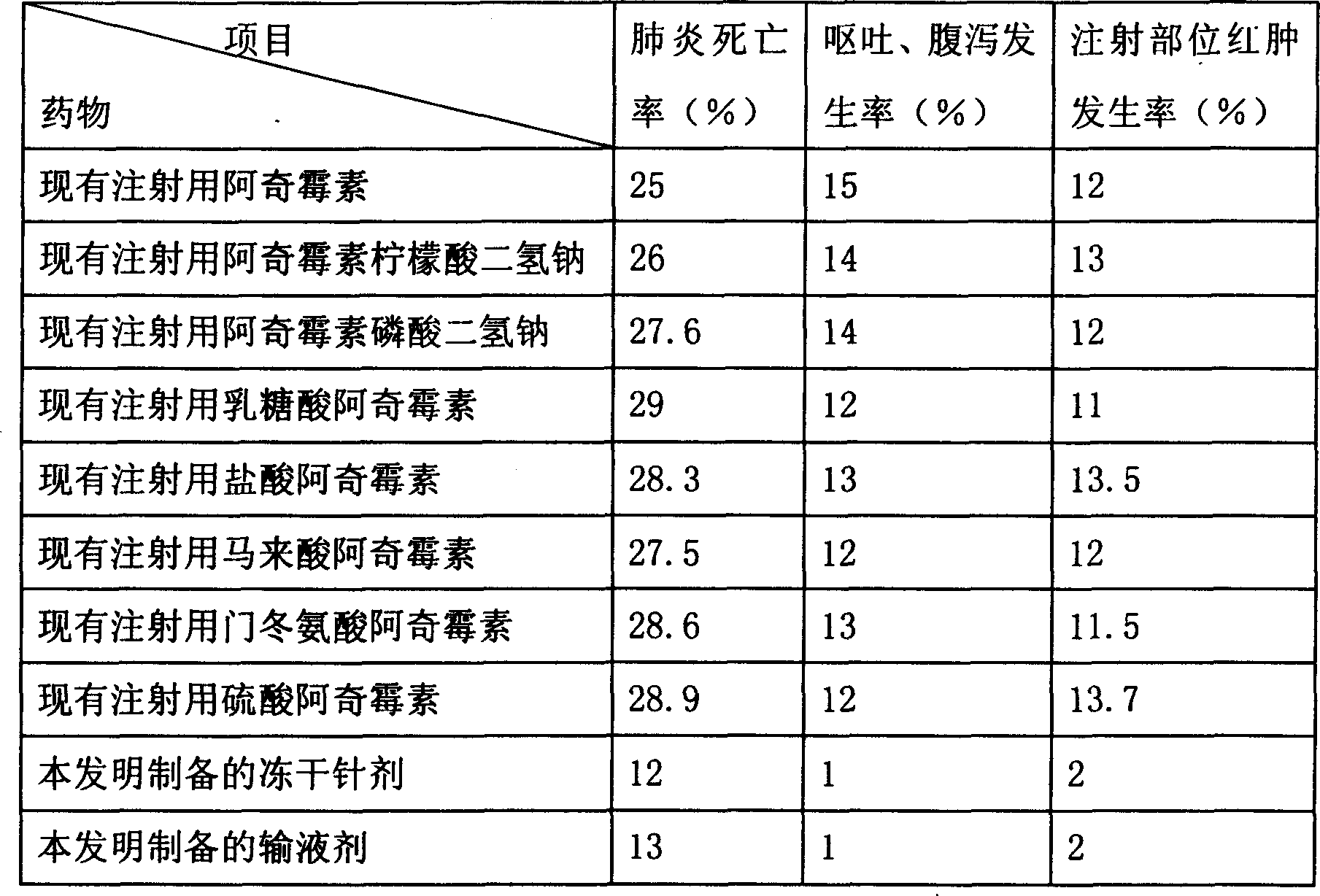
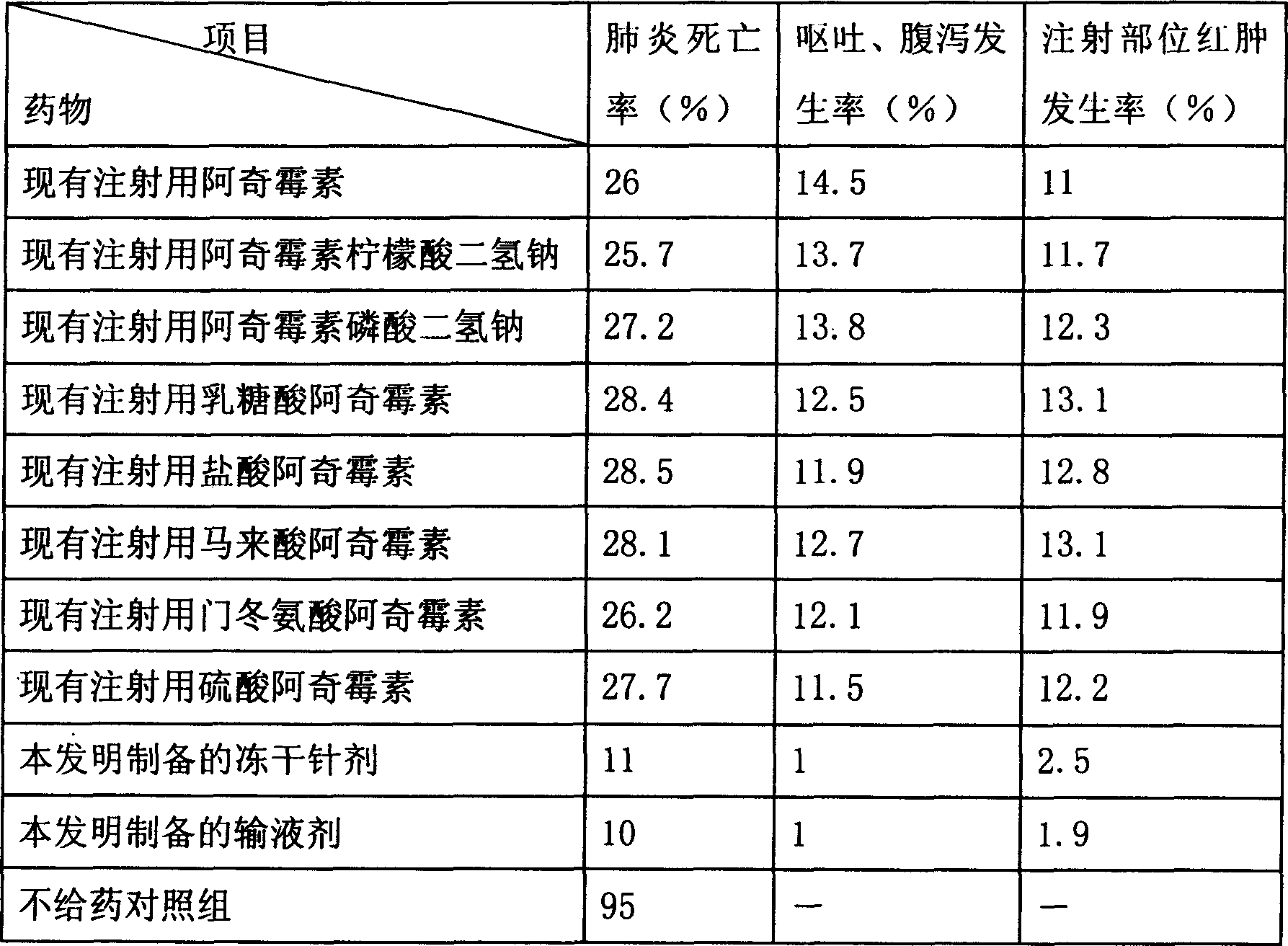
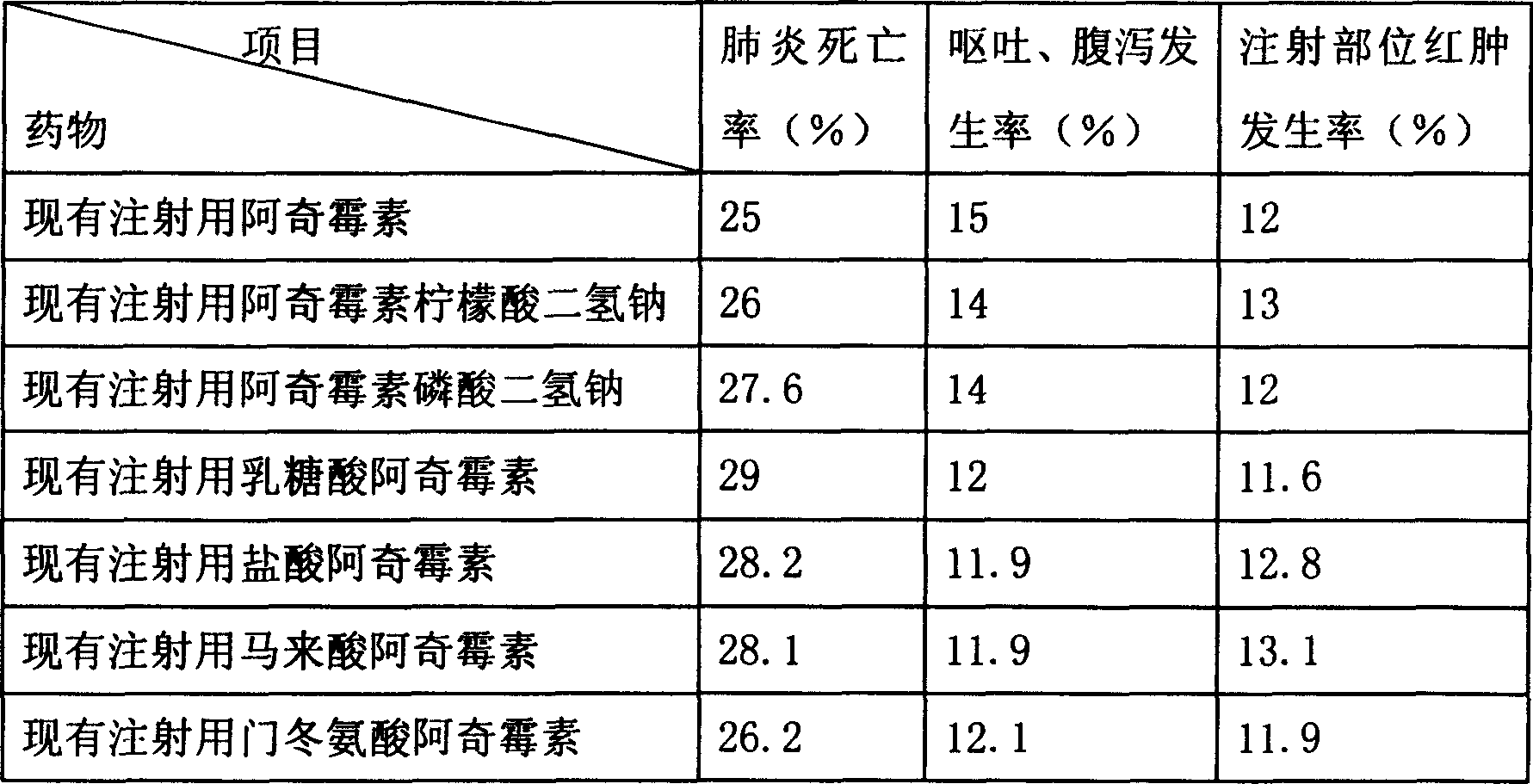
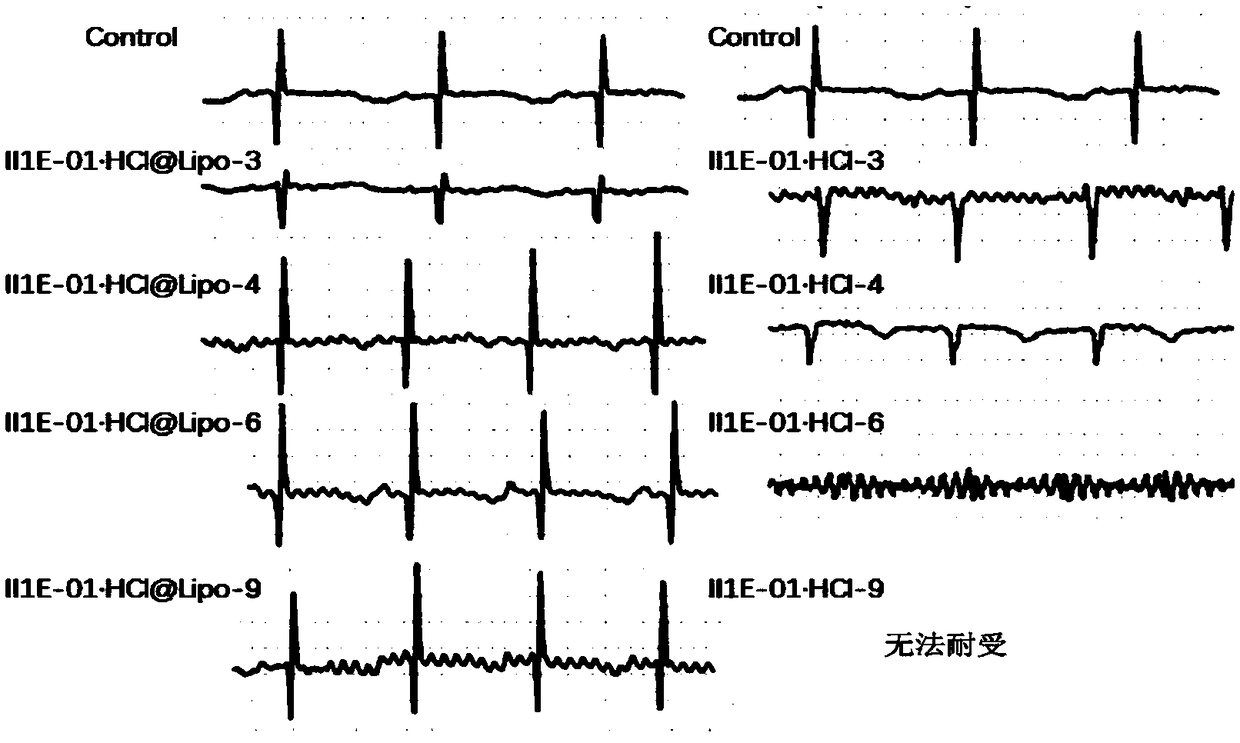
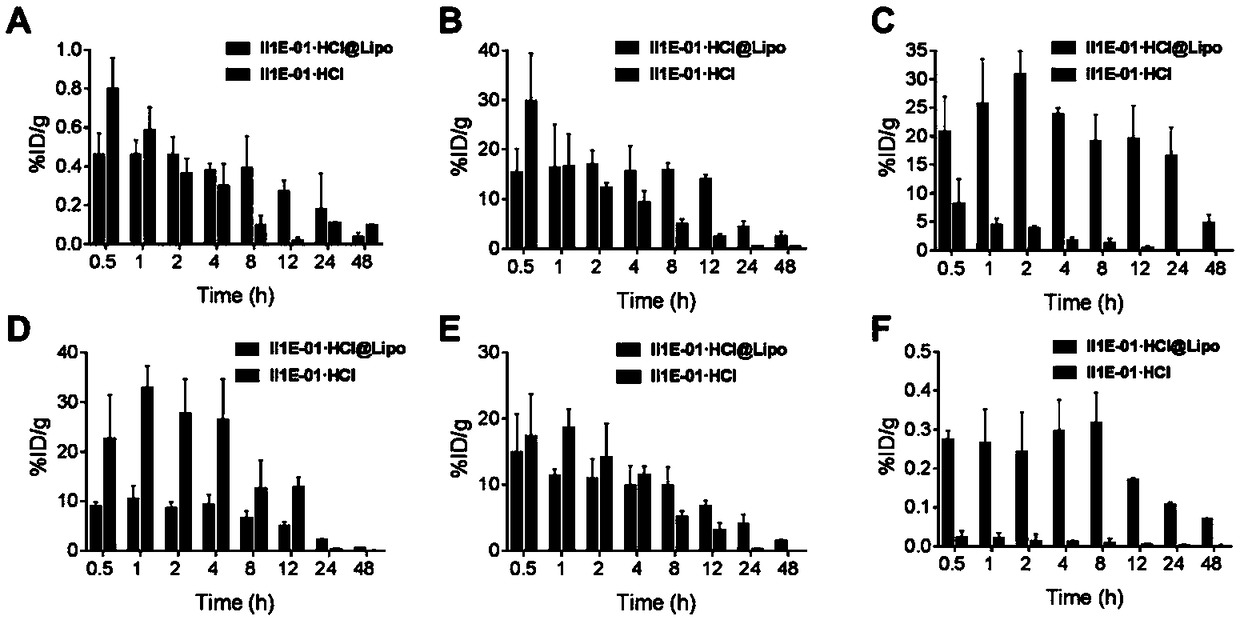
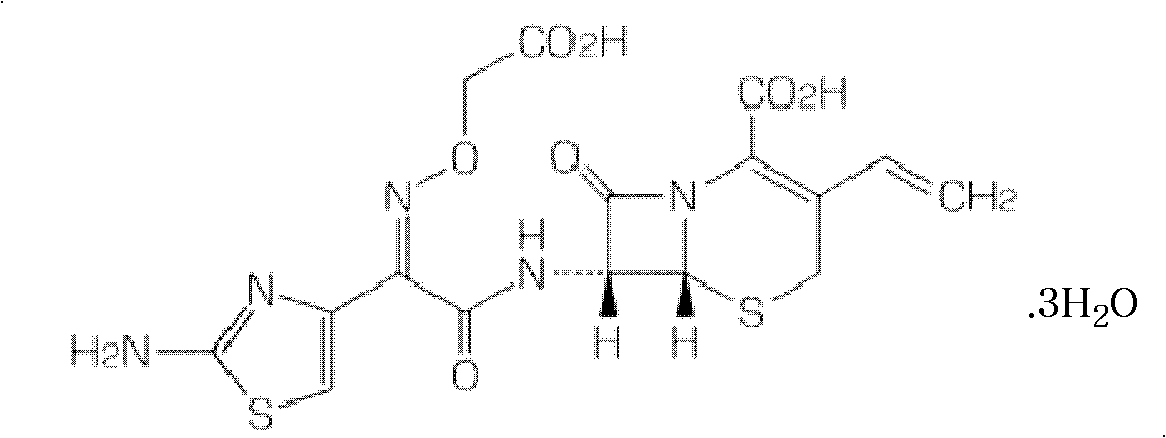
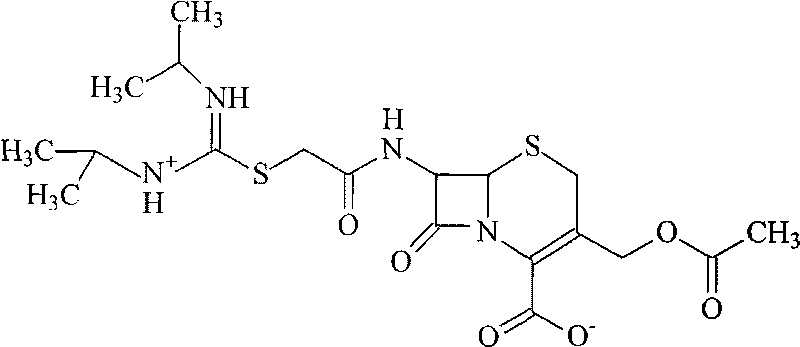
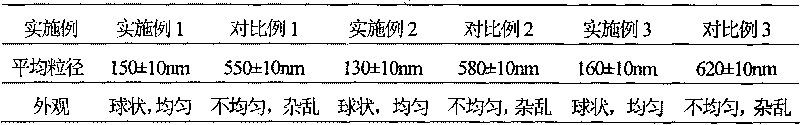
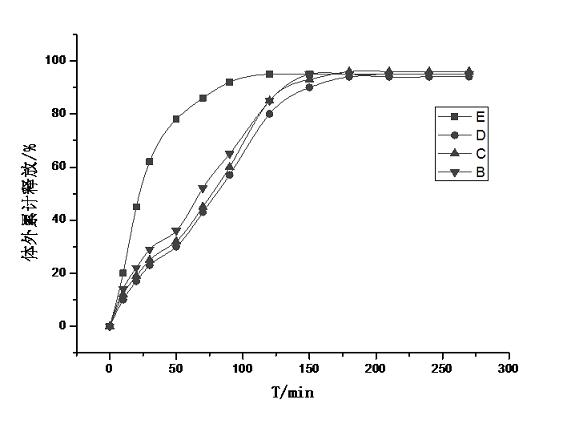
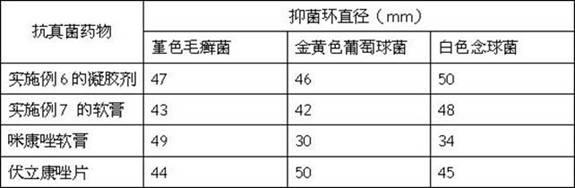
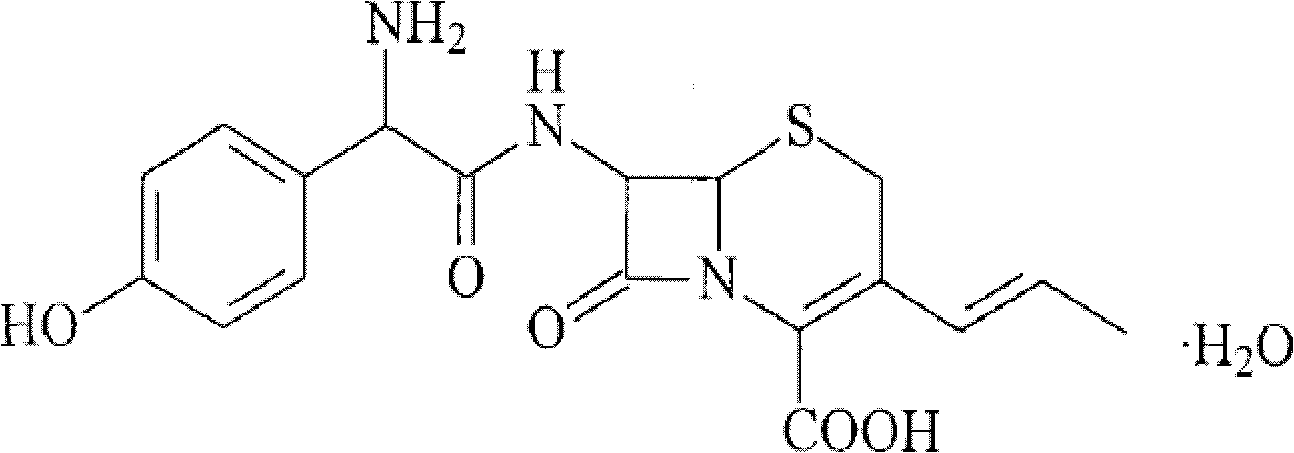
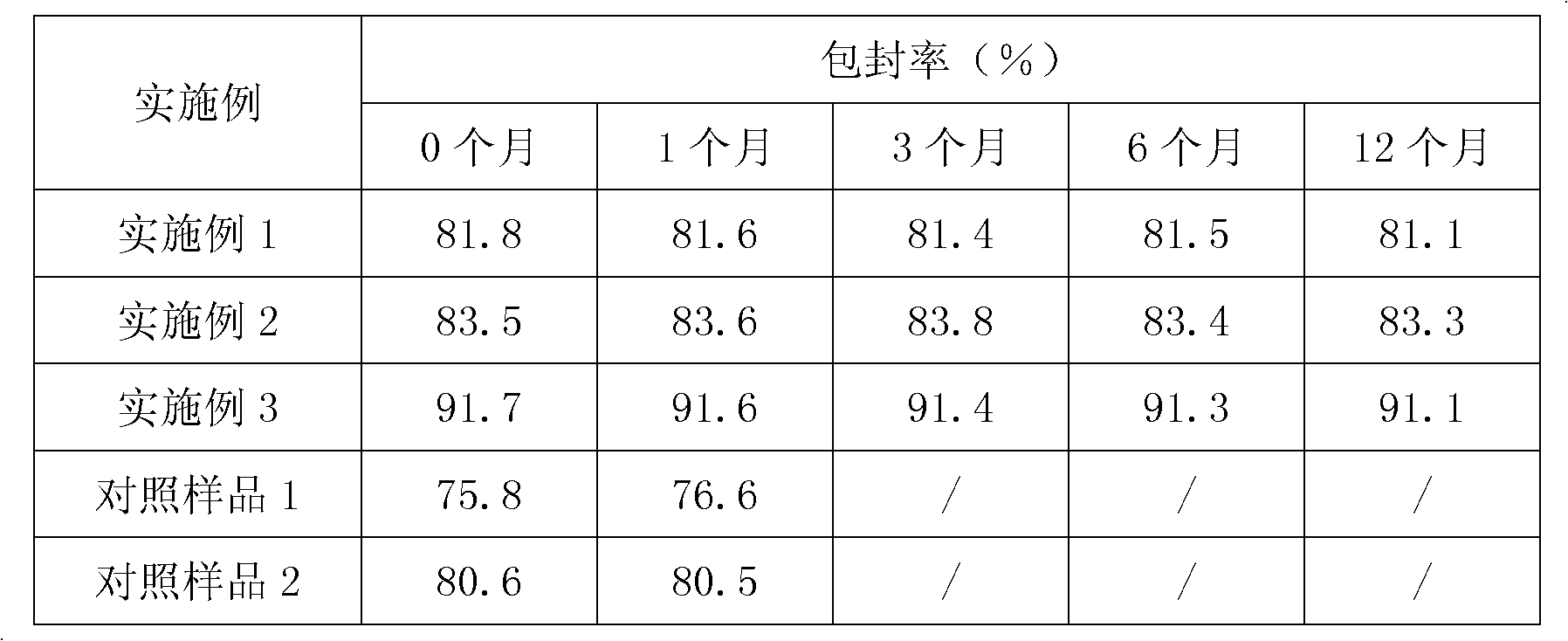
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
77 results about "HYDROGENATED SOYBEAN LECITHIN" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Liposome injection based on drug combination of mezlocillin sodium and sulbactam sodium
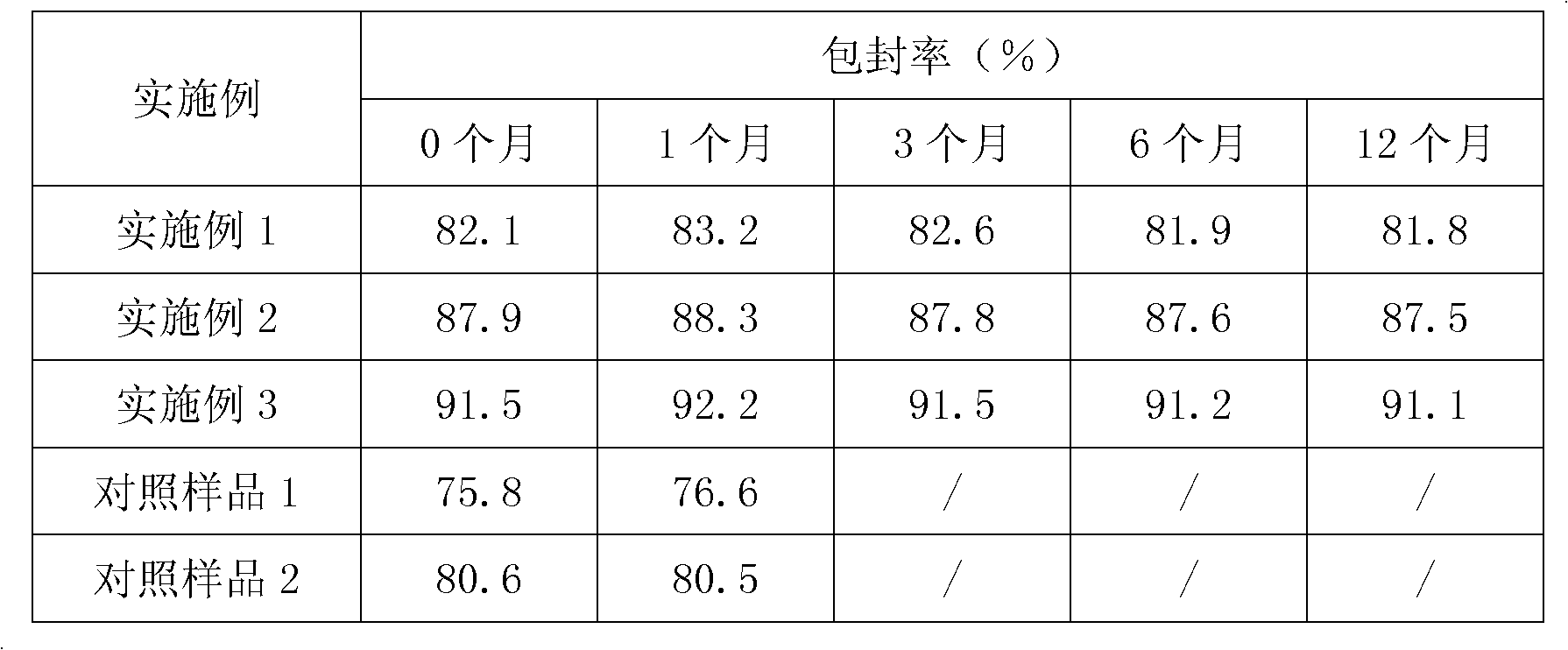
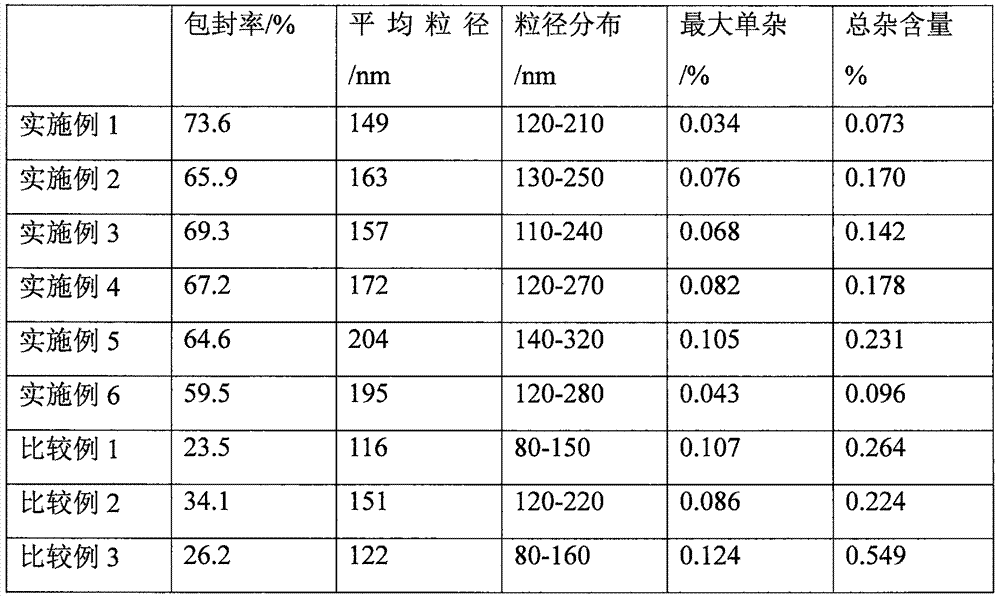
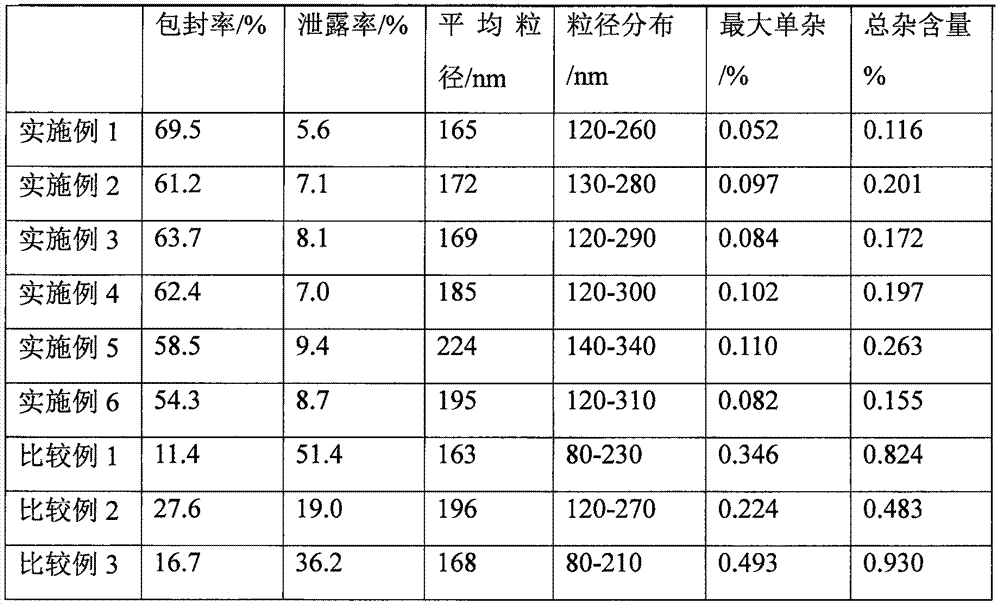
InactiveCN101804052AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPharmaceutical non-active ingredientsAntioxidantDissolution
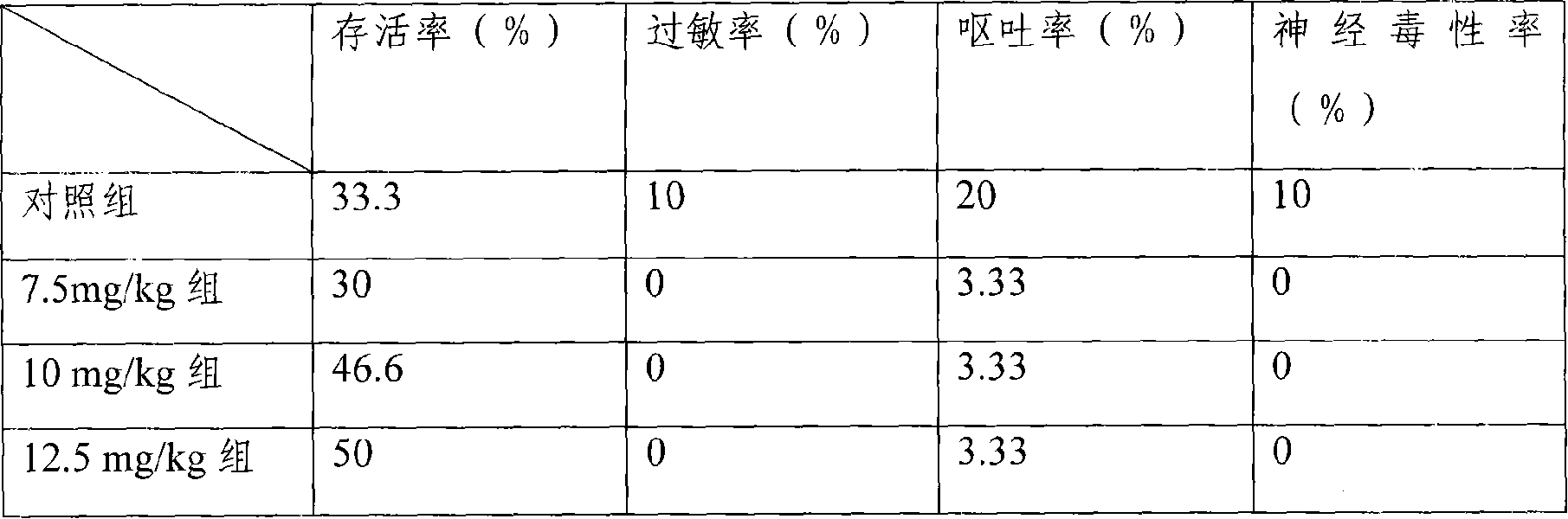
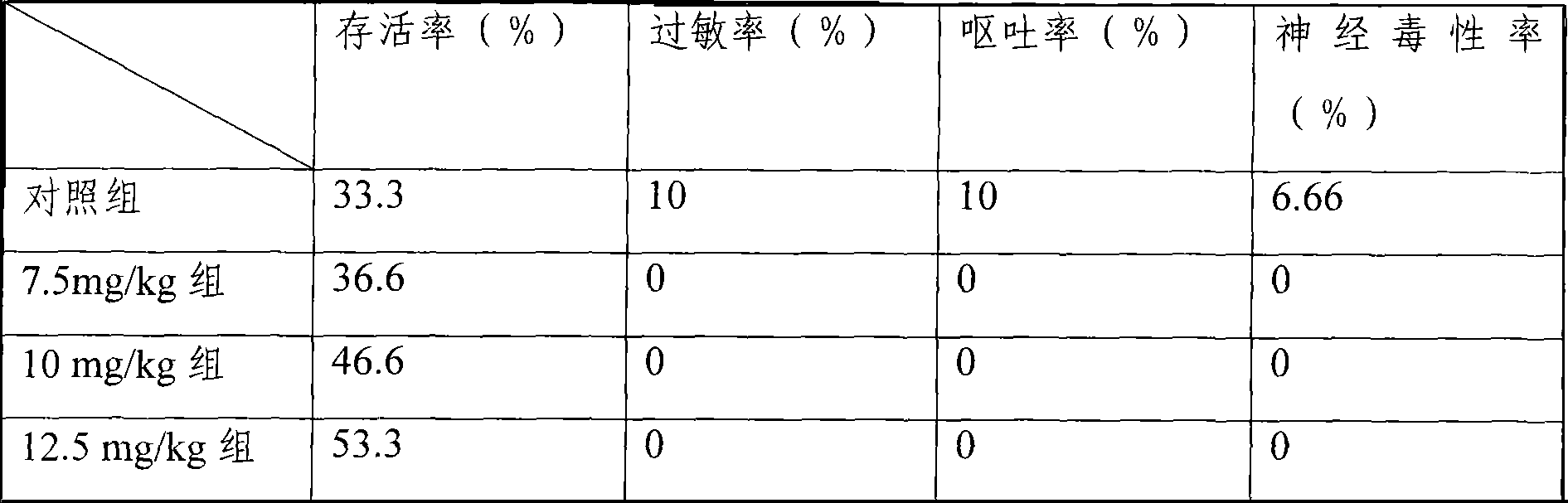
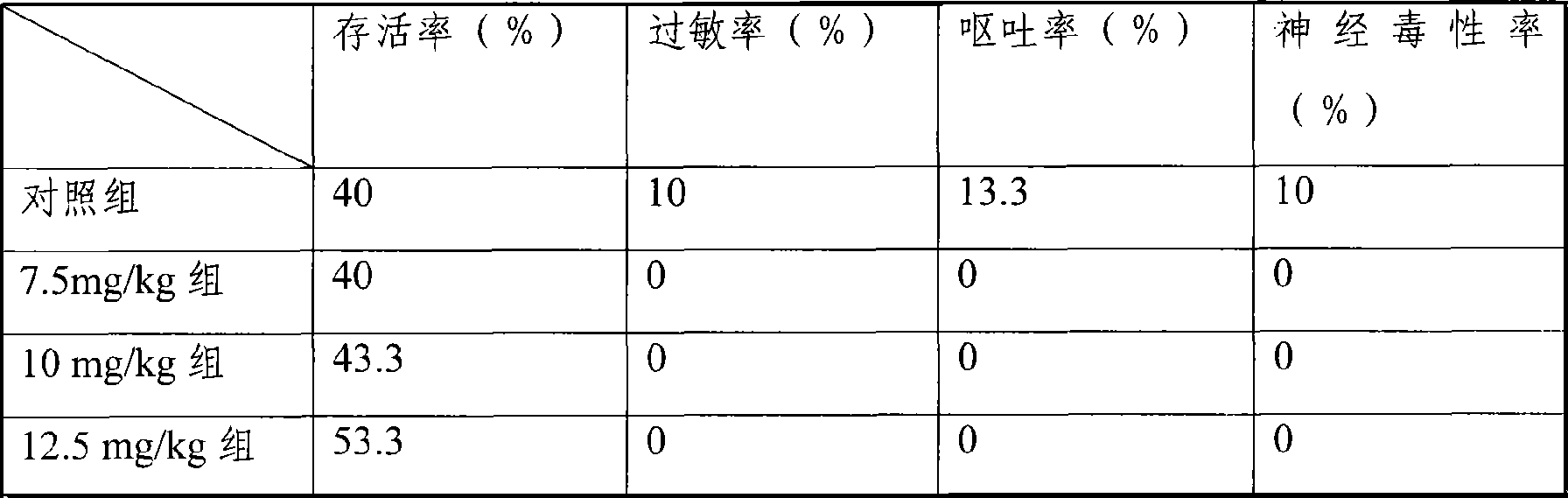
The invention provides a liposome injection based on a drug combination of mezlocillin sodium and sulbactam sodium. The liposome injection comprises the following components: mezlocillin sodium, sulbactam sodium, liposome carriers, frozen and dried supporting agent and optional existing antioxidant, wherein the liposome carriers are particularly hydrogenated soybean phosphatidylcholine and octadecylamine. The liposome injection of the invention has good preparation stability, prevents the liposome from being cracked under the action of dehydration, fusion and ice crystal generation in the freezing-drying process, and keeps good entrapment rate of the liposome after re-dissolution through hydration.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Sunburn protecting and repairing type cosmetic composition and preparation method thereof
