Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
122 results about "Venlafaxine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Venlafaxine is used to treat depression, anxiety, panic attacks, and social anxiety disorder (social phobia).
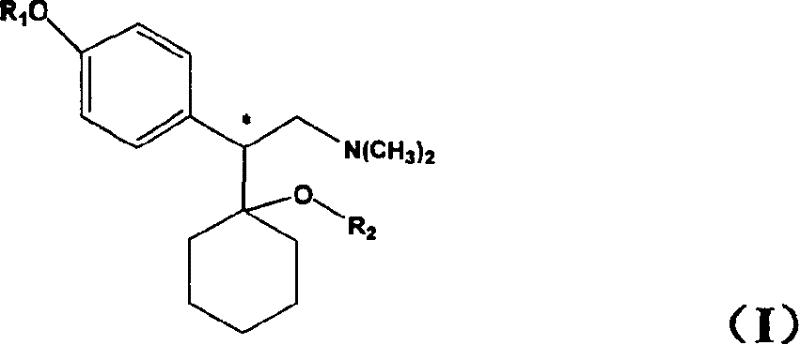
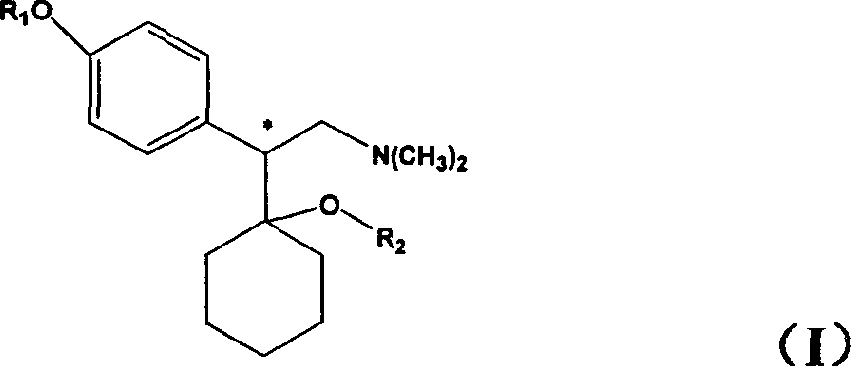
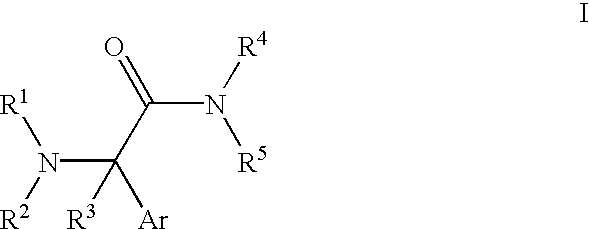
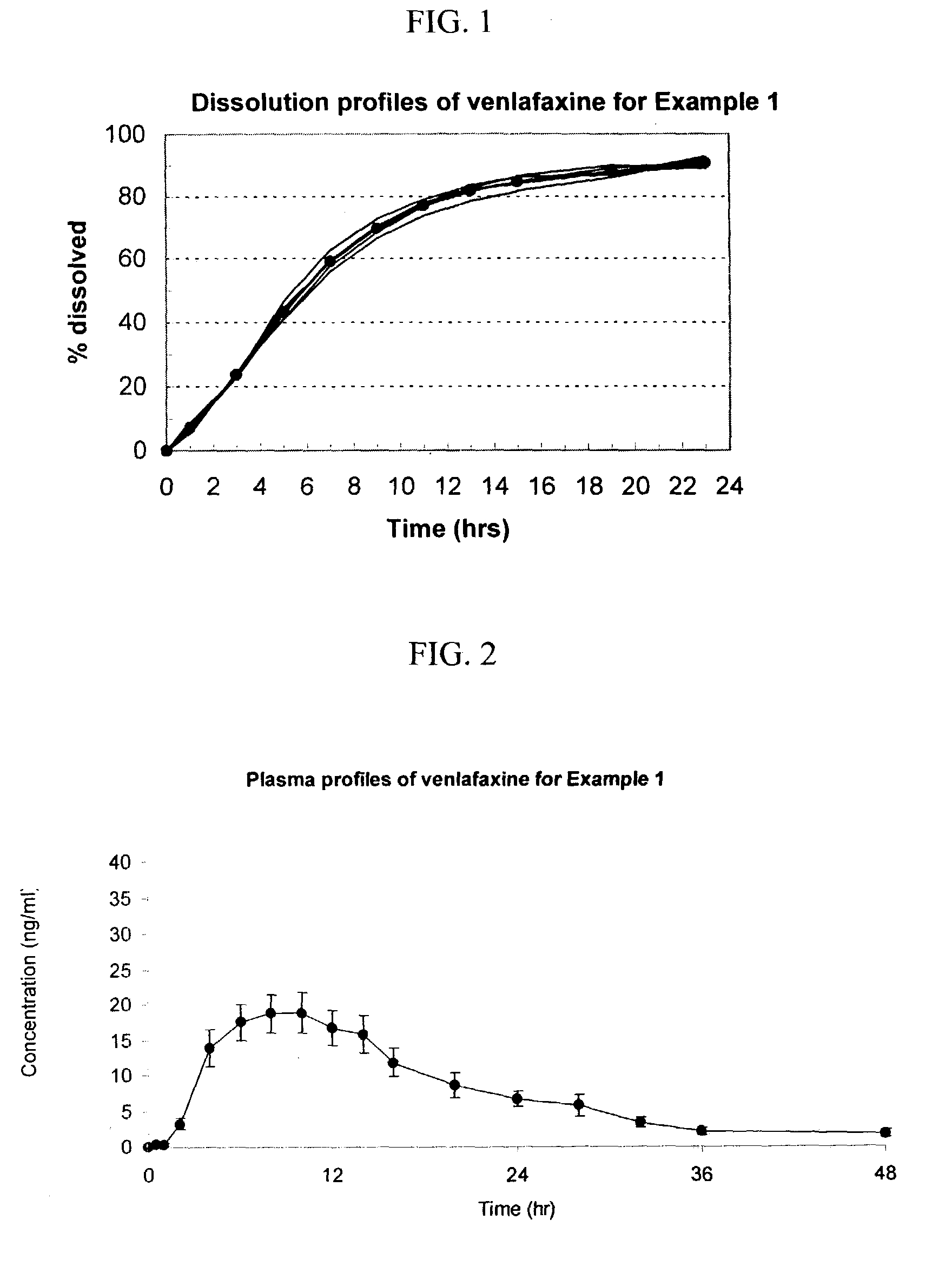
Medicine precursor containing long chain fatty acyl group substituted venlafaxine and its prepn and use
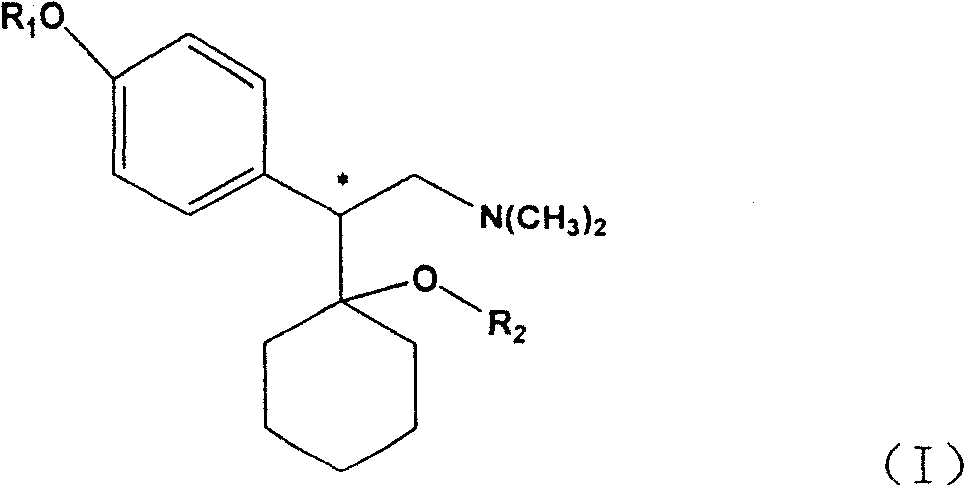
The present invention discloses one kind of medicine precursors containing long chain fatty acyl group substituted venlafaxine in the structure as shown in general expression I and its medicinal salt and hydrate. The present invention also discloses the preparation process of the medicine precursors and their application in preventing and treating diseases of central nerve system. The medicine precursors of the present invention has half life near or over 10 hr, and compared with venlafaxine, they has excellent long-acting effect.
