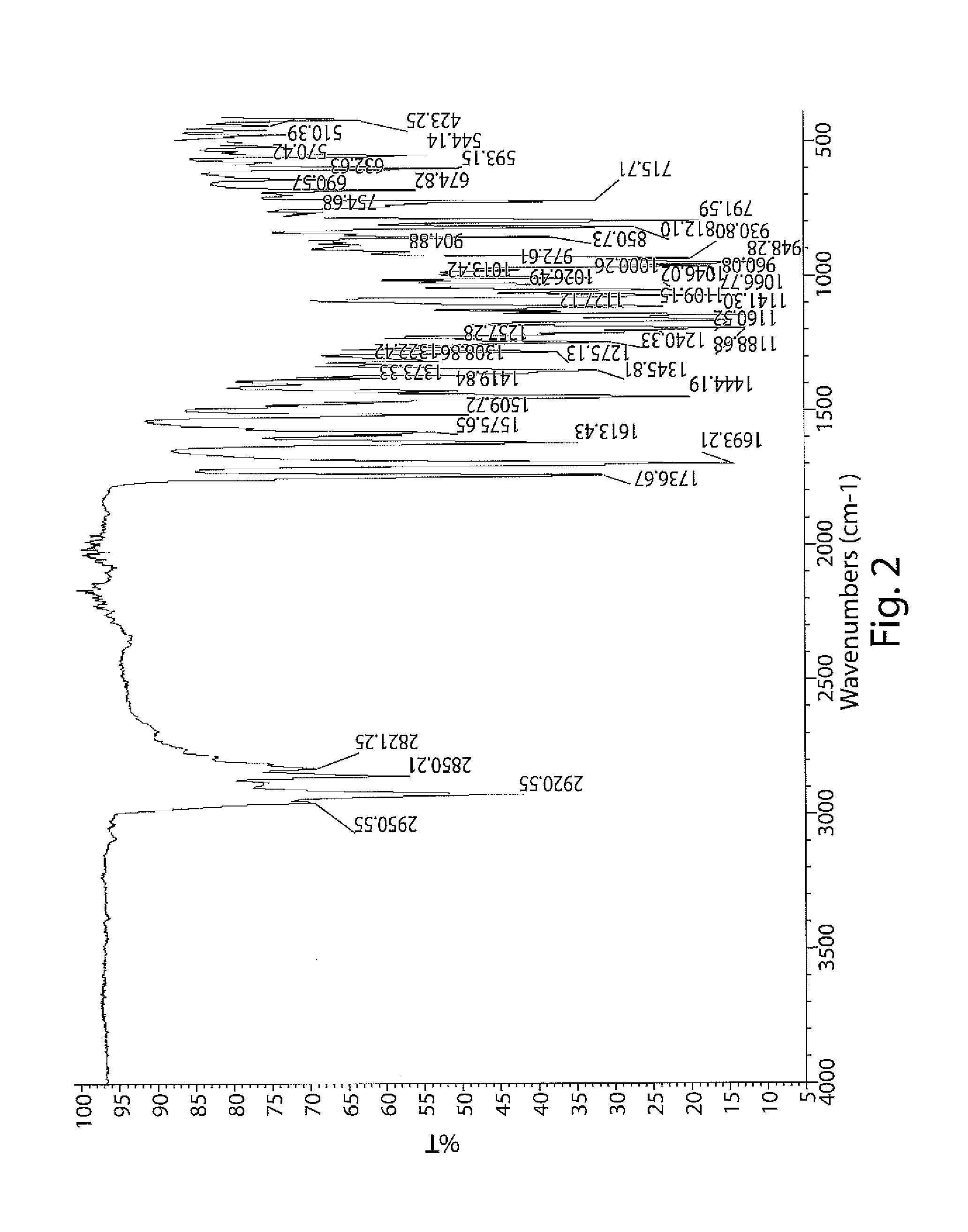
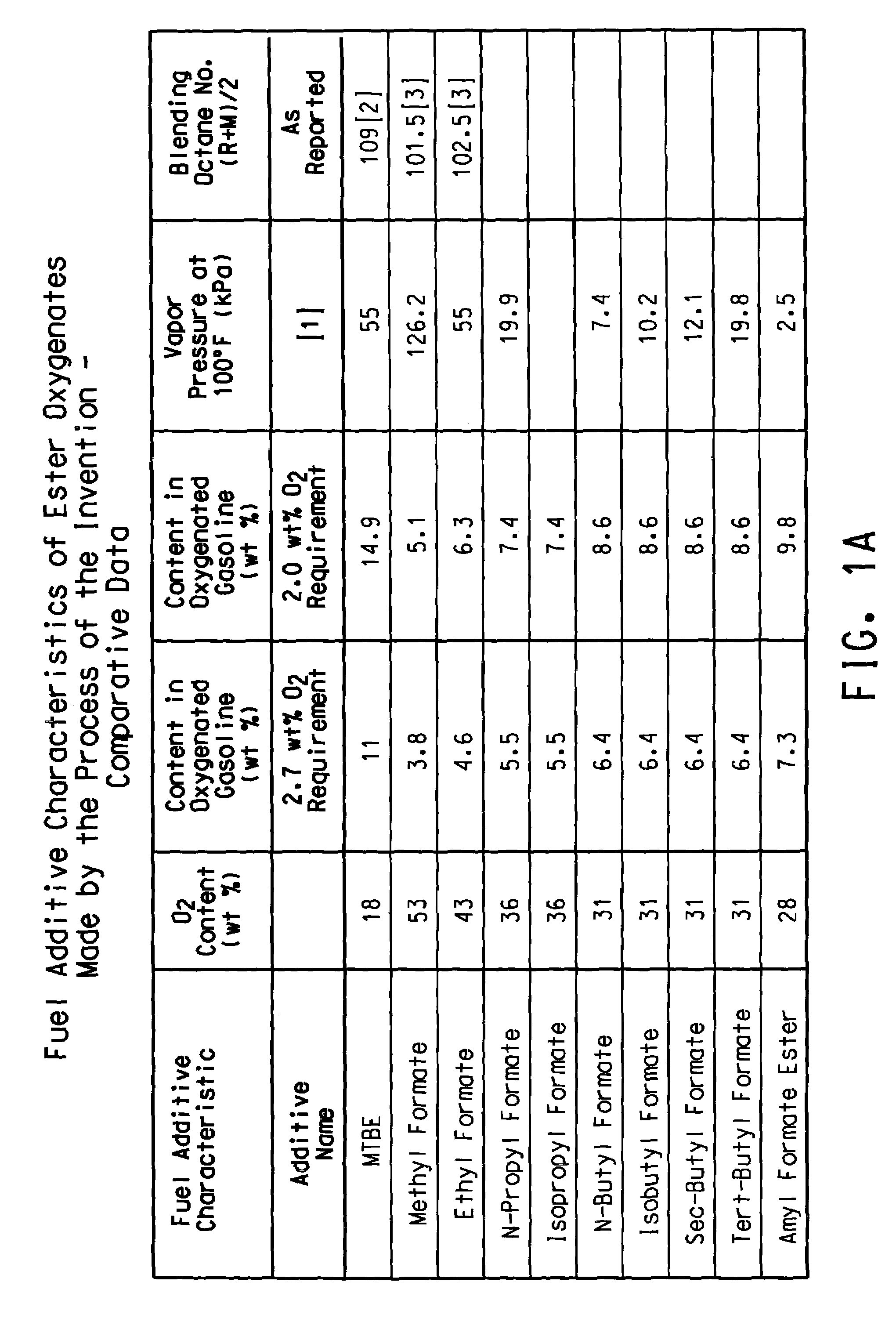
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
244 results about "Formic Acid Esters" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Formate (IUPAC name: methanoate) is the anion derived from formic acid. Its formula is represented in various equivalent ways: CHOO− or HCOO− or HCO2−. It is the product of deprotonation of formic acid. It is the simplest carboxylate anion. A formate (compound) is a salt or ester of formic acid.
