / W2 or S / O / W2 composite pre-emulsion, 4, carrying out a filter pressing process on the W1 / O / W2 or S / O / W2 composite pre-emulsion through a millipore membrane to obtain W1 / O / W2 or S / O / W2 composite emulsion, and 5, removing the organic solvent in the W1 / O / W2 or S / O / W2 composite pre-emulsion, carrying out solidification, centrifugal washing and freeze drying of the organic solvent-free W1 / O / W2 or S / O / W2 composite emulsion. The rhGH long-acting sustained-release microcapsule obtained by the preparation method has the advantages of even size, high encapsulation efficiency, high activity, low burst release quantity, good repeatability, simpleness of operation, and benefit to drug effect activity keeping and industrialized mass production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI
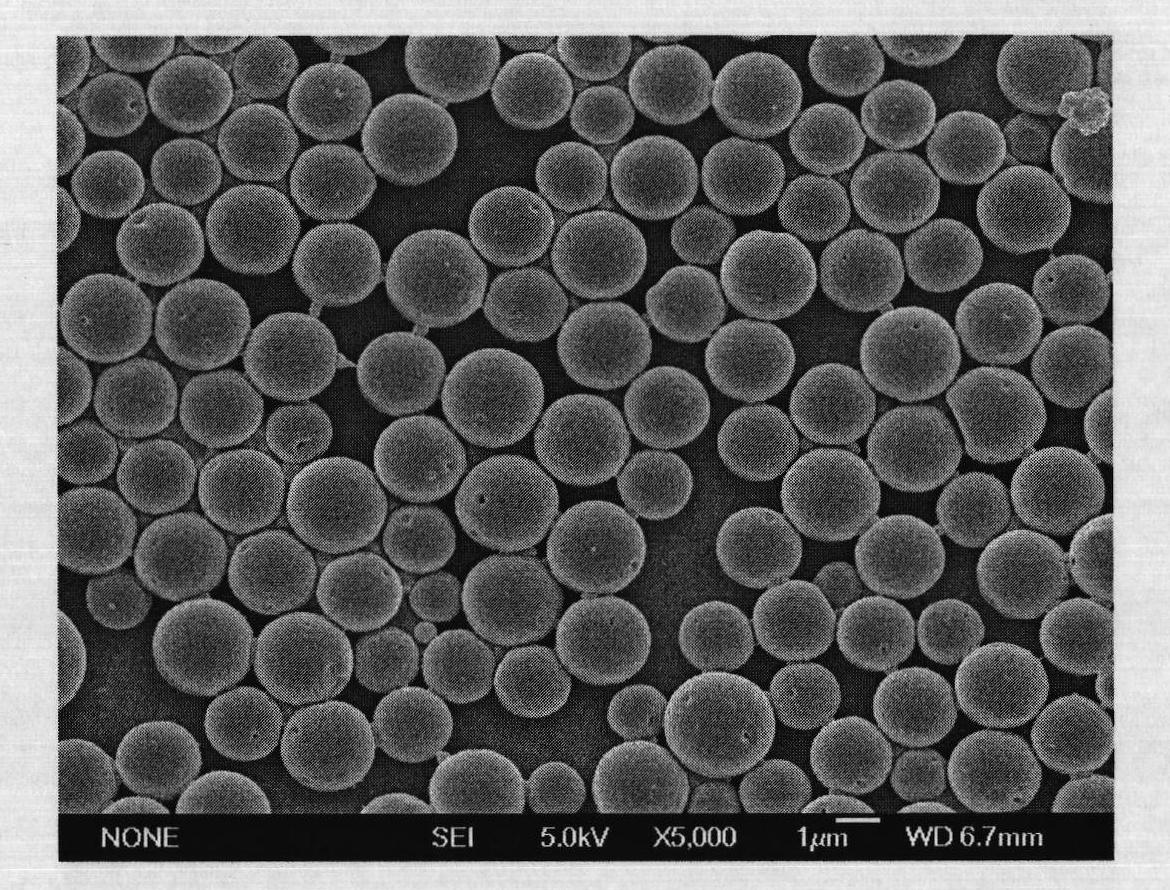
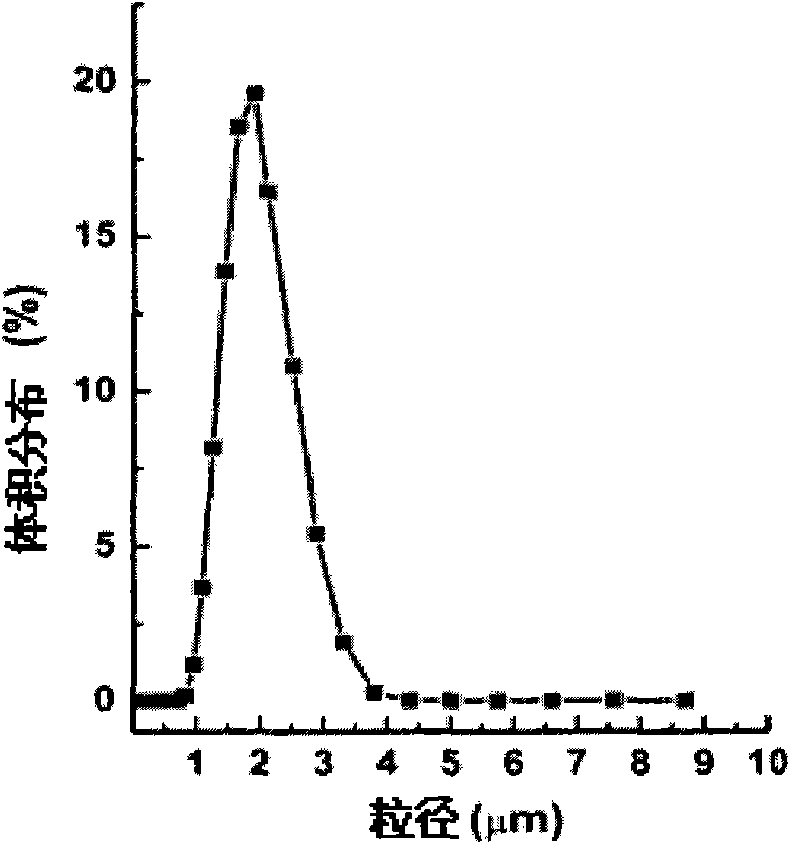
Narcotic analgesic-loaded sustained-release microsphere and preparation method thereof and application thereof
ActiveCN109010307AGuaranteed repeatabilityGood for stabilityAntipyreticAnalgesicsDrugAnalgesic agents
The invention discloses a narcotic analgesic-loaded sustained-release microsphere and a preparation method thereof and application thereof. The narcotic analgesic-loaded sustained-release microspherecan be continuously released for 1 to 7 days, the burst release rate is less than 20% within 0.5 hour, and the drug embedding rate is higher than 80%, so that high drug embedding rate and low burst release rate and sustained release can be realized. The method has simple process, the obtained product has uniform particle size, batches of products have good repeatability, and the preparation methodis easy for industrial production.
Owner:INST OF PROCESS ENG CHINESE ACAD OF SCI +2
Method of preparing simvastatin sustained-release microsphere carried series
InactiveCN101219119ARelease stabilityLarge adjustment rangeOrganic active ingredientsSurgeryMicrospherePolyvinyl alcohol
The invention relates to a method that a functional drug is enveloped into a polymeric material with biodegradability to form a nano-micron microsphere system. The method comprises that the polymeric material and simvastatin are dissolved in an organic liquor to form uniform dispersion which is then added into an liquor containing emulsifier Tween 80 and biologically nontoxic electrolytic polyvinyl alcohol or sodium dodecyl benzene sulfonate (SDBS), and then the obtained liquor is stirred, evaporated at a reduced