Cubic liquid crystal in situ gel injection of local anesthetic and preparation method thereof
A technology of local anesthetics and gel injections, applied in the field of pharmaceutical preparations, can solve the problems of complex preparation process of PLGA microspheres, short action time of local anesthetics, and restrictions on the application of PLGA microspheres, so as to facilitate stability and storage, and inhibit breeding The effect of simple and easy reproduction and preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] This embodiment provides a bupivacaine hydrochloride cubic liquid crystal in situ gel injection and a preparation method thereof, the preparation method comprising the following steps:
[0037] 1) Heating a certain amount of glycerol monooleate (GMO) in a water bath at 45°C to melt, adding a certain amount of absolute ethanol so that the mass ratio of GMO and absolute ethanol is 8:2, and vortexing to obtain in situ gel precursor solution;
[0038] 2) Weigh a certain mass of bupivacaine hydrochloride and add it to the in-situ gel precursor solution obtained in step 1), so that the mass concentration of bupivacaine hydrochloride is 5% (w / w), and vortex to mix , ultrasonically dispersed to obtain an in situ gel injection containing 5w / w% bupivacaine hydrochloride.
[0039] The following experiment was carried out to the bupivacaine hydrochloride cubic liquid crystal in situ gel injection of the present embodiment:
[0040] (1) In vitro gelation test: a small amount of wa...
Embodiment 2
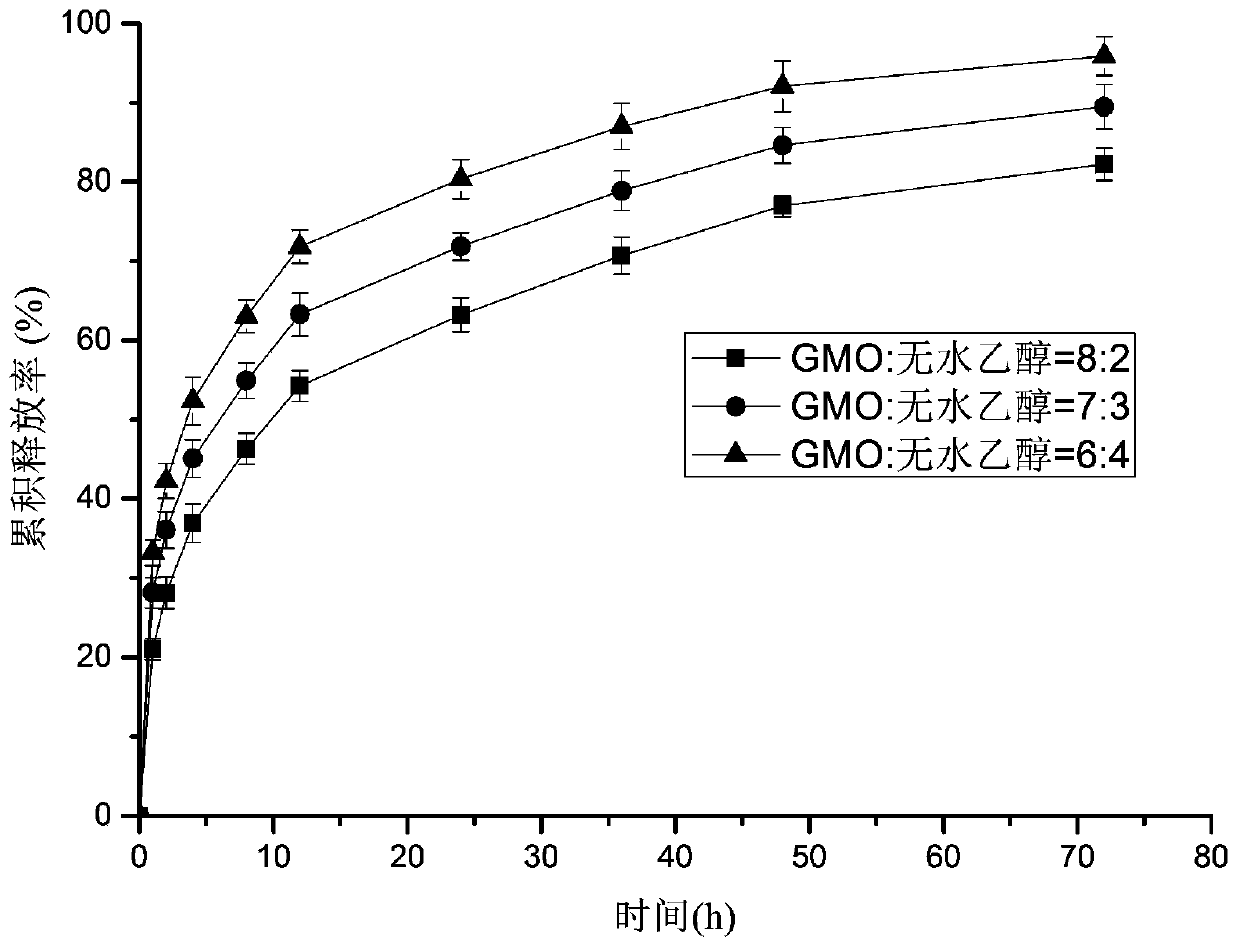
[0045] This embodiment provides the bupivacaine hydrochloride cubic liquid crystal in situ gel injection prepared by GMO and absolute ethanol in different mass ratios, and its preparation method comprises the following steps:
[0046] 1) Heat a certain amount of glycerol monooleate (GMO) in a 45°C water bath until it melts, and add a certain amount of absolute ethanol so that the mass ratios of GMO and absolute ethanol are 6:4, 7:3, and 8, respectively. :2, vortex mixing to obtain the corresponding in situ gel precursor solution;
[0047] 2) Weigh a certain mass of bupivacaine hydrochloride and add them to the in-situ gel precursor solution obtained in step 1), so that the concentration of bupivacaine hydrochloride is 5% (w / w), and vortex to mix , and ultrasonically dispersed to obtain in situ gel injections containing 5w / w% bupivacaine hydrochloride, respectively.
[0048] Three kinds of bupivacaine hydrochloride cubic liquid crystal in situ gel injections prepared in this e...
Embodiment 3
[0056] The bupivacaine hydrochloride cubic liquid crystal in situ gel injection and its preparation method provided in this embodiment, the preparation method comprises the following steps:
[0057] 1) Heating a certain amount of glycerol monooleate (GMO) in a water bath at 45°C to melt, adding a certain amount of absolute ethanol so that the mass ratio of GMO and absolute ethanol is 8:2, and vortexing to obtain Corresponding in situ gel precursor solution A;
[0058] 2) Tocopheryl acetate was added to the in-situ gel precursor solution respectively to make the mass concentrations 10%, 20%, and 30%, respectively, to obtain in-situ gel precursor solutions B with different contents of tocopherol acetate.
[0059] 3) Weigh a certain mass of bupivacaine hydrochloride and add them to the in-situ gel precursor solution B obtained in step 2), so that the concentration of bupivacaine hydrochloride is 5% (w / w), and vortex to mix Uniform, ultrasonic dispersion, respectively to obtain b...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com