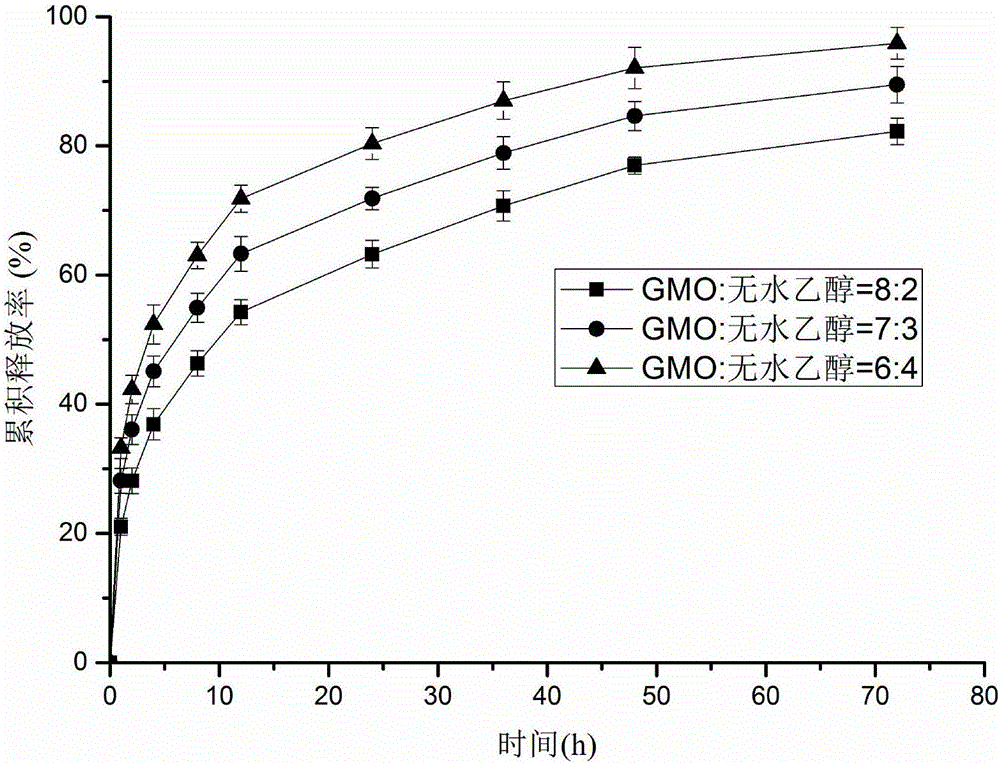
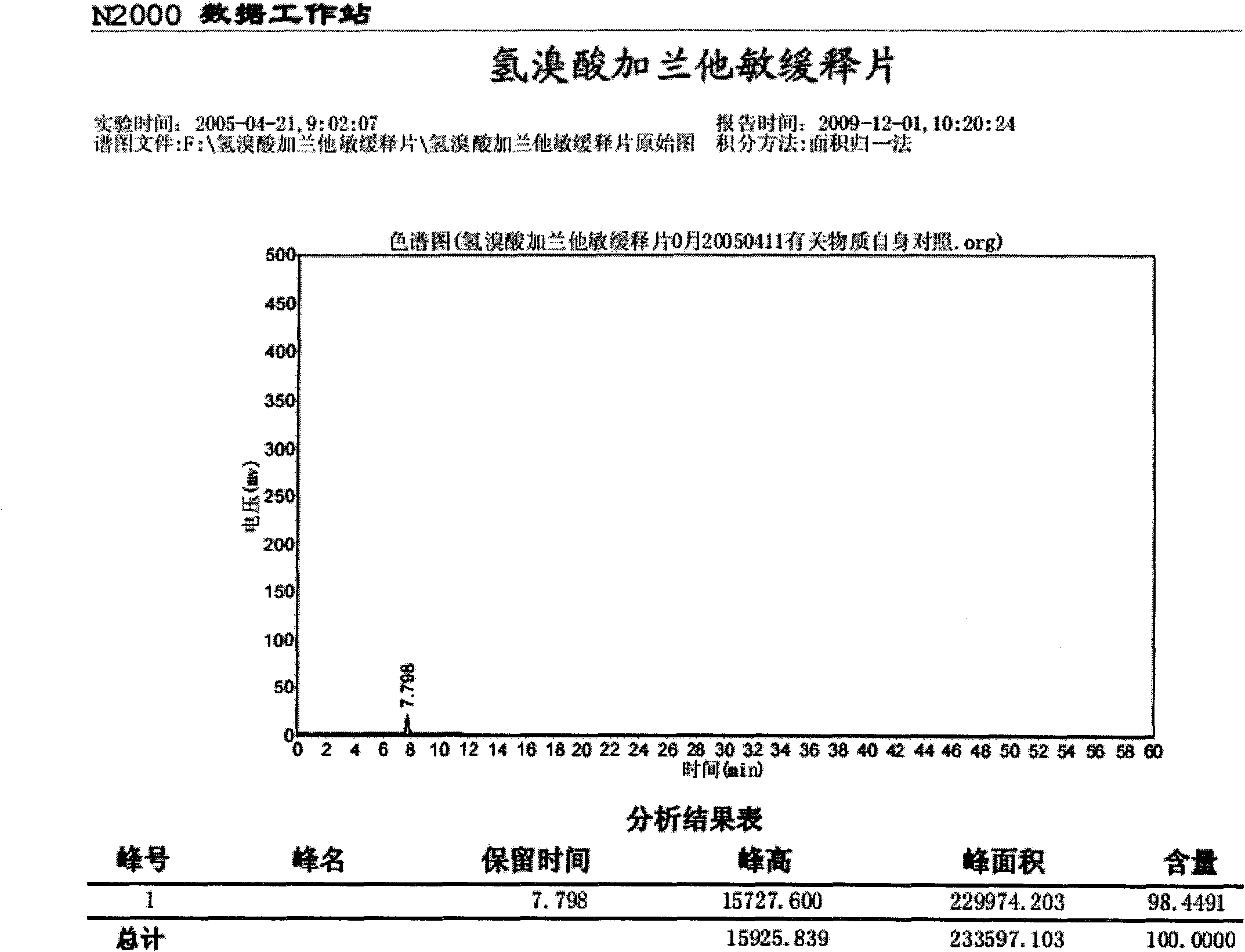
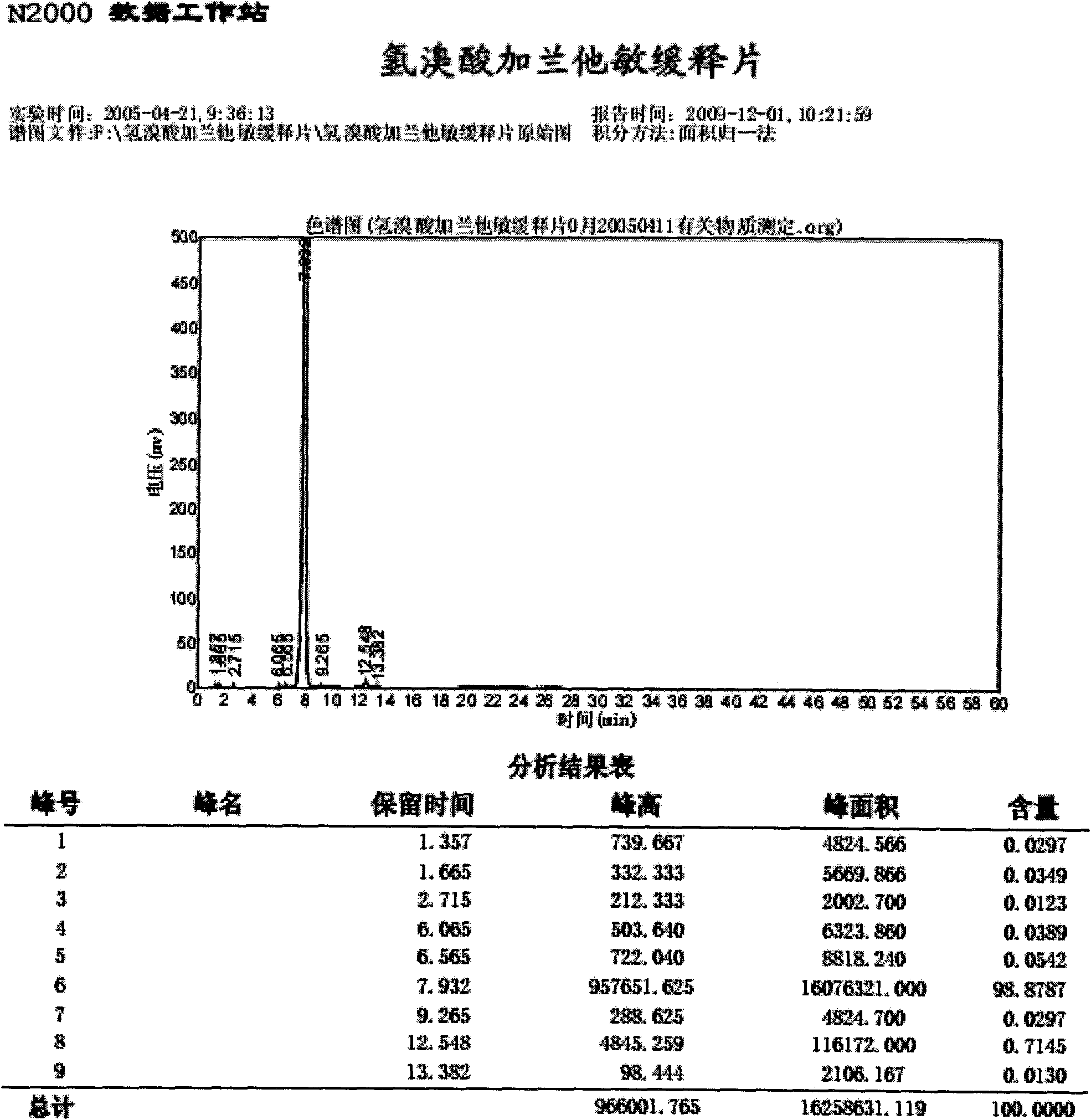
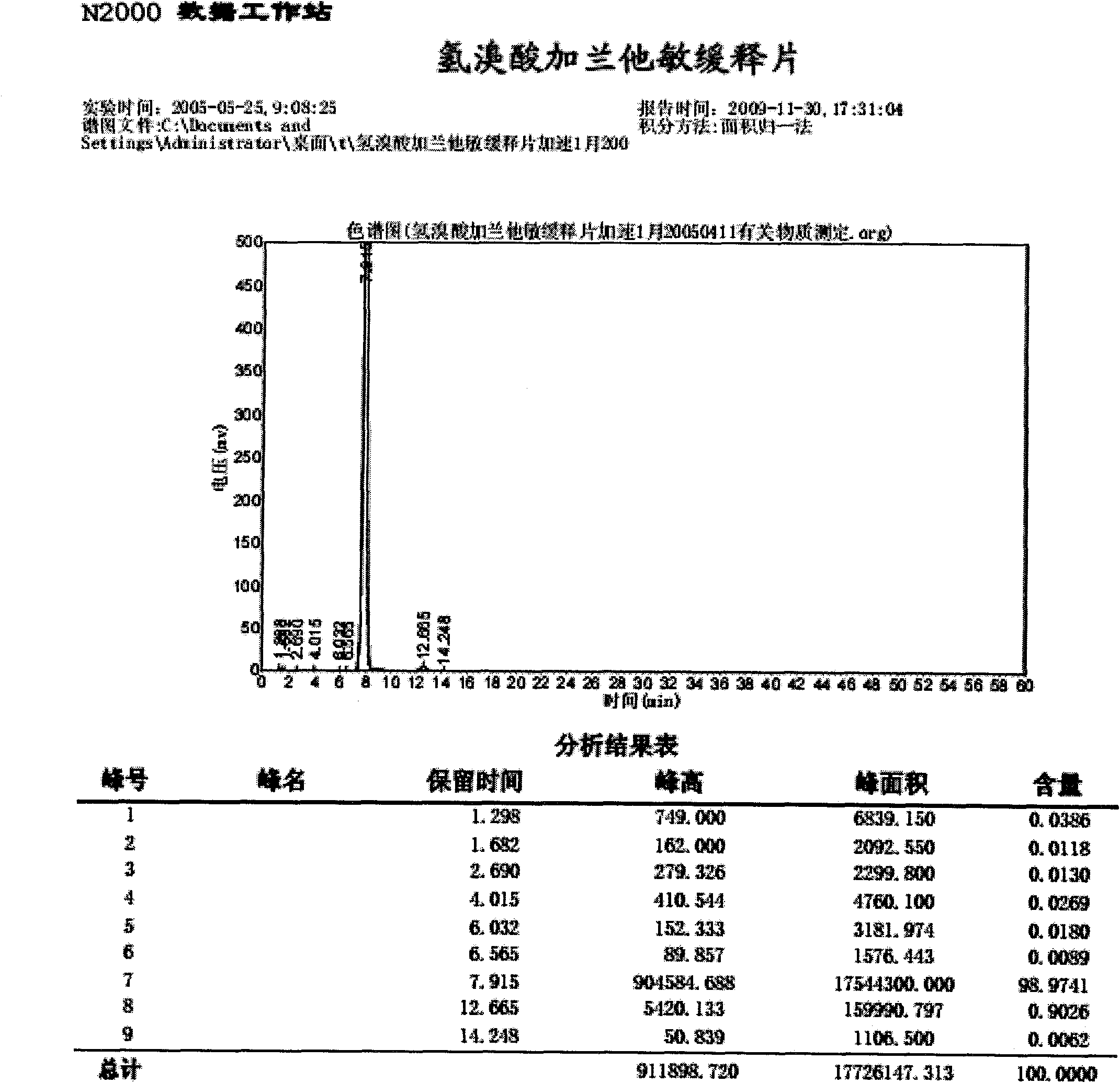
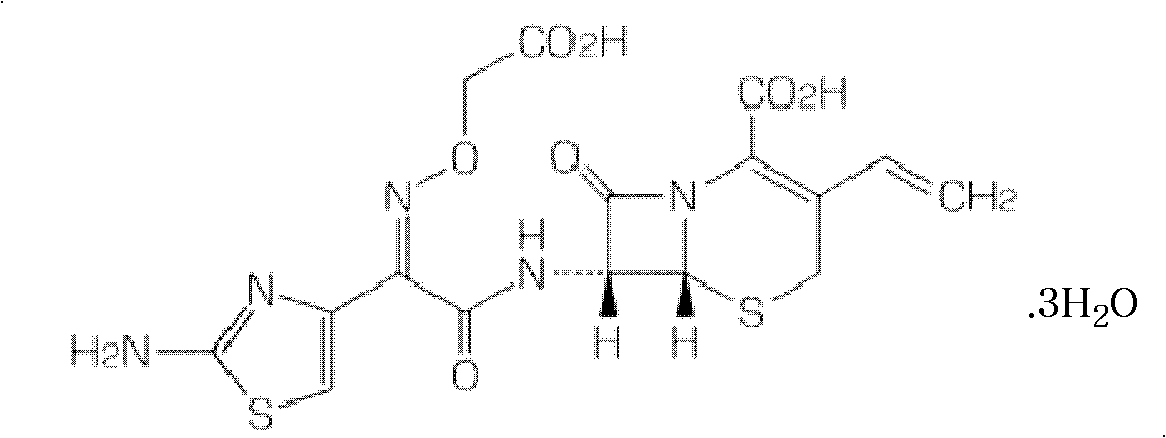
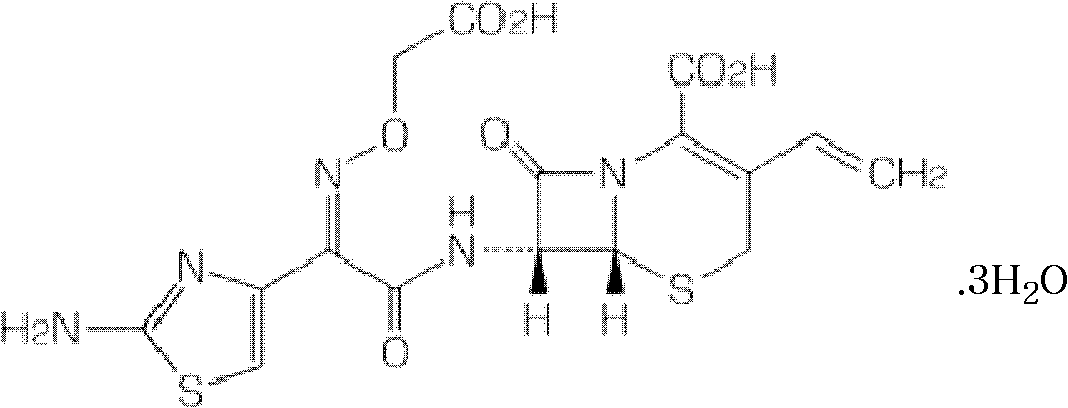
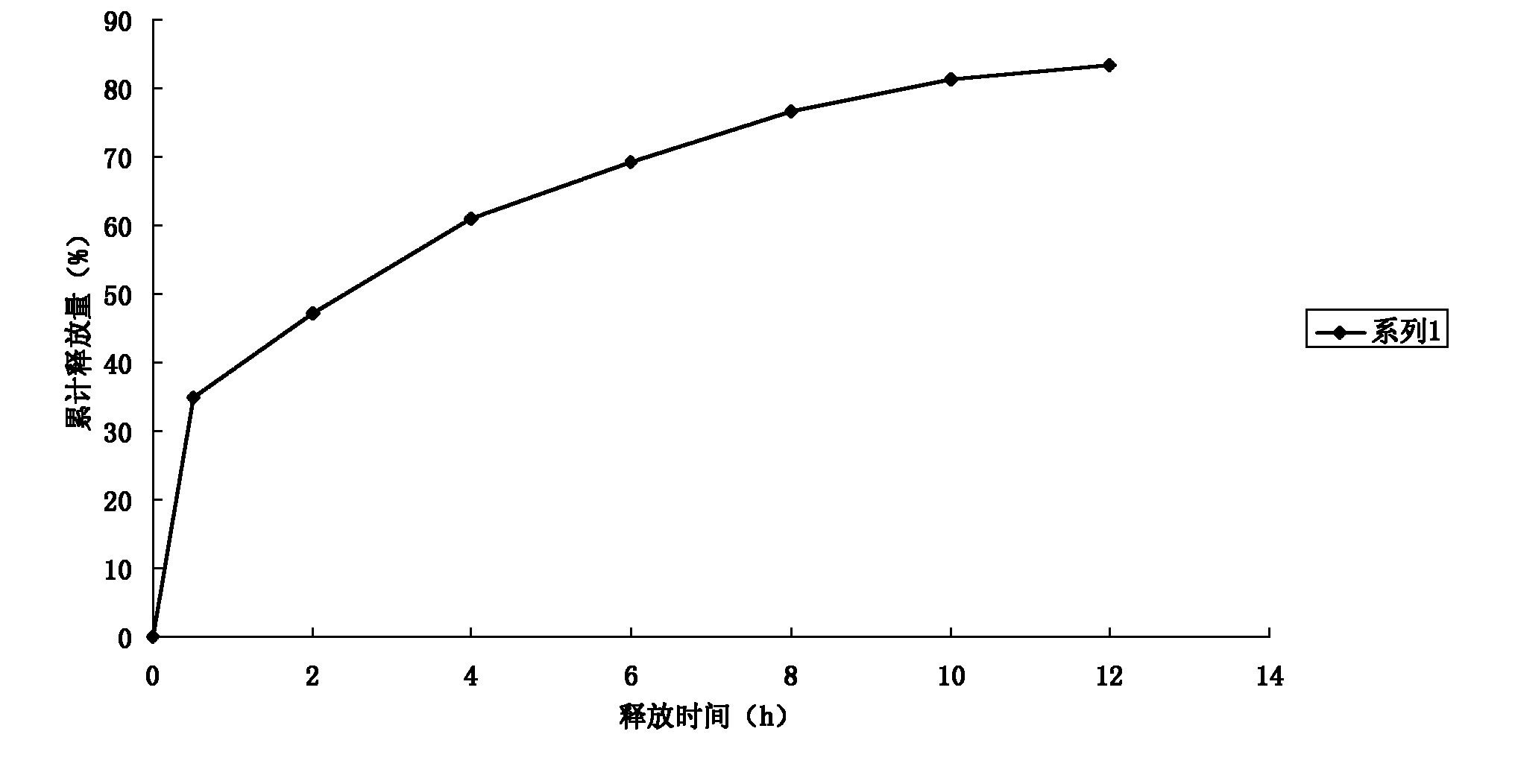
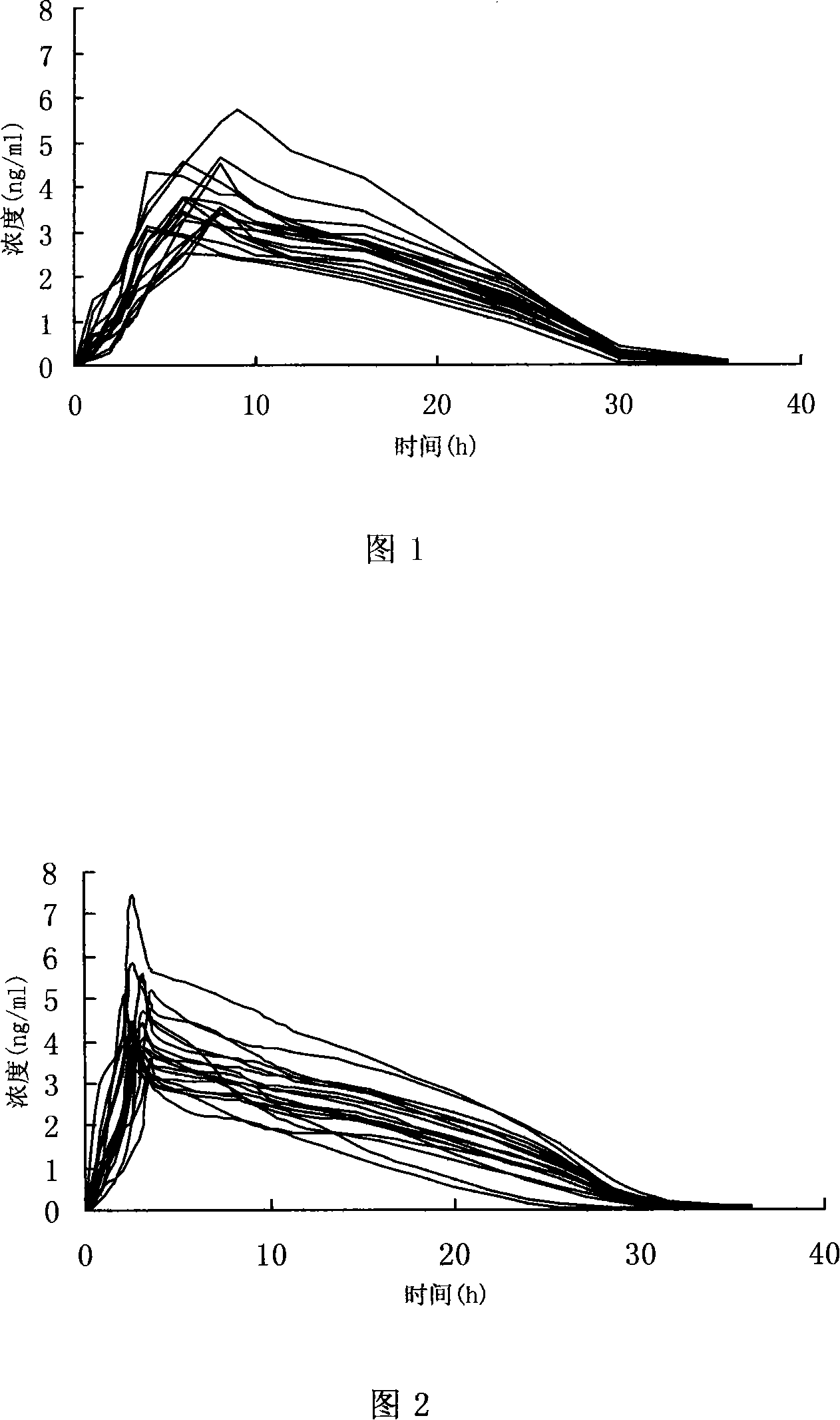
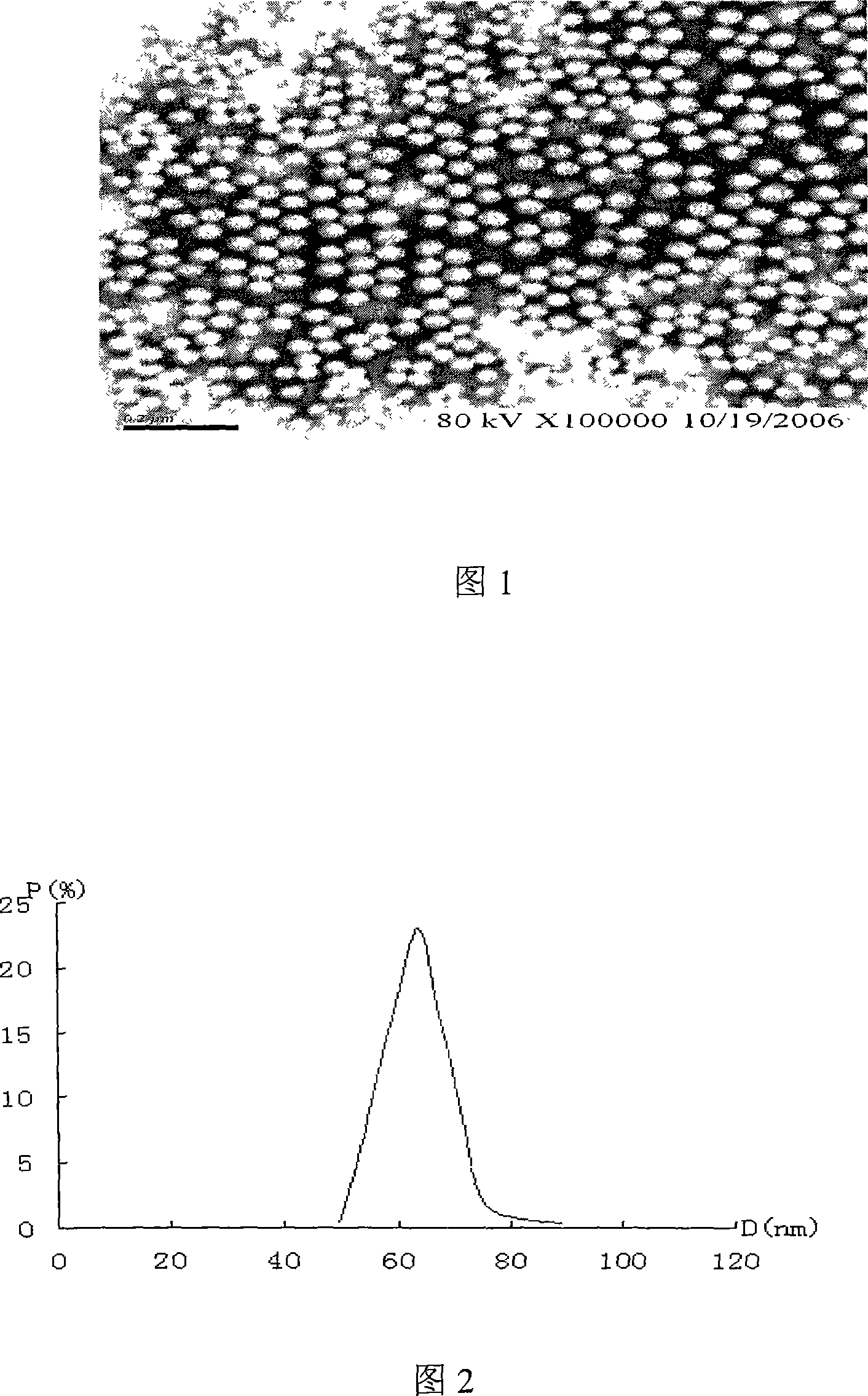
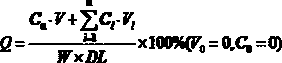Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
96results about How to "Avoid peaks and valleys" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
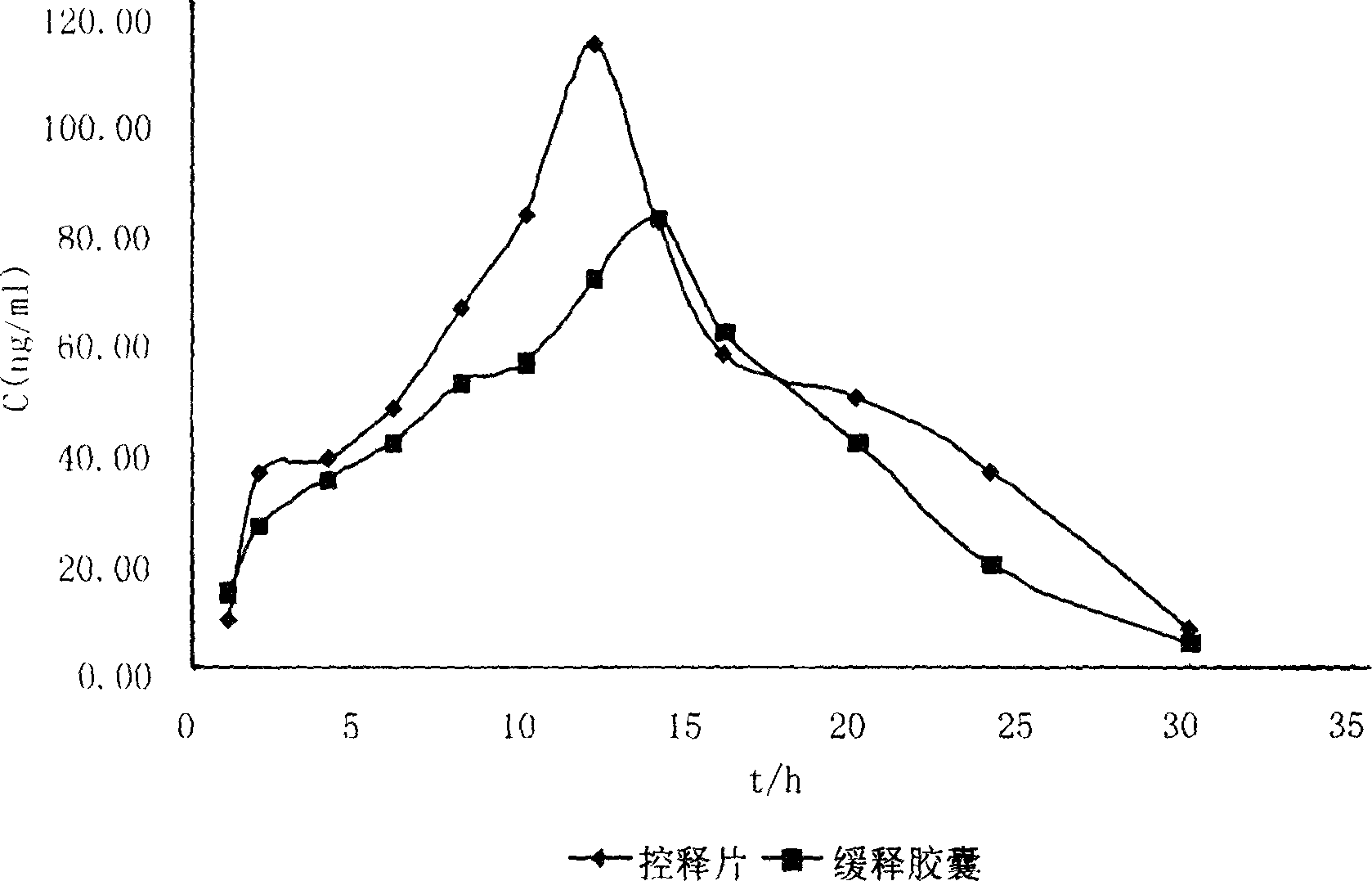
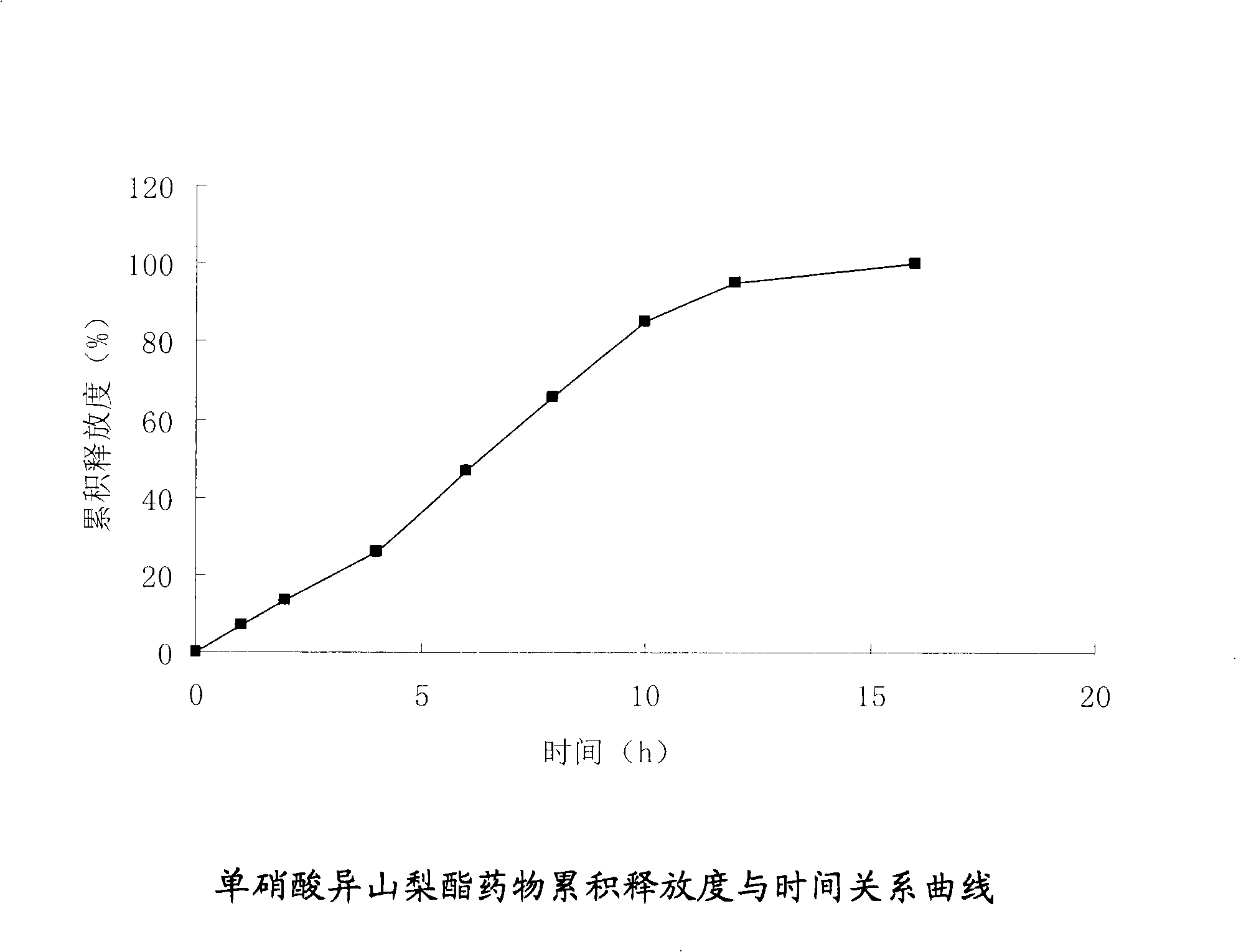
Tablet of isosorbide mononitrate
ActiveCN101732276AEasy to pumpStable release ratePill deliveryHeterocyclic compound active ingredientsMedicineSemipermeable membrane
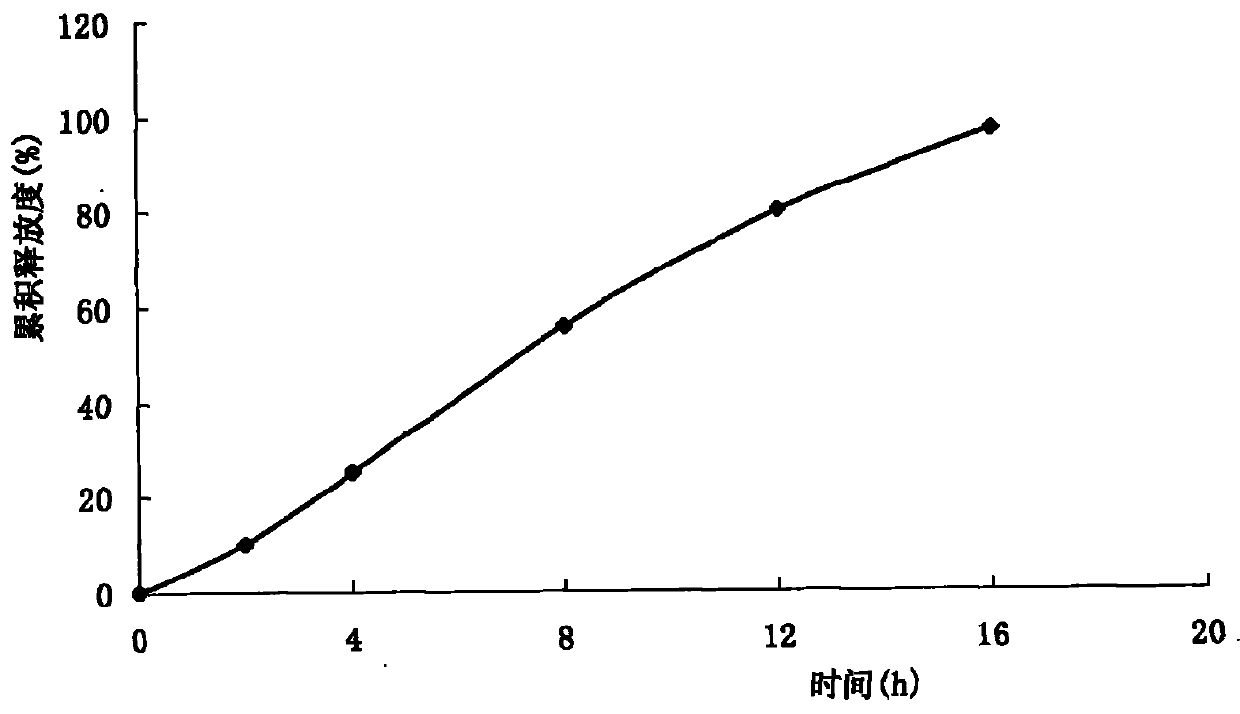
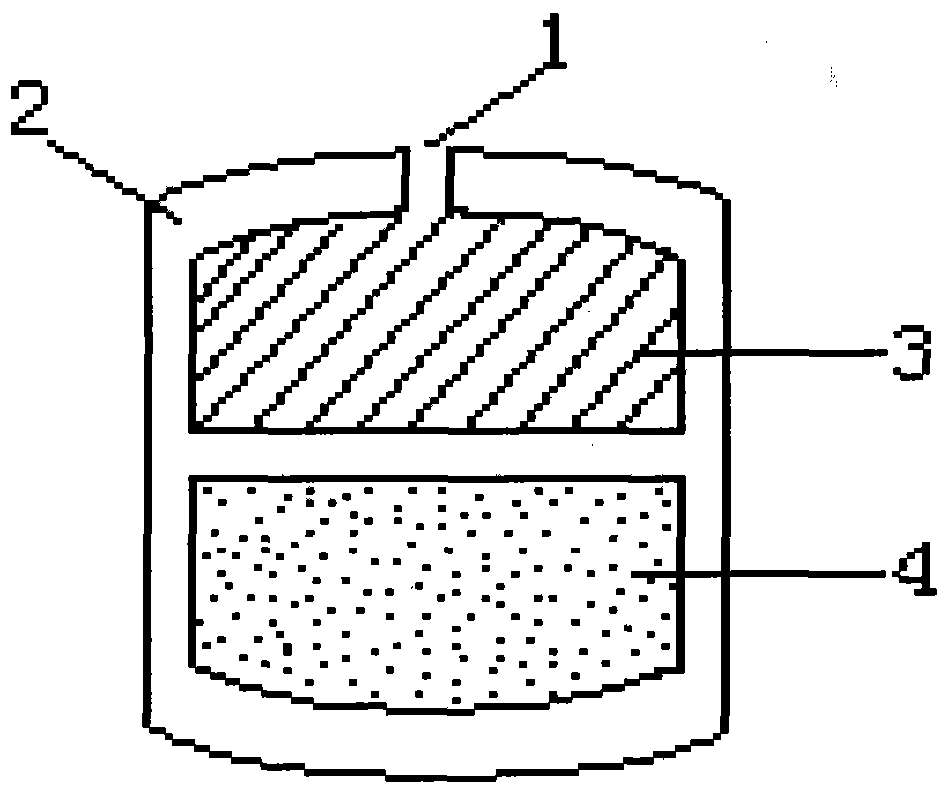
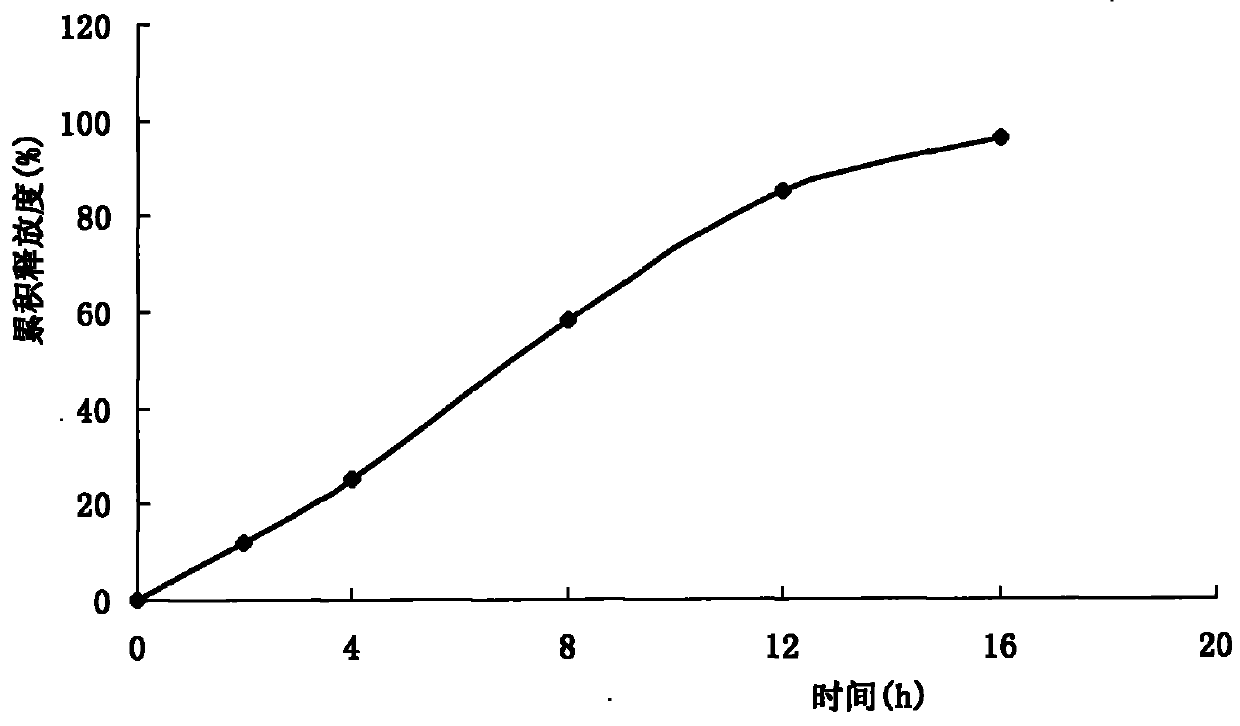
The invention relates to a tablet of isosorbide mononitrate, in particular to a double-layer osmotic pump controlled release tablet of isosorbide mononitrate, which belongs to the field of medicine preparation. A single-chamber double-layer