pressure, centrifugalized, washed and vacuum dried, finally the simvastatin-contained delayed-release microsphere system is obtained. The surface of the microsphere is smooth and round, the granule thereof is regular without conglutination, and the granule diameter, the drug-loading rate (1-10 percent) and the encapsulation rate (above 40 percent) are all controllable, and the delayed-release time exceeds 2 months. The prepared simvastatin-contained delayed-release microsphere system can be processed into various preparations used in bony tissue absorption or bony defect parts, the microsphere system is degraded at a proper speed, thus the simvastatin can be further released; the degradation of the polymer can provide bony tissue with subsequent recuperating space to complete the repair of the bony defect parts.
Owner:JILIN UNIV
Sustained-release microgranules, method for preparing same and application of sustained-release microgranules
ActiveCN105878191AHigh encapsulation efficiencyHigh drug loading rateOrganic active ingredientsPeptide/protein ingredientsPolymer scienceWater insoluble
The invention discloses a method for preparing sustained-release microgranules. The method includes steps of 1), preparing solid dispersion of biodegradable and biocompatible water-insoluble polymer and water-soluble drug; 2), dissolving the prepared solid dispersion in an organic solvent C to form solid dispersion emulsion; 3), injecting the obtained solid dispersion emulsion into oil solution with surfactants to form uniform emulsion; 4), curing microgranules in the emulsion by means of solvent evaporation or solvent extraction, and collecting, washing and drying the microgranules to obtain the sustained-release microgranules. The invention further discloses the sustained-release microgranules prepared by the aid of the method and application of the sustained-release microgranules to implantable sustained-release pharmaceutical compositions. The method, the sustained-release microgranules and the application have the advantages that full procedures for preparing the sustained-release microgranules by the aid of the method are carried out at the normal temperature or the low temperatures, and accordingly the method is quite favorable for preparing polymer matrix compositions from high-temperature-sensitive drugs; excellent similarly zero-level sustained-release effects can be realized by the prepared sustained-release microgranules, and the drugs are stable in concentration in sustained-release period.