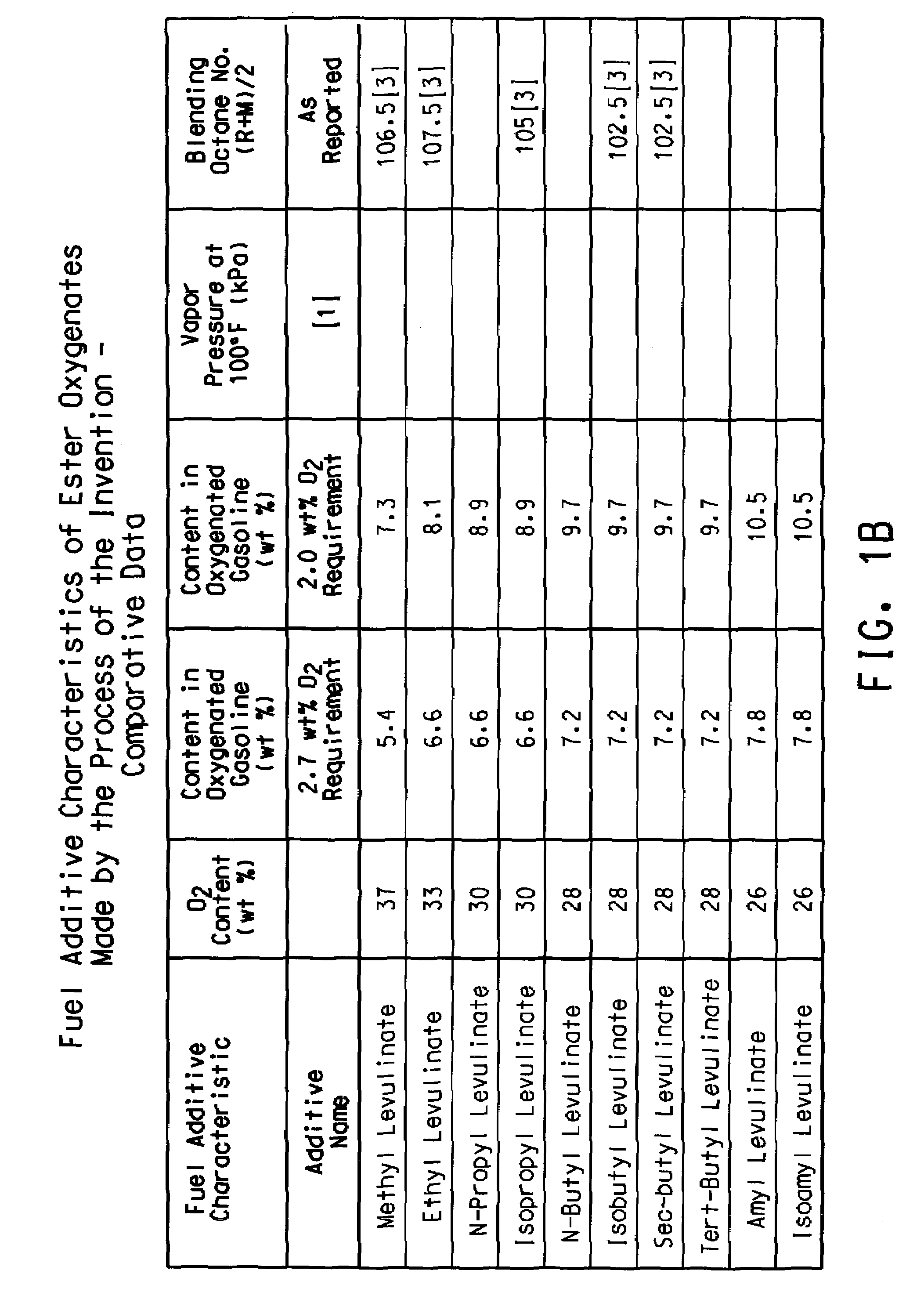
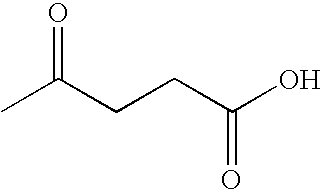
Preparation of levulinic acid esters and formic acid esters from biomass and olefins
This invention relates to a process for producing a mixture of levulinic acid esters and formic acid esters from biomass and olefins, and the composition prepared therefrom. This invention also relates to usage of the mixture of these esters as fuel and as fuel additives for gasoline fuel, diesel fuel, and biofuel.