Owner:RADIOLOGY INST ACAD OF MILITARY MEDICINE SCI PLA
Combination treatment for impaired motor function in parkinson's disease
InactiveUS20060063810A1Shorten the timeIncreased “ on ” timeBiocideNervous disorderNR1 NMDA receptorNMDA receptor
The invention provides a method, and dosage form therefor, of treating impaired motor function associated with Parkinson's disease, anti-Parkinson's drug treatment, e.g. L-Dopa therapy, and / or dementia associated with Parkinson's disease. The invention includes the combined administration of an NMDA receptor antagonist and an antidepressant, e.g., the combination of amantadine and citalopram or venlafaxine, or an NMDA receptor antagonist and an anxiolytic agent, e.g., amantadine and buspirone or trazodone, for the amelioration of undesired tremors, akinesia, dyskinesia, or bradykinesia associated with one or more different disorders or diseases. The drugs can be included in a single dosage form. One embodiment includes a combination dosage form containing each drug in controlled release forms. Another embodiment includes a combination dosage form providing a controlled release of an NMDA receptor antagonist and a rapid release of a neuroactive agent after administration to a subject.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
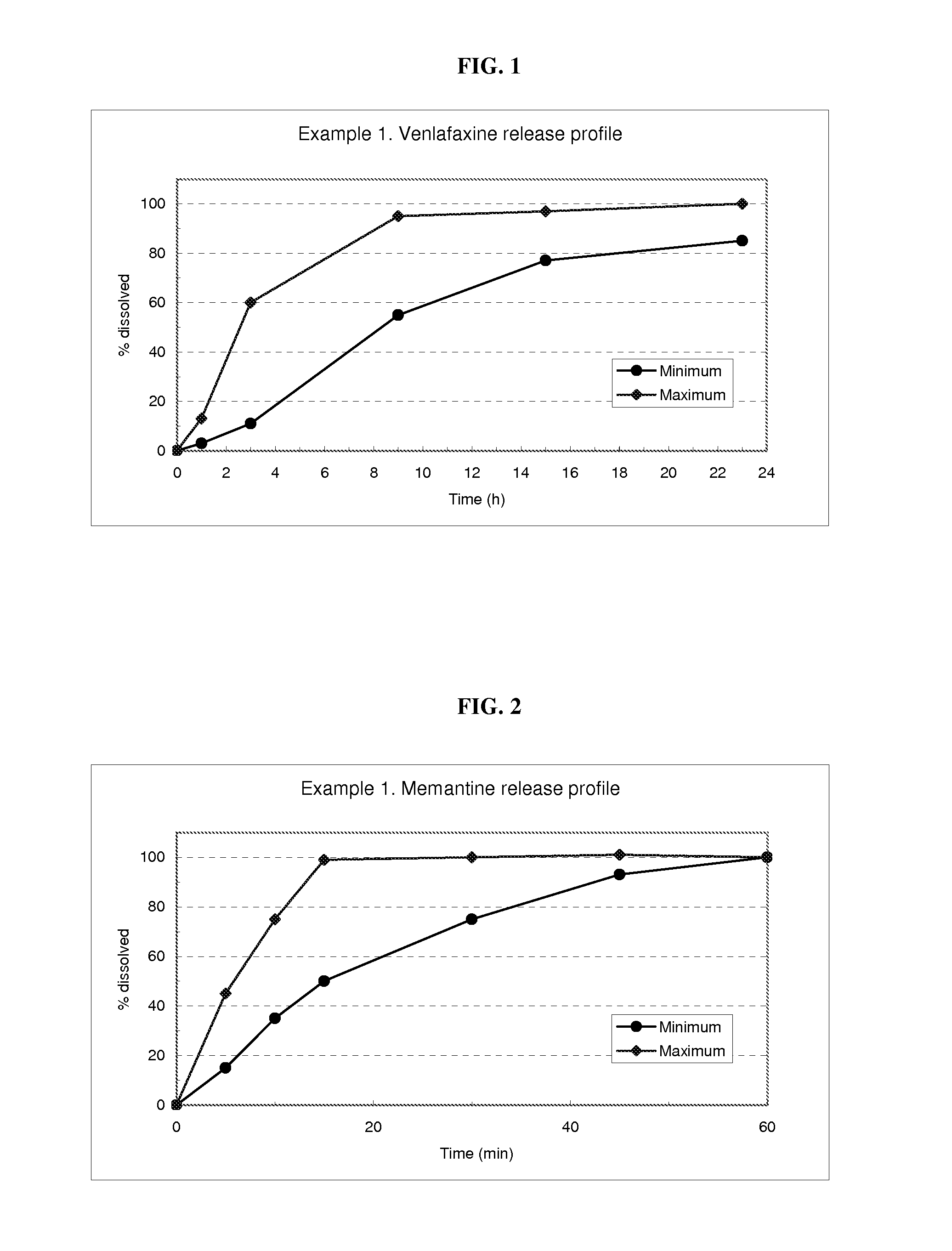
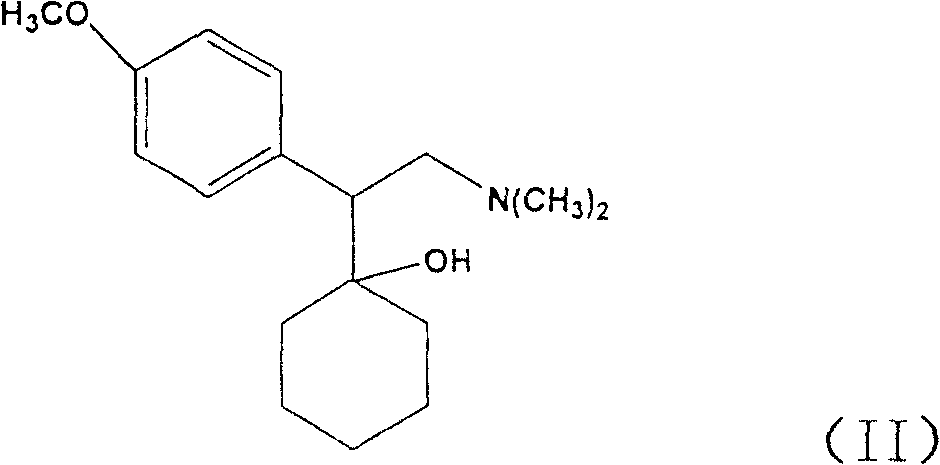
Delivery device containing venlafaxine and memantine and methods of use thereof
The present invention provides an osmotic device containing controlled release venlafaxine in the core in combination with an anti-Alzheimer's or an anti-Parkinson's drug in a rapid release external coat. Memantine is used as an anti-Alzheimer's drug or an anti-Parkinson's drug. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray-coated rather than compression-coated onto the device. The device is useful for the treatment of depression in Alzheimer's disease and / or Parkinson's disease patients. The device and method can also be used to treat or ameliorate other symptoms associated with Alzheimer's disease, Parkinson's disease or any other neurological disorder. Other dosage forms that provide a controlled, sustained or extended release of venlafaxine in combination with a rapid or immediate release of memantine are useful in the invention.
Owner:OSMOTICA CORP
Treatment of chronic pain associated with drug or radiation therapy
Methods for treating chronic widespread pain associated with drug therapy or radiation therapy are described. The method generally involves administering a therapeutically effective amount of a dual or tri reuptake inhibitor of a specific type or a pharmaceutically acceptable salt thereof. Preferably the compound is a non-tricyclic dual reuptake inhibitor. The most preferred compound is milnacipran or a bioequivalent or pharmaceutically acceptable salt thereof. Other preferred compounds are duloxetine and venlafaxine or a bioequivalent or pharmaceutically acceptable salt thereof. In yet another embodiment, a therapeutically effective amount of a non-tricyclic triple reuptake inhibitor (“TRI”) compound of a specific type, or a pharmaceutically acceptable salt thereof, is administered. The TRI compounds are characterized by their ability to block the reuptake (and, hence, increase central concentrations of) the three primary brain monoamines: serotonin, noradrenaline, and dopamine.
Owner:CYPRESS BIOSCI
Method for treating tension-type headache
Tension-type headache is treated by interacting smith neuronal transmission in relation to pain in connection with headache in a way which prevents or decreases sensitization of second order nociceptive neurons. In particular, treatment is performed by administration of an effective amount of a substance which prevents or decreases central sensitization. Important examples of such substances are substances which interact with glutamate neurotransmission, such as glutamate receptor antagonists, such as NMDA receptor antagonists, such as MK-801 or Amitriptylline or Imipramine or Desipramine or Mirtazaprine or Venlafaxine. Other examples are substances which interact with nitric oxide, such as nitric oxide synthase (NOS) inhibitors, such as L-NMMA or L-NAME or L-NIO or L-NNA. According to a broader aspect of the invention tension-type headache is treated by administration of substances which are effective in preventing or decreasing pain in connection with tension-type headache, such as the substances mentioned above. An additional aspect of the invention relates to treatment of tension-type headache by administration of substances which substantially inhibit the activity of nitric oxide synthase (NOS), such as NOS inhibitors, such as L-NMMA or L-NAME or L-NIO or L-NNA.