Owner:AC PHARMA CO LTD
Preparation method of sustained-release microparticles
ActiveCN105963258AImprove complianceHigh encapsulation efficiencyCyclic peptide ingredientsPharmaceutical non-active ingredientsMicroparticleDrug release
The invention provides sustained-release microparticles. According to the invention, a preparation method of the sustained-release microparticles is conducted at normal temperature or low temperature in a whole course, which is quite conducive to high-temperature sensitive drugs, in particular compositions which polymer matrixes prepared from protein, nucleic acid and peptide drugs. Compared with public technologies, the preparation method disclosed by the invention can keep bioactivity of active substances to the greatest extent in the entire technological process; meanwhile, the prepared sustained-release microparticles have an excellent sustained-release effect close to a zero level, and drug concentration is kept stable in a sustained-release period, so that shortcomings of microparticles, which are prepared by a conventional S / O / W (solid-in-oil-in-water) process which needs to prepare the drug microparticles in advance, that drug release fails to occur in an early stage while rapid drug release happens in a late stage are overcome; and moreover, the sustained-release microparticles are relatively high in drug loading ratio and drug entrapment efficiency.
Owner:AC PHARMA CO LTD
Composite micro-sphere co-carrying adriamycin nanoparticles and ginsenoside rh2 and preparation method thereof
ActiveCN108553447AReduce dosageRestore sensitivityOrganic active ingredientsPharmaceutical non-active ingredientsAdriamycin HydrochlorideMicrosphere
The invention relates to a composite microsphere for co-loading adriamycin nanoparticles and ginsenoside rh2 and a preparation method thereof, wherein the preparation method comprises the following steps: (1) preparing adriamycin hydrochloride and a nanoparticle carrier material into doxorubicin polyelectrolyte nanoparticles; (2) dispersing the adriamycin polyelectrolyte nanoparticles in water toform a nano-particle suspension, and dispersing the nano-particle suspension in an organic solution containingginsenoside rh2 and a high-molecular polymer to prepare S / W1 / O colostrum; (3) adding the S / W1 / O colostrum into an aqueous solution containing an emulsifier to prepare S / W1 / O / W2 complex milk; (4) solidifying the S / W1 / O / W2 complex emulsion into microspheres, collecting the microspheres, washing and drying. The microsphere prepared by the method can achieve the sequential release, slow release of doxorubicin hydrochloride and ginsenoside rh2,and has the advantages of low burst release rate and high encapsulation rate.
Owner:SUN YAT SEN UNIV
Preparing method of microcapsule for controlled-releasing spice for smoke
ActiveCN107212459ALarge adsorption capacityChemically stableTobacco treatmentControl releasePorous starch
The invention provides a preparing method of microcapsule for controlled-releasing spice for smoke. The microcapsule is mainly composed of modified porous starch and an airtight material. The microcapsule solves the insufficiencies that after prescriptions are directly added into the spice for smoke, the spice is volatile and poor in stability, and the microcapsule has a controlled-release effect on the spice for smoke. The adopted porous starch is modified porous starch, through introducing of hydrophobic groups, the capacity of the starch in adsorbing hydrophobic substances is improved, thus the application range of the starch is expanded, and the airtight material is utilized to cover the surface of the starch. The microcapsule can be fixed in cigarette paper at the outer layer through the adhesiveness of the airtight material, and the microcapsule can be used for conducting sealing, packaging, printing trademarks and the like.
Owner:SOUTHEAST UNIV
Thymus gland peptide alpha1sustained-release microsphere preparation for injection and preparation thereof
ActiveCN101244259AHigh encapsulation efficiencyRelease stabilityPeptide/protein ingredientsGranular deliverySide effectMicrosphere
The invention provides a slow release microsphere agents, comprising thymosin Alpha 1 of 0.5% to 10% of the microsphere weight, degradable pharmaceutical macromolecular accessories with a molecular weight of 5,000 to 500,000 Dalton and of 70% to 99.5% of the macroshpere weight, and 0% to 10% of other pharmaceutically acceptable accessories. The invention also provides the preparation method of the slow release microsphere agents. The slow release microsphere agent of thymosin Alpha 1 for injection has the advantages that the poison and side effect of thymosin Alpha 1 is reduced; the bioavailability is enhanced; times for drug taking is reduced and the drug can be conveniently taken by patients.
Owner:CHENGDU DIAO JIUHONG PHARMA FACTORY
Exenatide composite microspheres and preparation method thereof
InactiveCN108404120ALow burst rateImprove biological activityPeptide/protein ingredientsMetabolism disorderOrganic solventNanoparticle
The invention provides exenatide composite microspheres. The exenatide composite microspheres are characterized in that lecithin wraps exenatide and a protecting agent simultaneously, and after exenatide lipid nanoparticles are formed, a high-molecular polymer carrier encapsulates the exenatide lipid nanoparticles to prepare the exenatide composite microspheres; and the molecular weight of the high-molecular polymer carrier is 10000-90000, and the high-molecular polymer carrier is dissolved in a mixing organic solvent. The prepared composite microspheres are uniform in grain size, the encapsulation efficiency of the exenatide is high, the microspheres are spherical and have smooth surfaces, the mean grain size is between 1 mu m and 50 mu m, and by the characteristics of smooth surfaces, high encapsulation efficiency and uniform grain size, the releasing speed of the microspheres is stable, the microspheres can release for a long time, therefore, the drug-delivering frequency can be reduced, and the compliance of patients is good.