Owner:EI DU PONT DE NEMOURS & CO
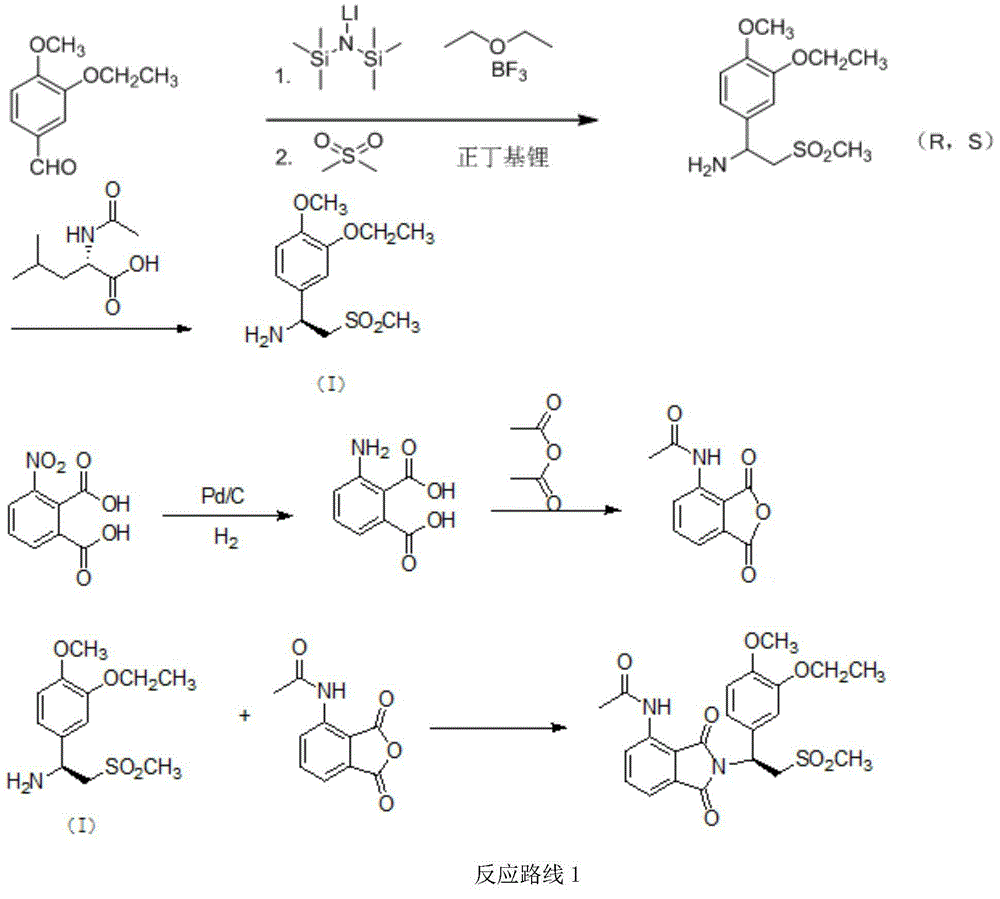
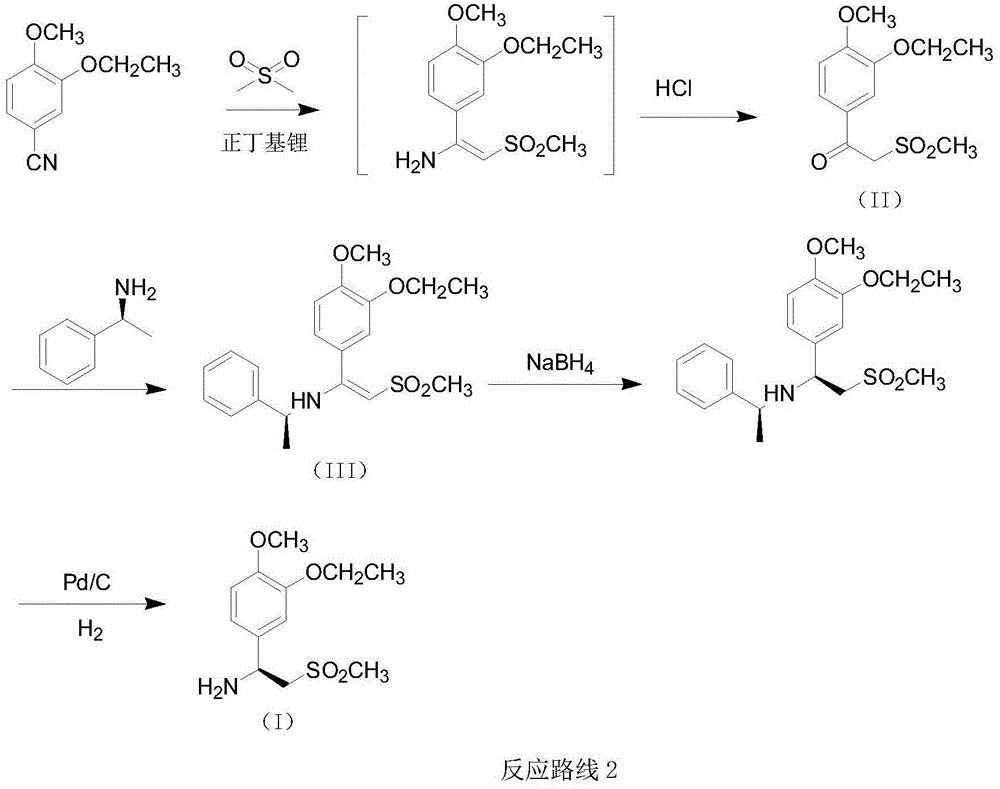
Preparation method for synthesizing apremilast intermediate
ActiveCN104447445AStable and cheapReaction is easy to controlOrganic chemistryOrganic compound preparationPhenyl groupSulfone
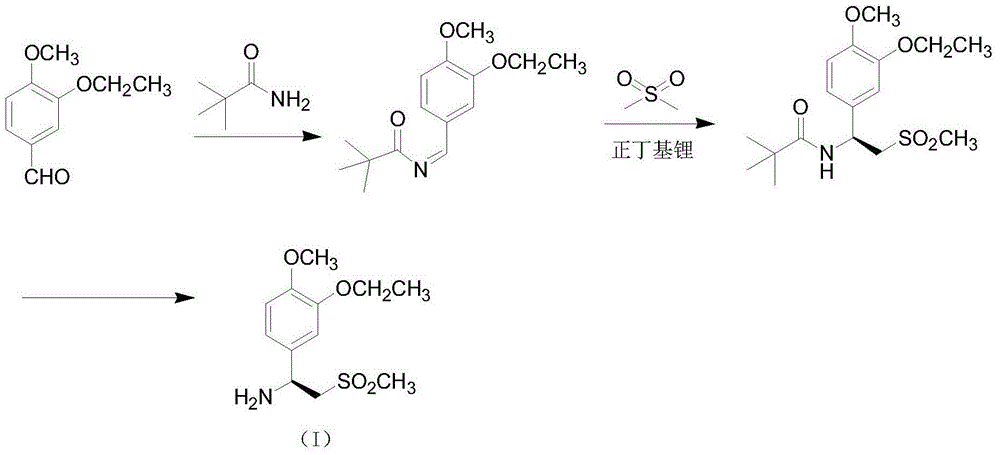
The invention relates to a preparation method for synthesizing an apremilast intermediate. The preparation method comprises the following steps of: carrying out condensation reaction on 3-ethoxyl-4-methoxyl-benzoate and dimethyl sulfone under an alkaline condition to generate 2-(3-ethoxy-4-methoxyphenyl)-1-methylsulfonyl acetone; reacting the compound II and chiral amine in the presence of an acidic catalyst to obtain 1-N-substituted amino-1-(3-ethoxyl-4-methoxyl) phenyl-2-methylsulfonyl ethylene (III), and directly hydrogenating the obtained compound III in the presence of a hydrogenation catalyst without separating the compound III to obtain a product (S)-1-(3-ethoxyl-4-methoxyl) phenyl-2-methanesulfonyl ethylamine (I), namely the apremilast intermediate, wherein the apremilast intermediate can be further prepared into N-acetyl L-leucinate. The invention also provides a preparation method of apremilast. The preparation method disclosed by the invention has the advantages of simple process flow, safety, environmental friendliness and low cost and is favorable to clean industrialized production.
Owner:XINFA PHARMA
Method of Preparing Cyclic Carbonates, Cyclic Carbamates, Cyclic Ureas, Cyclic Thiocarbonates, Cyclic Thiocarbamates, and Cyclic Dithiocarbonates
A method of preparing a cyclic monomer, comprising: forming a first mixture comprising a precursor compound, bis(pentafluorophenyl) carbonate, and a catalyst; wherein the precursor compound has a structure comprising a) two or more carbons, and b) two functional groups selected from the group consisting of primary amine, secondary amine, thiol group, hydroxyl group, and combinations thereof; and agitating the first mixture at a temperature effective to form a second mixture comprising the cyclic monomer, the cyclic monomer selected from the group consisting of a cyclic carbonate, a cyclic carbamate, a cyclic urea, a cyclic thiocarbonate, a cyclic thiocarbamate, and a cyclic dithiocarbonate.
Owner:IBM CORP
UV-Curable Aqueous Emulsion, Preparation Thereof And Solventless Coating Composition Comprising The Same