Owner:NEURAXON INC
Phosphates of secondary alcohols
InactiveUS20060281715A1High metabolismReduced bioavailabilityBiocideNervous disorderAlcoholVenlafaxine
According to the invention, there is provided a phosphate derivative of a compound having a secondary hydroxy group. The compound having a secondary hydroxyl group may, for example, be chosen from pravastatin, atorvastatin venlafaxine, their derivatives and mixtures thereof.
Owner:VITAL HEALTH SCIENCES PTY LTD
Controlled porous osmotic pump tablets of high permeable drugs and the preparation process thereof
The present invention relates to controlled porous osmotic pump tablets of high permeable drugs and the preparation process thereof. The controlled porosity osmotic pump tablets do not need to be drilled by laser, but provides controlled porosity for drug release by adding a suitable quantity of pore-forming agents into the semipermeable membrane. In specific embodiments, the present invention relates to controlled porous osmotic pump tablets comprising venlafaxine or metoprolol or pharmaceutically acceptable salts thereof.
Owner:COSCI MED TECH CO LTD
Venlafaxine osmotic device formulation
InactiveUS20070077301A1Reduced food effectReducing food effectBiocideOrganic active ingredientsImmediate releaseNeurological disorder
The present invention provides an osmotic device containing controlled release venlafaxine in the core, wherein the osmotic device exhibits a reduced food effect as compared to a reference controlled release capsule formulation. Some embodiments include venlafaxine in controlled release form in combination with an anti-Alzheimer's or an anti-Parkinson's drug in a rapid release external coat. Memantine is used as an anti-Alzheimer's drug or an anti-Parkinson's drug. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray-coated rather than compression-coated onto the device. The device is useful for the treatment of depression in Alzheimer's and / or Parkinson's patients. The device and method can also be used to treat or ameliorate other symptoms associated with Alzheimer's disease, Parkinson's disease or any other neurological disorder. Other dosage forms that provide a controlled, sustained or extended release of venlafaxine in combination with a rapid or immediate release of memantine are useful in the invention.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO
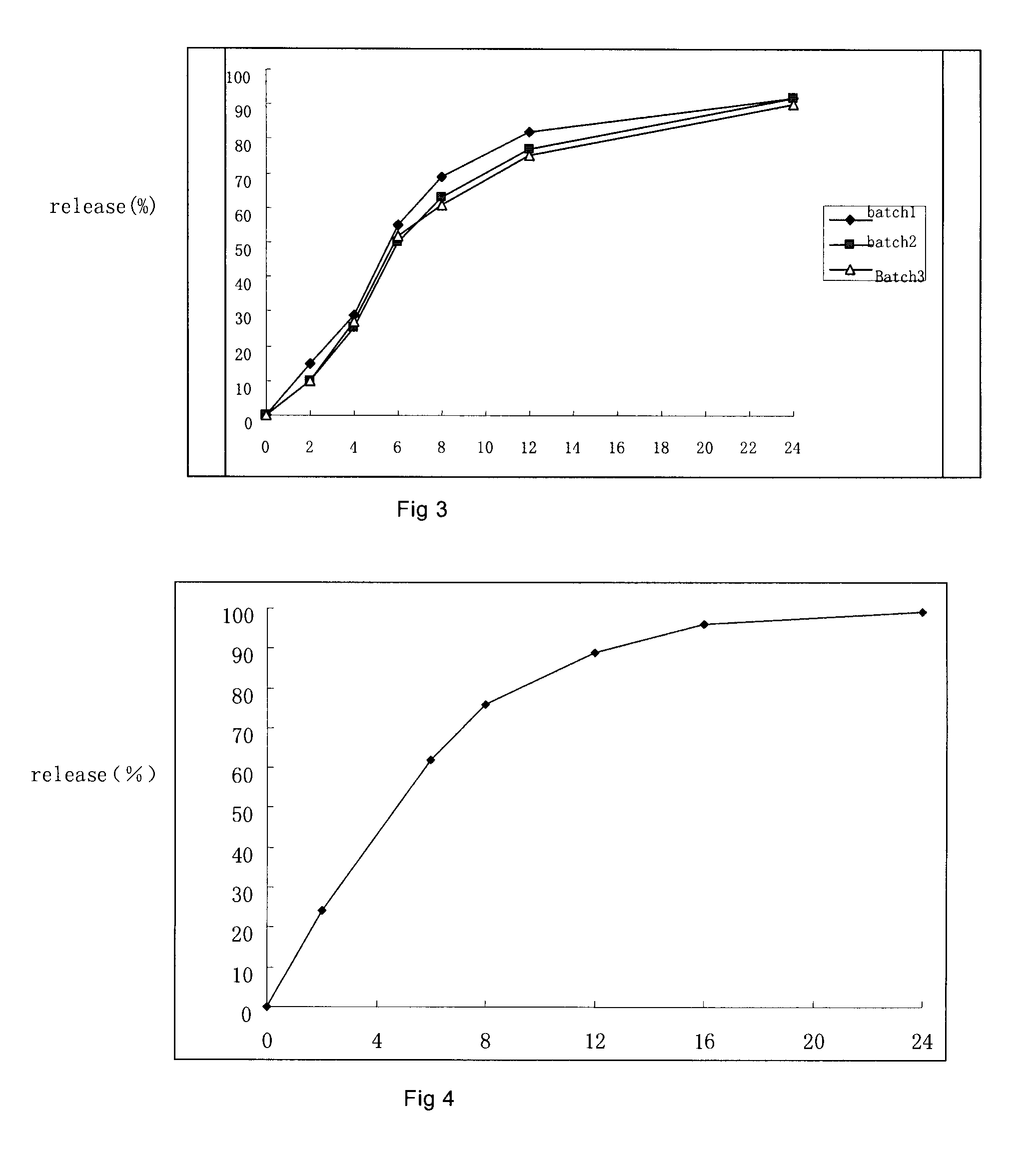
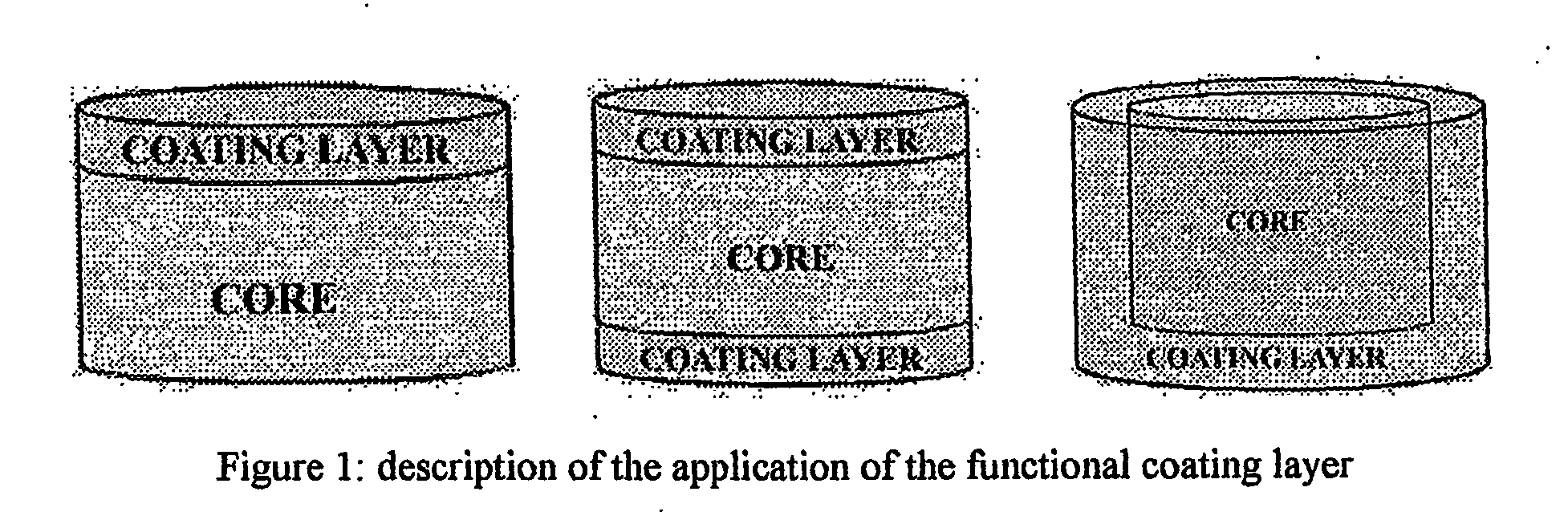
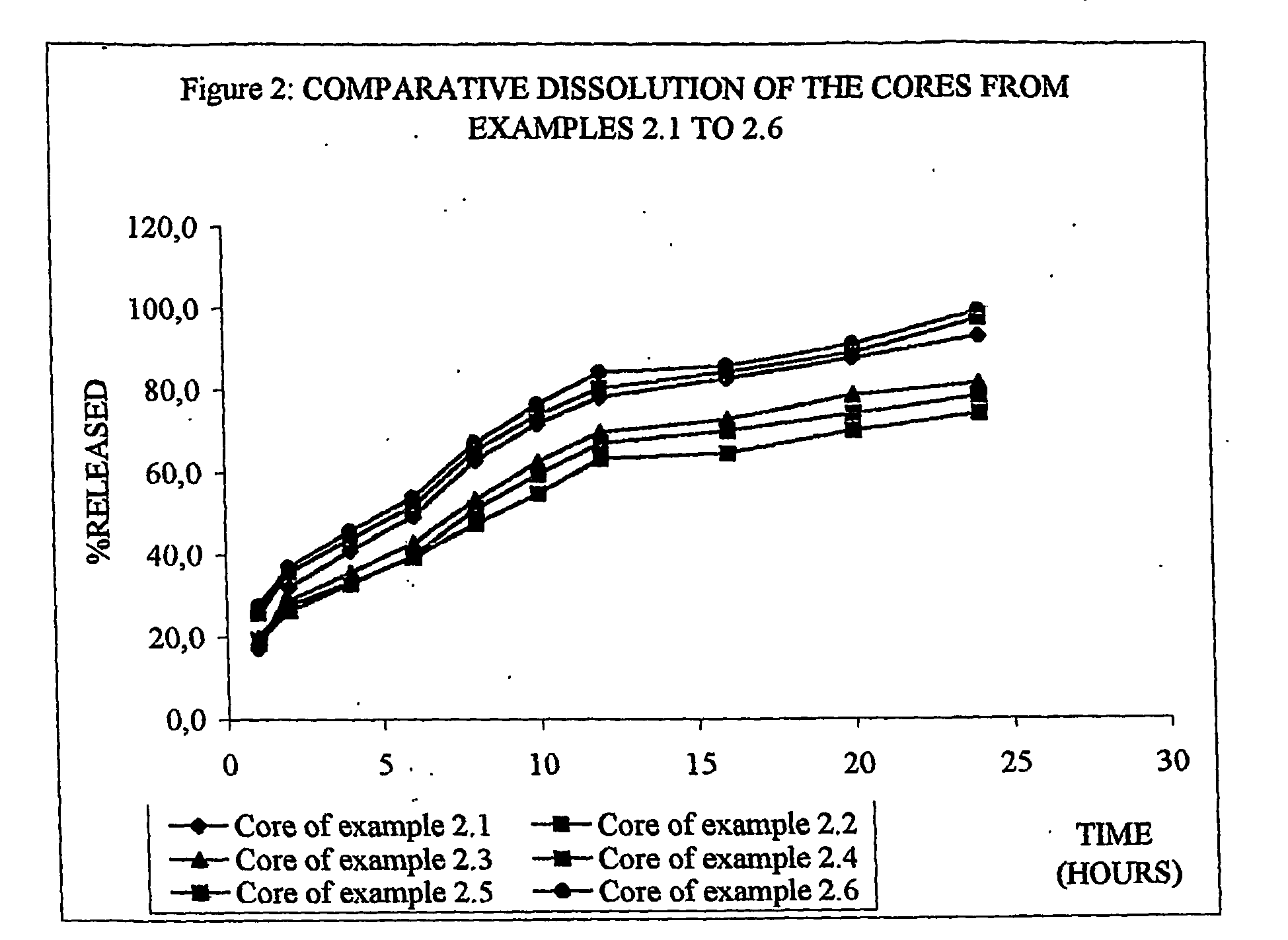
Sutained release formulation for venlafaxine hydrochloride
InactiveUS20060182797A1Quick releaseOrganic active ingredientsNervous disorderSustained Release CapsuleMini tablets
The invention provides a sustained release composition that; 1. Is free of initially increased drug delivery that occurs (in sustained release systems containing the water soluble drug venlafaxine HCl, known as burst phenomenon, by using a functional core partially or totally coated by a functional coating layer or film. 2. Delivers the drug substance within 24 hours and is therefore suitable for once daily administration of the said drug substance. 3. Exhibits linearity between the strength dosage form and the (total mass of the dosage form, by proportional increase of the amounts of the drug substance and the excipients in the formulation. 4. Is possible to be divided in smaller doses, without affecting the release of the drug substance. The invention provides a sustained release capsule formulation containing an appropriate number of functional complex mini tablets comprising of: I. A functional core comprising the active ingredient, especially the water-soluble drug Venlafaxine HCl and appropriate excipients. 2. A functional coating layer or film that reduces the initial surface of the core that is available for the release of the water-soluble drug Venlafaxine HClt phenomenon.
Owner:PHARMATHEN
Osmotic device containing venlafaxine and an anti-psychotic agent
The present invention provides an osmotic device containing controlled release venlafaxine in the core in combination with an anti-psychotic agent in a rapid release external coat. A wide range of anti-psychotic agents can be used in this device. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray-coated rather compression-coated onto the device. The device with spray-coated external core is smaller and easier to swallow than the similar device having a compression-coated external coat. The device is useful for the treatment of depression, anxiety or psychosis related disorders.