Owner:SUN YAT SEN UNIV
Cubic liquid crystal in-situ gel injection of local anesthetic, and preparation method of injection
ActiveCN106491519AAvoid breedingImprove stabilityAerosol deliveryOintment deliveryMass ratioAmide local anesthetics
The invention relates to cubic liquid crystal in-situ gel injection of local anesthetic, and a preparation method of the injection. The injection is prepared from the local anesthetic, a liquid crystal material and an organic solvent and / or a release regulator; the local anesthetic is amide local anesthetic and the mass concentration of the local anesthetic is 0.1 to 8 w / w%; the organic solvent is an organic solvent which is mutually soluble with water, the liquid crystal material is mono-oleic acid glycerolipid, and the mass ratio of the liquid crystal material to the organic solvent is (1-9):1; and the release regulator is selected from at least one of medium chain triglyceride, oleic acid, tocopherol and tocopheryl acetate, and the mass concentration of the release regulator is 0 to 30 w / w%. The cubic liquid crystal in-situ gel injection of the local anesthetic has very good long-acting sustained release effect, low sudden release raten high compliance and small adverse effect, can reduce administration times and can avoid the peak valley phenomenon.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Protein polypeptide drug long-acting microspheres and preparation method thereof
ActiveCN108403643AEasy to prepareMild preparation methodPeptide/protein ingredientsPharmaceutical non-active ingredientsDrugs solutionPeristaltic pump
The invention relates to protein polypeptide drug long-acting microspheres and a preparation method thereof. The preparation method comprises the steps of (1) dissolving a protein or polypeptide water-soluble drug and a protein protectant to obtain an aqueous drug solution; dissolving a macromolecular carrier material in an organic solvent to obtain an oil phase macromolecular solution; (2) addingthe aqueous drug solution into the oil phase macromolecular solution, and using a cell disrupter or a high-speed shear apparatus to prepare W / O emulsion; (3) feeding the W / O emulsion into a superfineparticle preparation system through a peristaltic pump so as to obtain the protein polypeptide drug long-acting microspheres. The protein polypeptide drug long-acting microspheres prepared via the preparation method have the advantages of good preparation process simplicity, controllable parameters, good reproducibility, and high production efficiency. The protein polypeptide drug long-acting microspheres prepared herein have the advantages of high drug encapsulating rate, low sudden release agent, stable and lasting release speed of drug, smooth microsphere surface, good spherical shape integrity, and homogenous particle size.
Owner:SUN YAT SEN UNIV
Battery cell connecting piece welding mechanism and welding method thereof
PendingCN109483055AReduce adverse effectsGuaranteed performance qualityLi-accumulatorsCell component detailsVacuum pumpingSlag
The invention discloses a battery cell connecting piece welding mechanism. The welding mechanism comprises a welding table, a laser welding assembly and a dust removal cover; the dust removal cover comprises a battery cell accommodating chamber and a welding chamber; an air inlet hole and an air suction hole are formed in the welding chamber, the air inlet hole is provided with an air blowing pipecommunicating with the welding chamber, and the air suction hole is provided with an air suction pipe communicating with the welding chamber. The mechanism is provided with the dust removal cover, and the welding chamber of the dust removal cover is in communication with a protective gas conveying device and a vacuum generating device through the air blowing pipe and the air suction pipe respectively; when the laser welding assembly is used for carrying out laser welding on a connecting piece of a battery cell and a pole column of a top cover, the protective gas conveying device introduces protective gas into the welding chamber at the same time, the vacuum generating device is used for sucking metal spatters produced by welding and welding slag formed by the metal spatters from the welding chamber in a negative pressure vacuum pumping mode, so that the purpose of eliminating the welding slag is achieved, adverse effects on the battery cell caused by the welding slag are avoided, andtherefore the safety of a battery is improved while the performance quality of the battery is guaranteed.
Owner:JIANGSU ZENIO NEW ENERGY BATTERY TECH CO LTD
Preparation method of slow-released microgranules, prepared slow-released microgranules and application thereof
ActiveCN105878190AHigh encapsulation efficiencyHigh drug loading ratePeptide/protein ingredientsPharmaceutical non-active ingredientsOrganic solventEmulsion
The invention discloses a preparation method of slow-released microgranules. The preparation method of the slow-released microgranules includes the following steps: (1) preparing solid dispersion of biodegradable and biocompatible water-insoluble polymer and water-soluble drug; (2) dissolving the prepared solid dispersion in an organic solvent C to form solid dispersion emulsion; (3) injecting the obtained solid dispersion emulsion in an oil solution which contains a surfactant to form uniform emulsion; and (4) solidifying microgranules in the emulsion by solvent volatilization or solvent extraction, collecting the microgranules, and washing and drying to obtain the slow-released microgranules. The invention further discloses slow-released microgranules prepared by the preparation method and an application of the slow-released microgranules in an implantable slow-released pharmaceutical composition. By the preparation method of the slow-released microgranules, the temperature is normal or low in the whole process, and the preparation method is quite beneficial to preparation of a compound of a polymer matrix by high-temperature-sensitive drug; and meanwhile, the prepared slow-released microgranules have excellent slow-released effect close to zero level, and the concentration of the drug is stable in a slow release period.