ActiveUS20090012201A1Good environmental acceptabilitySatisfactory coatingImpression capsPlantingPolyesterCarbamate
The UV-curable aqueous coating composition is characterized by using a UV-curable aqueous emulsion obtained by urethane reaction with a mixture including a poly-carbonate polyol or polyester polyol; an isocyanate compound; a urethane-reactive carboxylic acid; a UV-curable acrylic oligomer having 2 to 9 functional acrylate groups; a UV-curable acrylic monomer; and a urethane-reactive acrylate without an organic solvent. The aqueous composition provides good environmental acceptability and satisfactory film properties comparable to those of the prior oily UV-curable coating composition and, therefore, it is useful for coating various plastics.
Owner:AKZO NOBEL COATINGS INT BV
Method for synthesizing formic ester and specific catalyzer thereof
InactiveCN101134163AStable in natureRealize three-dimensional free rotationPreparation by carbon monoxide or formate reactionMetal/metal-oxides/metal-hydroxide catalystsNano catalystLiquid medium
The present invention discloses nanometer metal catalyst catalyzed formic acid ester synthesizing process, in which alcohol is catalyzed for caronylation reaction in the presence of nanometer metal catalyst and CO. The nanometer metal catalyst with particle size of 1-10 nm is prepared through mixing metal salt and macromolecular stabilizer, dispersing in liquid medium and reducing with reductant. The nanometer metal catalyst has the following advantages: capacity of realizing 3D free rotation, excellent low temperature activity capable of catalyzing formic acid ester synthesis at 60-200 deg.c, high conversion efficiency and high selectivity of formic acid ester, stable performance, environment friendship, etc. The nanometer metal catalyst catalyzed formic acid ester synthesizing process of the present invention has broad application foreground.
Owner:PEKING UNIV
(-)-meptazinol carbamate derivative and/or its salt and their prepn and use
InactiveCN101020661AImprove therapeutic indexSmall side effectsNervous disorderOrganic chemistryCarbamateStereochemistry
The present invention is (-)-meptazinol carbamate derivative as shown and / or its salt and their preparation process and use in preparing medicine for treating dementia including Alzheimer disease. The said compound, extracorporeal experiment shows, has very high AChE and BChE inhibiting activity, hundreds times higher than that of available medicine.