InactiveCN107929152AReduce generationAvoid damageCosmetic preparationsToilet preparationsIrritationRaw material
The invention relates to a sunburn protecting and repairing type cosmetic composition and a preparation method thereof. The cosmetic composition comprises the following raw materials and components inpercentage by weight: 1-5% of plant extractive, 1-5% of hydrogenated lecithin, 1-5% of an emulsifier, 1-5% of a co-emulsifier, 5-10% of a humectant, 0.1-1% of a thickening agent, 5-10% of an emollient, 0.5-10% of a functional additive, and 60-80% of water, wherein the plant extractive includes white perilla leaf extractive, pomegranate blossom extractive and terminalia ferdinandiana fruit extractive; the hydrogenated lecithin is hydrogenated soybean lecithin containing 60-80% of phosphatidylcholine. The cosmetic composition is capable of protecting skin from UV radiation and visible light andeven UV radiation mediated delayed sunlight induced irritation; skin can be effectively repaired at night, and the ageing of the skin is delayed.
Owner:广州智媛生物科技有限公司
Granular formulation containing cefixime liposomes and preparation method thereof
InactiveCN101966154AHigh encapsulation efficiencyReduce leak rateOrganic active ingredientsAntiinfectivesYolkCholesterol
The invention relates to a cefixime liposome, a preparation method and a granular formulation containing the cefixime liposome. The granular formulation comprises the cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome is prepared from the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 2.5-10 parts of cholesterol and 0.1-4.5 parts of polysorbate 80. The granular formulation not only accords with the requirement on Chinese pharmacopoeia, but also has the advantages of stabler storage and rapider drug effect exertion and remarkably improved bioavailability compared with the common pharmaceutical cefixime composition at a room temperature.
