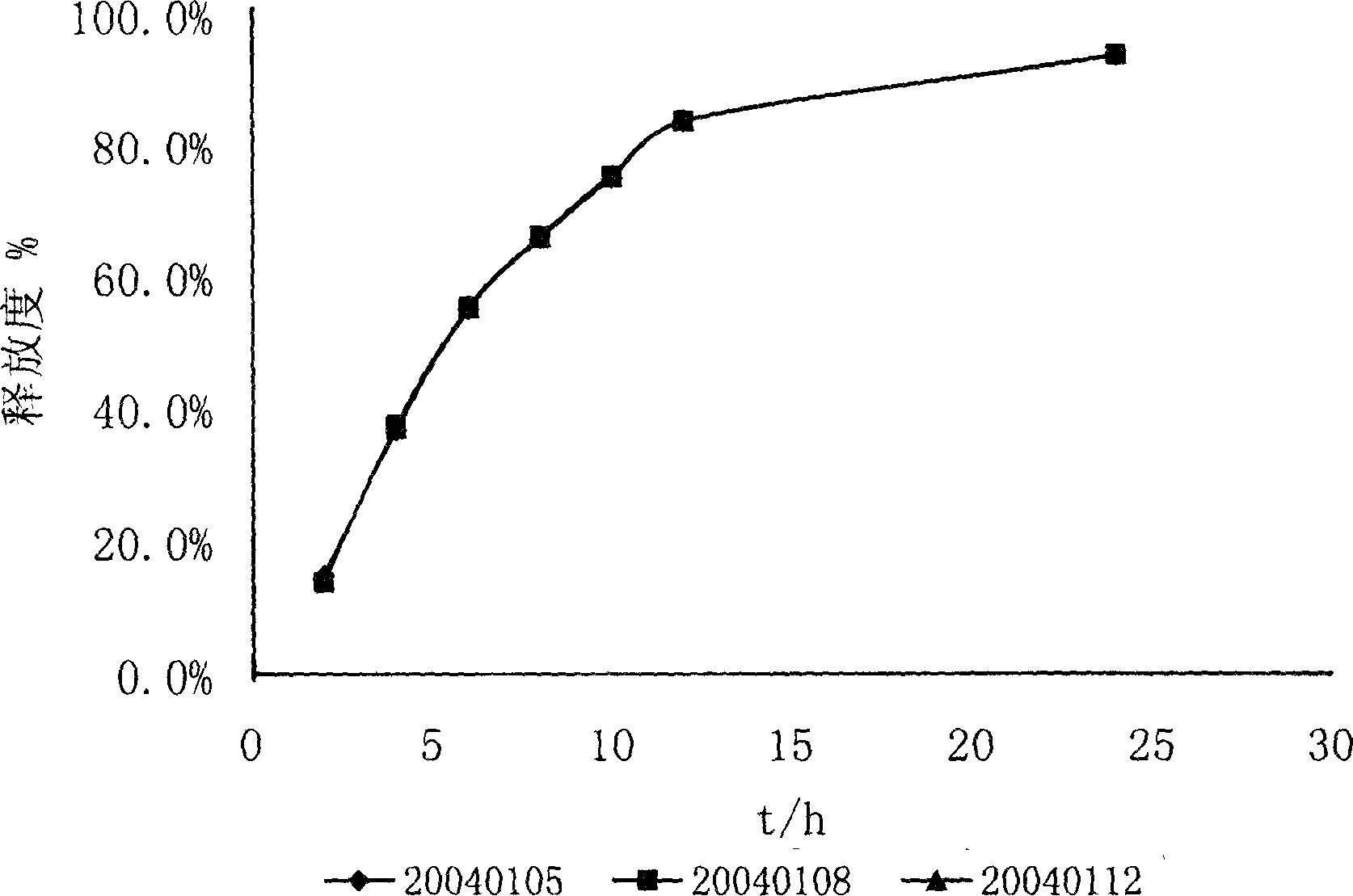
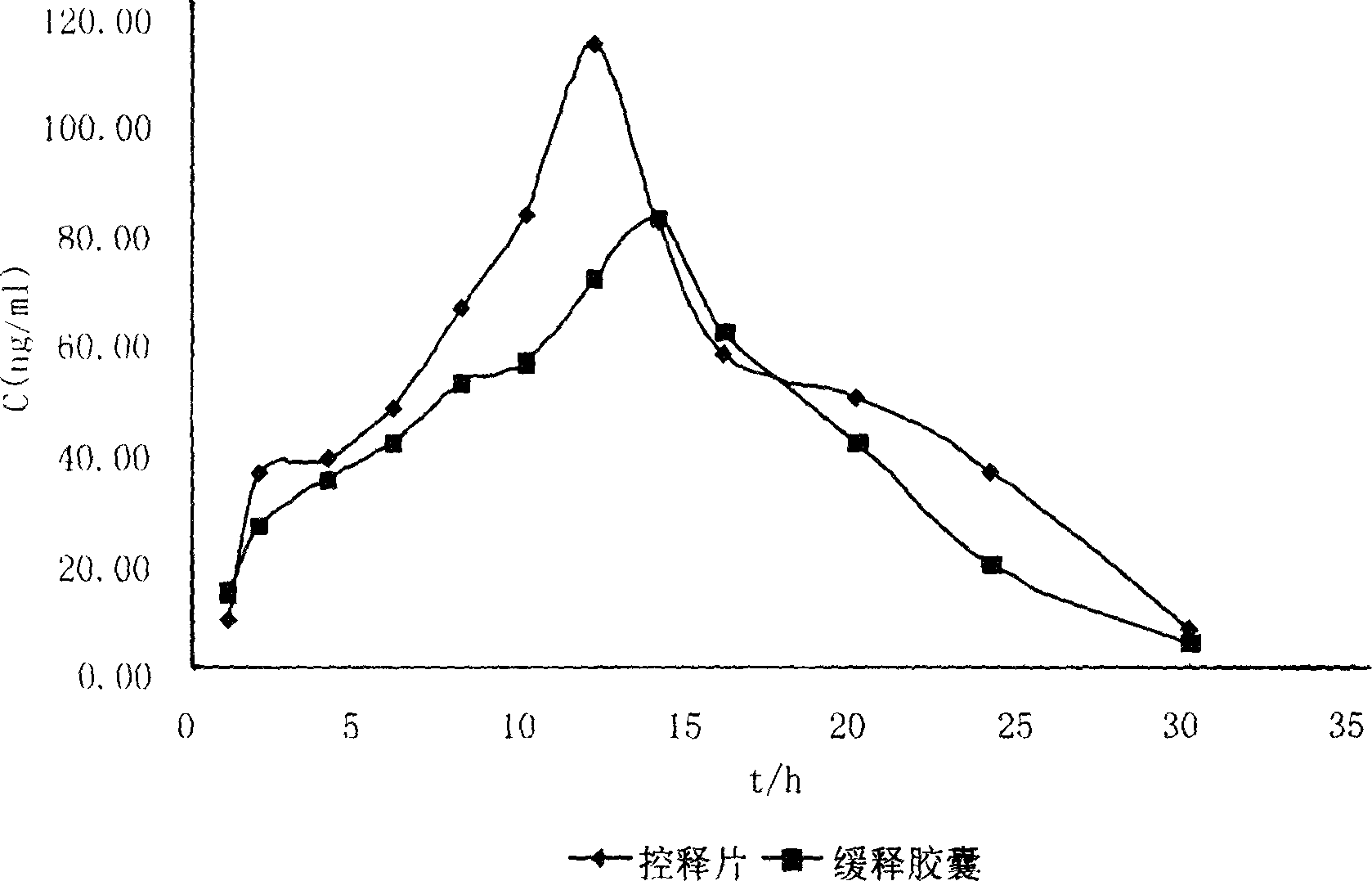
Control releasing venlafaxine hydrochloride tablet and its prepn
A technology of venlafaxine hydrochloride and controlled-release tablets, which is used in pill delivery, pharmaceutical formulations, and medical preparations containing active ingredients, etc., can solve the problems of patients with large side effects, fluctuations in blood drug concentration, and poor compliance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The venlafaxine hydrochloride controlled-release tablet preparation with a weight ratio of drug tablet core and semipermeable film coating of 1:0.035, wherein:
[0052] The composition of the drug tablet core is: 75 parts of venlafaxine hydrochloride, 114 parts of microcrystalline cellulose, 4 parts of sodium chloride, 6 parts of povidone K30 and 1 part of magnesium stearate.
[0053] The composition of the semipermeable film coating was: 9 parts of cellulose acetate, 0.78 parts of diethyl phthalate and 0.22 parts of polyethylene glycol 1500.
[0054] The preparation method of above-mentioned venlafaxine hydrochloride controlled-release tablet preparation, comprises the following steps:
[0055] (1) Preparation of drug tablet core: get the venlafaxine hydrochloride, microcrystalline cellulose, sodium chloride and povidone K30 of the proportioning, mix well, add 95% ethanol solution to make soft material in right amount, pass 20 Mesh sieve granulation, drying at 50°C, g...
Embodiment 2
[0060] The venlafaxine hydrochloride controlled-release tablet preparation with a weight ratio of drug tablet core and semipermeable film coating of 1:0.05, wherein:
