Thymus gland peptide alpha1sustained-release microsphere preparation for injection and preparation thereof
A technology for sustained-release microsphere preparations and thymosin, which is applied to medical preparations with inactive ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, can solve the problems of reduced encapsulation rate, waste of cost, loss, etc. The effect of stable release, reduced administration frequency and low burst release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
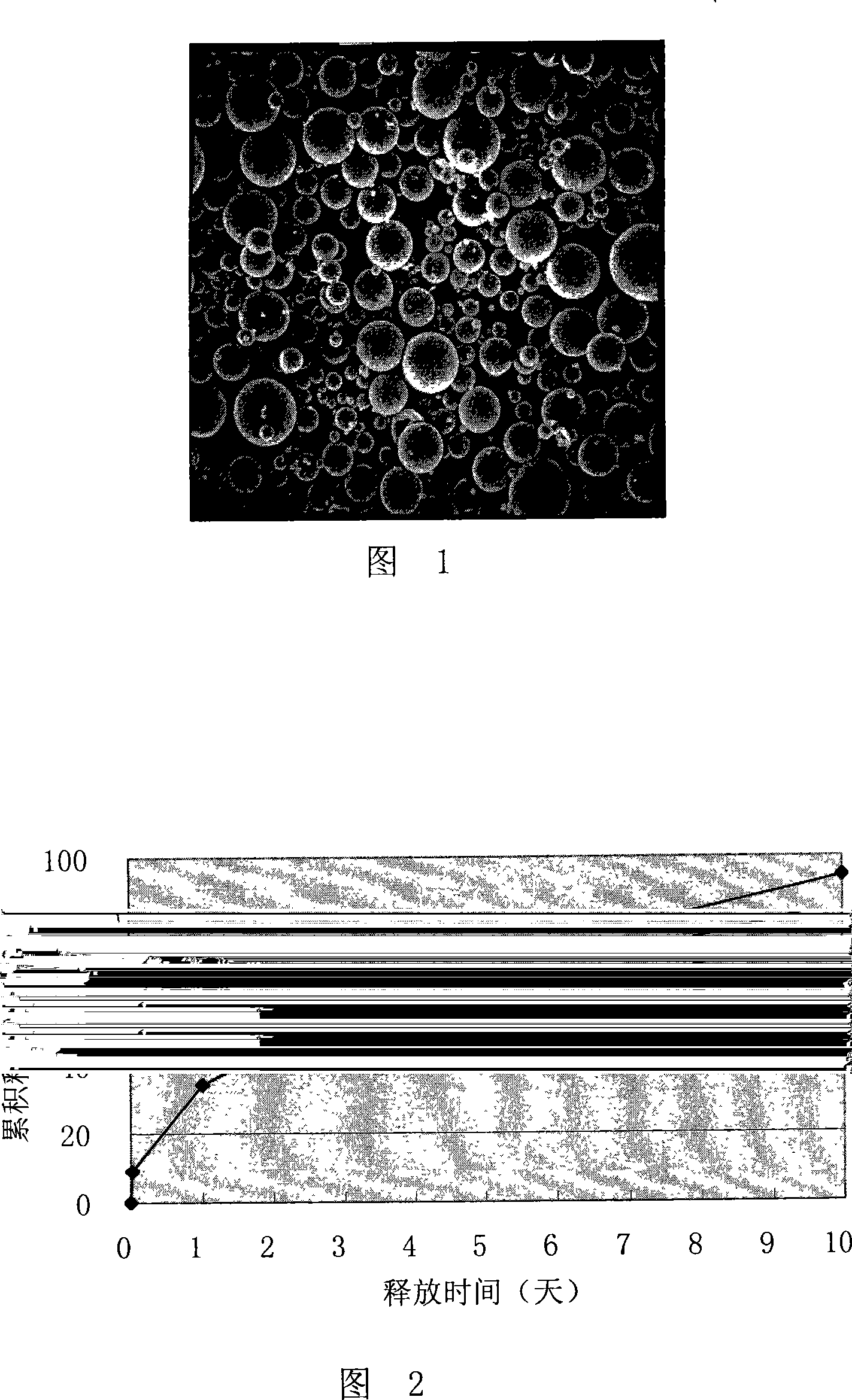
Image
Examples
Embodiment 1
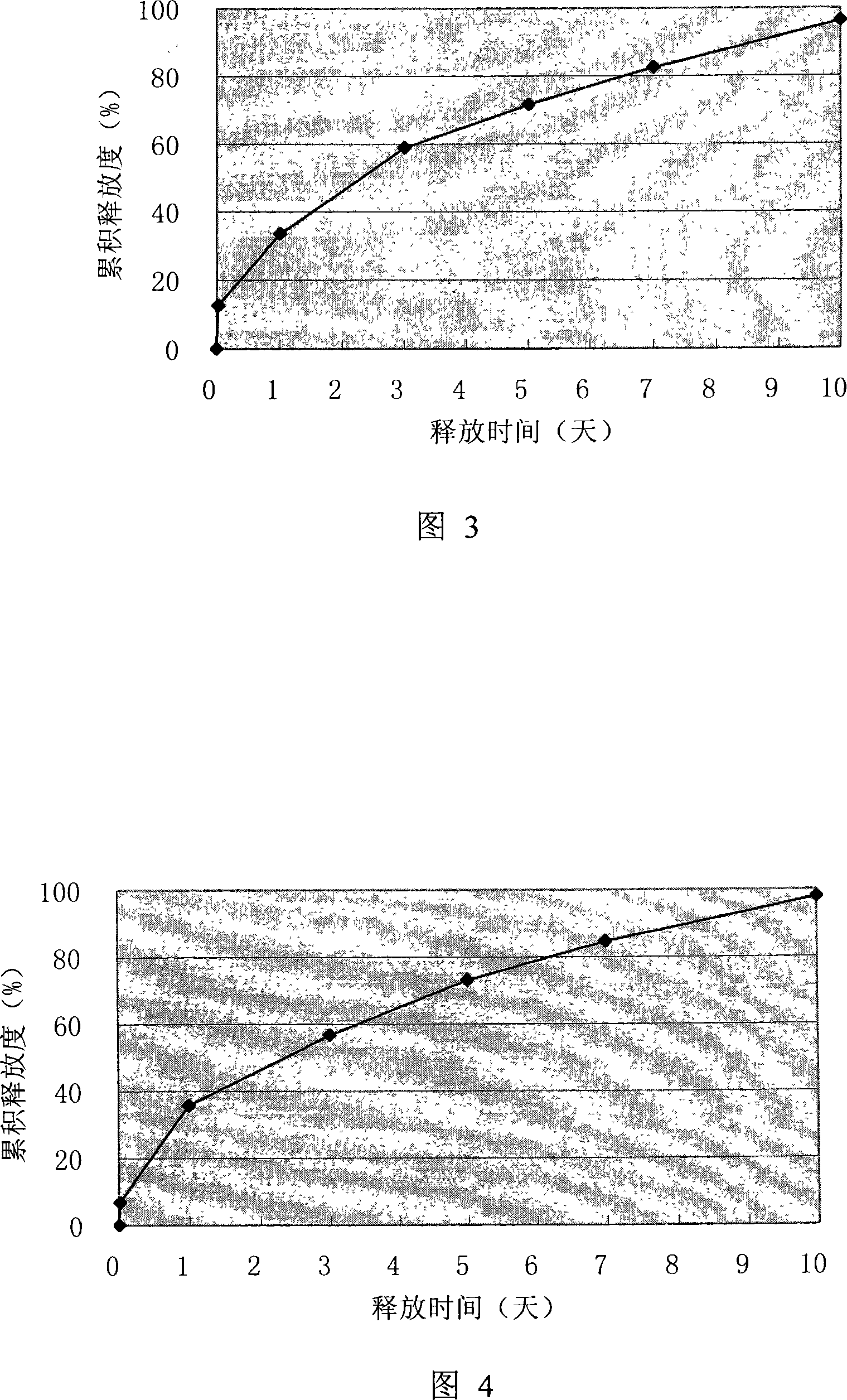
[0063] Example 1: Thymosin α for injection prepared by drying method in double emulsion-liquid 1 slow release microspheres
[0064] Thymosin alpha 100mg 1 , 0.7g of PEG-6000 was dissolved in 6ml of pH7.4 phosphate buffer, 6g of polyglycolide-lactide (glycolide:lactide=50:50) (intrinsic viscosity 0.45dl / g: 0.55dl / g=2: 3) and be dissolved in 130ml of dichloromethane, the water phase is poured into the oil phase under the effect of high-shear homogenizer rotating speed 2000r / m, emulsify under 2500r / m rotating speed -8min to get w / o emulsion, pour the w / o emulsion into 1000ml ice-bath containing 2%PVA, 1%NaCl external aqueous solution, stirring speed 700r / m, time 2-3 minutes, then add 1500ml The external aqueous phase solution was stirred at a stirring speed of 550r / m for 4 hours, the organic solvent was evaporated, filtered with suction, washed 3 times with distilled water, and freeze-dried for 4 days to obtain a white powder with good fluidity. The encapsulation efficiency wa...