Owner:王丽燕
Composition of liposome, and preparation method
InactiveCN1915222AInhibit transferRegulate immunityOrganic active ingredientsNervous disorderYolkDisease
A liposome composition used to prepare the medicines for treating tumor, cardiovascular and cerebrovascular disease, altitude ischemia and insomnia is prepared from the mixture of aspirin, prostaglandin E1, antioxidant, 2-hydroxypropyl-beta-dextrin and gamma-dextrin, the mixture of hydrogenated soybean lecithin and yolk lecithin, and the mixture of soybean sterol, polyethanediol-2000, VC and glycine. Its preparing process is also disclosed.
Owner:江苏仲德医药科技有限公司
Dry suspension containing cefprozil liposome and preparation method thereof
InactiveCN101953789AStable storageImprove bioavailabilityAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefprozil liposome, a preparation method thereof and a dry suspension containing the cefprozil liposome. The cefprozil liposome comprises cefprozil, hydrogenated soya bean lecithin, hydrogenated yolk lecithin, cholesterol and vitamin E in the weight ratio of 1:(1.25-5):(1.25-5):(2.5-10):(0.1-3). The dry suspension containing the cefprozil liposome not only conforms to the requirements of Chinese pharmacopoeia, but also has the advantages of more stable storage at normal temperature, rapider effect taking and obviously increased bioavailability compared with common cefprozil medicine compositions.
Owner:王丽燕
Sophoridine nano liposome medicament and preparation of the same
InactiveCN101152178ANo difficultyReduce ineffective lossOrganic active ingredientsPowder deliveryCholesterolViral Myocarditis