Owner:FUDAN UNIV
Wetting and dispersing agent, production method and use thereof
ActiveUS20140012036A1Low viscosityGood storage stabilityCarbamic acid derivatives preparationOrganic compound preparationIsocyanate compoundWetting
The invention relates to a wetting and dispersing agent that can be produced by carrying out step (1): wherein a) an organic polymer containing primary and / or secondary amino groups and h) at least one branched polyhydroxymonocarboxylic acid comprising, in addition to a carboxyl group, at least two hydroxy groups, one of which is not bound to the main chain of the molecule, are reacted by condensation reactions, forming amide linkages; and step (2): wherein at least some of the hydroxy groups of the hydroxy-functional reaction product obtained in step (1) are reacted with at least one organic monoisocyanate, forming urethane linkages.
Owner:BYK CHEM GMBH
Low viscosity allophanates containing actinically curable groups
ActiveUS20070191570A1Low residual monomer contentOrganic chemistryPolyurea/polyurethane coatingsAllophanePhosphonium salt
The present invention relates to a process for preparing radiation-curing binders containing allophanate groups by reacting at temperatures of ≦130° C. A) one or more compounds containing uretdione groups with B) one or more OH-functional compounds which contain groups which react, with polymerization, with ethylenically unsaturated compounds on exposure to actinic radiation, (radiation-curing groups), C) optionally NCO-reactive compounds other than B), in the presence D) a catalyst containing at least one tetrasubstituted ammonium or phosphonium salt of an aliphatic or cycloaliphatic carboxylic acid, to form allophanate groups by opening the uretdione ring. The present invention also relates to the binders obtained by the process of the invention.
Owner:ALLNEX NETHERLANDS BV
S-(5-substituted-1, 3, 4-thiadiazole)-(5-substituted phenyl)-2-furancarbothioic acid ester compound, preparation method and application thereof
ActiveCN106008496ANovel structureImprove biological activityOrganic active ingredientsBiocideBiotechnologyHuman leukemia
The invention discloses an S-(5-substituted-1, 3, 4-thiadiazole)-(5-substituted phenyl)-2-furancarbothioic acid ester compound, a preparation method and application thereof. The compound has a structural general formula shown as formula I. The S-(5-substituted-1, 3, 4-thiadiazole)-(5-substituted phenyl)-2-furancarbothioic acid ester compound has an obvious control effect on potato late blight, cucumber fusarium wilt, Botrytis cinerea Pers, Pepper Phytophthora blight, rice sheath blight, wheat scab, Colletotrichum musae, asparagus stem blight and the like, and can be used as an agricultural fungicide in control of plant diseases. At the same time, the compound has an inhibitory effect on HL-60 human leukemia cells, MCF-7 human breast cancer cells, BGC-823 human gastric cancer cells, Bel-7402 human hepatocellular carcinoma cells, and KB human nasopharyngeal carcinoma cells, etc., and has application prospects in the aspect of anti-tumor activity. (formula I).
Owner:SOUTH CHINA AGRI UNIV
Carbamate mercaptan epoxy curing agent as well as preparation method and application thereof
ActiveCN107141446AFast curingGood flexibilityEpoxy resin adhesivesEpoxy resin coatingsThiolCarbamate
The invention discloses a carbamate mercaptan epoxy curing agent as well as a preparation method and application thereof. According to the preparation method, sulfhydryl pentacyclic carbonate and polyamine are taken as raw materials, and the carbamate mercaptan epoxy curing agent is prepared by one-step open-loop reaction. The carbamate mercaptan epoxy curing agent prepared by the preparation method disclosed by the invention is a novel mercaptan compound with a carbamate structure. By utilizing the carbamate mercaptan epoxy curing agent prepared by the preparation method disclosed by the invention, the flexibility and the mechanical properties of the curing agent are effectively increased.
Owner:广州飞思合成材料有限公司
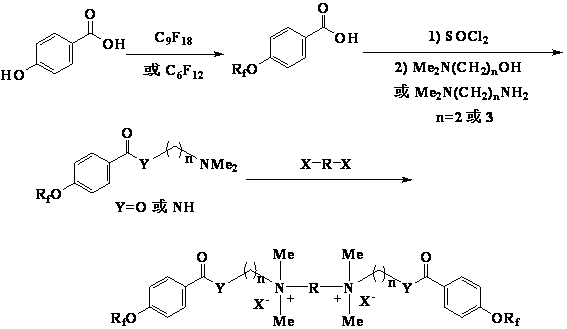
Cationic type gemini fluorinated surfactant based on perfluorinated nonene and perfluorinated hexene and preparation method of cationic type gemini fluorinated surfactant
ActiveCN102908937AHigh reactivityReactive and expensiveOrganic compound preparationTransportation and packagingBenzoic acidPolymer science
The invention discloses a cationic type gemini fluorinated surfactant based on perfluorinated nonene and perfluorinated hexene and a preparation method of the cationic type gemini fluorinated surfactant. The preparation method comprises the following steps of: with the perfluorinated nonene or perfluorinated hexene as a raw material, condensing the perfluorinated nonene or perfluorinated hexene with p-hydroxybenzoic acid, and carrying out chlorination on a matter obtained after condensation and thionyl chloride to prepare perfluorinated alkene oxyl benzoyl chloride; condensing the perfluorinated alkene oxyl benzoyl chloride without separation with 2-dimethylamino ethanol, 3-dimethylamino propanol, 2-dimethylamino ethylamine and 3-dimethylamino propylamine to obtain perfluorinated alkene oxyl benzoate or perfluorinated alkene oxyl benzamide with tertiary amine at an alkanol part or amine part; and finally, condensing the perfluorinated alkene oxyl benzoate or perfluorinated alkene oxyl benzamide with 1,2-dihaloethane, 1,3-dihalopropane, 1,4-dichlorobutane, 1,5-dichloropentane or 3-oxa-1,5-dichloropentane to prepare a quaternary ammonium gemini fluorinated surfactant. The synthesized compound is high in surface activity and is low in critical micelle concentration, has the characteristics of simpleness in synthesization, low cost and the like and has good application prospect.