osmotic pump tablet of the isosorbide mononitrate is characterized in that a semi-transparent coating film cover a double-layer core body consisting of a medicine-containing layer and a boosting layer; and the coating film is provided with a medicine releasing pore on the surface of the medicine-containing layer. The gastrointestinal tract water enters a double-layer tablet chip through the semi-transparent film; the medicines forms a mixed suspension liquid or solution when contacting water in the medicine-containing layer; a penetration enhancer enables the solution of the medicine-containing layer to be hypertonic so that an osmotic pressure difference exists between the inner side and the outer side of the film, which is beneficial to pumping the medicines out; and the pressure is generated in the boosting layer through water absorption, dissolution and expansion of a penetrating agent so as to further boost a medicine liquid to eject the pore.
Owner:LUNAN BETTER PHARMA
Stone needle energy mud matrix, stone needle energy mud containing matrix and preparation method of stone needle energy mud
ActiveCN105708612APrevent recurrenceEliminate stomach painHeavy metal active ingredientsElectrotherapySide effectRadix Aconiti
The invention relates to a stone needle energy mud matrix, stone needle energy mud containing the matrix and a preparation method of the stone needle energy mud. The matrix consists of the following raw materials in parts by weight: 200-300 parts of stone needle powder, 100-200 parts of volcanic mud, 50-100 parts of far-infrared ceramic powder, 50-100 parts of tourmaline powder, 500-800 parts of beeswax, 10-50 parts of an exothermal agent, 50-100 parts of rosin and 50-100 parts of glycerol; the stone needle energy mud consists of 1010-1750 parts of the stone needle energy mud matrix as well as the following Chinese herbal medicines: 20-30 parts of black ants, 5-10 parts of roasted pangolin scales, 15-20 parts of radix aconiti preparata and the like; and and the preparation method comprises the following steps: crushing the Chinese herbal medicines and uniformly stirring the crushed Chinese herbal medicines with the stone needle energy mud matrix. The stone needle energy mud matrix disclosed by the invention has the beneficial effects that the stone needle energy mud matrix can improve a medicine availability, avoid side effects caused by gastrointestinal administration, enhance therapeutical effect and achieve point transdermal application so as to rapidly and effectively reach focuses.
Owner:河北鹊神生物科技有限公司
Ivermectin transdermal liniment for animals
InactiveCN102440944APromote percutaneous absorptionReduce decomposition rateOrganic active ingredientsPharmaceutical delivery mechanismIrritationEthyl acetate
The invention discloses an ivermectin transdermal liniment for animals, which is prepared from medicinal liquor and ivermectin, wherein the medicinal liquor is prepared from the following components in percentage by volume: 20-40% of ethyl acetate, 2-5% of azone and 55-75% of propylene glycol; and the ratio of ivermectin to medicinal liquor is (0.5-1g):100mL. The novel transdermal accelerator selected in the formula can enhance the absorption of skin for medicines, does not have toxicity or irritation on the skin and body, does not have pharmacological action, and does not react with the medicines and other additives. The invention is simple to prepare and convenient to use, has high stability, and can achieve favorable anthelminthic effect.