Owner:OSMOTICA KERESKEDELMI & SZOLGALTATO +1
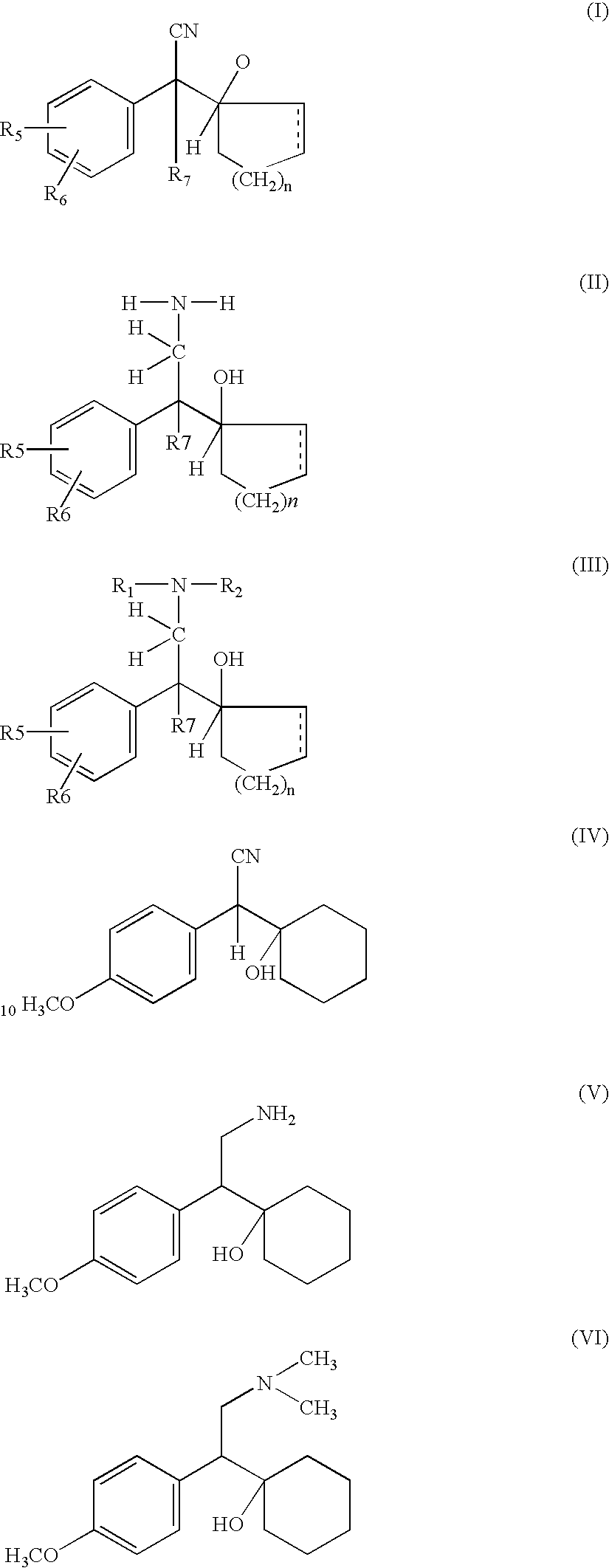
Manufacture of phenyl ethylamine compounds, in particular venlafaxine
InactiveUS7026513B2Reduce the cyanocarbinol most effectivelyPreparation by oxidation reactionsOrganic compound preparationPhenyl groupEthylamine
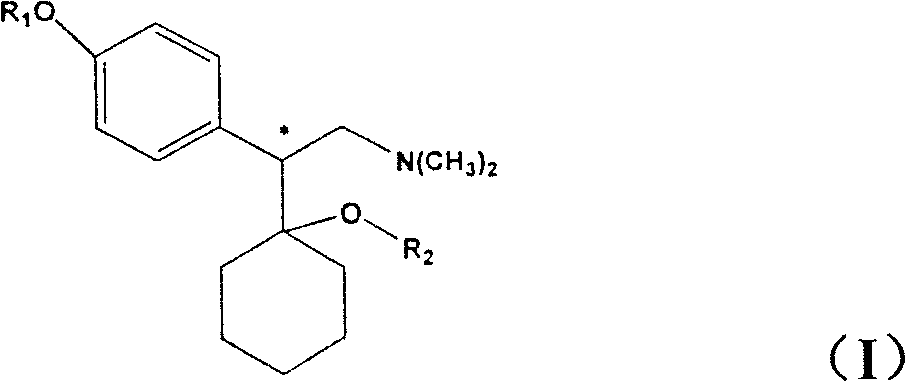
A process for the preparation of hydroxy(cycloalkane / cyclokene) phenylethyl amine of the general formula (III) comprising alkylation of its precursor amine of general formula (II) which is in turn produced by an effective reduction process from its precursor cyanide having the general formula (I) using Raney Nickel (CORMIII) as catalyst where, either of R5 and R6 independently could be in meta or para position and R5, R6 are independently hydrogen, hydroxyl, alkyl, alkanoyloxy, cyano, nitro, alkylmercapto, amino, alkylamino, allkanamido, halo, trifluoromethyl, or taken together methylenedioxy, n is 0, 1, 2, 3, 4, R7 is hydrogen of alkyl of 1–7 carbon atom, R1 IH or alkyl of 1–3 carbon atom and R2 is alkyl 1–3 carbon atom, the dotted line represents optional unsaturation. Compounds of formulae IV, V and VI are respectively derivatives of compounds I, II and III respectively.
Owner:NICHOLAS PIRAMAL INDIA LTD
Venlafaxine hydrochloride sustained-release tablet preparation and preparation method thereof
InactiveCN101584674AGood sustained release effectOrganic active ingredientsNervous disorderSustained Release TabletAdhesive
The invention discloses a venlafaxine hydrochloride sustained-release tablet preparation which comprises venlafaxine hydrochloride, framework material, diluent, lubricant, adhesive and coating material. The experiments demonstrate that the venlafaxine hydrochloride sustained-release tablet achieves better sustained-release effect, can reduce the dosing frequency and is convenient for the patients to use. The invention also provides a preparation method thereof.
Owner:上海医药科技发展有限公司
Controlled release venlafaxine formulations
InactiveUS20060193911A1Reduce dosageEliminate side effectsOrganic active ingredientsPill deliveryCross-linkControlled release
In certain embodiments, the present invention is directed to a controlled release oral solid dosage form comprising a matrix comprising a therapeutically effective amount of venlafaxine, an active metabolite of venlafaxine, or a pharmaceutically acceptable salt thereof, dispersed in a cross-linked gelling agent, the matrix providing a controlled release of venlafaxine, active metabolite of venlafaxine, or salt thereof to provide 24 hour therapeutic plasma levels after oral administration to human patients.
Owner:PENWEST PHARMA CO
Modified release composition of at least one form of venlafaxine
The present invention relates to a modified release composition of at least one form of venlafaxine, which is a delayed controlled release composition. The composition comprises a core comprising at least one form of venlafaxine selected from the group consisting of venlafaxine, an active metabolite of venlafaxine, a pharmaceutically acceptable salt of venlafaxine, a pharmaceutically acceptable salt of an active metabolite of venlafaxine, and combinations thereof, less than 10% of a gelling agent and a pharmaceutically acceptable excipient. The composition further comprises a modified release coating which substantially surrounds the core which provides a delayed controlled release of the at least one form of venlafaxine.
Owner:BIOVAIL LAB INT SRL
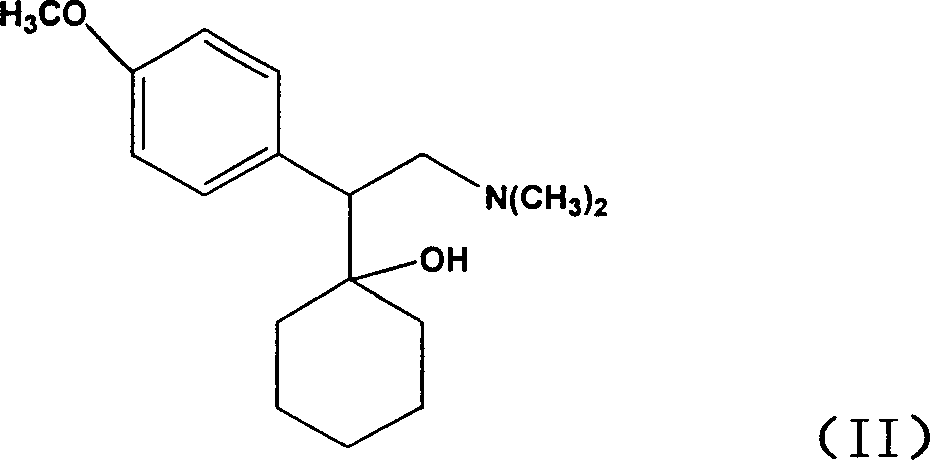
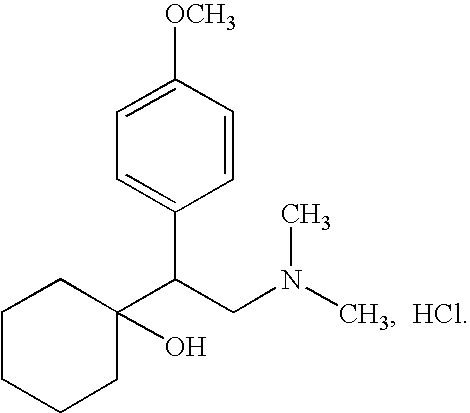
Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol
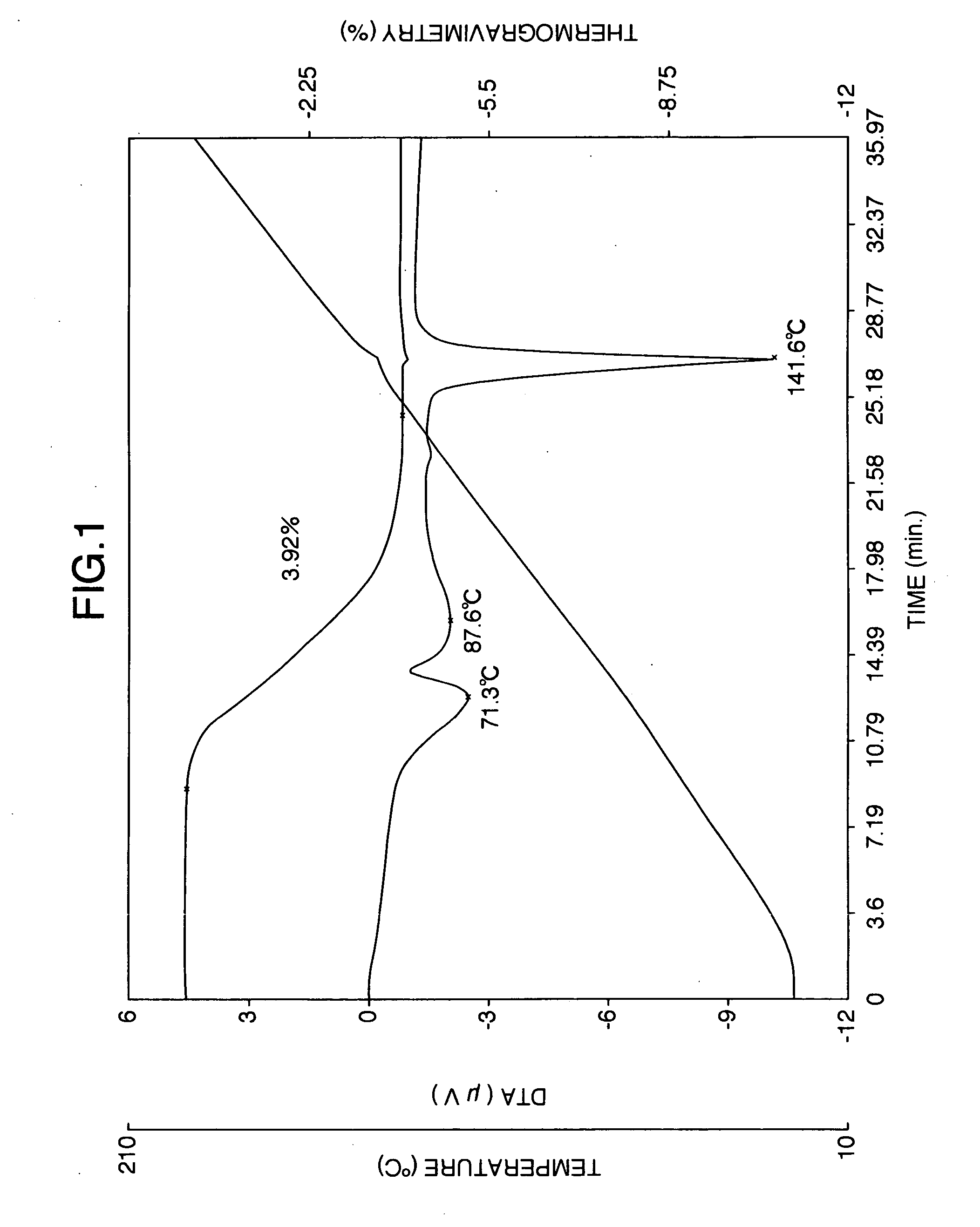
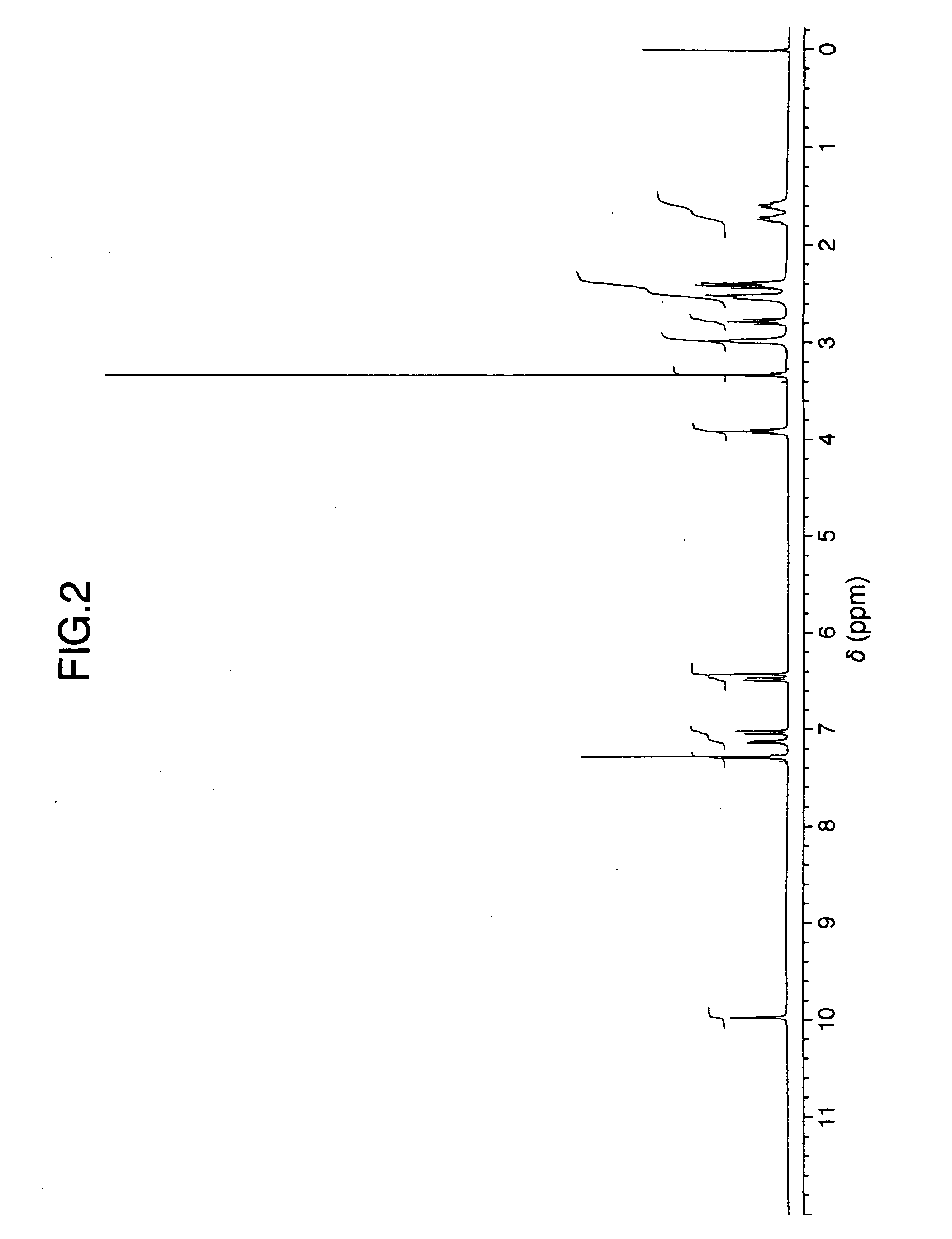
InactiveCN101503365ASuitable for industrial productionMild reaction conditionsNervous disorderMetabolism disorderPotassium borohydrideVenlafaxine
The invention discloses a method for preparing venlafaxine intermediate 1, (2- amino-1-(4- methoxyphenyl) ethide cyclohexanol. The method is characterized in that 1- cyano-((4- methoxyphenyl) methyl) cyclohexanol is deacidized by sodium borohydride or potassium borohydride under the catalysis of elemental iodine to obtain the 1, (2- amino-1-(4- methoxyphenyl) ethide cyclohexanol. The method has the advantages of mild reaction condition, simple post treatment, high yield, good product purity and low cost, and is suitable for commercial process.
