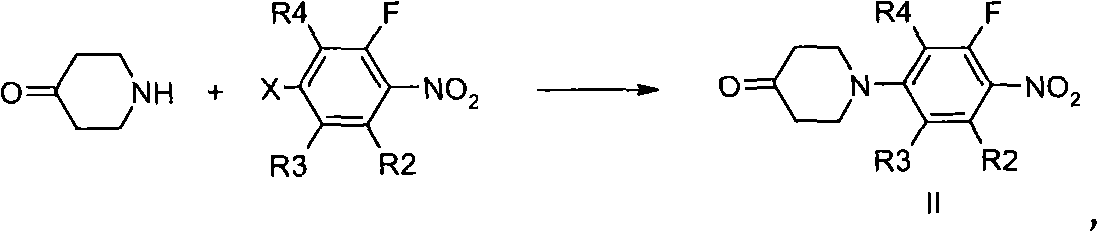Method and technology for synthesizing and producing antibiotic medicament namely 1-(o-fluorophenyl) dihydropyridone
A synthesis method and technology of production process are applied in the field of synthesis, production and process of antibiotic drug 1-(o-fluorophenyl)dihydropyridone, which can solve the problem of insufficient antibacterial activity, achieve low bone marrow suppression, Strong antibacterial activity, low monoamine oxidase inhibitory effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0160] Example 1. Compound structure
[0161]
[0162] Compound synthetic route of embodiment 1:
[0163]
[0164]Intermediate 1. 94.7g (0.486mol) of 2,3,4,5-tetrafluoronitrobenzene and 82g (0.534mol) of 4-piperidone hydrochloride were dissolved in 110mL of N-methylpyrrolidone (NMP), The solution was cooled to 5°C with an ice-water bath. Then slowly add 156.8g (0.712mol) of N,N-diisopropylethylamine (DIEA) dropwise into the above solution under stirring, control the temperature between 0 and 10°C, react for 30 minutes, and then rise to Room temperature, reaction overnight, TLC detection, the raw material completely disappeared. The reaction solution was slowly poured into 1.5L of water, a yellow solid product was precipitated, filtered, the filtered solid was washed 2-3 times with water, and then dried to obtain 140 g of intermediate 1 compound, yield: 90%. 1 H NMR (400MHz, CDCl 3 ): 7.74 (m, 1H); 3.73 (t, J = 6.0Hz, 4H); 2.66 (t, J = 6.0Hz, 4H). MS (m / z): 275 [M+H]...
Embodiment 2
[0174] Example 2. Compound structure
[0175]
[0176] Compound synthetic route of embodiment 2:
[0177]
[0178] Intermediate 6. 12.6g (36.8mmol) of the compound of Example 1 and 15.4mL (110.4mmol) of triethylamine were dissolved in 150mL of dichloromethane (DCM), cooled to 0°C in an ice bath under stirring, and slowly added dropwise to 5.7mL ( 73.6 mmol) of methanesulfonyl chloride. After the dropwise addition was completed, stirring was continued for 1 hour under an ice bath, 100 mL of saturated brine was added to the reaction solution, and the dichloromethane layer was separated. The dichloromethane layer was washed once with 100 mL of saturated ammonium chloride solution, the combined dichloromethane layers were washed once with 100 mL of saturated brine, dried over anhydrous magnesium sulfate, filtered, and most of the solvent was removed under reduced pressure. The wet solid was filtered, crushed and washed with 30 mL of ethyl acetate, stirred at 15 °C for one...
Embodiment 3
[0181] Example 3. Compound structure
[0182]
[0183] Embodiment 3 compound synthetic route:
[0184]
[0185] Intermediate 8
[0186] Method A. Under nitrogen protection, 187mg (1.00mmol) of N-Boc-3-aminoisoxazole was dissolved in 1mL of DMF, and then it was slowly added dropwise to NaH (content 60%, 48mg, 1.20mmol) in DMF (2mL) In the solution, the temperature was raised to 35°C, and the reaction was stirred for 15 minutes. Intermediate 6 (357 mg, 0.85 mmol) was dissolved in 1 mL of DMF, and then slowly added dropwise to the above reaction solution, and the system was heated to 50° C. for 1.5 hours. The reaction mixture was poured into 30 mL of ethyl acetate and washed with 10% NH 4 It was washed with aqueous Cl solution (2×30 mL), then washed with saturated brine, and dried over anhydrous sodium sulfate. The solvent was distilled off under reduced pressure, and the product was separated through a silica gel column (2% methanol / dichloromethane) to obtain 151 mg o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com