Synthesis method of lornoxicam
A technology of lornoxicam and synthetic method, which is applied in the field of drug synthesis, can solve the problems of destroying the stability of 2-aminopyridine, the decrease of yield, and the increase of reaction by-products, so as to improve production efficiency and product yield, and reduce activation Can, avoid the effect of coking
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
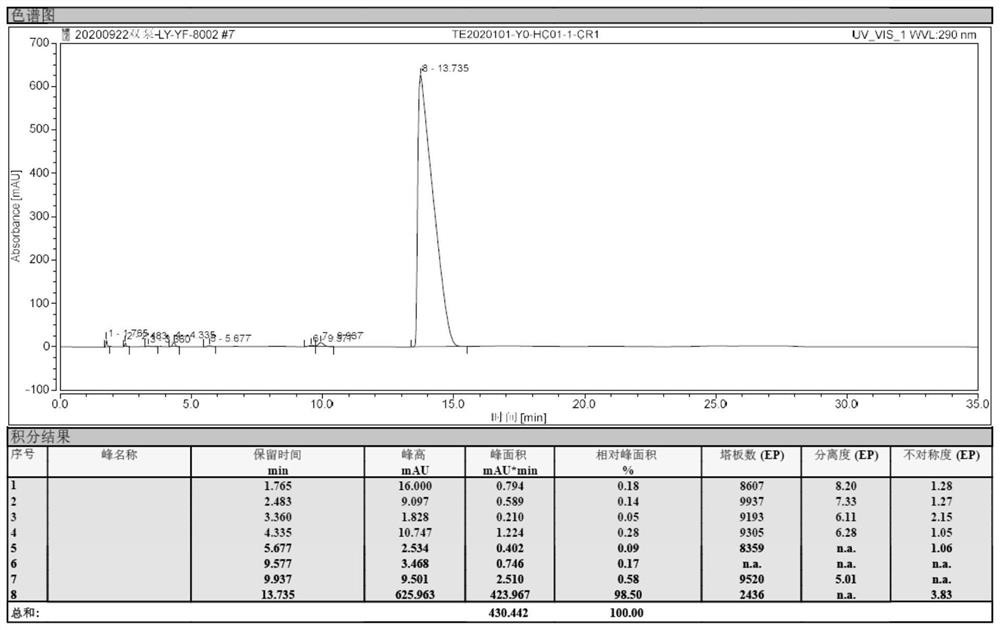
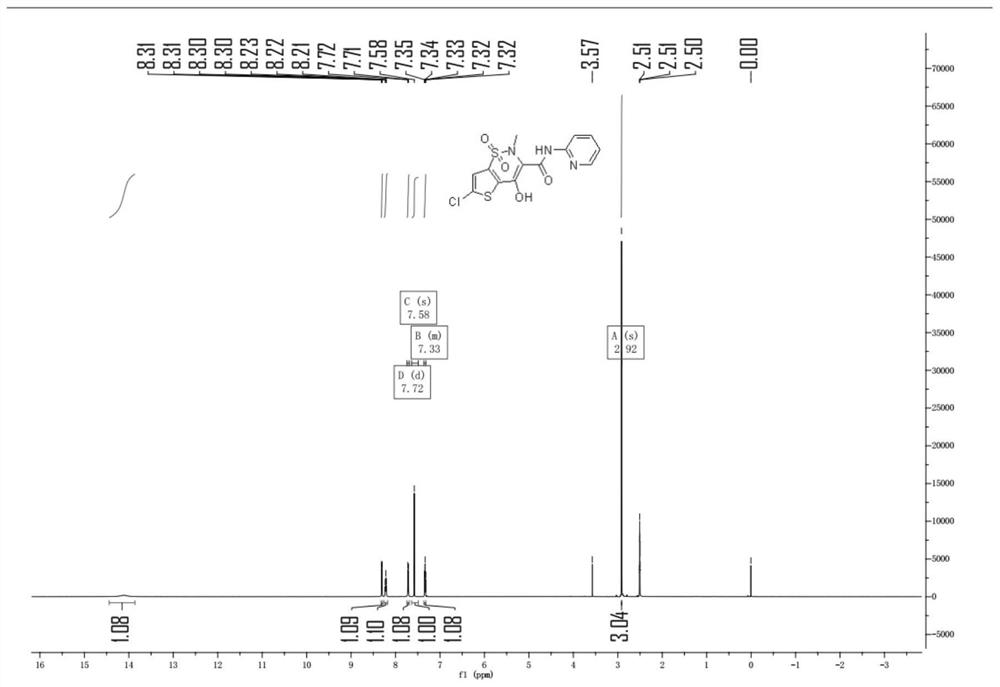
[0045]Under nitrogen protection, xylene was added 200ml, 6-chloro-4-hydroxy-2-methyl-2-H-thiophene and [2,3-E] -1,2-thiazine carboxarboxes were added to 500 ml of four-mouth flasks. Ester-1,1-dioxide 10g, 2-aminopyridine 3.65 g, 0.6 g of methylbenzenesulfonic acid. Heat until 130 ° C insulation ammonia reaction for 4 hours. Sampling HPLC detection, when the reaction feedstock 6-chloro-4-hydroxy-2-methyl-2-H-thiophene and [2,3-E] -1, 2-thiazine carboxylate-1, 1- When the content of the dioxide is ≤ 2%, the reaction is stopped.
