Recombinant human growth hormone (rhGH) long-acting sustained-release microcapsule and preparation method thereof
A human growth hormone, sustained-release technology, applied in the field of medicine, can solve the problems of aggravating side effects, producing immunogenicity, low bioavailability, etc., and achieves the effect of maintaining sustainable release, maintaining biological activity, and improving the encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
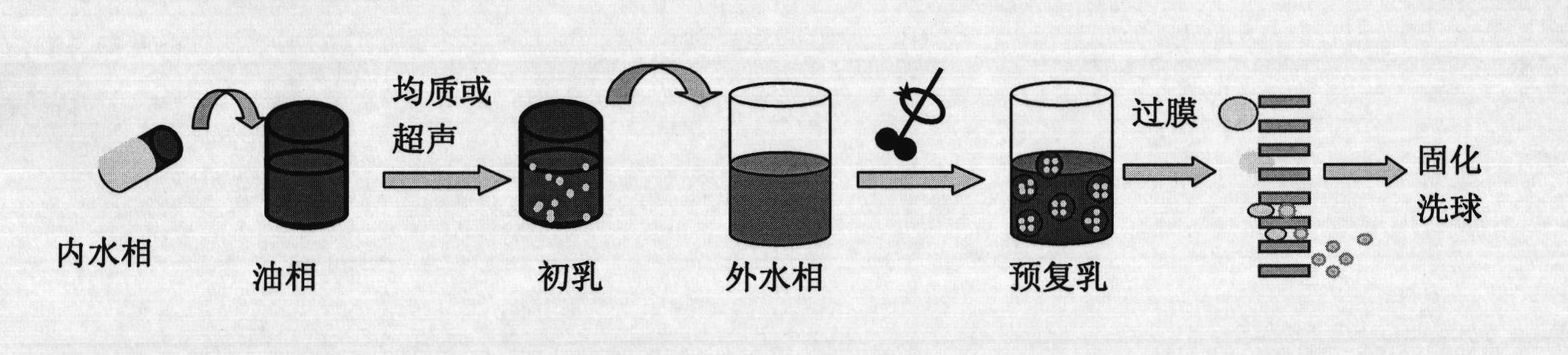
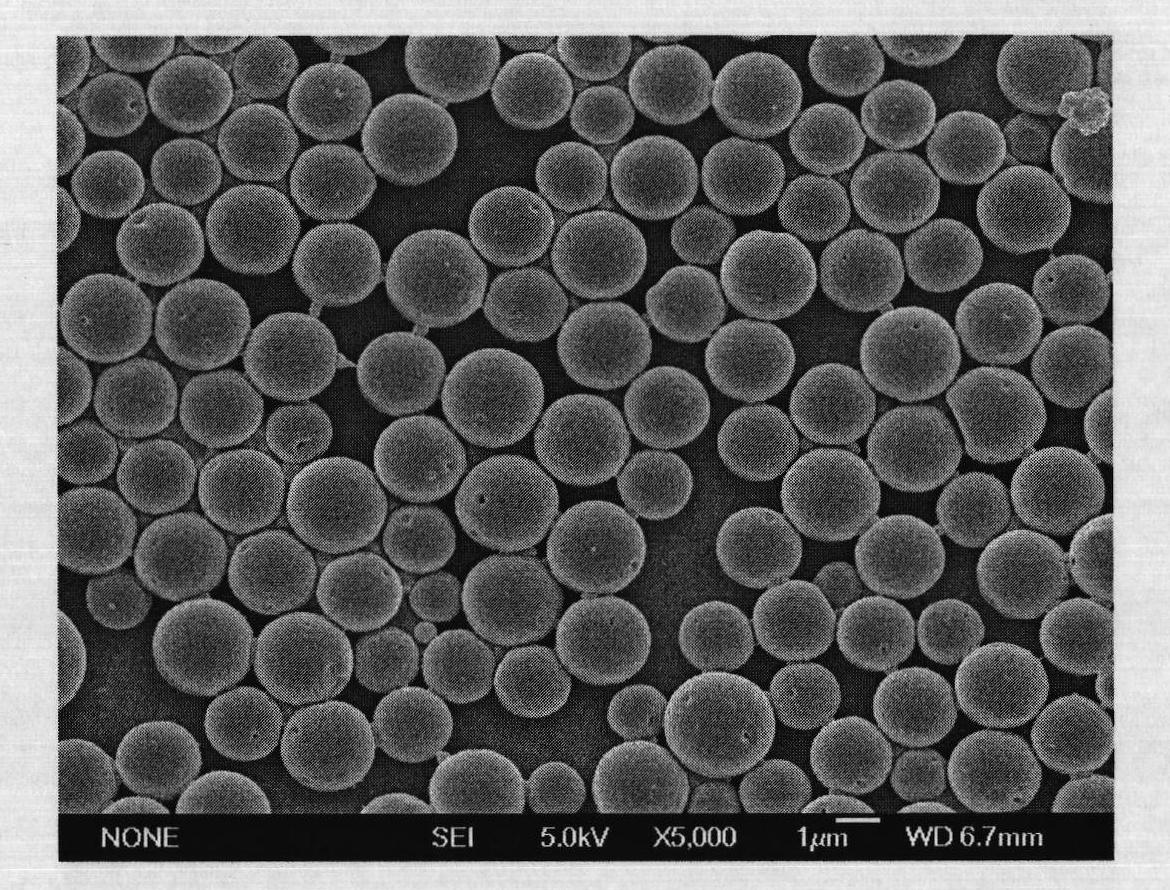
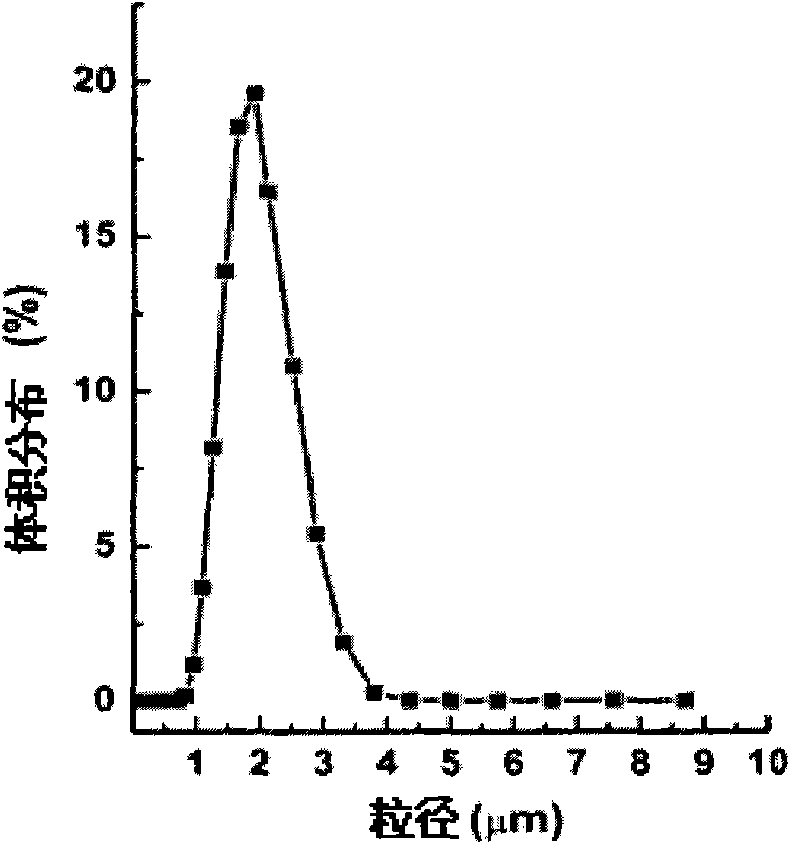
[0063] The hydrophilic SPG membrane with a pore size of 5.2 μm was soaked in water to fully wet the porous membrane. 0.8mL concentration is 40mg / mL recombinant human growth hormone (rhGH) aqueous solution as inner water phase (W 1 ), 0.4 g of polylactic acid-polyethylene glycol copolymer (PELA) with a molecular weight of 30,000 was dissolved in 8 mL of ethyl acetate as the oil phase (O). Mix the inner water phase and the oil phase, homogeneously emulsify for 30s, and get W 1 / O colostrum. The colostrum was added to 48 mL of 1% wt polyvinyl alcohol (PVA) aqueous solution (W 2 ), magnetically stirred at 300rpm for 1min to prepare pre-double emulsion (W 1 / O / W 2 ), and then press the pre-complex emulsion under the operating pressure of 300kPa through the microporous membrane device (such as figure 1 ) to obtain a multiple emulsion, the emulsion film-passing time is less than 10s, then the multiple emulsion is stirred at room temperature with 0.9% NaCl of 1L for 4h to extract...
Embodiment 2