Owner:AC PHARMA CO LTD
Exenatide long-acting microsphere preparation and preparation method thereof
InactiveCN104043113AReduce the rate of contaminationSimple prescriptionPeptide/protein ingredientsMetabolism disorderPolyesterPeristaltic pump
The invention relates to a preparation method of an exenatide microsphere preparation. The method comprises the steps of: (1) weighing 5-50wt% of exenatide and 95-50wt% of polyester, which is one of or a mixture of PLGA and PLA at an ester group terminal or carboxyl terminal, with the molecular weight of PLGA being 3.0*10<3>-7*10<4> Dalton and the molecular weight of PLA being 4.0*10<3>-7*10<4> Dalton; (2) dissolving the polyester material in an organic solvent completely to obtain a clear solution, then putting exenatide into the clear solution, and conducting mixing and stirring evenly to obtain a homogeneous oil solution; and (3) supplying the homogeneous oil solution into a high speed rotating disc of an ultra-particle preparation system's (UPPS) droplet generation device by a peristaltic pump to form droplets, and curing the droplets to obtain the microsphere preparation. The microsphere preparation prepared by the method has excellent sustained release performance, a drug loading rate of 35%, an encapsulation rate of above 95%, low burst release rate, a sustained release period of more than 2 weeks, and no release lag period.
Owner:天津新济复兴药业科技有限公司
Preparation method of sustained-release microparticles
ActiveCN105963257AImprove complianceHigh encapsulation efficiencyOrganic active ingredientsPeptide/protein ingredientsMicroparticleEntrapment
The invention provides sustained-release microparticles. According to the invention, a preparation method of the sustained-release microparticles is conducted at normal temperature or low temperature in a whole course, which is quite conducive to high-temperature sensitive drugs, in particular compositions which polymer matrixes prepared from protein, nucleic acid and peptide drugs. Compared with public technologies, the preparation method disclosed by the invention can keep bioactivity of active substances to the greatest extent in the entire technological process; meanwhile, the prepared sustained-release microparticles have an excellent sustained-release effect close to a zero level, and drug concentration is kept stable in a sustained-release period, so that shortcomings of microparticles, which are prepared by a conventional S / O / W (solid-in-oil-in-water) process which needs to prepare the drug microparticles in advance, that drug release fails to occur in an early stage while rapid drug release happens in a late stage are overcome; and moreover, the sustained-release microparticles are relatively high in drug loading ratio and drug entrapment efficiency.
Owner:AC PHARMA CO LTD
Method of preparing simvastatin sustained-release microsphere carried series
InactiveCN100579524CAppropriate degradationPromote growthOrganic active ingredientsMetabolism disorderMicrospherePolyvinyl alcohol
The invention relates to a method that a functional drug is enveloped into a polymeric material with biodegradability to form a nano-micron microsphere system. The method comprises that the polymeric material and simvastatin are dissolved in an organic liquor to form uniform dispersion which is then added into an liquor containing emulsifier Tween 80 and biologically nontoxic electrolytic polyvinyl alcohol or sodium dodecyl benzene sulfonate (SDBS), and then the obtained liquor is stirred, evaporated at a reduced pressure, centrifugalized, washed and vacuum dried, finally the simvastatin-contained delayed-release microsphere system is obtained. The surface of the microsphere is smooth and round, the granule thereof is regular without conglutination, and the granule diameter, the drug-loading rate (1-10 percent) and the encapsulation rate (above 40 percent) are all controllable, and the delayed-release time exceeds 2 months. The prepared simvastatin-contained delayed-release microsphere system can be processed into various preparations used in bony tissue absorption or bony defect parts, the microsphere system is degraded at a proper speed, thus the simvastatin can be further released; the degradation of the polymer can provide bony tissue with subsequent recuperating space to complete the repair of the bony defect parts.
Owner:JILIN UNIV
Low-sudden-release-rate semaglutide microspheres and preparation method thereof
ActiveCN110101846AImprove securityReduce dosing frequencyPeptide/protein ingredientsMetabolism disorderLong actingChemistry
The invention provides low-sudden-release-rate semaglutide microspheres and a preparation method thereof. The preparation is a long-acting injection prepared from the active ingredient semaglutide with the weight being 1-20% of the weight of the microspheres, a biocompatible polymer substrate with the weight being 60-99% of the weight of the microspheres, and other pharmaceutically acceptable auxiliary materials with the weight being 0-20% of the weight of the microspheres. The semaglutide microspheres prepared by using the method are high in encapsulation efficiency, low in sudden release rate and capable of being slowly continuously released, the slow-release effect can last for 20-60 days, the effect on reducing blood sugar can last for at least 2-4 weeks, it only takes two weeks to onemonth or longer to conduct injection administration one time, the bioavailability of the polypeptide drug semaglutide is obviously improved, the metabolic half-life of the semaglutide is prolonged, the semaglutide administration frequency is further reduced, and the drug application compliance of a patient is further improved; because there are few drugs on the surface and the superficial layer of the preparation, the preparation is little released in the incipient administration stage, and therefore, the adverse drug sudden release effect is avoided.