The invention relates to a sophoridine nanoliposome drug and the preparation method. The invention provides a sophoridine nanoliposome drug and the material and the content of the material are as follows: 350-500 compound of HSPC for injection and Vitamin E with a mass ration 9.8: 0.2; 20 to 30 compound of cholesterol and Beta-sitosterol with a mass ratio 1 to 3:1; sophoridine 10 to 25; 125 to 200 compound of dextran 40 and mannitol with a mass ratio 1: 7 to 9; 5 to 10reduced glutathione; and poloxamer F-6835-40. The invention also provides the preparation method of the sophoridine nanoliposome. The drug provided in the invention can be used to treat adenocarcinoma, inflammation, gout, progressive disease of the central nervous system, to relieve pain and treat viral myocarditis.
Owner:江苏德伦生物制药有限公司
Azithromycin liposome composition medicine and its prepn process
InactiveCN1887271ASmooth releaseReduce stimulationAntibacterial agentsOrganic active ingredientsYolkVitamin C
The present invention relates to azithromycin liposome composition medicine and its preparation process. The azithromycin liposome composition medicine consists of azithromycin 0.8-1.2 (in molar portions, the same below), mixture of hydrogenated soybean lecithin and / or yolk lecithin in 8 to 1 molar ratio 8-12, mixture of soyasterol and cholesterol in 1 to 1-2 molar ratio 8-12, vitamin E 0.8-1.2, PEG2000 0.1-0.2, and alpha-mercapto glycin 0.1-0.2; and has vitamin C in the amount of 4-8 % added as excipient. The present invention also provides the preparation process of the azithromycin liposome composition medicine. The medicine of the present invention has high and stable curative effect and less adverse reactions.
Owner:刘祥华 +1
Tablet containing cefixime liposome and preparation method thereof