Owner:INNER MONGOLIA AGRICULTURAL UNIVERSITY
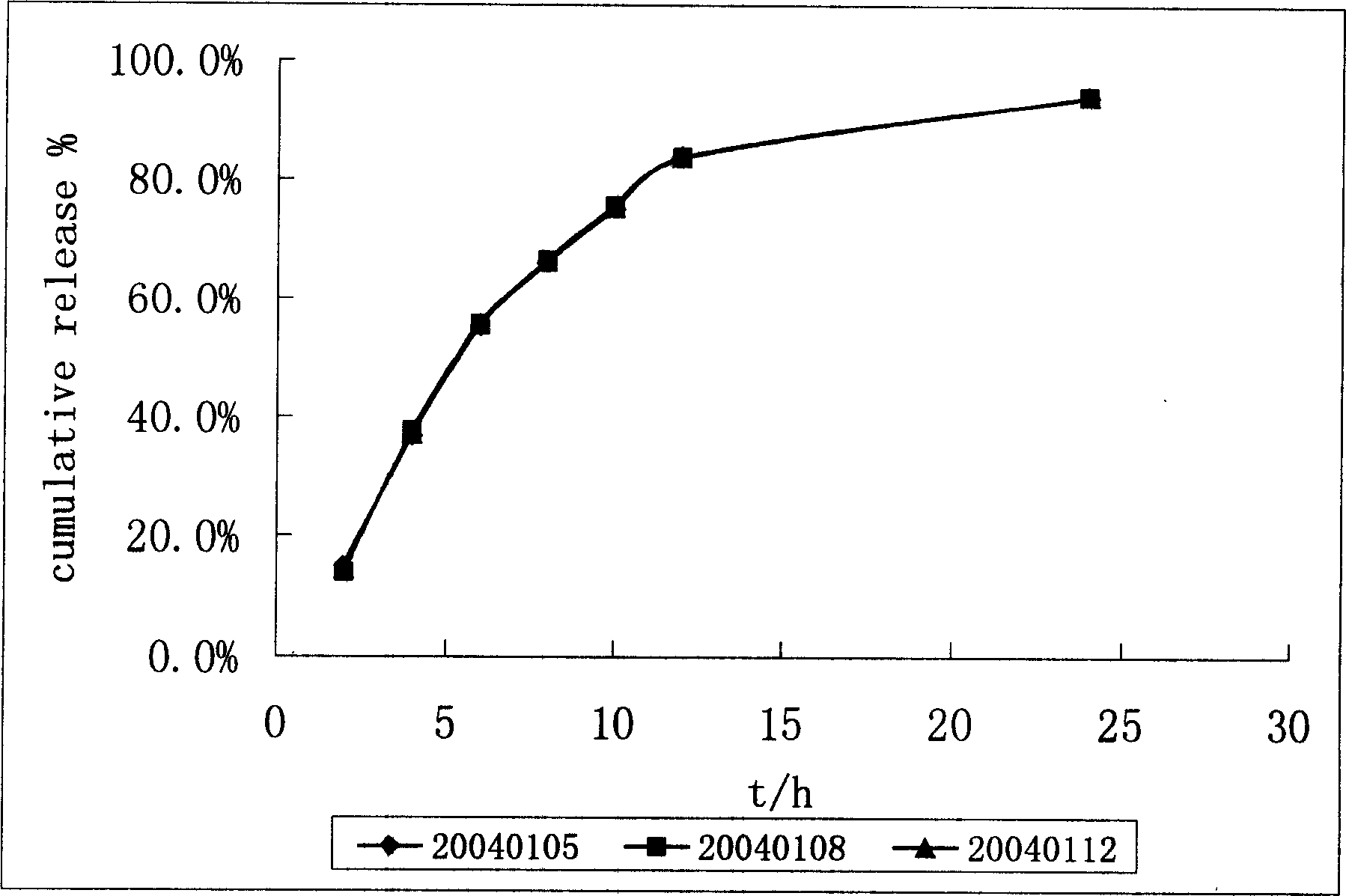
Control releasing venlafaxine hydrochloride tablet and its prepn
ActiveCN1771921ALittle side effectsGuaranteed curative effectOrganic active ingredientsNervous disorderDrugSide effect
The present invention discloses one kind of control releasing venlafaxine hydrochloride tablet and its preparation process. The control releasing venlafaxine hydrochloride tablet consists of medicine core of venlafaxine hydrochloride and semi-permeable coating with pores. The medicine core consists of venlafaxine hydrochloride 75 weight portions, stuffing 30-250 weight portions, osmotic pressure promoter 1-50 weight portions, adhesive 1-20 weight portions and lubricant 0.1-20 weight portions. The preparation has reasonable composition, excellent control releasing effect, long effective time, less side effect on gastrointestinal tract, less differential personal effect, high safety and good compliance.
Owner:CHENGDU KANGHONG PHARMA GRP
Isosorbide mononitrate osmotic pump type controlled release formulation and preparation method thereof
InactiveCN101342151AImprove complianceThe effect of small individual differencesPharmaceutical delivery mechanismCardiovascular disorderSide effectExcipient
The invention relates to an isosorbide mononitrate osmotic pump type controlled release preparation and a preparation method thereof. The osmotic pump type controlled release preparation is composed of a tablet core and semipermeable film coating with medicine releasing holes; wherein, the tablet core is composed of isosorbide mononitrate, penetration enhancer, bond and other excipient, and the semipermeable film includes coating material and plasticizer; the punching mode includes mechanical drilling and laser drilling. The preparation of the invention can effectively control the constant speed release rate of medicine and has the advantages of few administration times, less side effect, long efficacy lasting time, avoidance of medicine tolerance, etc.
Owner:INST OF PHARMACOLOGY & TOXICOLOGY ACAD OF MILITARY MEDICAL SCI P L A
Control released permeation bump tablet of venlafaxine hydrochloride and its prepn process
InactiveCN1846693ALittle side effectsGuaranteed curative effectOrganic active ingredientsNervous disorderPlasticizerControlled Release Tablet
The present invention discloses one kind of osmotic pump controlled release tablet of venlafaxine hydrochloride. The tablet consists of medicine core of venlafaxine hydrochloride and semipermeable film coating with small holes. The medicine core consists of venlafaxine hydrochloride in 75 weight portions, stuffing in 30-250 weight portions, osmotic pressure promoter in 1-50 weight portions, adhesive in 1-20 weight portions and lubricant in 0.1-20 weight portions. The semipermeable film coating consists of semipermeable film coating material in 5-15 weight portions and plasticizer in 1 weight portion. The tablet has excellent release controlling effect, high safety and good patient dependence. The present invention also discloses the preparation process of the osmotic pump controlled release tablet of venlafaxine hydrochloride.
Owner:潘卫三 +2
Acarbose solid sustained-release preparation and preparing method thereof
InactiveCN101606921AImprove complianceSmall toxicityOrganic active ingredientsMetabolism disorderIn vitro testBiochemistry
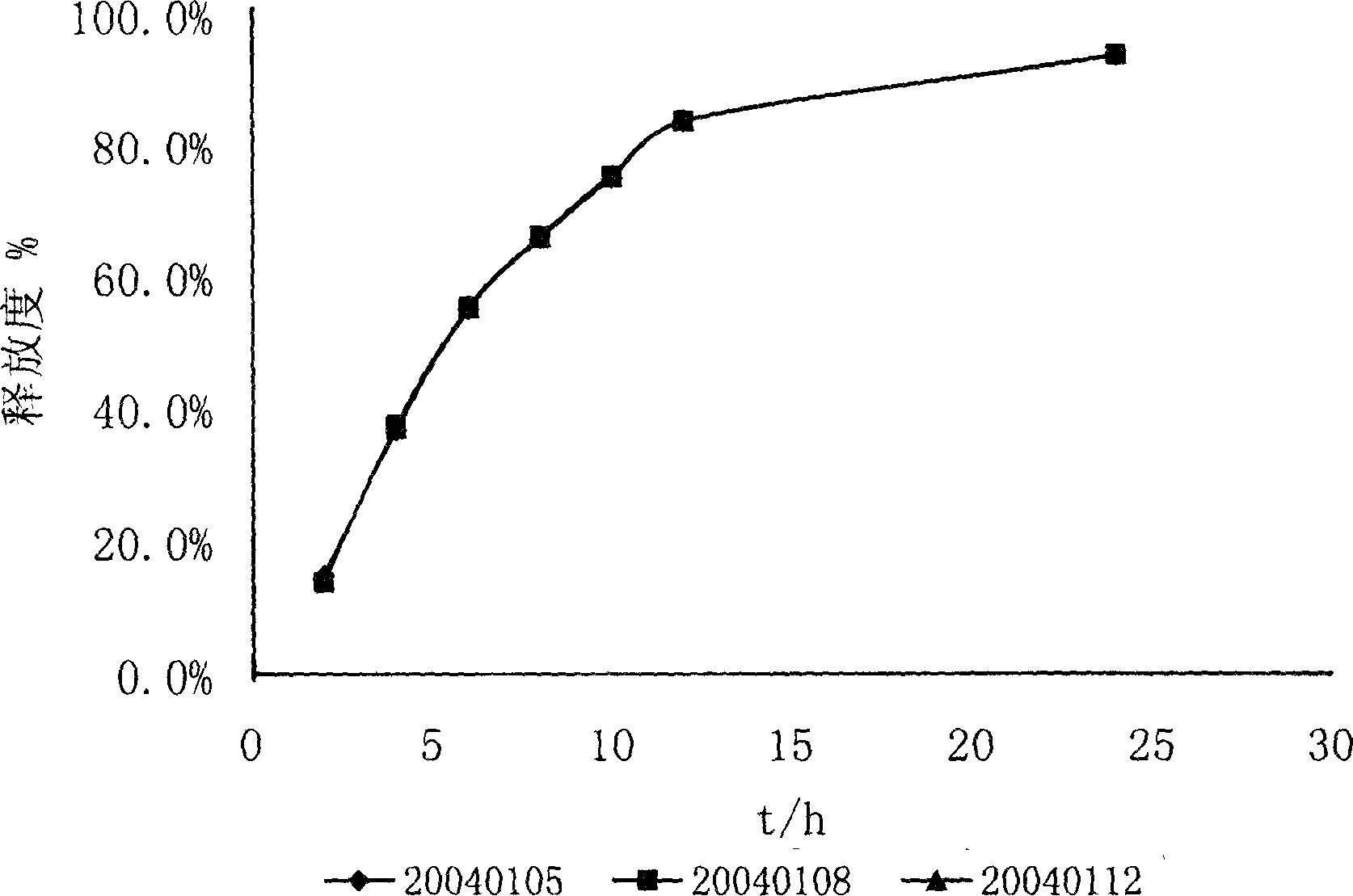
The invention discloses an acarbose solid sustained-release preparation which is prepared by pulverizing and mixing acarbose, sustained release retarder and pharmaceutic excipient, palletizing and then filling in capsules or tabletting by a tabletting machine. The acarbose solid sustained release preparation completely meets the requirement of sustained release. In vitro test indicates that the release rate of the preparation is 20-40 percent after two hours, 40-65 percent after four hours, and over 75 percent after eight hours.
Owner:QINGDAO HANHE PHARMA
Granular formulation containing cefixime liposomes and preparation method thereof
InactiveCN101966154AHigh encapsulation efficiencyReduce leak rateOrganic active ingredientsAntiinfectivesYolkCholesterol
The invention relates to a cefixime liposome, a preparation method and a granular formulation containing the cefixime liposome. The granular formulation comprises the cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome is prepared from the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 2.5-10 parts of cholesterol and 0.1-4.5 parts of polysorbate 80. The granular formulation not only accords with the requirement on Chinese pharmacopoeia, but also has the advantages of stabler storage and rapider drug effect exertion and remarkably improved bioavailability compared with the common pharmaceutical cefixime composition at a room temperature.