Embodiment 2
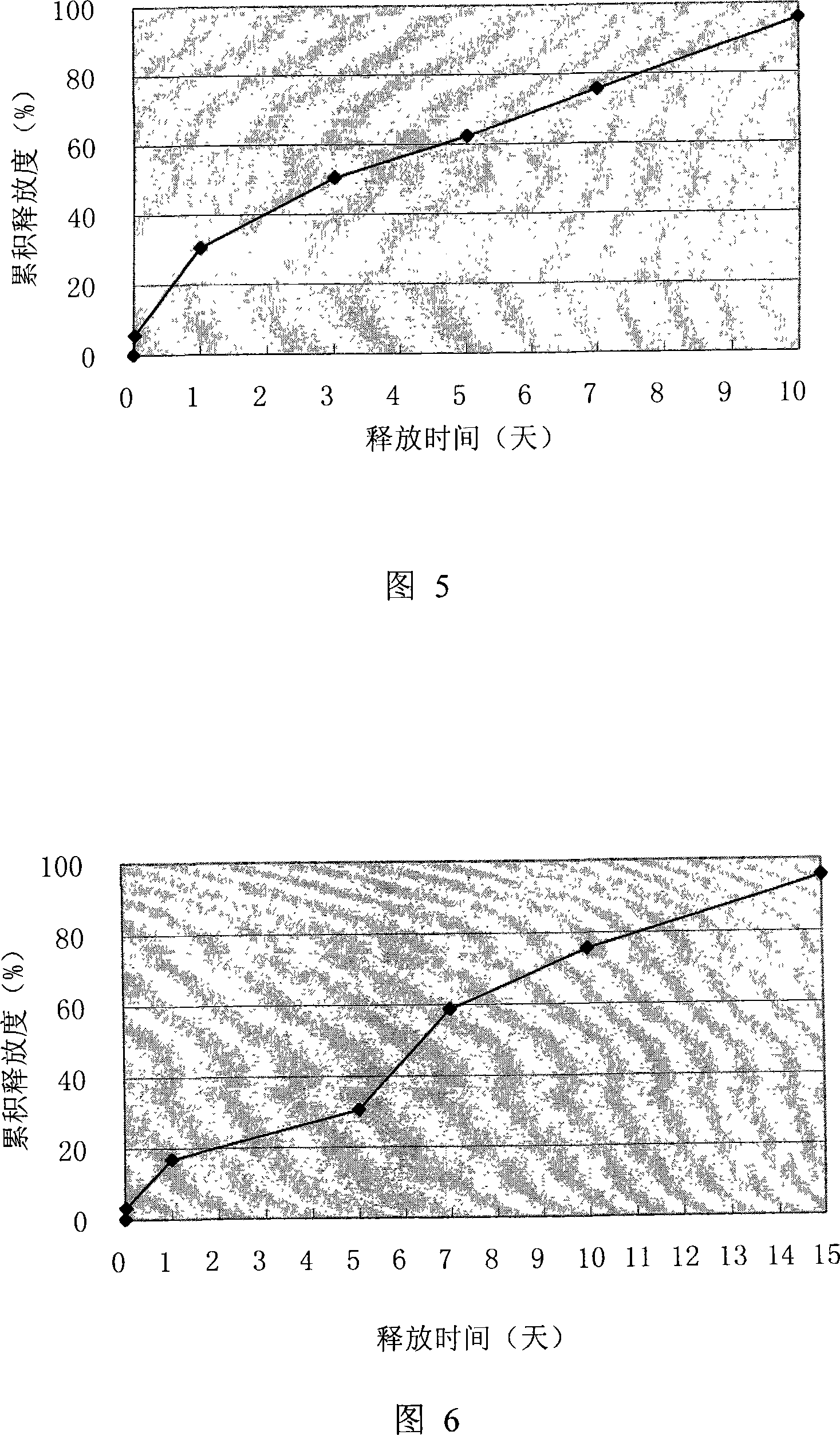
[0065] Example 2: Preparation of Thymosin α for Injection by Drying Method in Double Emulsion-Liquid 1 sustained-release microspheres
[0066] Thymosin alpha 100mg 1 The mannitol of 1.5g is dissolved in the phosphate buffered saline solution of the pH7.4 of 6ml, the polyglycolide-lactide of 6g (glycolide: lactide=50: 50, intrinsic viscosity 0.35dl / g: 0.89 dl / g=1:3) was dissolved in 130ml of dichloromethane, the water phase was poured into the oil phase under the action of a high-shear homogenizer at a speed of 2000r / m, and emulsified at a speed of 2500r / m for 5-8min to obtain w / o emulsion, pour the w / o emulsion into 1000ml ice-bath containing 2%PVA, 1%NaCl external water phase solution, stirring speed 700r / m, time 2-3 minutes, then add 1500ml external water phase solution , the stirring speed was 550r / m, the stirring time was 4 hours, the organic solvent was evaporated, suction filtered, washed 3 times with distilled water, and freeze-dried to obtain a white powder with goo...
Embodiment 3
[0067] Example 3: Preparation of thymosin α for injection by dry method in double emulsion-liquid 1 sustained-release microspheres
[0068] Thymosin alpha 500mg 1Dissolve in 20ml of pH7.4 phosphate buffer, 33g of polyglycolide-lactide (glycolide:lactide=50:50, intrinsic viscosity 0.65dl / g:0.80dl / g=1 : 1) and PEG-60003g were dissolved in 600ml of dichloromethane, the water phase was poured into the oil phase under the effect of high-shear homogenizer rotating speed 2000r / m, emulsified under 2500r / m rotating speed for 5-8min to obtain w / o Emulsion, pour w / o emulsion into 4000ml ice-bath containing 2%PVA, 1%NaCl external water phase solution, stirring speed 700r / m, time 2-3 minutes, then add 1000ml external water phase solution, The stirring speed was 550r / m and the stirring time was 5 hours. The organic solvent was evaporated, suction filtered, washed 3 times with distilled water, and freeze-dried to obtain a white powder with good fluidity. The average particle size of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com