InactiveCN101966159AStable storageImprove bioavailabilityAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefixime liposome and a preparation method thereof as well as a tablet containing the cefixime liposome. The tablet comprises a cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome comprises the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated yolk lecithin, 1.25-10 parts of cholesterol and 0.1-3 parts of polysorbate 80. The tablet not only meets the requirement on Chinese pharmacopoeia but also has the advantages of more stable storage at normal temperature, more obvious curative effect and obvious improvement of bioavailability compared with the traditional cefixime medicament composition.
Owner:王丽燕
Cardiac glycoside active compound lipidosome and preparation method thereof
InactiveCN109453117AWith sustained releaseLong-actingOrganic active ingredientsPharmaceutical non-active ingredientsSide effectCholesterol
The invention discloses a liposome preparation of a cardiac glycoside active compound. According to the liposome, hydrogenated soybean lecithin, polyethylene glycol phosphatide and cholesterol are used as lipid materials; firstly, a blank liposome is prepared, and then an ammonium sulfate gradient method is used to actively carry a drug. The prepared drug-carried liposome is good in biocompatibility and has a uniformly distributed particle size of 120 to 240 nm. The liposome can prolong the circulation time of the cardiac glycoside active compound in the body and reduce the number of administrations, and can increase the drug concentration in tumor tissues by using the high permeation and long-term retention effect of the tumor tissues, reduce toxic and side effects on other tissues and organs, especially the heart and improve the anti-tumor effect and the clinical adaptability of the cardiac glycoside active compound.
