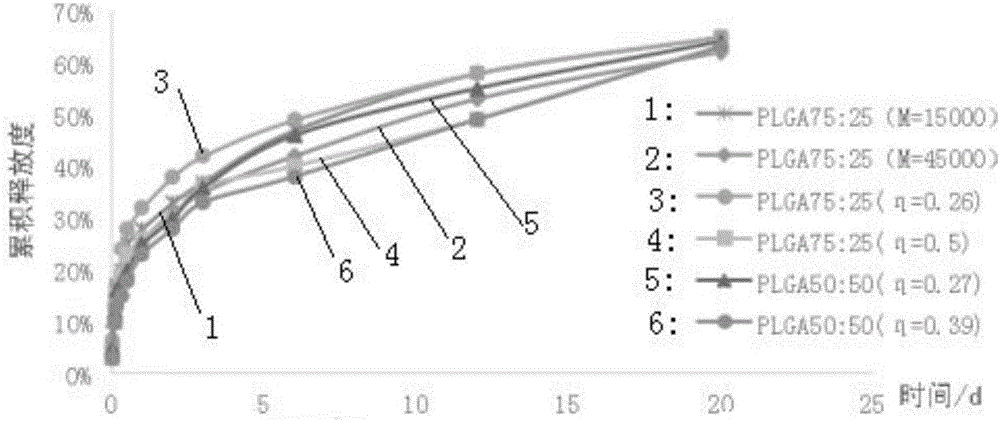
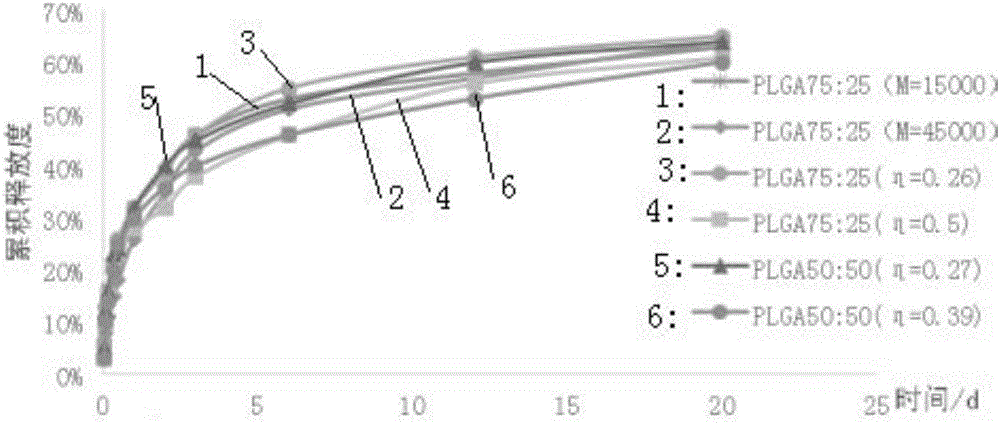
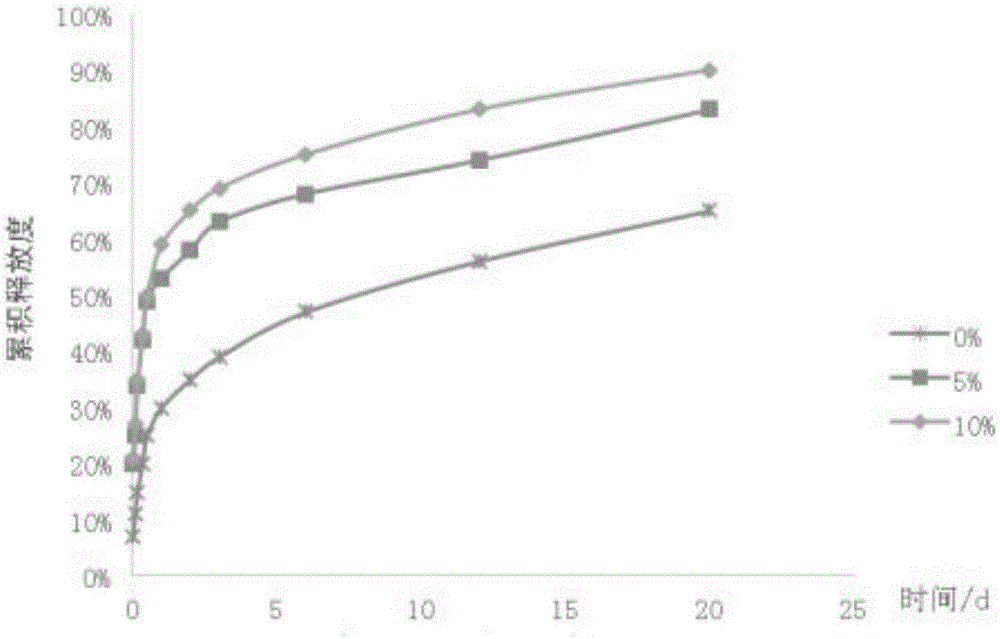
Injectable in-situ gel capable of replacing surgical catgut for acupuncture and moxibustion embedding
A technology of in-situ gel and catgut, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, organic active ingredients, etc., can solve the problems of limited toxic and side effects, low bioavailability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Composition: PLGA 30g, HMPC 2g, PEG400 10g and NMP 54g.
[0118] Process: After mixing PEG400 and NMP, add HPMC, after complete mixing, add PLGA, vortex shake for 24 hours, sterilize with Co60, and store in the dark at 4°C to obtain the product.
[0119] Usage and dosage: acupoint injection; dosage: 16mg once for adults; dosing interval: once a week.
Embodiment 2
[0121] Composition: PLGA 45g, HMPC 3g, PEG400 20g and NMP 65g.
[0122] Process: After mixing PEG400 and NMP, add HPMC, after complete mixing, add PLGA, vortex for 28 hours, sterilize with Co60, and store in the dark at 4°C to obtain the product.
[0123] Usage and dosage are the same as in Example 1.
Embodiment 3
[0125] Composition: PLGA 15g, HMPC 1g, PEG400 1g and NMP 40g.
[0126] Process: After mixing PEG400 and NMP, add HPMC, and after mixing completely, add PLGA, vortex for 20 hours, sterilize with Co60, and store in the dark at 4°C.
[0127] Usage and dosage are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com