Preparing technology for cyclohexanol derivatives used to prepare the intermediate of Venlafaxine
A preparation process and a derivative technology, applied in the field of preparation technology of cyclohexanol derivatives, can solve problems such as explosion and industrialized production impact, and achieve the effects of reducing production cost and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
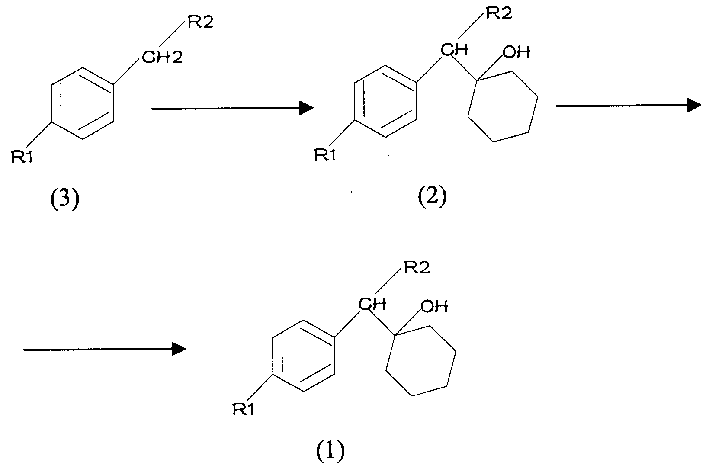
[0025] Example 1: Preparation of 1-[cyano(p-methoxyoxy)methyl]cyclohexanol (ie general formula 2) (intermediate (I)).
[0026] method 1
[0027] Add 10ml of n-hexane, 10ml of toluene and 710mg of NaH (60%, 17.8mmol) successively in a dry round bottom flask to make it a suspension, stir at room temperature for 15 minutes, slowly add 2.5g of p-methoxyphenylacetonitrile (17.0mmol) , Added within 10 minutes, stirred at room temperature for 50 minutes. Cool to -5 degrees Celsius, add dropwise 2.18g (22.0mmol) of cyclohexanone, complete the addition within 10 minutes, stir for 5 hours, TLC (developing solvent: petroleum ether: ethyl acetate = 4: 1) monitors until the reaction is complete (only a spot). Add 5% dilute hydrochloric acid dropwise thereto to adjust the pH to 6-7, stir for 15 minutes, filter with suction, wash the filter cake with water, and dry to obtain a crude product. Recrystallize with 3 times the amount of toluene (weight to volume ratio) to obtain 3.33 g of the ...
Embodiment 2
[0030] Example 2: Preparation of 1-[2-amino-1-(p-methoxyphenyl)ethyl]cyclohexanol and its hydrochloride (ie intermediate (II)).
[0031] 1. The preparation of the compound of general formula (1)
[0032] method 1
[0033]In a 50ml round bottom flask, intermediate (I) 2.45g (10mmol) and RaneyNi catalyst 1.0g were dispersed in 30ml methanol, and the air in the bottle was replaced with hydrogen more than 3 times, vigorously stirred at normal temperature and pressure, TLC It takes about 15 hours to monitor until no more hydrogen is absorbed. Stop stirring, let it stand, filter to remove the catalyst, and wash with methanol three times, combine the methanol solution, recover methanol, and obtain 2.50 g of crude product. with silica gel column chromatography (CH 2 Cl 2 : MeOH=30:1, volume ratio), 2.06g of the product was obtained, and the yield was 83%. TLC (developing solvent: methylene chloride: methanol = 30: 1, volume ratio) detected only one main spot, and the product coul...
Embodiment 3
[0038] Embodiment 3: Preparation of venlafaxine hydrochloride
[0039] Transfer the concentrated solution prepared by Method 2 of Example 2 to a 5000ml three-necked flask, add 3050ml of water, 332ml of formaldehyde and 438ml of formic acid, reflux for 12 hours, concentrate under reduced pressure until the raffinate is about 2000ml, add water to dilute to 4000ml, and wash with concentrated hydrochloric acid Adjust pH to 2, extract with ethyl acetate 3 times (300ml each time) to remove impurities (pink), treat the aqueous solution with concentrated KOH solution until alkaline, and extract 3 times with ethyl acetate. Concentrate the extract, acidify with isopropanol-HCl, add an appropriate amount of ether, and stand to obtain a white crystalline precipitate, which is filtered and dried to obtain 260-300 g of crude venlafaxine, with a yield of 80% and a melting point of 212-214 degrees Celsius. The crude product is recrystallized once with methanol-ethyl acetate to obtain the fini...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com