Owner:NANJING UNIVERSITY OF TRADITIONAL CHINESE MEDICINE
Montelukast sodium liposome solid preparation
InactiveCN102085187BWell mixedImprove stabilityPharmaceutical non-active ingredientsRespiratory disorderSide effectMANNITOL/SORBITOL
The invention provides a montelukast sodium liposome solid preparation which is prepared from raw and auxiliary materials comprising the following components in parts by weight: 5 parts of montelukast sodium, 15-30 parts of hydrogenated soybean phosphatidylcholine (HSPC), 8-20 parts of cholesterol, 4-12 parts of Tween 80, 20-50 parts of mannitol and 1-3 parts of soyasterol. The montelukast sodiumliposome solid preparation has high stability, is stable in light and heat environments, is convenient to store, has the advantages of simple preparation method, high encapsulation rate and uniform particle size and can be preserved in a body for a long time, thereby improving the product quality of the preparation and reducing the toxic side effects.
Owner:HAINAN MEIDA PHARMA
Capsule containing cefixime liposome and preparation method thereof
InactiveCN101966166AHigh dissolution rateThe drug works quicklyAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefixime liposome and a preparation method thereof as well as a capsule containing the cefixime liposome. The capsule comprises the cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome is prepared from the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 1.25-10 parts of cholesterol and 0.1-2.5 parts of polysorbate 80. The capsule not only accords with the requirement on Chinese pharmacopoeia, but also has the advantages of stabler storage and rapider drug effect exertion and remarkably improved bioavailability compared with the common pharmaceutical cefixime pharmaceutical composition at a room temperature.
Owner:王丽燕
Dispersible tablet containing cefixime liposome and preparation method thereof
InactiveCN101966160AHigh encapsulation efficiencyReduce leak rateAntibacterial agentsOrganic active ingredientsCholesterolNiosome
The invention relates to a cefixime liposome, a preparation method thereof and a dispersible tablet containing the cefixime liposome. The dispersible tablet comprises the cefixime liposome and a pharmaceutically acceptable carrier, wherein the cefixime liposome comprises the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 1.25-10 parts of cholesterol and 0.2-3.5 parts of polysorbate 80. The dispersible tablet not only conforms to the requirements of a Chinese pharmacopoeia, but also has the advantages of more stable storage under normal temperature and remarkable improvement of bioavailability compared with the ordinary cefixime medicament composition, and can take effect more rapidly.
Owner:王丽燕
Cefathiamidine prosoma liposome preparation
InactiveCN101693010AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPowder deliverySide effectAdditive ingredient
The invention provides a cefathiamidine prosoma liposome preparation, which comprises the following ingredients by shares: 1 share of cefathiamidine, 3-20 shares of hydrogenated soya bean lecithin, 2-12 shares of poloxamer 188-, 0.5-10 shares of deoxysodium cholate and 1-30 shares of proppants. The invention further provides a preparation method and usage of prosoma liposome. The prosoma liposome of the invention has the advantages of high stability, high entrapping efficiency, even grain diameters, small side effects and the like.
Owner:HAINAN LINGKANG PHARMA CO LTD
Granules comprising cefprozil lipidosome and preparation method thereof
InactiveCN101953790AStable storageThe drug works quicklyAntibacterial agentsOrganic active ingredientsYolkHYDROGENATED SOYBEAN LECITHIN
The invention relates to cefprozil lipidosome, a preparation method thereof and granules comprising the same. The cefprozil lipidosome comprises the following components in proportion by weight: 1 cefprozi, 1.25-5 hydrogenated soybean lecithin, 1.25-5 egg yolk lecithin, 2.5-10 cholesterin and 0.1-3 vitamin E. The cefprozil granules not only conform to the requirements of Chinese pharmacopoeia, but also has the advantages of more stable storage at normal temperature, faster drug effect-taking, and obviously improved bioavailability compared with the common cefprozil pharmaceutical composition.
Owner:王丽燕
Hydrogenated soybean lecithin and preparation method and application thereof
ActiveCN105732702AImprove reaction efficiencyReduce manufacturing costGroup 5/15 element organic compoundsPharmaceutical non-active ingredientsEvaporationPhospholipid
The invention discloses hydrogenated soybean lecithin.A preparation method includes: using acetone to pulp powdery soybean lecithin, filtering, using alcohol to wash solid, combining alcohol solutions, using activated carbon for decoloring, drying by evaporation, continuing using a dichloromethane / acetone system for purification, methylating a crude product after purification, adding the crude product after methylation into a reaction kettle, adding a solvent and a catalyst in a certain amount, sealing the reaction kettle, and feeding hydrogen to certain pressure; reacting in a heat-collecting-type constant-temperature heating magnetic stirrer at constant temperature for certain time; after reaction is finished, separating a product from the catalyst, removing the solvent, and using a complexation method for purification to obtain the hydrogenated soybean lecithin.The preparation method is suitable for pilot-scale production, and the hydrogenated soybean lecithin prepared by the method can be used as a pharmaceutical excipient to be used in various drugs like injections, tablets and capsules.
Owner:苏州东南药业股份有限公司
Production method of injection-use hydrogenated soybean lecithin
ActiveCN103130829AHigh catalytic activityGood choicePharmaceutical delivery mechanismPharmaceutical non-active ingredientsHydrogenHYDROGENATED SOYBEAN LECITHIN
The invention provides a preparation method of injection-use hydrogenated soybean lecithin. The method comprises specific processes that: soybean lecithin and a certain amount of a catalyst are added into a reaction vessel; a solvent is added, and the materials are dissolved; the reaction vessel is sealed; hydrogen is delivered in until a certain pressure is reached; constant-temperature reaction is carried out for a certain period of time in a collector-type constant temperature heating magnetic stirrer; when the reaction is finished, the product is separated from the catalyst; active carbon is added for discoloration; filtering is carried out, and the solvent is removed; and column chromatography purification is carried out, such that the hydrogenated soybean lecithin product is obtained. The method provided by the invention is suitable for process large-scale productions. The prepared hydrogenated soybean lecithin product can be used in medical injection liquid.
Owner:艾伟拓(江苏)医药科技有限公司
Montelukast sodium liposome solid preparation
InactiveCN102085187AImprove qualityImprove stabilityPharmaceutical non-active ingredientsRespiratory disorderCholesterolHYDROGENATED SOYBEAN LECITHIN
The invention provides a montelukast sodium liposome solid preparation which is prepared from raw and auxiliary materials comprising the following components in parts by weight: 5 parts of montelukast sodium, 15-30 parts of hydrogenated soybean phosphatidylcholine (HSPC), 8-20 parts of cholesterol, 4-12 parts of Tween 80, 20-50 parts of mannitol and 1-3 parts of soyasterol. The montelukast sodium liposome solid preparation has high stability, is stable in light and heat environments, is convenient to store, has the advantages of simple preparation method, high encapsulation rate and uniform particle size and can be preserved in a body for a long time, thereby improving the product quality of the preparation and reducing the toxic side effects.