Owner:江苏超至和新材料有限公司
Bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex and preparation method and application thereof
ActiveCN106279253AHigh anticancer activityLow costTin organic compoundsAntineoplastic agentsStructural formulaCoordination complex
The invention discloses a bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex and a preparation method and application thereof. The structural formula of the complex is shown in the description. The invention further discloses a preparation method for the bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex and application of the bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex in preparing antitumor drugs.
Owner:HENGYANG NORMAL UNIV
Method to improve humidity resistance of phenolic urethane foundry binders
InactiveCN1344187AImprove moisture resistanceHigh strengthFoundry mouldsFoundry coresFoundryHydrofluoric acid
An embodiment of the present invention provides a method for improving the tensile strength of foundry cores and molds. More particularly, an embodiment of the present invention provides an improved binder for foundry cores and molds that include a fluoride bearing acid in combination with an inorganic silicon compound. Alternately, the improved binder may include a fluoride bearing acid in combination with a boron compound. In a preferred embodiment, a modified part 1 binder component includes a combination of hydrofluoric acid and an inorganic silicon compound.
Owner:MOMENTIVE SPECIALTY CHEM RES BELGIUM
Coating compositions for inorganic casting molds and cores, comprising formic acid esters, and use thereof
Owner:ASK CHEM LP
Prodrugs of NH-Acidic Compounds
ActiveUS20140221653A1Reduce polarityReduce solubilityOrganic active ingredientsNervous disorderFormic Acid EstersCarbamate
Owner:ALKERMES PHARMA IRELAND LTD
Synthetic method of cyclic enamine-3-formic acid ester compound
ActiveCN106831542AEmission reductionReduce the burden onOrganic chemistryFormic Acid EstersOrganic synthesis
The invention discloses a synthetic method of a cyclic enamine-3-formic acid ester compound and belongs to the field of organic synthesis technology. The main point of the technical scheme is as shown in the specification. In comparison with the prior art, the invention has the following advantages: (1) by the adoption of one-pot cascade reaction, tedious intermediate separation purification process is avoided, waste emission is decreased, and environmental burden is reduced; (2) raw materials are easily available; (3) the reaction is carried out at the temperature of minus 100 DEG C, condition is mild, and operation is simple; (4) the application range of a substrate is wide; and (5) atom economy of the reaction is high.
Owner:HENAN NORMAL UNIV
Hydrogen peroxide detecting fluorescent probe and application thereof
InactiveCN106243123AGood choiceEasy to synthesizeOrganic chemistryFluorescence/phosphorescenceFormic Acid EstersStructural formula
The invention discloses a hydrogen peroxide detecting fluorescent probe ACR, and belongs to the technical field of analytical chemistry. The hydrogen peroxide detecting fluorescent probe uses a rhodamine derivative as a parent and 2-azide methyl aromatic formic acid ester as a switch, and the chemical structural formula of the hydrogen peroxide detecting fluorescent probe is shown in the specification. The hydrogen peroxide detecting fluorescent probe is simple to synthesize and easy to use, can be specifically reacted with hydrogen peroxide to release fluorescence, and has good hydrogen peroxide selectivity, and in the hydrogen peroxide detection process in the presence of other related oxides, the hydrogen peroxide detecting fluorescent probe has good anti-interference performance, and can detect hydrogen peroxide in living cells.
Owner:UNIV OF JINAN
Process for producing optically active 3-hydroxypropionic ester derivative
InactiveUS20060166342A1Low costReducing the 2-formylacetic ester derivativeOrganic compound preparationCarboxylic acid esters preparationFormic Acid EstersPropanoic acid
The present invention is to provide a process for simply producing an optically active 3-hydroxypropionic ester derivative useful as a medicament intermediate from an inexpensive material. More specifically, the present invention is directed to a process for producing an optically active 3-hydroxypropionic ester derivative comprising reacting an acetic ester derivative available at low cost with abase and a formic ester, thereby converting the acetic ester derivative into a 2-formylacetic ester derivative, and thereafter, stereospecifically reducing the formyl group of the derivative by use of an enzymatic source capable of stereoselectively reducing the formyl group of the derivative.
Owner:KANEKA CORP
Method for testing trace carbofuran
ActiveCN102841067AWide variety of sourcesLow pricePreparing sample for investigationColor/spectral properties measurementsFormic Acid EstersCarbofuran
The invention relates to the technical field of chemical pesticide test, and particularly relates to a method for testing carbofuran in carbamic acid ester pesticides, in particular to a method for testing trace carbofuran. With flour, zinc meso-tetraphenylporphine solution and phosphate buffer with the pH value of 6.5 to 7.25 as materials, the method respectively prepares zinc meso-tetraphenylporphine-plant esterase compound solution and sample mixed solution, then respectively tests the absorption spectrum curves of the zinc meso-tetraphenylporphine-plant esterase compound solution and the sample mixed solution on light waves with the wavelength of 300nm to 750nm, and compares the two tested spectrum curves; if the two spectrum curves are identical, then N, N-dimethylformamide cleaner sample to be tested does not contain carbofuran, or the content of carbofuran exceeds the test limit of the method; and if the two spectrum curves are not identical, then the sample to be tested contains carbofuran. The test cost of the method is low, the method is applicable to a wide range of regions, the precision, repeatability and stability of the test result are high, field tests can be carried out as well, the method is easy to operate, and moreover, the affection of the environment on the test result is little.
Owner:LUZHOU PINCHUANG TECH CO LTD +1
Stabilized activated derivatives of carbamic acid, their process of preparation and their use for the preparation of ureas
Owner:CENT NAT DE LA RECHERCHE SCI +1
Method and technology for synthesizing and producing antibiotic medicament namely 1-(o-fluorophenyl) dihydropyridone
ActiveCN101798302AAntibacterial agentsGroup 4/14 element organic compoundsFormic Acid EstersOrtho position
The invention relates to a method and technology for synthesizing and producing an antibiotic medicament namely 1-(o-fluorophenyl) dihydropyridone. The type of compound can be used for treating infections of mammals. An entire preparation process comprises the following steps: i) transforming O-silanized N-(o-fluorophenyl)-4-piperidone into substituted 4-[4-(2,3-alkene) pyridine-1-]-radical-2-fluoronitrobenzene, wherein the latter can be reduced and acylated to generate carbamic acid ester; and ii) performing a reaction of the carbamic acid ester and an epoxy compound or chlorohydrin to generate corresponding 5-hydroxymethyl or substituted 5-aminomethyl oxazolidinone, wherein 5-aminomethyl oxazolidinone can be further transformed into antibiotic oxazolidinone with a structure I, and the definition of each group is as shown by the specification.
