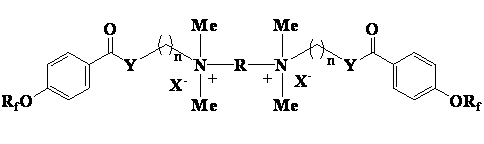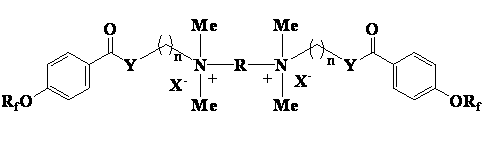Cationic type gemini fluorinated surfactant based on perfluorinated nonene and perfluorinated hexene and preparation method of cationic type gemini fluorinated surfactant
A technology of fluorosurfactant and perfluorononene, which is applied in the field of cationic gemini surfactant and its preparation, can solve the problems of insufficient surface activity of surfactants, achieve improved decontamination and washing ability, high bactericidal effect, Low toxicity and low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
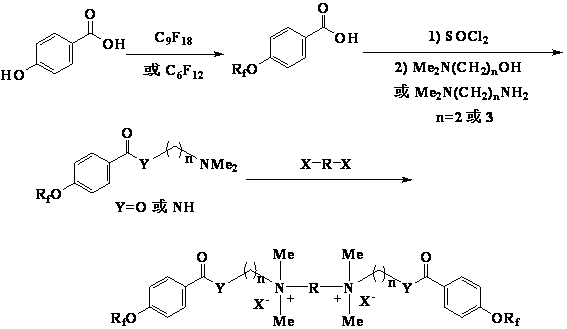
[0018] One, the preparation of perfluorononenyloxybenzoic acid intermediate
[0019] Add 90g (0.2mol) of perfluorononene, 27.6g (0.2mol) of p-hydroxybenzoic acid and 200mL of N,N-dimethylformamide into a four-necked flask, and dropwise add 40.4g (0.4 mol), after 40 minutes of dripping, the reaction was continued for 8 hours after dripping, the reaction solution was poured into dilute hydrochloric acid to precipitate a white solid, filtered, washed with water, and dried to obtain 110.5 g of a white solid, with a yield of 97.3%.
[0020] 2. Preparation of dimethylaminoethanol perfluorononenyloxybenzoate
[0021] Stir and mix 28.4 g (0.05 mol) of perfluorononenyloxybenzoic acid and 84.5 g (0.71 mol) of thionyl chloride, slowly raise the temperature to 45°C, keep the temperature for 5 hours, and absorb the tail gas with lye at the same time. After the reaction was completed, excess thionyl chloride was recovered by distillation under reduced pressure to obtain a light yellow ...
Embodiment 2
[0025] One, the preparation of perfluorononenyloxybenzoic acid intermediate
[0026] With embodiment 1.
[0027] 2. Preparation of N-(3-dimethylaminopropyl) perfluorononenyloxybenzamide
[0028] 28.4g (0.05mol) of perfluorononenyloxybenzoic acid and 84.5g (0.71mol) of thionyl chloride were stirred and mixed, and the temperature was slowly raised to 45°C, and the reaction was kept for 5 hours, while the tail gas was absorbed with lye. After the reaction was completed, excess thionyl chloride was recovered by distillation under reduced pressure to obtain a light yellow liquid. Add 50 mL of acetonitrile and 10.2 g (0.1 mol) of 3-dimethylaminopropylamine, and reflux for 5 h. Cool to room temperature, dilute with water, adjust the pH to above 10, let stand to separate the layers, extract the aqueous layer with acetonitrile, combine the organic phases, wash with water, dry, and precipitate to obtain a light yellow residue with a yield of 91.3%.
[0029] 3. Preparation of ...
Embodiment 3
[0032] One, the preparation of perfluorohexenyloxybenzoic acid intermediate
[0033] Add 60g (0.2mol) of perfluorohexene, 27.6g (0.2mol) of p-hydroxybenzoic acid and 200mL of N,N-dimethylformamide into a four-necked flask, and drop 40.4g (0.4 mol), after 40 minutes of dripping, the reaction was continued for 8 hours after dripping, the reaction solution was poured into dilute hydrochloric acid to precipitate a white solid, filtered, washed with water, and dried to obtain 79.8 g of a white solid, with a yield of 95.4%.
[0034] 2. Preparation of 3-dimethylaminopropanol perfluorohexenyloxybenzoate
[0035]20.9g (0.05mol) of perfluorohexenyloxybenzoic acid and 84.5g (0.71mol) of thionyl chloride were stirred and mixed, the temperature was slowly raised to 45°C, and the reaction was kept for 5 hours while the tail gas was absorbed with lye. After the reaction was completed, excess thionyl chloride was recovered by distillation under reduced pressure to obtain a light yellow l...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Surface tension | aaaaa | aaaaa |
| Surface tension | aaaaa | aaaaa |
| Surface tension | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com