Owner:CHENGDU QIAOFENG TECH DEV
Carbostyril derivatives and serotonin reuptake inhibitors for treatment of mood disorders
The pharmaceutical composition of the present invention comprises (1) a carbostyril derivative and (2) a serotonin reuptake inhibitor in a pharmaceutically acceptable carrier. The carbostyril derivative may be aripiprazole or a metabolite thereof, which is a dopamine-serotonin system stabilizer. The serotonin reuptake inhibitor may be fluoxetine, duloxetine, venlafaxine, milnacipran, citalopram, fluvoxamine, paroxetine, sertraline or escitalopram. The pharmaceutical composition of the present invention is useful for treating patients with mood disorders, particularly depression or major depressive disorder.
Owner:OTSUKA PHARM CO LTD
Pharmaceutical compositions containing venlafaxine
InactiveUS20060057204A1Improve bioavailabilityFlat and consistent concentration levelOrganic active ingredientsOrganic chemistryParticulatesVenlafaxine
The invention provides a delayed burst release formulation comprising a core formed as a compressed tablet and an outer coating that surrounds the core; said core comprising venlafaxine, or a pharmaceutically acceptable salt thereof, at least one burst controlling agent and a disintegrant; and said outer coating comprising a water insoluble hydrophobic carrier and water-insoluble but hydrophilic particulate matter.
Owner:DEXCEL PHARMA TECH
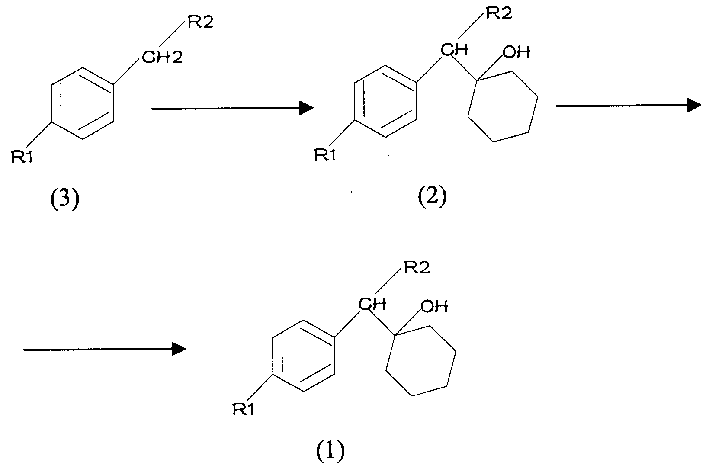
Preparing technology for cyclohexanol derivatives used to prepare the intermediate of Venlafaxine
InactiveCN1504456AMild preparation conditionsNo explosion hazardCarboxylic acid nitrile preparationOrganic compound preparationSolventEthylamine
The invention discloses a process for preparing hexahydrophenol derivative for making the intermediate of venlafaxime, a novel antidepressant drug, comprising the steps of, choosing suitable dissolvent and reaction condition, by the action of initiation nitriles or polyamides compounds having general formula (3) with alkali metal chloride hydrogenated or organic lithium derivatives, compound having general formula (2) can be obtained, under suitable dissolvent and reaction conditions, compounds having general formula (2) can be transformed into compounds having general formula (1) through aluminium-hydrogen compounds reduction or catalytic hydrogenation with nickel presence. The product is antidepressant drug with proven curative effects.
Owner:重庆凯林制药有限公司
Modified release composition of at least one form of venlafaxine
The present invention relates to a modified release composition of at least one form of venlafaxine, which is an enhanced absorption delayed controlled release composition. The composition comprises a core comprising at least one form of venlafaxine, less than 10% of a gelling agent and a pharmaceutically acceptable excipient. The composition further comprises a modified release coating which substantially surrounds the core which provides a delayed controlled release of the at least one form of venlafaxine.
Owner:BIOVAIL LAB INT SRL
Venlafaxine sustained-release capsule and preparation process thereof
ActiveCN103054835AReduce osmotic pressureReduce solubilityOrganic active ingredientsNervous disorderBiotechnologyCellulose
The invention discloses a venlafaxine sustained-release capsule and a preparation process thereof. Ethyl cellulose and enteric acrylic acid resin are compounded as an adhesive; an isolation layer is coated outside a blank pellet, so that the pellet is not released at all, the osmotic pressure inside a sustained-release pellet is reduced, and the release speed of the medicine is decelerated; and meanwhile the adhesive is used for a medicine-containing layer and a sustained-release protection layer, so that venlafaxine medicine layers are encapsulated in a sustained-release material, the dissolubility of the pellet is further reduced, and the stable release of the medicine is guaranteed to reach a standard requirement.