Owner:SHANGHAI MICURX LTD
Process for preparation of albendazole
The present invention discloses a novel, cost-effective process for preparation of a benzimidazole carbamates compound. Specifically, it relates to the process for the preparation of anti-parasite bulk drug albendazole. The process comprises a) thiocyanating 2-nitroaniline of formula VI with ammonium thiocyanated in presence of a halogen to obtain 2-nitro-4-thiocyanoaniline of formula V; b)propylating 2-nitro-4-thiocyanoaniline of formula V with propylbromide in presence of n-propanol and a base in absence of a phase transfer catalyst to obtain 4-propylthio-2-nitroaniline of formula III; C) reducing the nitro group of 4-propylthio-2-nitroaniline prepared in step b) by reacting an aqueous alkali metal sulphide or an alkaline metal sulphide to obtain 4-propylthio-o-phenylenediamine of formula II; and d)condensing 4-propylthio-o-phenylenediamine of formula II with alkali or alkaline earth metal salt of methylcyano carbamate in presence of an acid to form Albendazole of formula I.
Owner:SEQUENT SCI LTD
Method for producing chloroformate compound
ActiveCN109563020APromote safe productionPreparation from organic carbonatesPreparation from phosgene or haloformatesFormic Acid EstersAlcohol
Provided is a method for producing a chloroformate compound safely, with high efficiency and in a large quantity. A chloroformate compound can be produced by mixing a solution of triphosgene, an amineand an alcohol compound together in a flow-type reactor to cause the reaction of these components. Alternatively, a chloroformate compound can be produced by mixing a solution of triphosgene with a solution of an amine and an alcohol compound in a flow-type reactor to cause the reaction of these components. The amine is preferably tributylamine, and the amine is preferably used in an amount of 0.8 to 3 equivalents relative to the amount of the alcohol compound.
Owner:KANEKA CORP
Fluorescent probe for visual detection of Cu<2+> as well as preparation method and application of fluorescent probe
InactiveCN107629036AGood water solubilityTypical "push-pull" structureOrganic chemistryMaterial analysis by observing effect on chemical indicatorBenzaldehydeFluorescence
The invention discloses a fluorescent probe for visual detection of Cu<2+> as well as a preparation method and an application of the fluorescent probe. The fluorescent probe is 2-(4-picolinate styryl)-1,3,3-trimethyl-3H-indole iodide, and the structural formula is shown as formula (I) in the description. The preparation method comprises the following steps: (a) 4-picolinate benzaldehyde is prepared from p-hydroxybenzaldehyde and 2-picolinic acid through an esterification reaction; (b) the compound shown as the formula (I) is prepared from 4-picolinate benzaldehyde and 1 ,2 ,3 ,3-tetramethyl-3H-indole iodide through a condensation reaction. The fluorescent probe for visual detection of Cu<2+> has the advantages of high sensitivity, good selectivity, short response time and the like.
Owner:HEZHOU UNIV
Nitric oxide donor type hexadecadrol as well as preparation method and purpose thereof
ActiveCN105001296AIncreased anti-inflammatory activityLittle side effectsAntibacterial agentsOrganic active ingredientsNitric oxideChemistry
The invention relates to nitric oxide donor type hexadecadrol as well as a preparation method and application thereof. The nitric oxide donor type hexadecadrol as a compound is obtained by enabling nitrous acid ester to be connected with hexadecadrol through ethyl cyclohexanecarboxylate; the in-vitro antiinflammatory activity of the nitric oxide donor type hexadecadrol is better than that of the hexadecadrol; besides, the nitric oxide donor type hexadecadrol can restrain the growth of methicillin-resistant staphylococcus aureus (MRSA) in vitro, restrain the formation of MRSA biomembrane, and lead to the death of bacteria in the MRSA biomembrane. In addition, the compound can notably increase the survival rate of mice in MRSA sepsis lethal models, reduces the inflammation pathology damage of the mice in sub-sepsis models, decreases the constant value number of bacteria, and has notable advantages in the respect of preventing and treating MRSA sepsis.
Owner:ARMY MEDICAL UNIV
Reactive urethane compound having ether bond, curable composition, and cured material
ActiveUS8399569B2Maintain good propertiesSuperior sensitivity and developabilityOrganic chemistryPhotomechanical apparatusArylHydrogen atom
An object of the present invention is to provide a reactive urethane compound having superior curability, adhesion to substrates, transparency, molecular flexibility, and mechanical properties, a curable composition containing the compound, and a cured material formed from the composition. An ethylenically-unsaturated-group containing reactive urethane compound of the present invention is represented by formula (I):wherein R1 and R2 are each independently a hydrogen atom or an alkylene group; R3 is a hydrogen atom, a alkyl group, or an aryl group; R4 is a single bond or an alkylene group; R5 is a hydrogen atom or a methyl group; R6 is an oxygen atom, a sulfur atom, or an imino group; n is 2 to 12; m is 1 to 300; and X is an aliphatic, aromatic or heterocyclic compound residue.
Owner:RESONAC CORP
Processes for the preparation of N-heteroaryl-N-aryl-amines by reacting an N-aryl carbamic acid ester with a halo-heteroaryl and analogous processes
InactiveCN1761653ASimple reaction conditionsUrea derivatives preparationOrganic compound preparationFormic Acid EstersPtru catalyst
The present invention relates to processes for producing a diaryl amine compound of the formula (I); or a salt thereof, said process comprising the step of coupling a compound of formula (II) with an amine of formula (III) in the presence of an alkali metal salt or a transition metal catalyst, wherein: Ar1 and Ar2 are independently Q; wherein each Q is an aryl or heteroaryl ring system optionally fused to a saturated or unsaturated 5-8 membered ring having 0-4 heteroatoms; wherein Q is optionally substituted as defined in claim 1, wherein: X is a leaving group; and Y is -C(O)-O-Z; and Z is selected from C1-C6 aliphatic, benzyl, Fmoc, -SO2R' and Q, provided that Q is not substituted with X or alkyne; wherein R' is as defined in claim 1.