[0061] The composition of the drug tablet core is: 75 parts of venlafaxine hydrochloride, 120 parts of microcrystalline cellulose, 1 part of sodium chloride, 15 parts of povidone K30 and 0.1 part of magnesium stearate.
[0062] The composition of the semipermeable film coating was: 6 parts of cellulose acetate, 0.9 part of diethyl phthalate and 0.1 part of polyethylene glycol 1500.
[0063] The preparation method of above-mentioned venlafaxine hydrochloride controlled-release tablet preparation, comprises the following steps:
[0064] (1) Preparation of drug tablet core: get the venlafaxine hydrochloride, microcrystalline cellulose, sodium chloride and povidone K30 of the proportioning, mix well, add 90% ethanol solution to make soft material in right amount, pass 20 Mesh sieve granulation, drying at 40°C, gran...
Embodiment 3
[0069] A venlafaxine hydrochloride controlled-release tablet preparation in which the weight ratio of the drug tablet core to the semipermeable film coating is 1:0.02, wherein:
[0070] The composition of the drug tablet core is: 75 parts of venlafaxine hydrochloride, 100 parts of microcrystalline cellulose, 30 parts of sodium chloride, 1 part of povidone K30 and 10 parts of magnesium stearate.
[0071] The composition of the semipermeable film coating was: 12 parts of cellulose acetate, 0.6 part of diethyl phthalate and 0.4 part of polyethylene glycol 1500.
[0072] The preparation method of above-mentioned venlafaxine hydrochloride controlled-release tablet preparation, comprises the following steps:
[0073] (1) Preparation of drug tablet core: get the venlafaxine hydrochloride, microcrystalline cellulose, sodium chloride and povidone K30 of the proportioning, mix well, add 95% ethanol solution to make soft material in right amount, pass 20 Mesh sieve granulation, drying a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com