Owner:QILU PHARMA
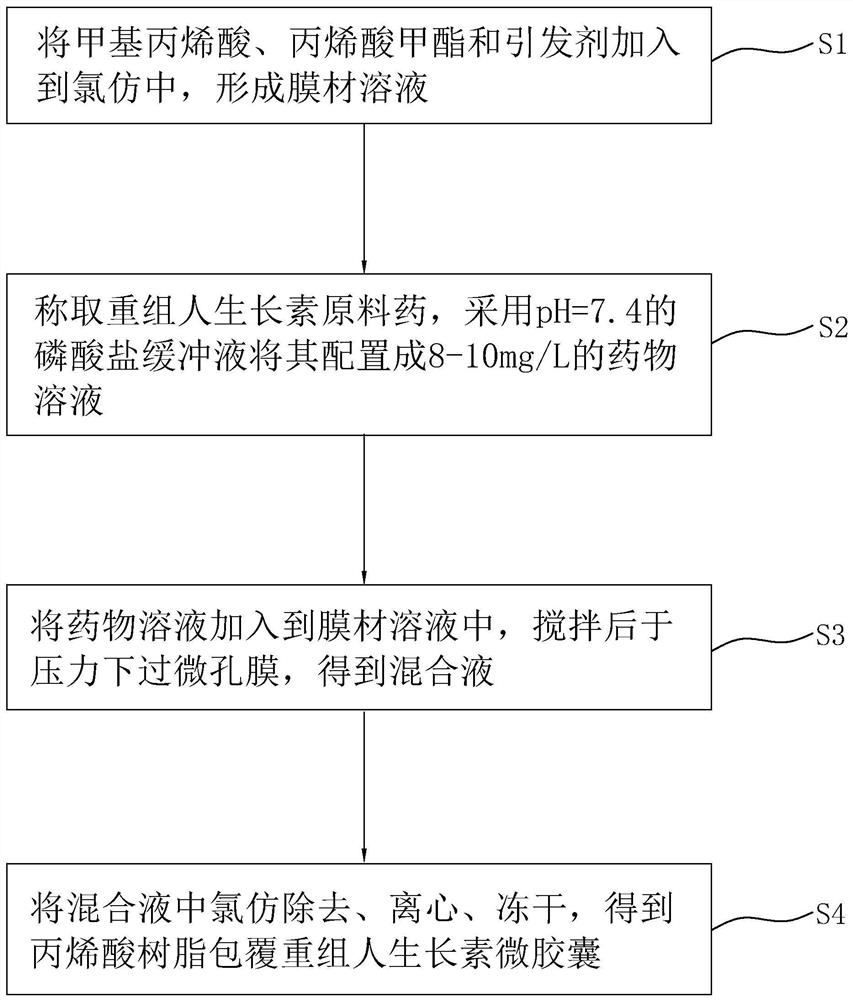
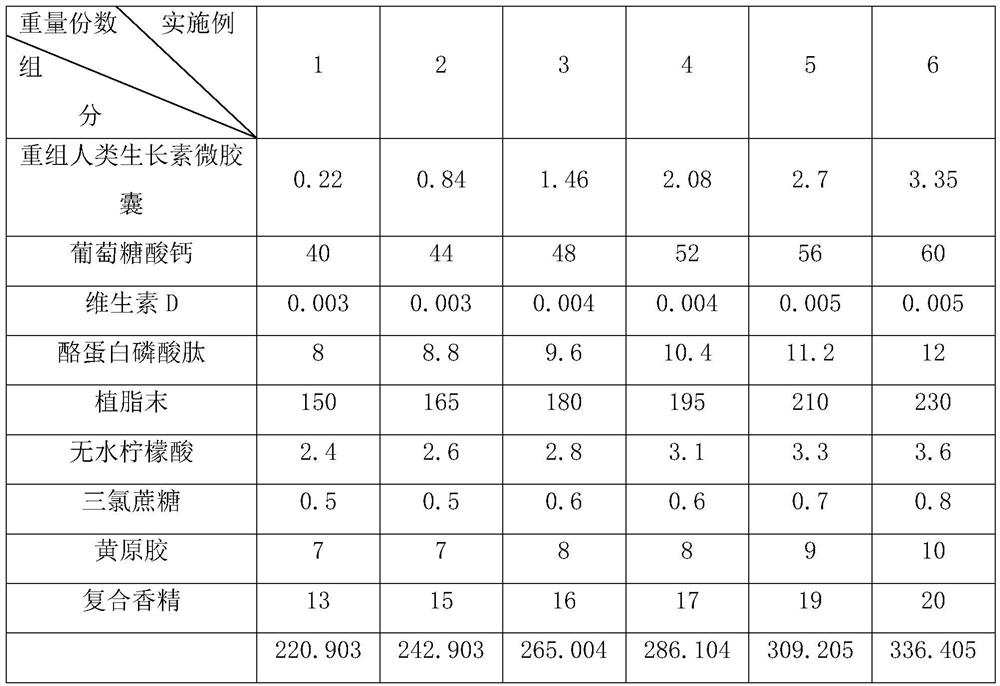
Solid beverage containing recombinant human auxin microcapsules and preparation method thereof
InactiveCN111758867AHigh nutritional valueHigh strengthPowder deliveryOrganic active ingredientsBiotechnologySucrose
The invention discloses a solid beverage containing recombinant human auxin microcapsules and a preparation method thereof, and relates to the technical field of solid beverages. The solid beverage containing the recombinant human auxin microcapsules is characterized by comprising the following components in parts by weight: 0.22-3.35 parts of recombinant human auxin microcapsules, 40-60 parts ofcalcium gluconate, 0.003-0.005 part of vitamin D, 8-12 parts of casein phosphopeptides, 150-230 parts of non-dairy creamer, 2.4-3.6 parts of anhydrous citric acid, 0.5-0.8 part of sucralose, 7-10 parts of xanthan gum and 13-20 parts of compound essence. The preparation method comprises the following steps: (1) screening; (2) weighing; (3) premixing; (4) mixing; (5) subpackaging and filling; and (6) packaging and warehousing. The solid beverage prepared by the method has the advantages of nutrition, health and improvement of bone strength; and by embedding the recombinant human auxin, the solidbeverage can be directly orally taken, and still has the advantages of high bioactivity and stability.