[0069] The hydrophilic SPG membrane with a pore size of 0.5 μm was soaked in water to fully wet the porous membrane. 1.0mL concentration is 100mg / mL recombinant human growth hormone (rhGH) aqueous solution as inner water phase (W 1 ), 0.2g of polylactic acid-polyethylene glycol copolymer (PELA) with a molecular weight of 10,000 and 0.2g of 20,000 was dissolved in 8mL of propyl acetate as the oil phase (O). Mix the inner water phase and the oil phase, homogeneously emulsify for 15s, and obtain (W 1 / O) type colostrum. This colostrum is added in the 1%wt PVA aqueous solution of 48mL, magnetic stirring 300rpm stirs 1min and prepares pre-double emulsion (W 1 / O / W 2 ), then press the pre-multiple emulsion through the microporous membrane device under the operating pressure of 2000kPa to obtain the multi-emulsion, the emulsion passing time is less than 10s, then under the room temperature of the multi-emulsion, stir 4h with 0.9% NaCl of 1L to extract ethyl acetate ester, and the...
Embodiment 3
[0071] The hydrophilic membrane with a pore size of 1.4 μm is soaked in water to fully wet the porous membrane. Dissolve 0.4g of polycaprolactone and polyethylene glycol copolymer with a molecular weight of 20,000 in 8mL ethyl propionate as the oil phase (O), mix 120mg of recombinant human growth hormone (rhGH) solid particles with the oil phase Mix and homogeneously emulsify for 15 seconds to obtain (S / O) type colostrum. This colostrum is added in the 1%wt PVA aqueous solution of 48mL, magnetic stirring 300rpm stirs 1min and prepares pre-double emulsion (S / O / W 2 ), and then press the pre-multiple emulsion through the microporous membrane device under the operating pressure of 800kPa to obtain the multi-emulsion, the emulsion passing time is less than 10s, then under the room temperature of the multi-emulsion, stir 4h with 0.9% NaCl of 1L to extract ethyl acetate ester, and then centrifuged to obtain drug-loaded microcapsules. The obtained microcapsules were vacuum-dried for...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com