Owner:珠海天翼医药技术开发有限公司
Method of manufacturing sustained release microbeads containing venlafaxine HCL
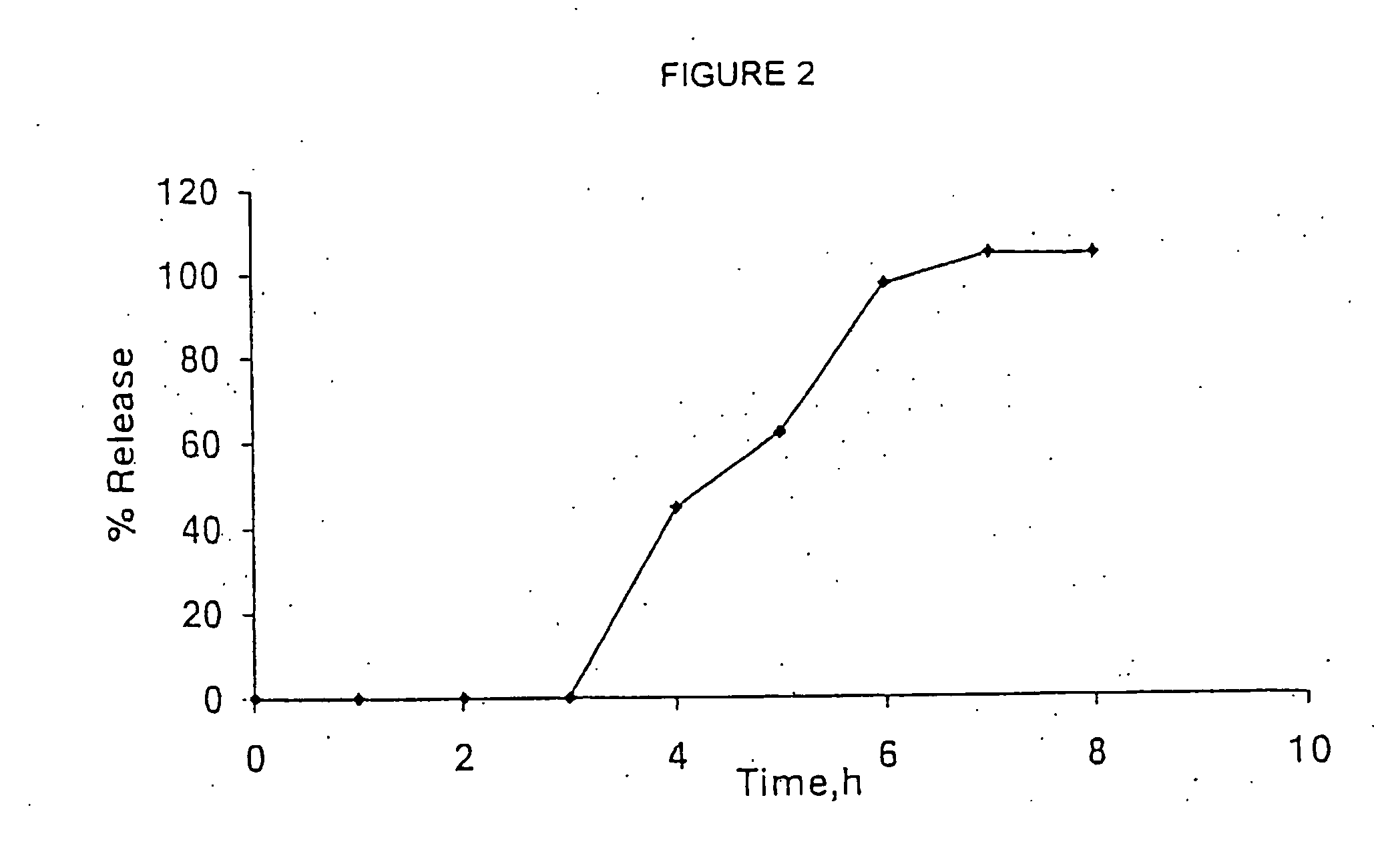
A sustained release Venlafaxine composition that includes a plurality of non-agglomerated, uniformly-shaped and sized microbeads of inert core particles having a first coating layer. The first coating layer includes an active agent of Venlafaxine or a pharmaceutical acceptable salt thereof, a binder, and an anti-tack agent. The active agent is present in the first coating layer in a concentration of at least about 5% to about 70% by weight of the composition, the binder is present in an amount of at least 35% by weight of the active agent, or in a further layer located upon or below the first coating layer, or as an alternating layer between plural first layers, wherein the binder is present in an amount of less than about 2.5% by weight of the composition, and the anti-tack agent is present in the first coating layer in a concentration of about 2.5% to about 20% by weight based on the weight of the active agent. The composition is also substantially free of organic acid.
Owner:THEMIS LAB PTE LTD
Venlafaxine hydrochloride sustained release capsule and preparation method thereof
ActiveCN102772390AReduce flammabilityLow hygroscopicityOrganic active ingredientsNervous disorderSustained release pelletsCellulose
The invention belongs to the field of pharmaceutical preparations, and discloses a venlafaxine hydrochloride sustained release capsule and a preparation method thereof. The content of the sustained release capsule is a sustained release micro pill, wherein the sustained release pill is successively provided with a pill core, an isolating layer and a sustained release layer from the interior to the exterior, the pill core is prepared from the following constituents according to weight percentage composition: 45-48% of venlafaxine hydrochloride, 15-18% of bulking agent, 3-3.5% of disintegrating agent, 4-6% of povidone K 30, 24-26% of absolute ethyl alcohol and the balance being water; the isolating layer is prepared from the following constituents according to weight percentage composition: 11-13% povidone K30, 83-86% of absolute ethyl alcohol and the balance being talcum powder; the sustained release layer is prepared from the following constituents according to weight percentage composition: 38-42% of ethocel suspension, 0.1-1.0% of polyethylene glycol-60000 and the balance being water, wherein the ethocel accounts for 20-30% of the ethocel suspension in mass; and the preparation method provided by the invention has the following steps of preparing the pill core, packing the isolating layer, packing the sustained release layer and filling a capsule, thus obtaining the sustained release capsule. The venlafaxine hydrochloride sustained release capsule provided by the invention adopts the ethocel, the flammability is low, the hygroscopicity is small, good film formation property can be achieved, the material is stable and is easy to control, and the venlafaxine hydrochloride sustained release capsule is safe and environment-friendly.
Owner:LEPU HENGJIUYUAN PHARMA CO LTD
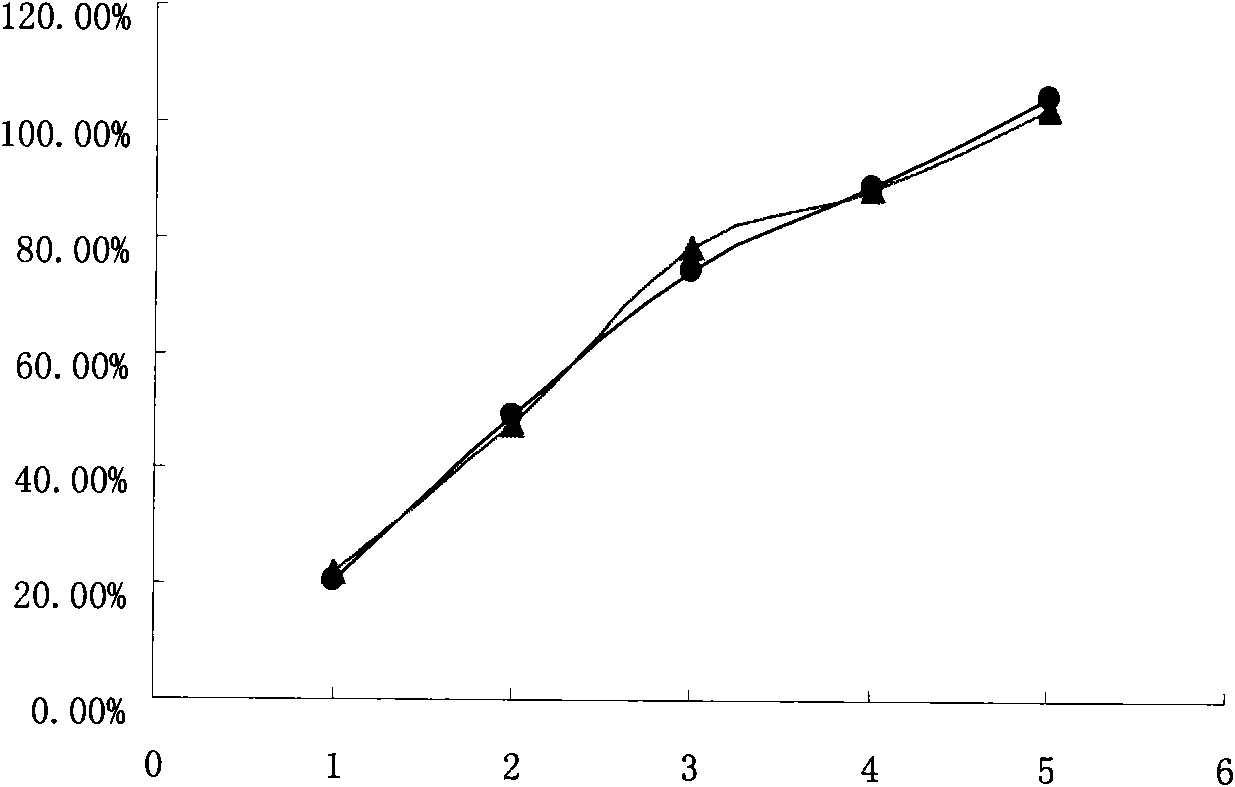
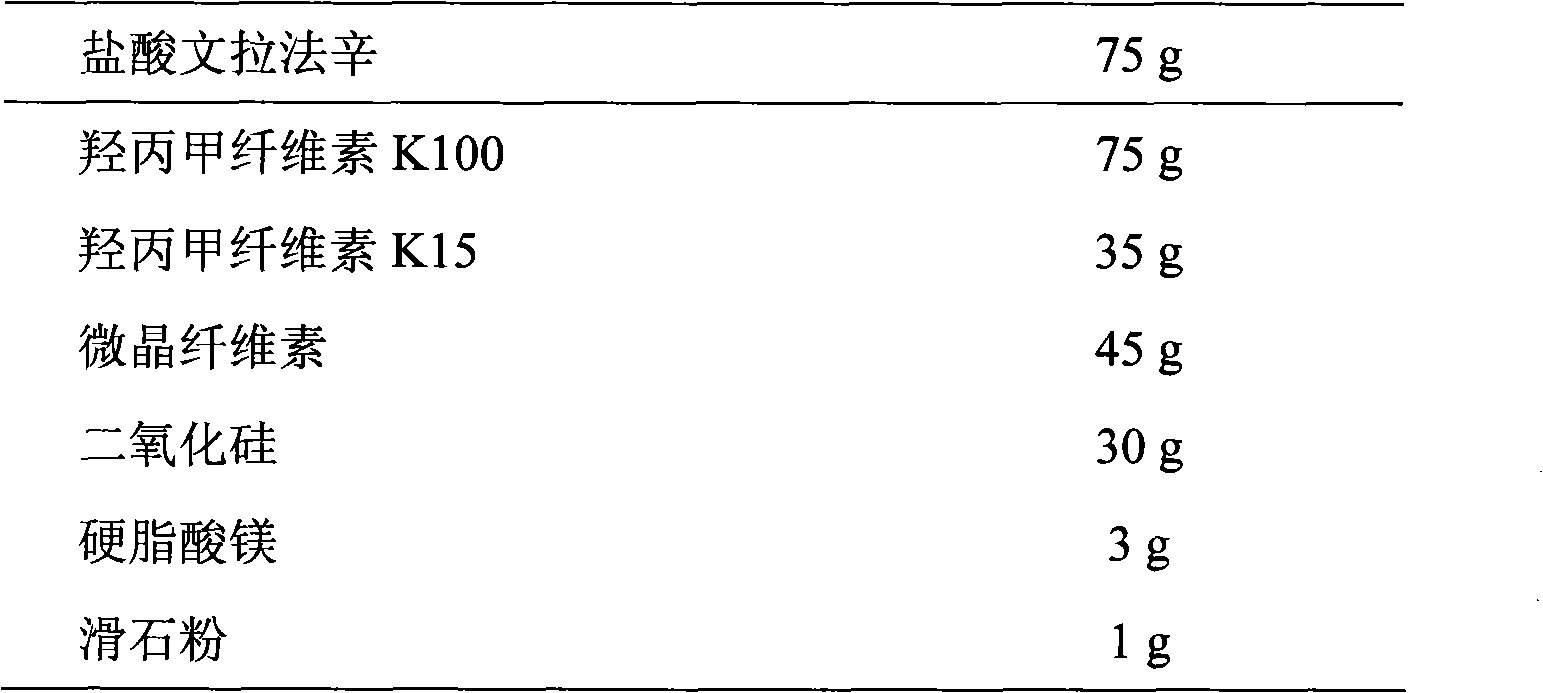
Sustained-release pharmaceutical composition containing venlafaxine hydrochloride as well as preparation method thereof
InactiveCN108210473ASave time and energyEasy to prepareOrganic active ingredientsNervous disorderSolventCalcium EDTA
The invention belongs to the field of a pharmaceutical composition for treating depression, more exactly relates to a new pharmaceutical composition of a venlafaxine hydrochloride sustained release tablet as well as a preparation method thereof. The pharmaceutical composition of the sustained release tablet consists of the following components according to weight proportion: each tablet contains 75 mg of venlafaxine hydrochloride, 25 to 250 mg of glyceryl behenate, 15 to 200 mg of hydroxypropyl methylcellulose, 20 to 200 mg of anhydrous calcium hydrogen phosphate, 1.8 to 7.2 mg of silicon dioxide and 1.8 to 7.2 mg of magnesium stearate; and the venlafaxine hydrochloride sustained release tablet is prepared by a melting granulation method, the sustained release tablet prepared by the methodcan continuously and stably release the medicine within 24 hours, stable blood concentration is maintained, the medicine use times of patients are reduced, and the medicine use safety and the compliance of the patients are improved. Compared with other granulation methods, the preparation method is time-saving and energy-saving, so the preparation process is simple, rapid and economic, solvent residues are avoided and the preparation method is suitable for industrialized production.