Owner:HAINAN MEIDA PHARMA
Preparation method of low-ester pectin stabilized composite phospholipid liposome
InactiveCN109498576AImprove stabilityImprove rigidityHydroxy compound active ingredientsAntinoxious agentsWater bathsVitamin C
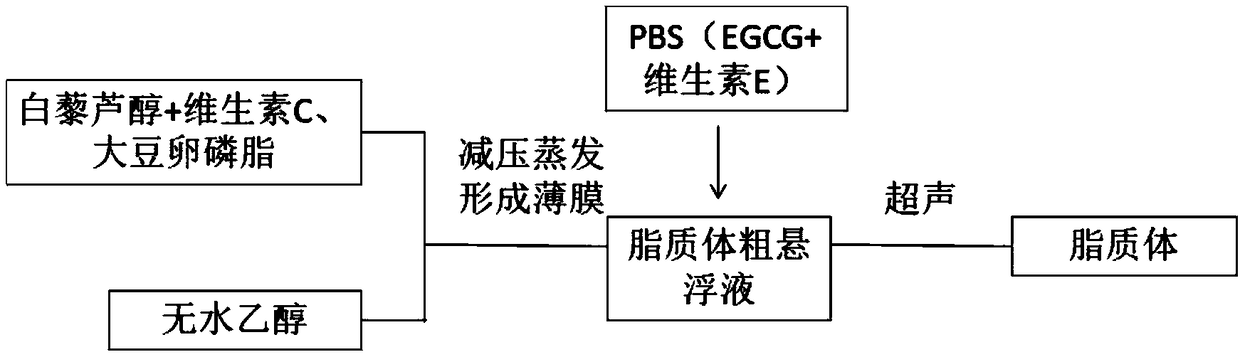
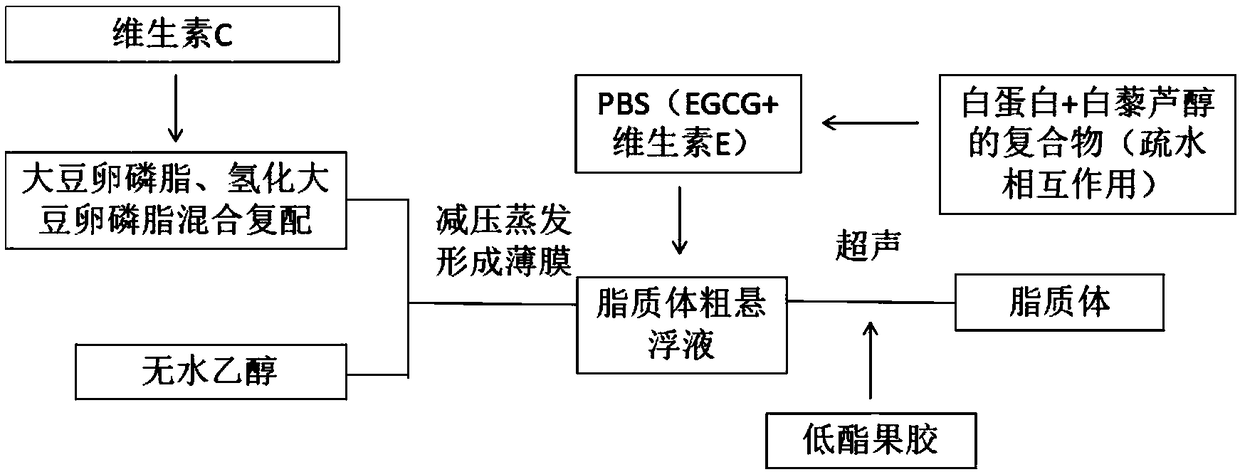
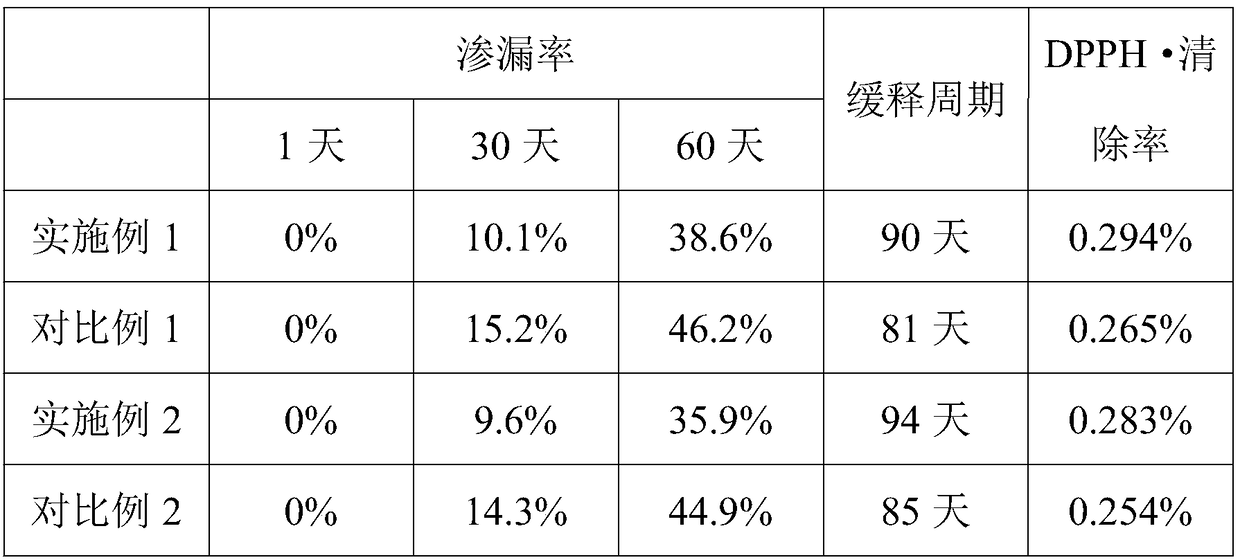
The invention discloses a preparation method of a low-ester pectin stabilized composite phospholipid liposome. The preparation method comprises the following steps: (1) weighing soybean lecithin, hydrogenated soybean lecithin, cholesterol and vitamin C, mixing, dissolving in absolute ethanol, and performing ultrasonic treatment in a water bath till the materials are dissolved completely to obtaina mixture; (2) performing rotary evaporation on the mixture in a water bath of 40 to 50 DEG C under reduced pressure to remove ethanol till a layer of uniform liposome film is formed on the wall of arotary evaporation bottle; (3) adding a phosphate buffer solution and a compound prepared from the EGCG (Epigallocatechin Gallate), vitamin E, albumin and resveratrol in a mass ratio of 1 to 2 to 1 to1 into the rotary evaporation bottle, and performing rotary hydration in a water bath of 40 to 50 DEG C for 30 to 60 min to obtain a liposome coarse suspension; (4) preparing a 0.1 to 0.6 g / 100 mL low-ester pectin aqueous solution, adding the liposome coarse suspension into the pectin solution dropwise, mixing and stirring in a volume ratio of 1 to 1, and finally performing ultrasonic treatment in an ice bath to obtain the low-ester pectin stabilized composite phospholipid liposome. By adopting the preparation method, the slow release performance and oxidization resistance of the liposome canbe improved effectively.
Owner:ZHEJIANG UNIV OF TECH
C20H18N6Na2O7S4 drug entity composition and preparation for children
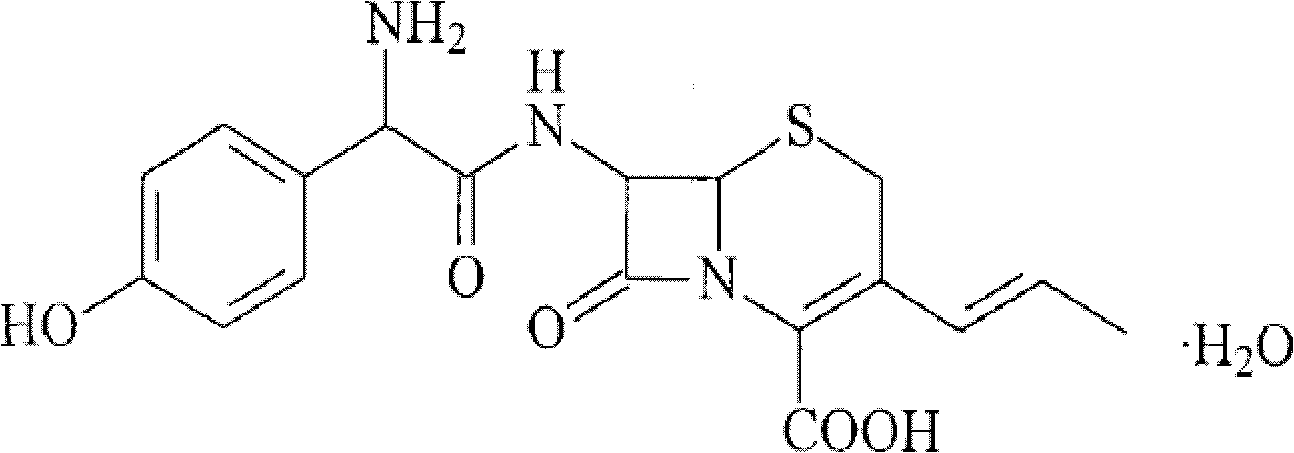
ActiveCN107412232AHigh encapsulation efficiencyReduce leak rateAntibacterial agentsOrganic active ingredientsHYDROGENATED SOYBEAN LECITHINSoybean Lecithin
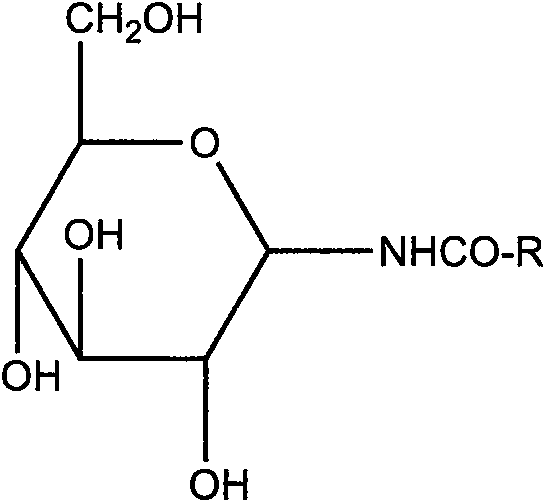
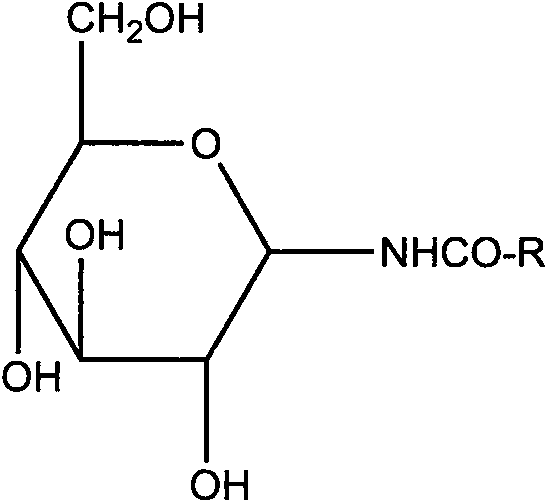
The invention discloses a C20H18N6Na2O7S4 drug entity composition and a preparation for children. Raw materials of the C20H18N6Na2O7S4 drug entity composition and the preparation for the children comprise hydrogenated soybean lecithin and cholesterin of a film forming substance and C20H18N6Na2O7S4, and the raw materials further comprise D-pyrane glucosamide serving as a film forming substance stabilizer.
Owner:广东金城金素制药有限公司
Composition of liposome, and preparation method
InactiveCN1915222BInhibit transferRegulate immunityOrganic active ingredientsNervous disorderDiseaseSterol
A liposome composition used to prepare the medicines for treating tumor, cardiovascular and cerebrovascular disease, altitude ischemia and insomnia is prepared from the mixture of aspirin, prostaglandin E1, antioxidant, 2-hydroxypropyl-beta-dextrin and gamma-dextrin, the mixture of hydrogenated soybean lecithin and yolk lecithin, and the mixture of soybean sterol, polyethanediol-2000, VC and glycine. Its preparing process is also disclosed.