Owner:VERTEX PHARMA INC
Preparation method of Sacubitril intermediate
ActiveCN110183357AEasy to synthesizeEfficient synthesisCarbamic acid derivatives preparationPreparation from carboxylic acid halideCarbamateSacubitril
The invention discloses a preparation method of an intermediate compound (R)-tert-butyl (1-((1,1'-diphenyl)-4-yl)-3-hydroxypropane-2-yl) carbamate. The preparation method is represented as the formulashown in the description. According to the preparation method, the steps are simple and short, the reaction yield is increased, reaction conditions are mild, most of the intermediates obtained in thereaction process are not required to be purified and can be subjected to the next reaction directly, large-batch synthesis is facilitated, chiral amine with stereospecificity is efficiently synthesized with aminotransferase, and the preparation method is more suitable for industrial production.
Owner:甘肃皓天医药科技有限责任公司
Steroid carbamates as potentiating agents
InactiveUS6046182AGood water solubilityBiocideOrganic active ingredientsMethyl carbamateCarboxylic acid
Novel potentiating steroid carbamates having the general formula (I) ST-OCONR1R2(I) wherein ST is a steroid or steroid derivative, of the structure (II) optionally containing double bonds and additional oxygen substituents; the carbamate moiety OCONR1R2 has lipophilic properties and is selected from N,N-dibutylcarbamate, N-(3-morpholin-4-yl-propyl)-carbamate, 4-benzyl-piperazine-1-carboxylic acid ester, N-(3-dibutylamino-propyl)-carbamate, N,N-dipropyl-carbamate, N-hexyl-carbamate, N-(1-benzyl-piperidine-4-yl)-carbamate, N-cyclohexylmethyl-carbamate, N-butyl-N-ethyl-carbamate, N-benzyl-carbamate, N-(3-dibenzazepin-1-yl-propyl)-N-methyl-carbamate, N-naphtalen-1-yl-methyl-carbamate, N-diphenylmethyl-carbamate, N,N-dibenzyl-carbamate, 3,4-dihydro-1H-isoquinoline-2-carboxylic acid ester, N,N-dipentyl-carbamate, N,N-diisobutyl-carbamate and N,N-bis-(4-fluoro-benzyl)-carbamate; and pharmaceutically acceptable salts thereof, in combination with radiation therapy and one or more cytotoxic drugs, optionally together with a pharmaceutically acceptable carrier and, if desired, other pharmacologically acceptable agents.
Owner:BATRA SATISH
Catalyst components for the polymerization of olefins
The present disclosure relates to a solid catalyst component for the (co)polymerization of olefins CH2═CHR, in which R is hydrogen or a hydrocarbyl radical with 1-12 carbon atoms, comprising Ti, Mg, and Cl, and optionally an electron donor compound selected from the group consisting of ethers, amines, silanes, carbamates ketones, esters of aliphatic acids, alkyl and aryl esters of optionally substituted aromatic polycarboxylic acids, diol derivatives chosen among monoesters monocarbamates and monoesters monocarbonates or mixtures thereof, comprising from 0.1 to 50% wt of Bi with respect to the total weight of the solid catalyst component.
Owner:BASSELL POLIOLEFINE ITAL SRL
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com






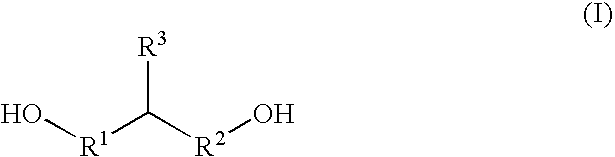
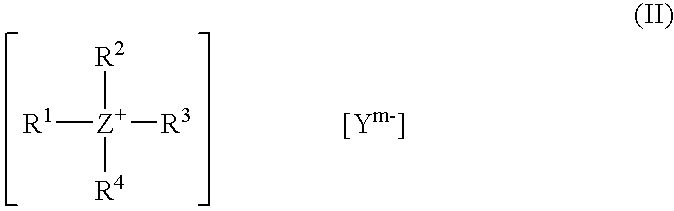
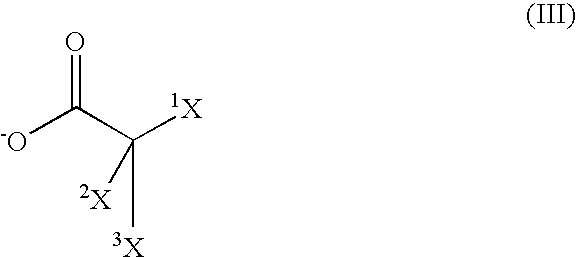




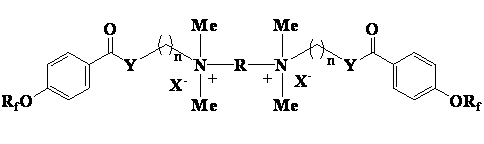
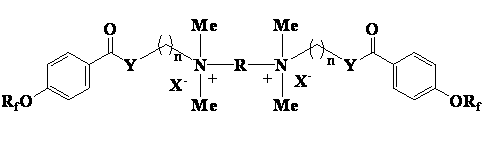
![Bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex and preparation method and application thereof Bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4d64092a-96ad-4a1d-bffd-3359d71970fc/1608150010361.png)
![Bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex and preparation method and application thereof Bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4d64092a-96ad-4a1d-bffd-3359d71970fc/1608150010362.png)
![Bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex and preparation method and application thereof Bis [tris (2-methyl-2-phenyl) propyl-tin] 5-nitro-isophthalate complex and preparation method and application thereof](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/4d64092a-96ad-4a1d-bffd-3359d71970fc/1608150010363.png)