Owner:SHANDONG ACADEMY OF PHARMACEUTICAL SCIENCES
Osmotic device containing a venlafaxine salt and a salt having an ion in common
ActiveUS8293799B2Low release rateModify release profileBiocidePill deliveryControl releaseVenlafaxine
The osmotic devices of the present invention include a single core comprising a salt of a drug and an osmotic salt, wherein the drug salt and the osmotic salt have a common ion. The release rate of the active drug is reduced, and the release profile of the active drug is modified, from a first order release profile to a zero order, pseudo-zero order, or sigmoidal release profile, by increasing the amount of the sodium chloride in the core of the device. In one embodiment the sodium chloride is used to modify a controlled release profile to a delayed and controlled release profile.
Owner:ACELLA HLDG LLC +1
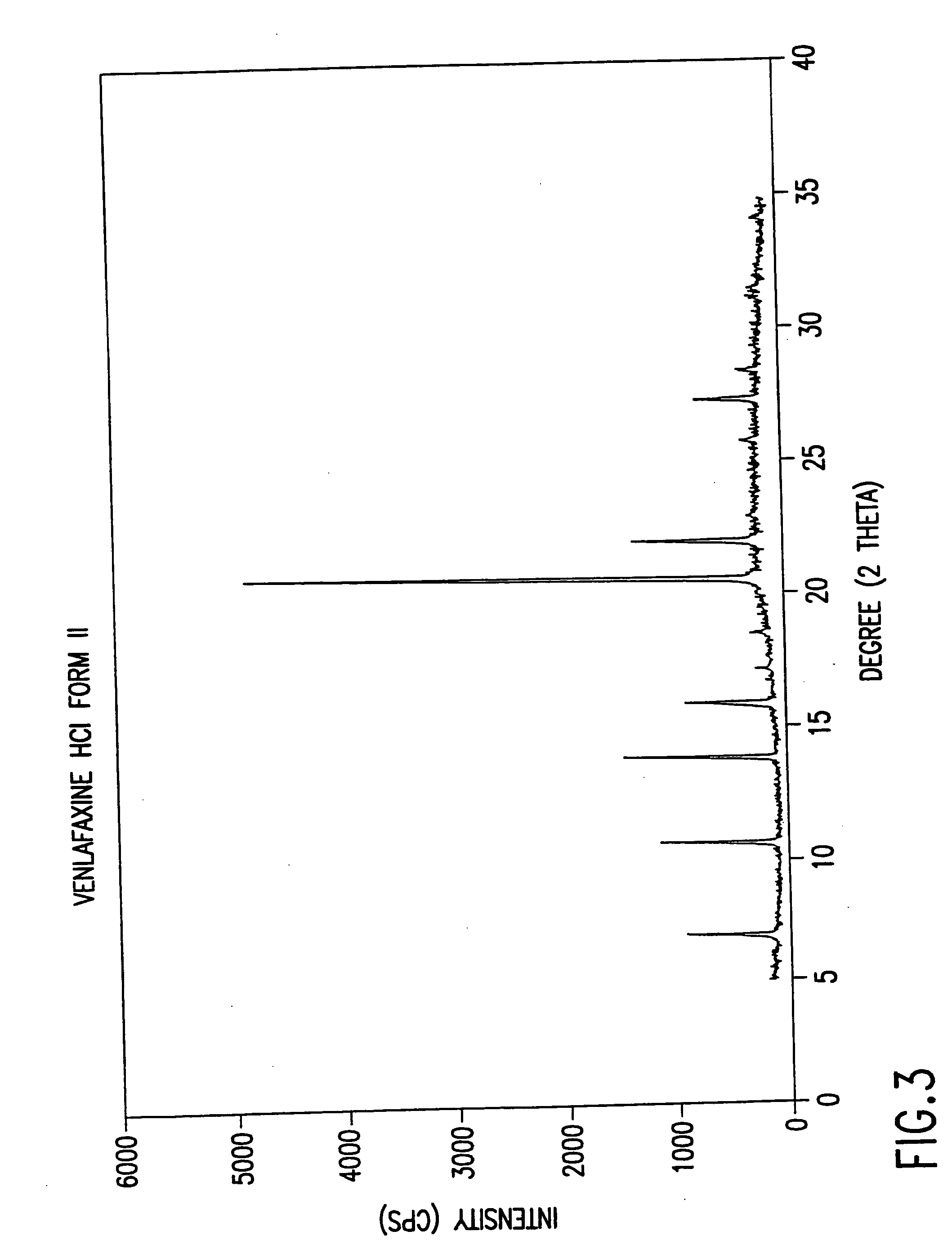
Novel crystalline polymorph of venlafaxine hydrochloride and methods for the preparation thereof
InactiveUS20060074131A1Easy to disassembleBiocideOrganic active ingredientsMedicinal chemistryVenlafaxine Hydrochloride
This invention relates to a highly thermally stable novel anhydrous crystalline polymorphic form of venlafaxine hydrochloride, methods for the preparation thereof, and its use.
Owner:WYETH LLC
Delivery device containing venlafaxine and memantine and method of use thereof
The present invention provides an osmotic device containing controlled release venlafaxine in the core in combination with an anti-Alzheimer's or an anti-Parkinson's drug in a rapid release external coat. Memantine is used as an anti-Alzheimer's drug or an anti-Parkinson's drug. Particular embodiments of the invention provide osmotic devices having predetermined release profiles. One embodiment of the osmotic device includes an external coat that has been spray-coated rather than compression-coated onto the device. The device is useful for the treatment of symptoms associate with Alzheimer's disease and / or Parkinson's disease patients. The device and method can also be used to treat or ameliorate other symptoms associated with Alzheimer's disease, Parkinson's disease or any other neurological disorder. Other dosage forms that provide a controlled, sustained or extended release of venlafaxine in combination with a rapid or immediate release of memantine are useful in the invention.
Owner:OSMOTICA CORP
Medicine precursor containing long chain fatty acyl group substituted venlafaxine and its prepn and use
The present invention discloses one kind of medicine precursors containing long chain fatty acyl group substituted venlafaxine in the structure as shown in general expression I and its medicinal salt and hydrate. The present invention also discloses the preparation process of the medicine precursors and their application in preventing and treating diseases of central nerve system. The medicine precursors of the present invention has half life near or over 10 hr, and compared with venlafaxine, they has excellent long-acting effect.
Owner:RADIOLOGY INST ACAD OF MILITARY MEDICINE SCI PLA
Venlafaxine hydrochloride slow-release capsule and preparation method thereof
InactiveCN103181916AGood sustained release effectAvoid stickingOrganic active ingredientsNervous disorderGas phasePolyethylene glycol
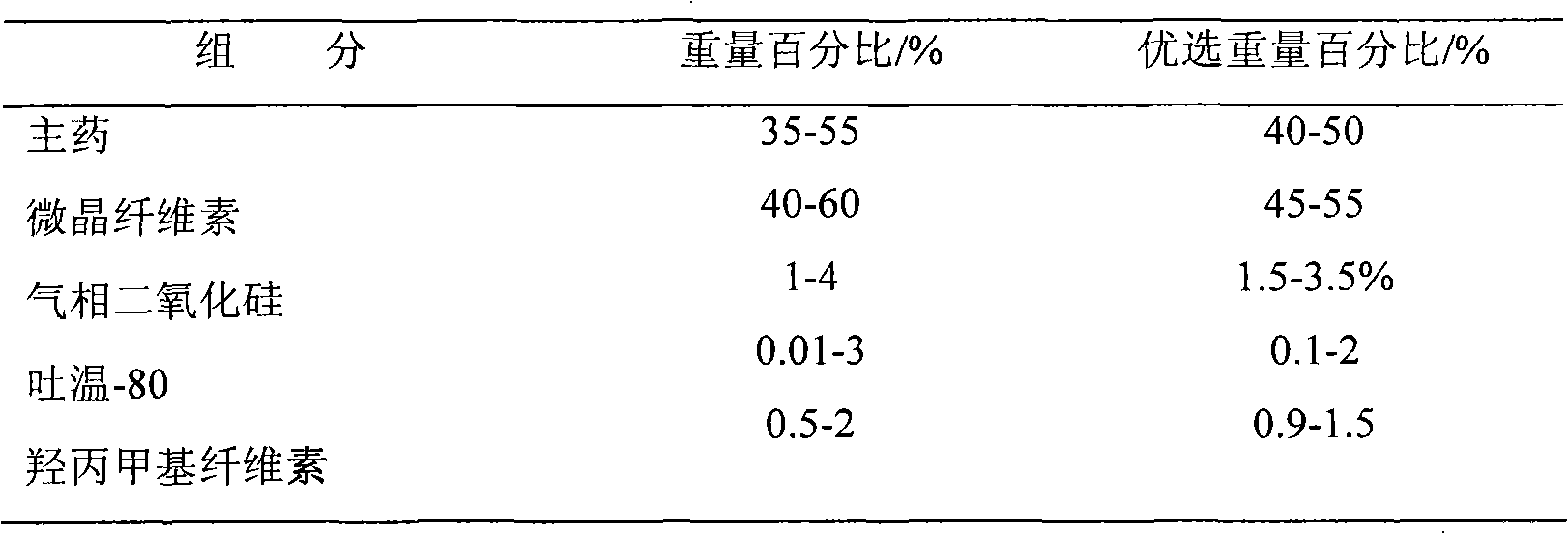
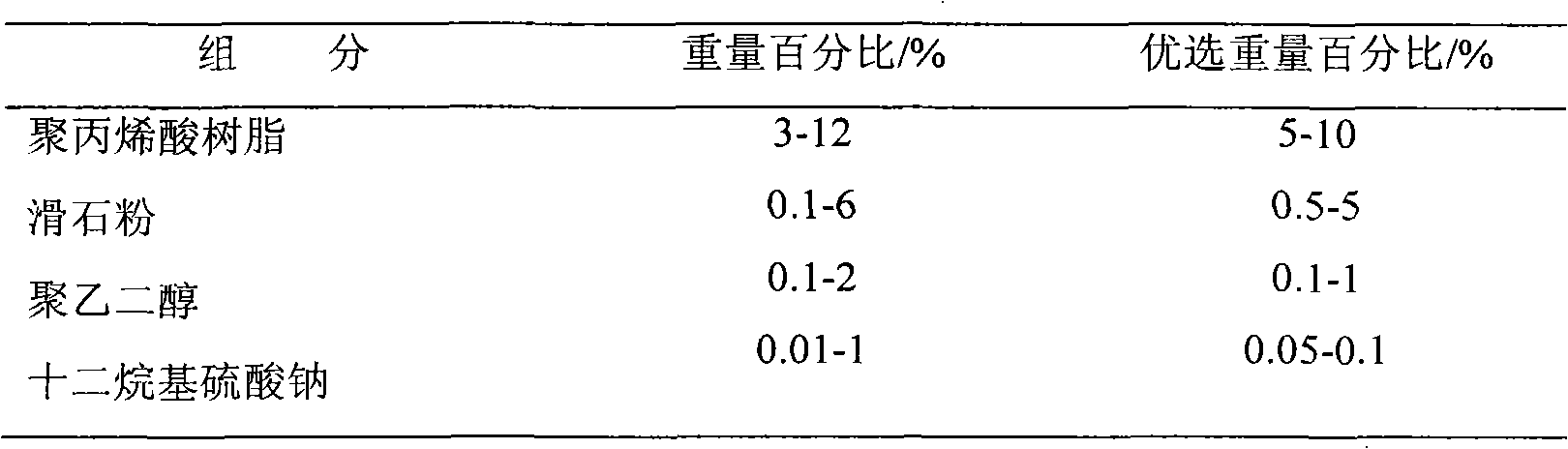