Owner:王丽燕
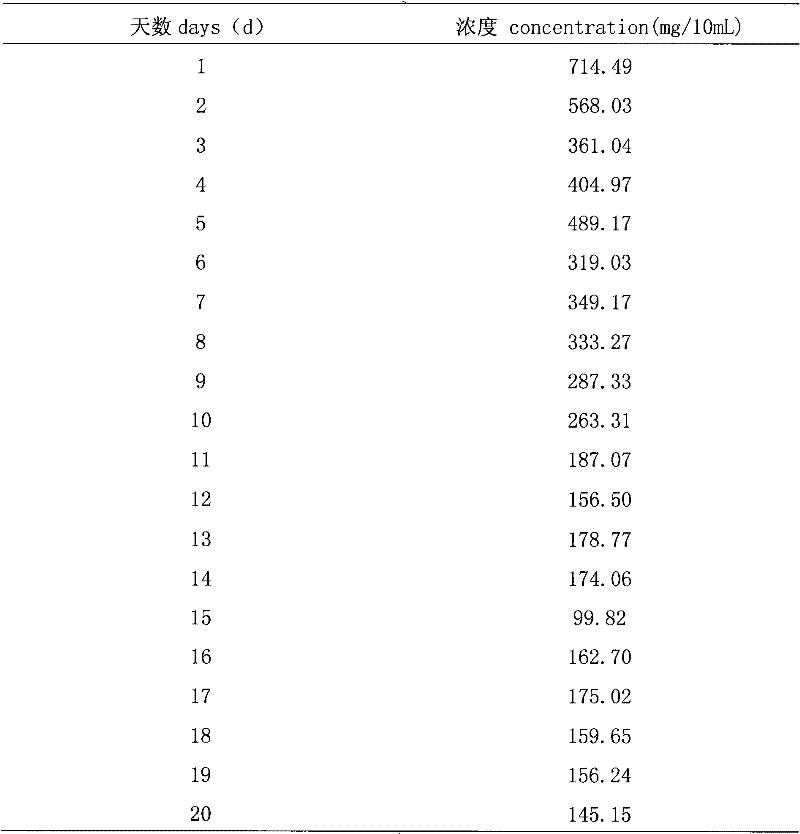
Iron-dextrin long-acting injection and preparation method thereof
InactiveCN102440952ASmall toxicityFacilitated releaseOrganic active ingredientsMetabolism disorderSide effectIron supplement
The invention relates to iron-dextrin long-acting injection and a preparation method thereof. The iron-dextrin long-acting injection is used for piglet iron supplement iron, and has the advantages that the release, absorption, distribution, metabolism and excretion processes of an iron preparation can be prolonged in vivo, so that the purpose of prolonging the action of a drug can be achieved, the number of the dosing times can be reduced, the complicance of livestocks is improved, the blood concentration can be stable, the peak and valley phenomena can be avoided, the side effects of the drug can be facilitated to be reduced, the total drug dosage is reduced, and further, the maximum drug effect by the minimum dosage is achieved. Thus, the iron-dextrin long-acting injection can completely play the role of long-acting iron supplement in the prevention and the cure of piglet iron-deficiency anemia.
Owner:TIANJIN RINGPU BIO TECH
Cubic liquid crystal in-situ gel injection of local anesthetic, and preparation method of injection
ActiveCN106491519AAvoid breedingImprove stabilityAerosol deliveryOintment deliveryMass ratioAmide local anesthetics
The invention relates to cubic liquid crystal in-situ gel injection of local anesthetic, and a preparation method of the injection. The injection is prepared from the local anesthetic, a liquid crystal material and an organic solvent and / or a release regulator; the local anesthetic is amide local anesthetic and the mass concentration of the local anesthetic is 0.1 to 8 w / w%; the organic solvent is an organic solvent which is mutually soluble with water, the liquid crystal material is mono-oleic acid glycerolipid, and the mass ratio of the liquid crystal material to the organic solvent is (1-9):1; and the release regulator is selected from at least one of medium chain triglyceride, oleic acid, tocopherol and tocopheryl acetate, and the mass concentration of the release regulator is 0 to 30 w / w%. The cubic liquid crystal in-situ gel injection of the local anesthetic has very good long-acting sustained release effect, low sudden release raten high compliance and small adverse effect, can reduce administration times and can avoid the peak valley phenomenon.
Owner:GUANGZHOU NEWORLD PHARMA CO LTD
Water soluble medicament sustained-release tablets and preparation method thereof
InactiveCN101829068ASmall toxicityImprove complianceNervous disorderPharmaceutical delivery mechanismCaptoprilDuodenal juice
The invention discloses water soluble medicament sustained-release tablets, which comprise the following components in part by weight: water soluble medicament 1-30, octadecanol 5-70, Eudragite L 100-55 2-50, talcpowder 2-30 and lactose 2-30. The water soluble medicament may be galanthamine hydrobromide, captopril, metoprolol tartaric acid or pseudoephedrine hydrochloride. In the invention, the octadecanol serving as a hydrophobic auxiliary material and the Eudragite L 100-55 sreving as a water soluble polymer auxiliary are adopted, so the release speed of the medicament can be controlled properly. The Eudragite L 100-55 is insoluble in gastric juice, but dissolves in duodenal juice to make high-viscosity sticky liquid, and thus the release and diffusion speed of the medicament can be reduced. The talcpowder which is a hydrophilic matter insoluble in water plays a porogen role in the sustained-release tablets. In the invention, the release speed of the medicament is regulated by regulating the mixing ratio of the medicament to the auxiliary material. Thus, the release speed of an active medicament is controlled.
Owner:XUZHOU PHOTOSYNTHETIC BIOLOGICAL NUTRIMENT
Dispersible tablet containing cefixime liposome and preparation method thereof
InactiveCN101966160AHigh encapsulation efficiencyReduce leak rateAntibacterial agentsOrganic active ingredientsCholesterolNiosome
The invention relates to a cefixime liposome, a preparation method thereof and a dispersible tablet containing the cefixime liposome. The dispersible tablet comprises the cefixime liposome and a pharmaceutically acceptable carrier, wherein the cefixime liposome comprises the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 1.25-10 parts of cholesterol and 0.2-3.5 parts of polysorbate 80. The dispersible tablet not only conforms to the requirements of a Chinese pharmacopoeia, but also has the advantages of more stable storage under normal temperature and remarkable improvement of bioavailability compared with the ordinary cefixime medicament composition, and can take effect more rapidly.
Owner:王丽燕
Traditional Chinese medicine composition for treating necrotic enteritis of chickens and preparation method thereof
ActiveCN105920268AImprove intestinal environmentImprove immunityAntibacterial agentsDigestive systemMonkshoodsMedicine
The invention discloses a traditional Chinese medicine composition for treating necrotic enteritis of chickens. The traditional Chinese medicine composition is mainly prepared from golden cypress, fructus aurantii, magnolia obavata, radix linderae, pericarpium arecae, rhizoma atractylodis macrocephalae, poria cocos, rhizoma dioscoreae, fructus crataegi, radix sophorae flavescentis, medicated leaven, radix sileris, herba patriniae, radix pulsatillae, moutan bark, selfheal, radix glycyrrhizae, nauclea officinalis, folium perillae, cassia occidentalis, radix astragali, pericarpium granati, monkshood, herba taraxaci, bunge corydalis herb, red triadica sebifera and radix scutellariae. A preparation method of the traditional Chinese medicine composition for treating necrotic enteritis of chickens comprises the following steps: superfine grinding, mixing in batches, extracting, combining and concentrating. The traditional Chinese medicine composition has the advantages that the curative effect is effective, the medicine resistance is avoided, the medicine residue is avoided, and the traditional Chinese medicine composition is capable of treating necrotic enteritis of chickens, improving intestinal environment of chickens and enhancing immunity of chickens.
Owner:RONGXIAN XIANGYI FARMING CO LTD
Nimesulide sustained-release pellets and preparation method thereof
ActiveCN102188386AGood slow releaseEasy to processAntipyreticAnalgesicsIcing sugarSustained release pellets
The invention provides nimesulide sustained-release pellets composed of basic pellets and sustained-release coatings, wherein the basic pellets comprise 40-80% of nimesulide, 5-20% of starch, 5-30% of powdered sugar and 5-20% of hydroxypropyl methylcellulose; and the sustained-release coatings comprise 3-40% of ethylcellulose, 20-80% of HP-55 and 3-40% of talcum powder. The invention also provides the preparation method of the nimesulide sustained-release pellets. The nimesulide sustained-release pellets provided by the invention have good sustained-release effect, convenience in processing and suitability for industrial mass production.
Owner:HAINAN HULUWA PHARMA GRP CO LTD
Phlegm dissolving and cough quenching paste
InactiveCN104225126AFlat drug concentration curveImprove the body's non-specific immunityRespiratory disorderSheet deliveryQuenchingAsthma
The invention discloses a phlegm dissolving and cough quenching paste and belongs to the technical field of medical preparations. The traditional Chinese medicine raw materials consist of the following substances in part by weight: 2-10 of sinapis alba, 1-6 of ephedra, 1-5 of euphorbia kansui, 1-4 of araceae, 0.5-4 of corydalis, 0.5-4 of asarum and 1-4 of castor bean oil. The phlegm dissolving and cough quenching paste is prepared by the method that traditional Chinese medicines in the traditional Chinese medicine raw materials except the castor bean oil are pulverized, blended with the castor bean oil, uniformly mixed and pasted on rubberized fabric. The phlegm dissolving and cough quenching paste has the functions of ventilating the lung, relieving cough, dispelling phlegm and preventing asthma and is mainly used for treating chronic obstructive pulmonary diseases and cough symptoms, such as cough, phlegm coughing and breath shortness.
Owner:STATE GRID CORP OF CHINA +1
Clonidine pamoate and preparation method thereof
The invention relates to clonidine pamoate and a preparation method thereof. The clonidine pamoate is formed by clonidine and pamoate in a solvent in a molar ratio of 2:1. The clonidine pamoate provided by the invention exists in three crystal forms, the solubility of each crystal form is lower, the solubility in water is about 0.02mg / ml and is equivalent to one hundredth of the solubility of clonidine (2.19mg / ml) and one thousandth of the solubility of clonidine hydrochloride (79.4mg / ml), the slow-release effect can be achieved without a complex preparation technology and is very stable, and crystal transformation does not occur under hot and humid and strong light conditions and under an accelerated test condition; and a production process is simple, and granularity can be easily controlled, so that the clonidine pamoate is applicable to enlarged production. The clonidine pamoate is applicable to being made into a long-acting slow-release preparation, can reduce mediation frequency, improves the medication compliance of a patient, can enable blood drug concentration to be balanced, avoids a peak valley phenomenon and reduces adverse reactions during medication.
Owner:力赛生物医药科技(厦门)有限公司 +1
Dry suspension containing cefixime liposome and preparation method thereof
InactiveCN101972231AStorage moreStorage stableOrganic active ingredientsAntiinfectivesPhysical ExertionsYolk
The invention relates to cefixime liposome and preparation method therefore as well as dry suspension containing the cefixime liposome. The dry suspension is composed of the cefixime liposome and pharmaceutically acceptable carriers, wherein the cefixime liposome comprises the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated yolk lecithin, 2.5-10 parts of cholesterol and 0.1-5 parts of polysorbate 80. The dry suspension not only accords with the requirements of Chinese pharmacopoeia, but also has the advantages of better storage stability in normal temperature, faster effect exertion and obviously improved bioavailability in comparison with the common cefixime medicaments.
