Novel enteric controlled-release tablet preparation and preparation method thereof
A controlled-release preparation and enteric-coated technology, applied in the field of pharmaceutical preparations, can solve the problems of inconvenient multi-component drugs and unstable drugs, etc., and achieve the effects of long effective duration, good patient compliance, and good safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
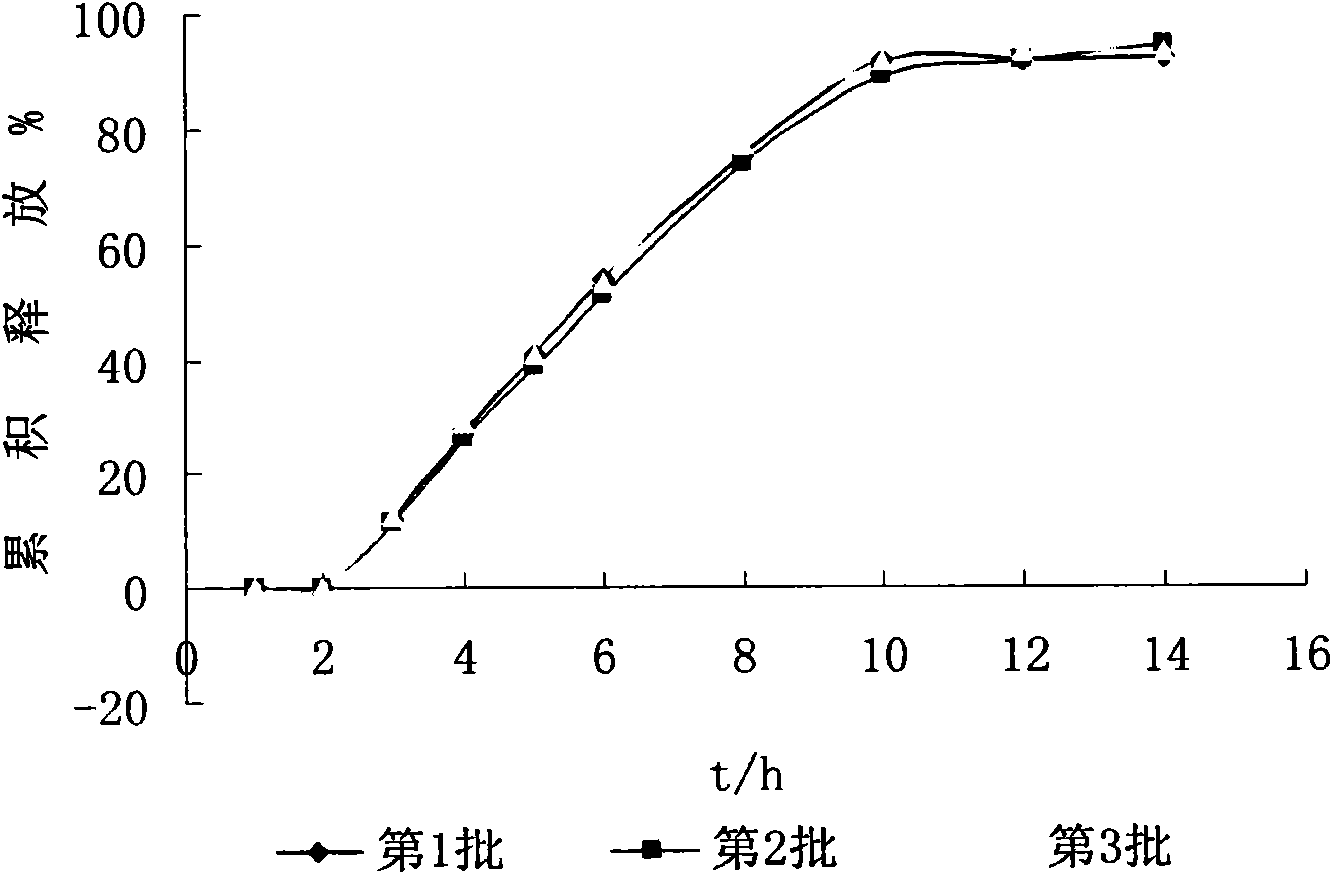
Image
Examples
Embodiment 1
[0052] The weight ratio of the drug tablet core to the semipermeable film coating is 1:0.035; the weight ratio of the drug tablet core to the semipermeable film coating to the enteric coating is 1:0.12. release tablet preparation, wherein:
[0053] The composition of the drug tablet core is: 22.5 parts of salvianolic acid B, 101 parts of microcrystalline cellulose, 30 parts of lactose, 60 parts of sodium chloride, 6 parts of povidone K30 and 1 part of magnesium stearate.
[0054] The composition of the semipermeable film coating is: 4 parts of cellulose acetate and 1 part of polyethylene glycol 1500.
[0055] The composition of the enteric coating is: 45 parts of acrylic resin No. I, 12 parts of liquid paraffin, 4 parts of diethyl phthalate, 12 parts of castor oil, 12 parts of Tween-80 and 15 parts of talcum powder.
[0056] The preparation method of the above-mentioned salvianolic acid B enteric-coated controlled-release tablet preparation comprises the following steps:
[...
Embodiment 2
[0064]The weight ratio of the drug tablet core to the semipermeable film coating is 1:0.05; the weight ratio of the drug tablet core to the semipermeable film coating to the enteric coating is 1:0.15. release tablet preparation, wherein:
[0065] The composition of the medicine tablet core is: 22.5 parts of salvianolic acid B, 105 parts of microcrystalline cellulose, 40 parts of lactose, 50 parts of sodium chloride, 6 parts of povidone K30 and 0.1 part of magnesium stearate.
[0066] The composition of the semipermeable film coating is: 3 parts of cellulose acetate and 1 part of polyethylene glycol 1500.
[0067] The composition of the enteric coating is: 55 parts of acrylic resin No. I, 10 parts of liquid paraffin, 5 parts of diethyl phthalate, 10 parts of castor oil, 10 parts of Tween-80 and 10 parts of talcum powder.
[0068] The preparation method of the above-mentioned salvianolic acid B enteric-coated controlled-release tablet preparation comprises the following steps: ...
Embodiment 3
[0076] The weight ratio of the drug tablet core to the semipermeable film coating is 1:0.02; the weight ratio of the drug tablet core to the semipermeable film coating to the enteric coating is 1:0.05. release tablet preparation, wherein:
[0077] The composition of the drug tablet core is: 22.5 parts of salvianolic acid B, 88 parts of microcrystalline cellulose, 25 parts of lactose, 80 parts of sodium chloride, 6 parts of povidone K30 and 10 parts of magnesium stearate.
[0078] The composition of the semipermeable film coating is: 12 parts of cellulose acetate and 1 part of polyethylene glycol 1500.
[0079] The composition of the enteric coating is: 50 parts of acrylic resin No. I, 9 parts of liquid paraffin, 6 parts of diethyl phthalate, 15 parts of castor oil, 8 parts of Tween-80 and 12 parts of talcum powder.
[0080] The preparation method of the above-mentioned salvianolic acid B enteric-coated controlled-release tablet preparation comprises the following steps:
[0...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com