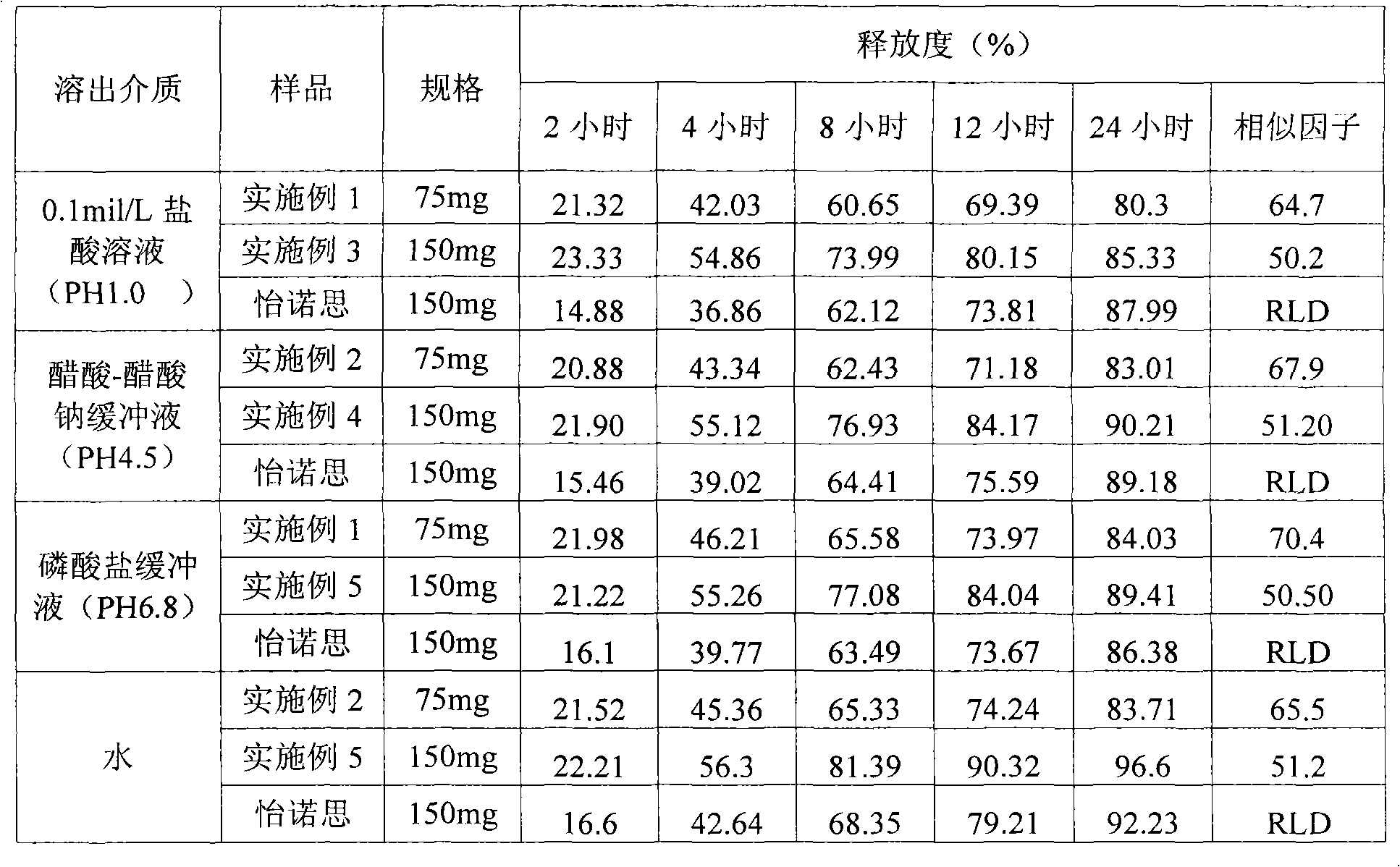
The invention relates to a venlafaxine hydrochloride slow-release capsule and a preparation method thereof. A drug-containing core of the slow-release capsule comprises venlafaxine hydrochloride, microcrystalline cellulose, gas phase silica, Tween-80, and hydroxypropyl methylcellulose; a slow-release coating comprises polyacrylic resin, talcum powder, polyethylene glycol and lauryl sodium sulfate. The invention realizes a pure water system for the capsule production by the addition of gas phase silica to change material flowing, and the selection of slow-release coating. The venlafaxine hydrochloride slow-release capsule prepared by the system is stable in drug release, has effective continuous release time of 24 hours, and is taken only once a day. The method is simple in process, mild in condition, free of any organic solvent, low in equipment requirements, and suitable for industrial production.
Owner:KUNMING JIDA PHARMA
Methods of treating affective disorders using derivatives of (-)- venlafaxine
InactiveUS20020086904A1Potent activityReducing and avoiding adverse effectOrganic active ingredientsAntipyreticClinical psychologyVenlafaxine
Methods of preparing, and compositions comprising, derivatives of (-)-venlafaxine are disclosed. Also disclosed are methods of treating and preventing diseases and disorders including, but not limited to, affective disorders such as depression, bipolar and manic disorders, attention deficit disorder, attention deficit disorder with hyperactivity, Parkinson's disease, epilepsy, cerebral function disorders, obesity and weight gain, incontinence, dementia and related disorders.
Owner:SUNOVION PHARMA INC
Polypeptide and application of polypeptide in preparation of drug used for treating depression
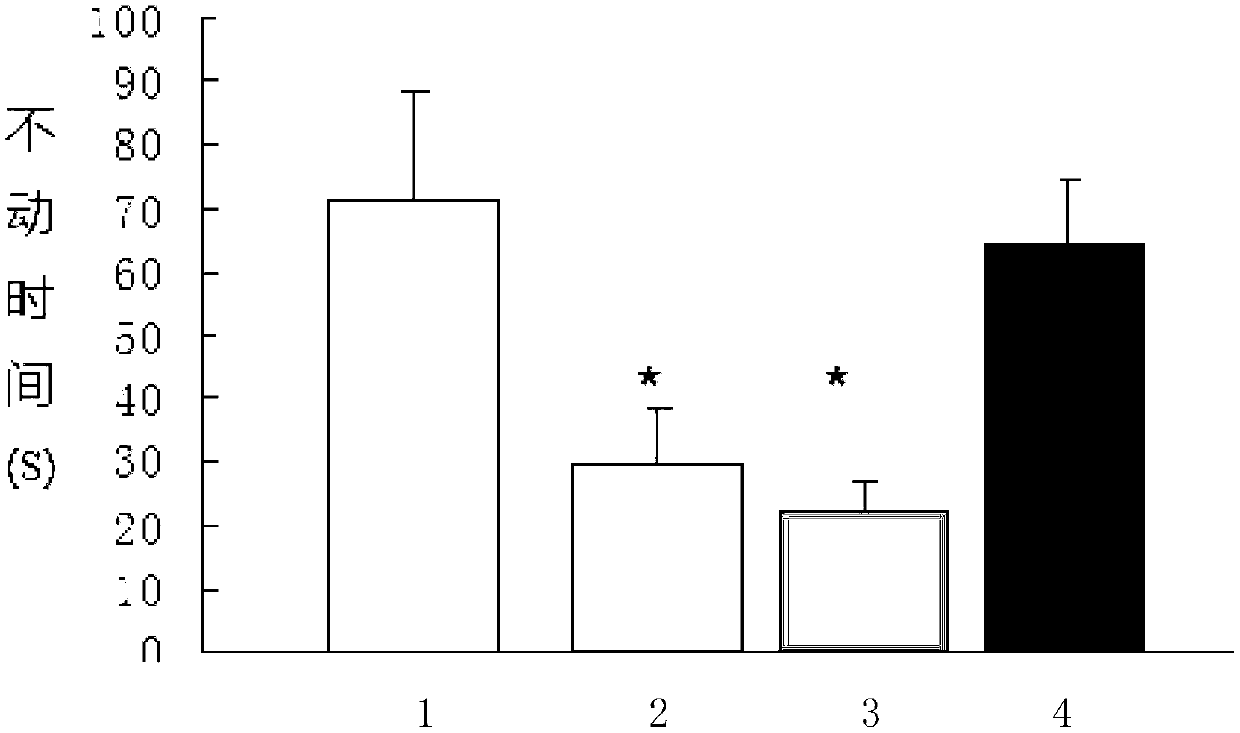
The invention discloses a polypeptide and an application of the polypeptide in preparation of a drug used for treating depression. The polypeptide is a peptide fragment shown as a sequence 1 or 2 in a sequence table. Experiments prove that once subcutaneous injection drug administration of the polypeptide shown as the sequence 1 or 2 in the sequence table is 100 micrograms / pc, in a rat forced swimming test, compared with a solvent control group and a venlafaxine group, dead time of a rat in forced swimming can be obviously shortened, and in a chronic unpredictability stress test, decrease of sweet water preference value caused by chronic unpredictability stress can be reversed. The polypeptide provided by the invention can immediately take effect after a single drug administration, duration of action is long, and the polypeptide still can treat depression-like behaviours tested on a 36th day after the drug administration. The invention provides a new drug and therapeutic method for preventing and / or treating depression and has a wide application prospect.
Owner:PEKING UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com








![Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28783494-7032-4a6b-8f4d-92fe3861cd01/a200910058269d00031.PNG)
![Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol Preparation of venlafaxine intermediate 1-[2-amino-1-(4-methoxy phenyl)ethyl] cyclohexanol](https://images-eureka-patsnap-com.libproxy1.nus.edu.sg/patent_img/28783494-7032-4a6b-8f4d-92fe3861cd01/a200910058269d00041.PNG)