Sustained-release drug containing active biological factors and preparation method thereof
A technology for sustained-release drugs and biological factors, which is applied in the directions of medical preparations containing active ingredients, pharmaceutical formulas, and medical preparations with non-active ingredients, etc. rate, burst release rate, etc., to achieve good sustained release effect, reduce the number of medication, low burst release rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: Preparation of chitosan sustained-release microspheres
[0031] In this field, chitosan microsphere carrier is generally prepared by spray drying method and emulsification crosslinking method. Considering that the active biological factor used belongs to protein / polypeptide, it has the characteristics of not being resistant to high temperature and easy to be inactivated and denatured. Therefore, this embodiment The chitosan microsphere carrier was prepared by emulsification and cross-linking method.
[0032] Existing studies have shown that the preparation of slow-release microsphere carriers with chitosan alone has the disadvantages of easy burst release of active substances and insufficient encapsulation efficiency. Sodium phosphate, albumin, collagen, sodium alginate and other microsphere carrier materials were mixed, and sodium tripolyphosphate was used as a crosslinking agent to prepare microspheres. The results showed that only when chitosan was mixe...
Embodiment 2
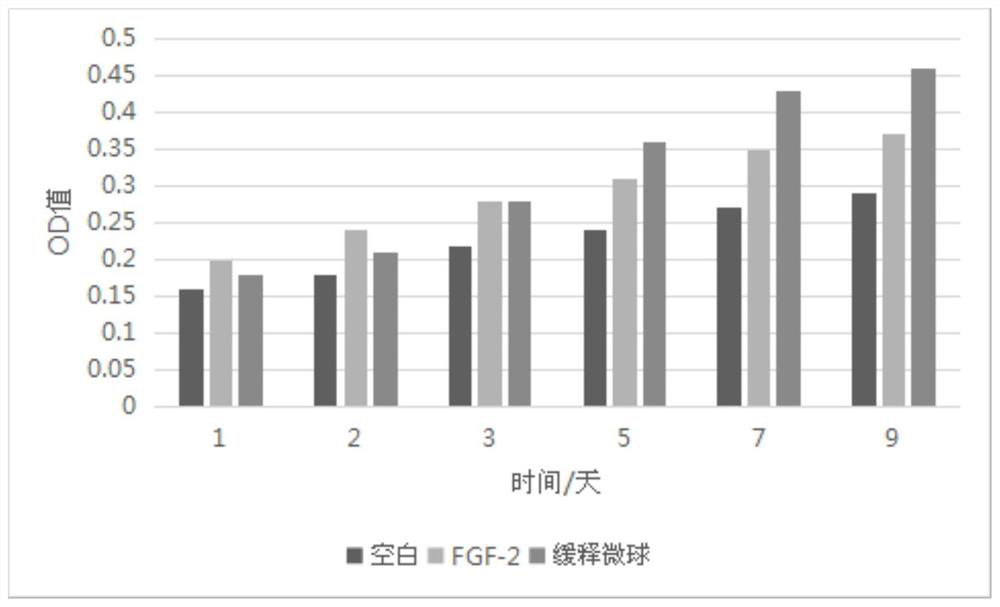
[0053] Example 2: Determination of cell proliferation activity of FGF-2 sustained-release microsphere carrier
[0054] Since the active FGF-2 has cell proliferation activity, the activity of the microsphere carrier was tested by the proliferative ability of the FGF-2 slow-release microsphere carrier on Balb / c3T3 cells.
[0055] Specific steps are as follows:
[0056] Get the Balb / c3T3 cells in the logarithmic growth phase and dilute them to 5-6×10 with 1640 medium containing 10% calf serum 4 100 μl per well was inoculated in a 96-well plate, no cells were added to the edge wells, only medium was added as a cell-free blank, 37°C, 5% CO 2 Cultivate in the incubator for 8 hours to allow the cells to adhere to the wall, then wash the 96-well plate cells twice with 1640 medium containing 0.5% calf serum, add 100 μl of 1640 medium containing 0.5% calf serum and culture for 48 hours, pass through the serum Starvation keeps cells in a quiescent state. During this period, the 1640 m...
Embodiment 3
[0058] Embodiment 3: FGF-2 sustained-release microsphere carrier stability
[0059] The purpose of this example is to study the influence of the FGF-2 slow-release microsphere carrier on the stability of FGF-2. The experiment is divided into the following three groups: placed at 4°C, placed at room temperature, and placed at 40°C. The samples were measured at 5, 10, 20, 30 days for each biological activity, and compare the assay results.
[0060] FGF-2 sustained-release microsphere carrier preparation method is as follows: get 150mg chitosan (100kDa) and 50mg starch (experimental group), or separate 150mg chitosan (kDa) (control group) is dissolved in the 20mL / L acetic acid of 9ml respectively In the solution, dissolve 10 μg FGF-2 in 1 ml of 4 mmol / L HCl solution, mix the above two solutions to obtain a mixed solution, slowly add the mixed solution to 90 mL of octanol solution containing 5 ml / l Tween-80, and magnetically Stir with a stirrer for 2 hours, add 100 g / L sodium tri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com