[0046]The reaction solution was cooled to 50 ° C, at this temperature, the pressure was -0.07 MPa, advanced decompression concentrated to the solvent, concentrated the reaction liquid to 30 mL, stop concentration; cool down to 20 ° C, mixed solution of dichloromethane and methanol 50 ml, wherein the volume ratio of dichloromethane and methanol is 4: 1; heating to 80 ° C, the heat preservation is 2 hours; after cooling to 30 ° C, it is obtained to obtain chlorovo...
Embodiment 2
[0049]Under nitrogen protection, xylene 400 ml of xylene, 6-chloro-4-hydroxy-2-methyl-2-H-thiophene [2,3-E] -1,2-thiazine is added to 1 L of 400 ml, 6-chloro-4-hydroxy-2-methyl-2-H-thiophene [2,3-E] -1,2-thiazine Acid methyl ester-1,1-dioxide 20g, 2-aminopyridine 7.3 g, 1.2 g of methylbenzenesulfonic acid. Heat until 115 ° C insulation ammonia reaction for 5 hours. Sampling HPLC detection, when the reaction feedstock 6-chloro-4-hydroxy-2-methyl-2-H-thiophene and [2,3-E] -1, 2-thiazine carboxylate-1, 1- When the content of the dioxide is ≤ 2%, the reaction is stopped.
[0050]The reaction solution was cooled to 65 ° C, at this temperature, the pressure was -0.09 MPa, first transdermally concentrated the solvent, concentrated the reaction solution to 100 ml, stop concentration; cool down to 25 ° C, mixed solution of dichloromethane and methanol 100 ml, wherein the volume ratio of dichloromethane and methanol is 4: 1; heating to 65 ° C, the heat preservation is 3 hours; then cool down to ...
Embodiment 3
[0053]Under nitrogen protection, xylene 800 ml, 6-chloro-4-hydroxy-2-methyl-2-H-thiophene [2,3-E] -1,2-thiazine carboxarboxes were added to a 4L four-mouth bottle. Acid methyl ester-1,1-dioxide 40g, 2-aminopyridine 14.6 g, 2.4 g of methylbenzenesulfonic acid. Heat until 110 ° C insulation ammonia reaction for 6 hours. Sampling HPLC detection, when the reaction feedstock 6-chloro-4-hydroxy-2-methyl-2-H-thiophene and [2,3-E] -1, 2-thiazine carboxylate-1, 1- When the content of the dioxide is ≤ 2%, the reaction is stopped.
[0054]The reaction liquid was cooled to 80 ° C, at this temperature, the pressure was -0.1MPa, advanced decompression concentrated to the solvent, concentrated the reaction liquid to 120 mL, stop concentration; cool down to 30 ° C, mixed solution of dichloromethane and methanol 200 ml, wherein the volume ratio of dichloromethane and methanol is 4: 1; heating to 50 ° C, the heat preservation is 5 hours; then cool down to 20 ° C to obtain chlorovoxic. The crude material...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com