Owner:王丽燕
Persimmon leaf general flavone sustained-release micropills and preparation method thereof
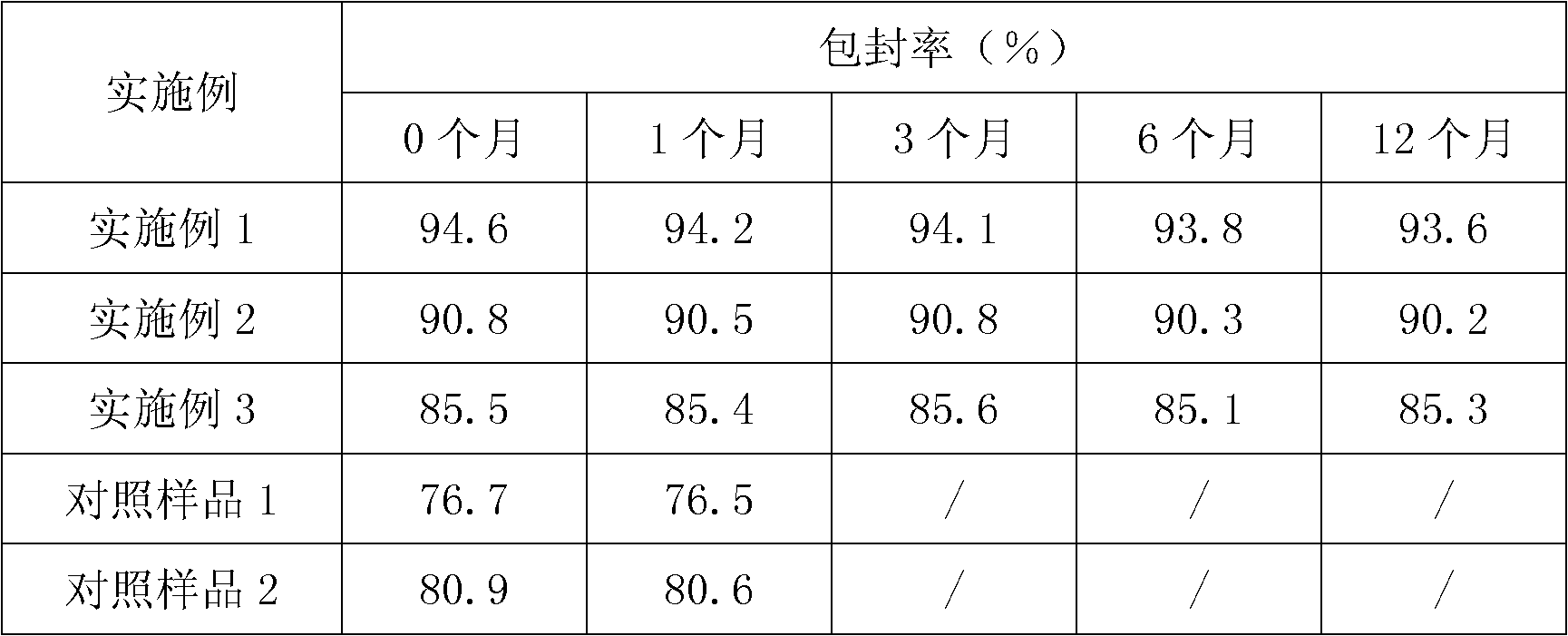
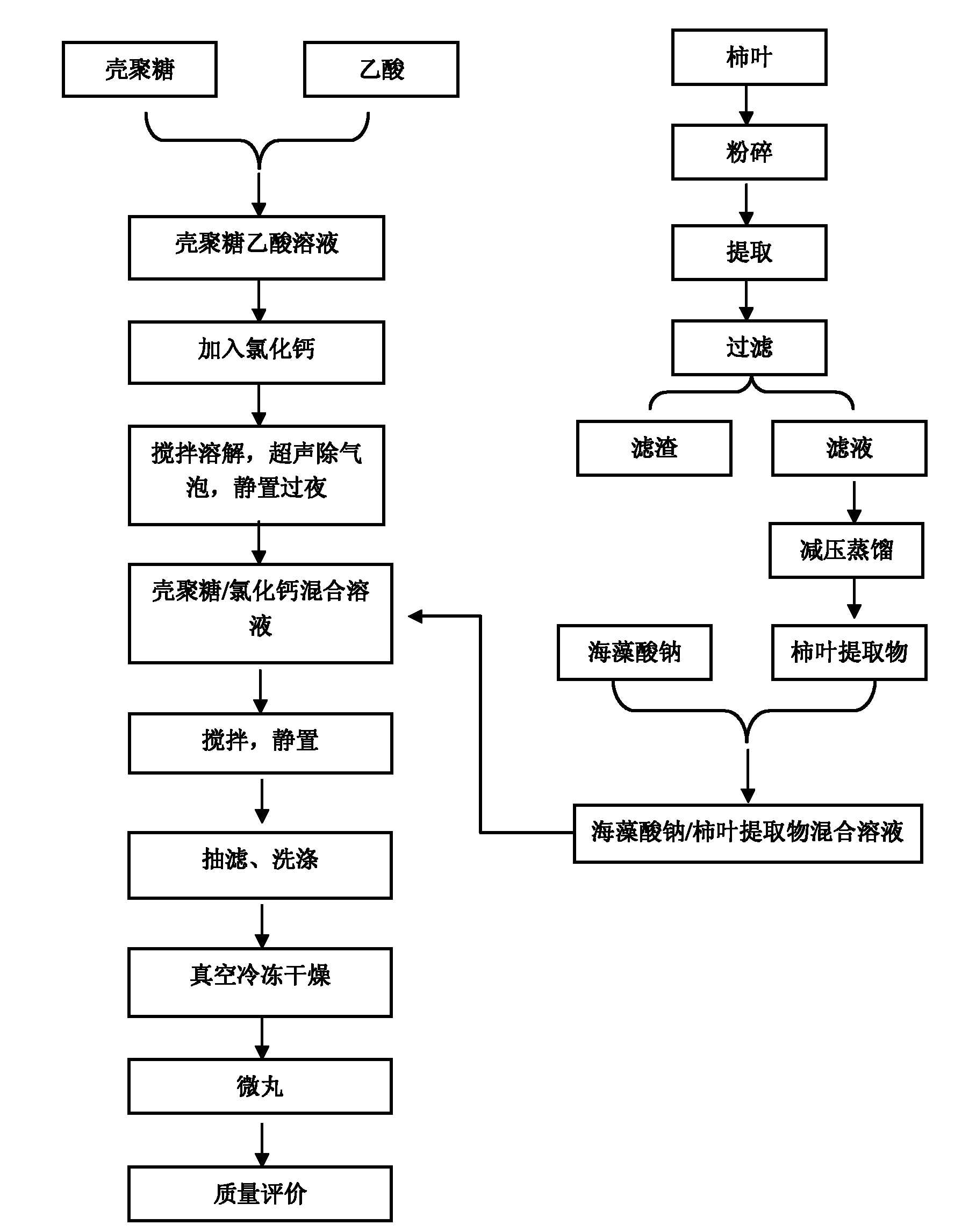
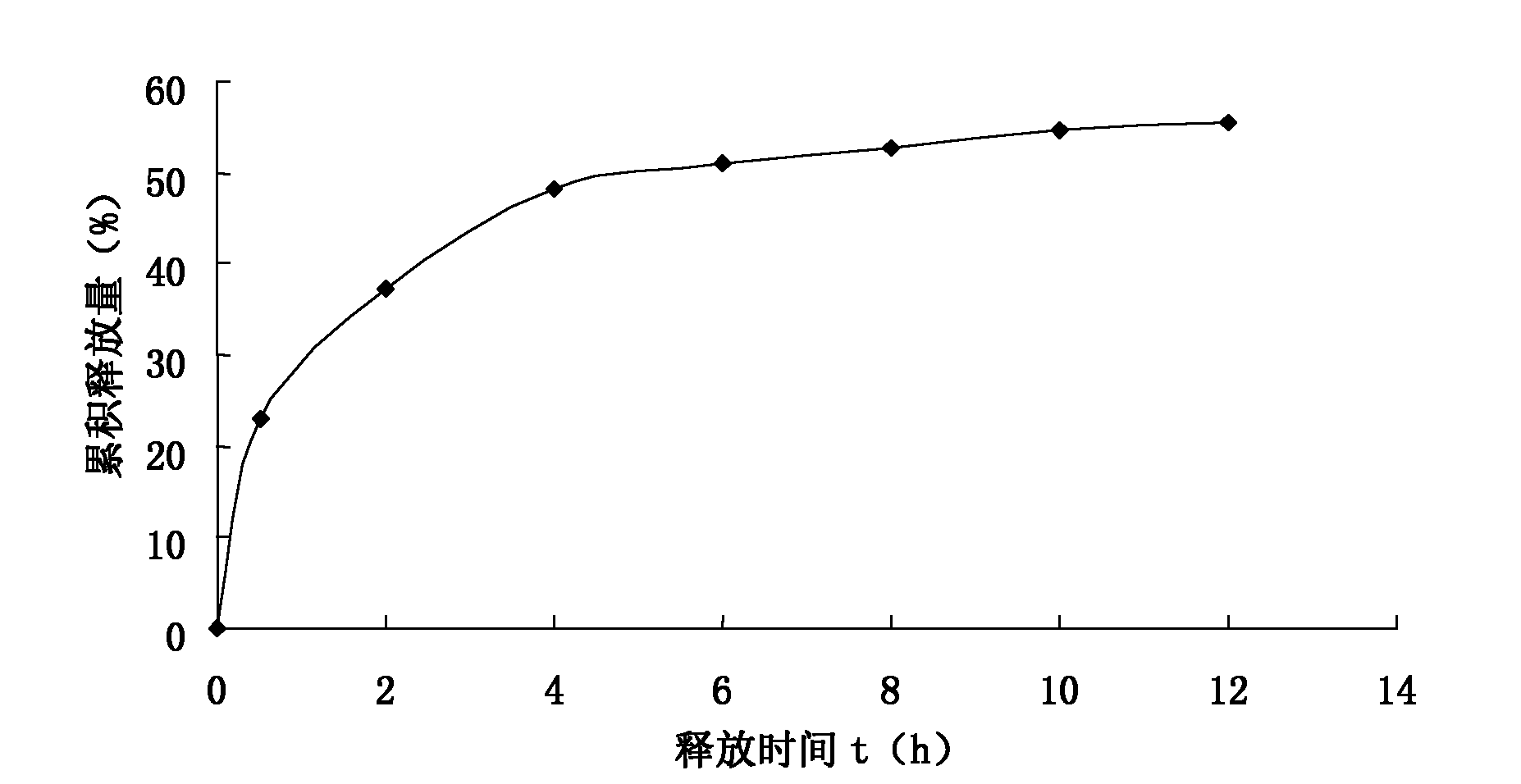
InactiveCN102178722AMeet the requirementsNo burst effectAntibacterial agentsMetabolism disorderBurst effectBULK ACTIVE INGREDIENT
The invention discloses persimmon leaf general flavone sustained-release micropills and a preparation method thereof. The persimmon leaf general flavone sustained-release micropills comprise the following components in percentage by mass: 1 to 25 percent of persimmon leaf general flavone, 10 to 50 percent of sodium alginate, 10 to 40 percent of chitosan, 10 to 50 percent of calcium chloride, 0.5 to 5 percent of acetic acid and the balance of water. The persimmon leaf general flavone sustained-release micropills take the persimmon leaf general flavone as active ingredients and are a novel Chinese medicinal sustained-release preparation. General flavone is used as a release amount index, the accumulative release amount of the general flavone of the persimmon leaf general flavone sustained-release micropills in artificial gastric juice and phosphate buffer saline (PBS) buffer solution is 22.97 percent and 34.93 percent respectively within 0.5 hour, does not exceed 45 percent and does not have a burst effect, and results meet the requirement on sustained-release preparations in Chinese pharmacopoeia (the guiding principle of sustained release, controlled release and delayed release in XIXD of 2010 version).
Owner:SHAANXI UNIV OF SCI & TECH
Lovastatin sustained-release tablets and method of producing the same
InactiveCN101129355ASimple preparation processGood cumulative releaseOrganic active ingredientsMetabolism disorderAlcoholSide effect
The invention discloses a Luovastatin slow release tablet and making method in the drug agent domain, which comprises the following parts per 1000g: 5-80g Luovastatin, 8-128g frame material, 56-896g filler, 7. 5-120mg anti-oxidant, 31-496ml binder alcohol solution with content at 0. 25%-16% and 0. 7-11. 2g lubricant. The invention has good accumulated releasing effect with good curative effect for irregular diet patients, which is beneficial for diabetic patient with little side effect.