Owner:上海圣岳生物科技有限公司
Low-bursting-rate pure titanium seamless pipe for metal corrugated pipe and production process of low-bursting-rate pure titanium seamless pipe
PendingCN112044979AIncreased elongation at room temperatureUniform tissueRoll mill control devicesFurnace typesTitaniumPipe
The invention relates to a low-bursting-rate pure titanium seamless pipe for a metal corrugated pipe and a production process of the low-bursting-rate pure titanium seamless pipe The seamless pipe ischaracterized in that the seamless pipe has the grain size of 6-7 levels, the tensile strength Rm is larger than or equal to 300 MPa and smaller than or equal to 400 MPa, the yield strength Rp0.2 is larger than or equal to 180 MPa and smaller than or equal to 310 MPa, the ductility is larger than or equal to 50%, the wall thickness is uniform and is + / -5%t, the limit flaring rate is larger than orequal to 60%, and the bursting rate is smaller than or equal to 3%. According to the pure titanium seamless pipe prepared through the method, the grain size reaches the 6-7 levels, the room-temperature ductility reaches 50% or above, the wall thickness deviation is controlled to be + / -5%t, the limit flaring rate reaches 60% or above, and the bursting rate in subsequent corrugated pipe preparationcan be 3% or below.
Owner:宁夏中色金航钛业有限公司
Huperzine A slow-release orally disintegrating tablets and preparation method thereof
InactiveCN108186590AImprove the disintegration effectShort disintegration timeOrganic active ingredientsPharmaceutical non-active ingredientsOrally disintegrating tabletMicrosphere
The invention relates to huperzine A slow-release orally disintegrating tablets and a preparation method thereof. The huperzine A slow-release orally disintegrating tablets are prepared from huperzineA slow-release microspheres and acceptable auxiliary materials in orally disintegrating tablets. The huperzine A slow-release microspheres are prepared from huperzine A and raw materials of a framework material, the mass ratio of the huperzine A to the framework material is (0.1-0.6):100, and the framework material comprises ethyl cellulose and poloxamer in the mass ratio being (10-50):1. The huperzine A slow-release orally disintegrating tablets have more ideal slow-release effect, lower sudden release rate, can stably release in 24 h, can realize the release effect of basically complete release in 24 h, have the accumulated release quantity in 24 h up to 90% or above and are expected to be prepared into an oral slow-release preparation taken once a day, the medicine taking times of patients are reduced, and the compliance of patients is improved.
Owner:SUN YAT SEN UNIV
Sustained-release drug containing active biological factors and preparation method thereof
PendingCN112089826ALow burst rateGood slow releasePeptide/protein ingredientsPharmaceutical non-active ingredientsDrug loading dosePharmaceutical Substances
The invention aims to provide a sustained-release drug containing active biological factors and a preparation method of the sustained-release drug. The drug contains a sustained-release carrier, and the sustained-release carrier is starch and / or chitosan, preferably chitosan and starch. The sustained-release drug is prepared through an emulsification crosslinking method, the preparation method issimple, and the obtained sustained-release drug can greatly reduce burst release rate of active biological factors, can obtain longer sustained-release capacity, has good drug loading capacity and encapsulation efficiency, is suitable for clinical popularization of the active biological factors, and has huge application value.
Owner:杭州生物医药创新研究中心
Huperzine A sustained release microspheres as well as preparation method and application thereof
InactiveCN108186580AGood slow releaseLow burst rateOrganic active ingredientsPharmaceutical non-active ingredientsSide effectMass ratio
The invention relates to huperzine A sustained release microspheres as well as a preparation method and an application thereof. The huperzine A sustained-release microspheres are prepared from huperzine A and raw materials of a framework material, the mass ratio of the huperzine A to the framework material is (0.1-0.6):100, and the framework material comprises ethyl cellulose and poloxamer in themass ratio being (10-50):1. The huperzine A sustained-release microspheres have more ideal sustained-release effects, lower sudden release rate, can stably release in 24 h, can realize the release effect of basically complete release in 24 h, have the accumulated release quantity in 24 h up to 90% or above, so that toxic and side effects caused by sudden drug release or drug concentration fluctuation can be effectively reduced, and the utilization rate and bioavailability of the drug can be effectively improved.