Owner:SHANDONG HUAXIN PHARMA
Tandospirone micropore osmotic pump preparation
ActiveCN104706614AProlong the action timeReduce the number of daily dosesOrganic active ingredientsNervous disorderDrug releaseOsmotic pump
The invention provides a tandospirone micropore osmotic pump preparation. The osmotic pump preparation can reach zero-level release, reaches 24-hour drug release, can effectively prolong the time of drug action, makes patients reduce the number of times of taking medicine daily, and improves compliance.
Owner:SICHUAN CREDIT PHARMA
Palmatine nano particle preparations and preparation thereof
InactiveCN101214246AImprove playbackAntibacterial activity did not decreaseAntibacterial agentsPowder deliveryWater bathsCyanoacrylate
The present invention discloses a palmatine nano-particle preparation which is that 3 to 5 portions of palmatine is added into the distilled water with proper quantity to be heated in water bath under constant temperature to be dissolved completely and confected as liquid A; 4 to 8 portions of surfactant is added into the rest distilled water to be confected as liquid B; the pH values of the liquid A and the liquid B are respectively adjusted to be acidic by hydrochloric acid; under the condition of stirring under constant temperature, 2 to 6 portions of alkyl cyanoacrylate (ACA) is dripped into the liquid B slowly to be stirred for minutes, the liquid A is added gradually to be stirred by an electromagnetic stirrer for a long time to be added with 3 to 9 portions of additive to be stirred for hours continuously; sodium hydroxide solution is used to adjust the pH value, and the reaction is finished; a microporous filtering film is used for filtering to obtain deposit after ultracentrifugation under low temperature, and the deposit is dispersed and diluted by the distilled water to obtain the palmatine nano-particle preparation which has low side effect, obvious curative effect and the functions of the slow-release and targeting.
Owner:NORTHWEST A & F UNIV
Ivermectin controlled-release capsule and preparation method and application thereof
InactiveCN107773554ARelease fullyHigh protection rateOrganic active ingredientsAntiparasitic agentsTherapeutic effectPhospholipid
The invention discloses an ivermectin controlled-release capsule and a preparation method and application thereof. The ivermectin controlled-release capsule comprises, by mass, 0.1-1% of ivermectin raw material, 20-60% of water-soluble carrier and 30-70% of enteric-soluble wrapping material, the water-soluble carrier is one or multiple of hydroxypropyl-beta-cyclodextrin, methyl-beta-cyclodextrin,HPMC, PVP, PEG, poloxamer 188, mannitol, D-alpha-tocopherol PEG 1000 succinate, cholate / phospholipid mixed micelle, polyethylenediamine dendritic polymer, phospholipid or cholesterol, and the enteric-soluble wrapping material is one or multiple of hydroxypropyl methyl cellulose phthalic acid, acrylic resin II, acrylic III, cellulose acetate phthalate, polyethylene diacetate phthalate or hydroxypropyl methyl cellulose acetate succinate. The ivermectin controlled-release capsule has the advantage of outstanding acid resistance, enables drug to reach intestinal tracts to be disintegrated and released, remarkably improves bioavailability and has remarkable treatment effect.
Owner:SOUTH CHINA AGRI UNIV
Enticawer release-controllable tablet and its preparation
InactiveCN101028270ASmall toxicityImprove complianceDigestive systemAntiviralsBlood concentrationDrug release
A release-controlled tablet of entecavir for treating hepatitis B and its preparing process are disclosed. Its advantages are smooth release, stable blood concentration and low by-effect.
Owner:SUNSHINE LAKE PHARM CO LTD
Compound sustained-release tablet of epalrestat and sitagliptin or pharmaceutically acceptable salt thereof and preparation method thereof
ActiveCN113925838AExtend the time of synergyReduce releaseOrganic active ingredientsNervous disorderProlonged-release tabletPatient compliance
The invention belongs to the field of pharmaceutical preparations, and particularly relates to a compound sustained-release tablet of epalrestat and sitagliptin or pharmaceutically acceptable salt thereof and a preparation method thereof. The compound sustained-release tablet contains epalrestat, sitagliptin or pharmaceutically acceptable salt thereof and / or hydrate of the salt, anhydrous calcium hydrophosphate, a framework sustained-release material and other pharmaceutical excipients. The compound sustained-release tablet can be a single-layer tablet or a double-layer tablet, and is used for treating type 2 diabetes, especially peripheral neuropathy caused by the type 2 diabetes. The compound composition is prepared into the sustained-release tablet, so that the synergistic interaction time of the two active ingredients can be exerted and prolonged, the stimulation effect of the medicine on gastrointestinal tracts can be reduced, the compliance of patients can be improved, the peak valley phenomenon of a common preparation in blood after the common preparation is taken is avoided, the occurrence of adverse reactions is reduced, and the medication safety is improved.
Owner:乐普制药科技有限公司
Novel enteric controlled-release tablet preparation and preparation method thereof
InactiveCN101658508AReasonable compositionGood controlled release effectOrganic active ingredientsPharmaceutical delivery mechanismSide effectPatient compliance
The invention discloses a novel controlled-release preparation based on osmotic pump preparation and a preparation method thereof. The controlled-release preparation comprises a medicine tablet core containing active medicine, a semipermeable film coating coated on the outer layer of the medicine tablet core and provided with small holes, and an enteric coating arranged at the outermost layer, wherein the medicine tablet core comprises 20-70 parts of danshinolic acid B, 50-250 parts of loading agent, 1-120 parts of osmotic pressure accelerant, 1-20 parts of adhesive and 0.1-20 parts of lubricants; the semipermeable film coating comprises 3-15 parts of semipermeable film coating material and 1 part of plasticizer; and the enteric coating comprises 35-65 parts of enteric coating material, 10-35 parts of plasticizer, 5-15 parts of surface active agent and 10-25 parts of antiplastering aid. The preparation has reasonable composition, better controlled-release effect, long effective duration, little gastrointestinal tract side effects, few influences of individual difference, high safety, and excellent patient compliance, and only needs to be taken twice one day. The invention also discloses a danshinolic acid B enteric controlled-release tablet preparation and a preparation method thereof.
Owner:TIANJIN UNIV OF TRADITIONAL CHINESE MEDICINE
Cardio-cerebral refreshing sustained-release soft capsules and preparation method thereof
ActiveCN102106891AReduce volumeEasy to useNervous disorderHydroxy compound active ingredientsMass ratioAdditive ingredient
The invention discloses cardio-cerebral refreshing sustained-release soft capsules and a preparation method thereof. The sustained-release soft capsules contain capsule shells and contents, wherein the contents contain the following active pharmaceutical ingredients in mass ratio: 390 parts of safflower oil, 3 parts of borneol, 17 parts of vitamin E and 65 parts of vitamin B; and the contents contain the following raw materials in parts by weight: 4.15 parts of active pharmaceutical ingredients and 2-8 parts of sustained-release coating material, wherein the sustained-release coating material is one or mixture of polyethylene glycol (PEG) and glyceryl monostearate based on an arbitrary proportion. The preparation method mainly comprises the following steps: grinding and evenly mixing 1 / 2-2 / 3 of the safflower oil in the active pharmaceutical ingredients and the other active pharmaceutical ingredients, and then, grinding and evenly mixing the residual safflower oil, the sustained-release coating material and a dispersing agent or a diluting agent; and evenly mixing the two obtained mixtures, and sieving with a 80-100 mesh sieve to obtain the capsule contents. The sustained-release soft capsules provided by the invention have the advantages that drug release can keep uniform and constant, drug action time can be prolonged, and a peak valley phenomenon can be avoided.
Owner:SHINEWAY PHARMA GRP LTD
Scutellaria baicalensis total flavonoid sustained release tablet and preparation method thereof
InactiveCN103933002ASlow and long-lasting drug releaseSignificant progressPharmaceutical delivery mechanismPharmaceutical non-active ingredientsSide effectRelease time
The invention discloses a scutellaria baicalensis total flavonoid sustained release tablet and a preparation method thereof, belonging to the field of Chinese medicine preparations. The scutellaria baicalensis total flavonoid sustained release tablet consists of the following components in percentage by weight: 20-50% of a scutellaria baicalensis total flavonoid extract, 20-40% of a sustained release framework material and 10-50% of pharmaceutical adjuvants, wherein the scutellaria baicalensis total flavonoid extract is determined through an ultraviolet spectrophotometry and contains not less than 60% of total flavonoid in terms of baicalin; a high performance liquid chromatography (HPLC) determines that content of a baicalin monomer is not less than 40%. In addition, the invention also discloses a method for preparing the scutellaria baicalensis total flavonoid sustained release tablet. The method mainly comprises the processes of extracting and separating the scutellaria baicalensis total flavonoid extract and preparing the sustained release tablet. Compared to a current commercial scutellaria baicalensis tablet, the scutellaria baicalensis total flavonoid sustained release tablet disclosed by the invention can significantly reduce a drug release speed, prolong release time and keep a stable blood concentration; moreover, the sustained release tablet can reduce administration time, reduce side effect and improve a medication compliance of patients.
Owner:LUNAN PHARMA GROUP CORPORATION
3D printing drug release capsules and preparation method and application thereof
ActiveCN111249257AImprove utilizationLow efficiencyBiocideAdditive manufacturing apparatusDrug utilisationPharmaceutical drug
The invention provides 3D printing drug release capsules and a preparation method and application thereof. Each of the 3D printing drug release capsules is characterized by comprising a base and a capsule body formed in the base, wherein at least two chambers with different outer wall thicknesses are arranged in the capsule body, and each chamber is stuffed with homogeneous medicines or differentmedicines. Because each capsule consists of the base and the chamber capsule body with a gradient wall thickness structure, wherein each chamber structure has different outer wall thicknesses, and each chamber is stuffed with homogeneous medicines or different medicines, so that drug release is controlled step by step along with different dissolving speed of different wall thicknesses of the capsules, the drug release capsules can durably and slowly release medicines within the long time when being taken / implanted in bodies, or placed in required environment as required, peak-to-valley phenomenon is avoided, the drug concentration can be maintained to the effective concentration range for a long time, the availability of drugs is improved, and the number of medication times can be reduced.
Owner:WUHAN UNIV
Pain-relieving plaster and preparation method thereof
InactiveCN1876154ASmall toxicityAvoid interferenceAntipyreticAerosol deliveryAconitum pendulumCremanthodium
The invention provides an externally-used analgesic plaster and process for preparation, wherein the preparation comprises the following raw materials (by weight portion): Aconitum pendulum Busch 400-600, Duyiwei 200-400, Oxytropis 200-400, Cremanthodium 200-400, curcuma longa 400-600, and camphor 60-100. The preparing process comprises the steps of alcohol extraction, water extraction and condensating into thick plaster.
Owner:西藏央科生物科技有限公司
Biogenic controlled-release sterilization liquid and preparation method and application thereof
InactiveCN103548851AControl release speedReduce dosageBiocideFungicidesSolventBULK ACTIVE INGREDIENT
The invention relates to biogenic controlled-release sterilization liquid and a preparation method and application thereof, belonging to the technical field of biopesticides. The biogenic controlled-release sterilization liquid is characterized by taking methyl alcohol as a solvent, wherein per kilogram of methyl alcohol contains the following active ingredients: 160-280g of sinomenine, 50-170g of kaempferol, 70-180g of astragalin, and 100-240g of gentiopicroside, and also contains 70-230g of penetrant, and 100-300g of controlled-release additive. The biogenic controlled-release sterilization liquid takes natural substances in a plant as the active ingredients, can control the release rate of pesticide by the pesticide release rate of the controlled-release agent, can resist bacteria continuously and efficiently, can reduce photolysis and losses of active substances in environment, avoids peak valley fluctuation of pesticide concentration, enables the pesticide to play a role smoothly and continuously so as to reduce the dosage of the pesticide and prolong the effective duration. The biogenic controlled-release sterilization liquid has the advantages of being convenient to manufacture, low in cost, stable and efficient.
Owner:武汉润之生环保科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com