Owner:SUN YAT SEN UNIV
Injectable in-situ gel capable of replacing surgical catgut for acupuncture and moxibustion embedding
InactiveCN106511261ASolve the sudden release problemReduce pathological damageAerosol deliveryOintment deliveryEphedrineMedicine
The invention discloses injectable in-situ gel capable of replacing a surgical catgut for acupuncture and moxibustion embedding. The injectable in-situ gel is mainly prepared from PLGA, HMPC, PEG400, NMP and alpha-asarin or ephedrine. The injectable in-situ gel is good in fluidity, curing forming performance and release degree and high in bioavailability. Meanwhile, the injectable in-situ gel has a good anti-asthma treatment effect, can be used for acupoint injection treatment on asthma and can replace surgical catgut for acupuncture and moxibustion embedding in treatment.
Owner:贵州中医药大学
Local used composition containing analgesic, and preparation method and use of local used composition
InactiveCN110420177AThe release rate is stable and long-lastingEasy to carryNervous disorderInorganic active ingredientsPharmacyAdditive ingredient
The invention discloses a local used medicine composition containing analgesic. The local used medicine composition is fundamentally prepared from the following components: opioid agents, ozone and acceptable local used medicine composition ingredients in pharmacy. According to the local used medicine composition, medicine packaging efficiency is improved, and up to more than 90%, the phenomenon of initial burst release of medicine is effectively reduced, the medicine releasing rate is stable and lasting, and a slow release period can be 2-3 days. The medicine composition is easy to smear, carrying is convenient, and compliance of a patient is improved; and a preparation method is simple, the condition is mild, the preparation process is simple, parameters can be controlled, production efficiency is high, continuous and large-scale production can be realized, the requirement of the storage condition is low, and the medicine composition is easy to package, transport and store.
Owner:YICHANG HUMANWELL PHARMA
Polypeptide drug sustained-release microsphere preparation and preparation method thereof
ActiveCN102688198BUniform particle sizeNarrow particle sizeMetabolism disorderGranular deliveryMicrosphereOrganosolv
A polypeptide-medicine-slow-releasing microsphere preparation. The preparation method for the preparation comprises the steps: dissolving a polylactic acid-glycolic acid polymer or a polylactic acid together with a protective agent and polypeptide medicine in an organic solvent to form a uniform mixed solution; adding the mixed solution to an oil phase to form an emulsion; removing the organic solvent, centrifuging, washing, and freezing and drying to obtain the polypeptide-medicine-slow-releasing microspheres. By using the 0 / 0 method, outward diffusion of the medicine into water phase is eliminated, and the medicine embedding rate is raised to 60% to 95%. The release time for a bioactive polypeptide medicine can last several weeks to several months, and in vitro release approximately matches zero-order release.
Owner:AC PHARMA CO LTD
Cubic liquid crystal in situ gel injection of local anesthetic and preparation method thereof
ActiveCN106491519BExtended release timeLong-acting slow-release effect is goodAerosol deliveryOintment deliveryEthylic acidGlycerol
The invention relates to cubic liquid crystal in-situ gel injection of local anesthetic, and a preparation method of the injection. The injection is prepared from the local anesthetic, a liquid crystal material and an organic solvent and / or a release regulator; the local anesthetic is amide local anesthetic and the mass concentration of the local anesthetic is 0.1 to 8 w / w%; the organic solvent is an organic solvent which is mutually soluble with water, the liquid crystal material is mono-oleic acid glycerolipid, and the mass ratio of the liquid crystal material to the organic solvent is (1-9):1; and the release regulator is selected from at least one of medium chain triglyceride, oleic acid, tocopherol and tocopheryl acetate, and the mass concentration of the release regulator is 0 to 30 w / w%. The cubic liquid crystal in-situ gel injection of the local anesthetic has very good long-acting sustained release effect, low sudden release raten high compliance and small adverse effect, can reduce administration times and can avoid the peak valley phenomenon.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Preparation method of sustained-release microparticles, prepared sustained-release microparticles and application thereof
ActiveCN105878190BHigh encapsulation efficiencyHigh drug loading ratePeptide/protein ingredientsPharmaceutical non-active ingredientsActive agentPharmaceutical Substances
The invention discloses a preparation method of sustained-release microparticles, which comprises the following steps: 1) preparing a solid dispersion of water-soluble medicine and a biodegradable and biocompatible poorly water-soluble polymer; 2) preparing the prepared solid dispersion Dissolve in organic solvent C to form a solid dispersion emulsion; 3) inject the obtained solid dispersion emulsion into an oil solution containing a surfactant to form a uniform emulsion; 4) solidify the particles in the emulsion by solvent volatilization or solvent extraction , collecting microparticles, washing and drying to obtain the sustained-release microparticles; the present invention also discloses the sustained-release microparticles prepared by the preparation method of the sustained-release microparticles and the application of the sustained-release microparticles in implantable sustained-release pharmaceutical compositions . The preparation method of the sustained-release microparticles of the present invention is at normal temperature or low temperature throughout the process, which is very beneficial for the preparation of polymer matrix compositions for high-temperature sensitive drugs; at the same time, the prepared sustained-release microparticles have an excellent sustained-release effect close to zero order, and the drug concentration is between Stable during sustained release.
Owner:AC PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com