Owner:江苏仲德医药科技有限公司
Anti-oxidant for baking food product and preparation thereof
InactiveCN101301082AAntioxidant hasWith hypolipidemicBakery product preservationFood preparationSide effectSucrose distearate
The invention provides an antioxidant of baking food and a preparation method thereof. The antioxidant includes 55-60 parts by weight of hydrogenized soya bean lecithin, 10-18 parts by weight of vitamin E, 5-10 parts by weight of soyabean protein polypeptide, 2-3 parts by weight of oligo-xylose, 1-5 parts by weight of sucrose monostearate, 2-3 parts by weight of xanthan gum and 11-20 parts by weight of baked milk powder. The preparation method includes extracting lecithin from the soya bean lecithin, separating catalyst, discoloring oxydol, removing solvent after the reaction of catalytic hydrogenation, obtaining hydrogenized soya bean lecithin with 20-30 of iodine number, uniform mixing 55-60 parts by weight of hydrogenized soya bean lecithin, 10-18 parts by weight of vitamin E, 5-10 parts by weight of soyabean protein polypeptide, 2-3 parts by weight of oligo-xylose, 1-5 parts by weight of sucrose monostearate, 2-3 parts by weight of xanthan gum and 11-20 parts by weight of baking milk powder to prepare the antioxidant for baking food. The invention has the advantages of strong antioxygenic property, pure natural property, no toxic and side effects, a plurality of healthcare functions.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
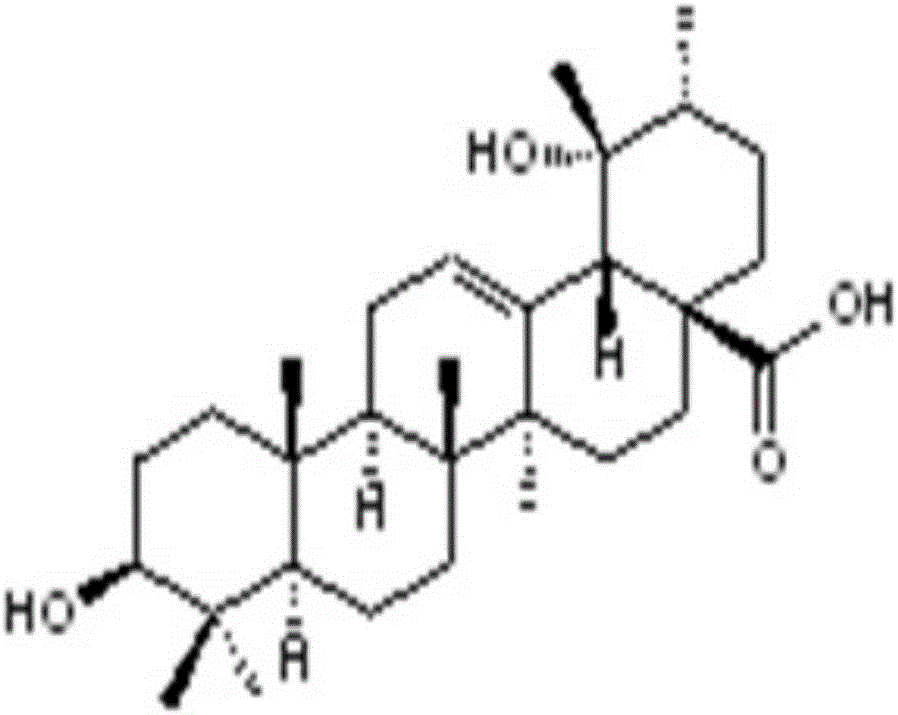
Sanguisorba officinalis aglycone lipidosome, and preparation method and purpose thereof
InactiveCN106580881AImprove bioavailabilityIncrease the number ofOrganic active ingredientsPharmaceutical non-active ingredientsAglyconeWhite blood cell number
The invention discloses a sanguisorba officinalis aglycone lipidosome, which is prepared from the following raw and auxiliary materials in proportion by weight: 1 part of sanguisorba officinalis aglycone and 2 to 40 parts of carrier materials, wherein the carrier materials are prepared from hydrogenated soybean phospholipids, distearoyl phosphoethanolamine-polyethyleneglycol 2000 and cholesterin according to a weight ratio of 5:(1 to 4):(1 to 2). The invention also provides a preparation method and a purpose of the lipidosome. The lipidosome has the advantages that the number of peripheral blood leucocytes, neutrophilic granulocytes, red cells, blood platelets, hemoglobin and bone marrow hematopoietic stem cells can be obviously increased; and the obvious effects of treating and / or preventing bone marrow suppression are achieved.
Owner:SICHUAN YINGLU WEITE PHARMA TECH CO LTD
A kind of pharmaceutical composition and preparation for children
ActiveCN107095874BHigh encapsulation efficiencyReduce leak rateAntibacterial agentsOrganic active ingredientsCholesterolPharmaceutical drug
The invention discloses a C18H16N8Na2O7S33.5H2O drug entity composition for children and a preparation thereof. The composition contains the following raw materials: hydrogenated lecithin, cholesterol, C18H16N8Na2O7S33 and D-glucopyranose amide, wherein hydrogenated lecithin and cholesterol are taken as film forming substances, and D-glucopyranose amide is taken as a film forming substance stabilizer.
Owner:广东金城金素制药有限公司
A kind of hydrogenated soybean lecithin and its preparation method and application
ActiveCN105732702BIncrease contentIncrease production costGroup 5/15 element organic compoundsPharmaceutical non-active ingredientsEvaporationSoybean Phospholipids
The invention discloses hydrogenated soybean lecithin.A preparation method includes: using acetone to pulp powdery soybean lecithin, filtering, using alcohol to wash solid, combining alcohol solutions, using activated carbon for decoloring, drying by evaporation, continuing using a dichloromethane / acetone system for purification, methylating a crude product after purification, adding the crude product after methylation into a reaction kettle, adding a solvent and a catalyst in a certain amount, sealing the reaction kettle, and feeding hydrogen to certain pressure; reacting in a heat-collecting-type constant-temperature heating magnetic stirrer at constant temperature for certain time; after reaction is finished, separating a product from the catalyst, removing the solvent, and using a complexation method for purification to obtain the hydrogenated soybean lecithin.The preparation method is suitable for pilot-scale production, and the hydrogenated soybean lecithin prepared by the method can be used as a pharmaceutical excipient to be used in various drugs like injections, tablets and capsules.
Owner:苏州东南药业股份有限公司
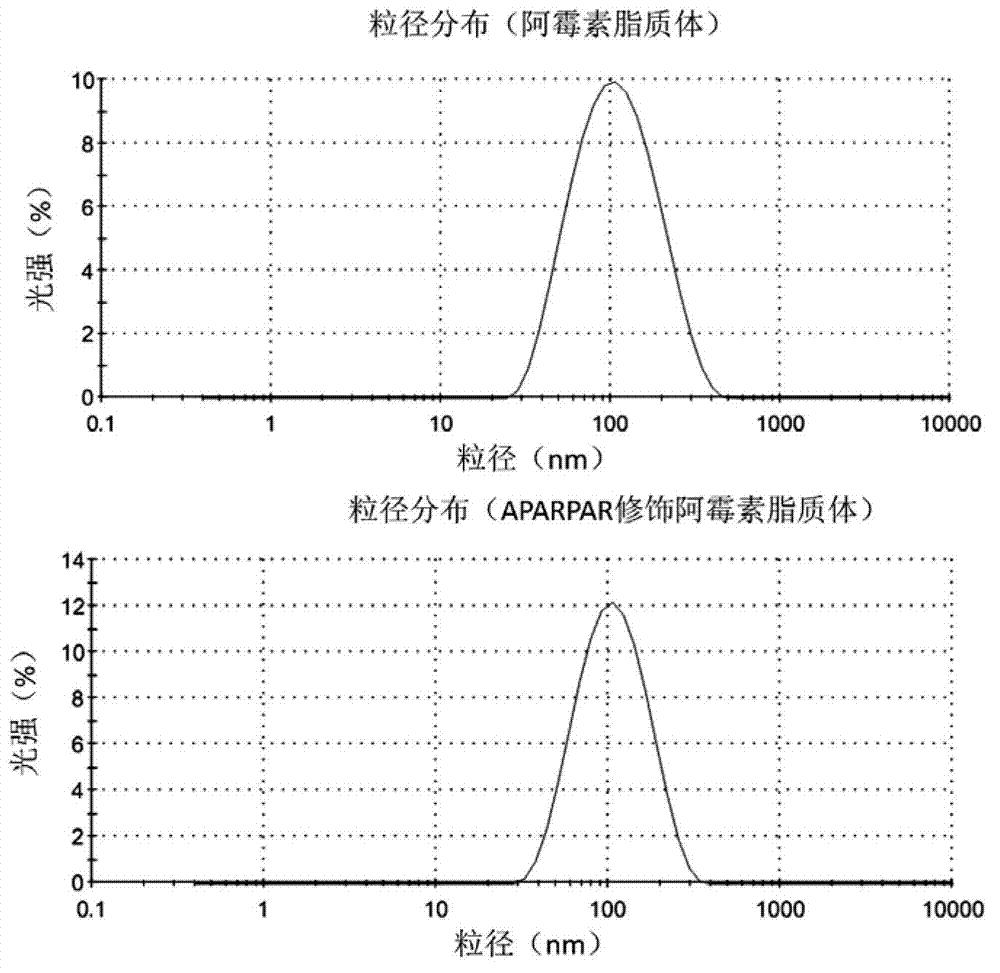
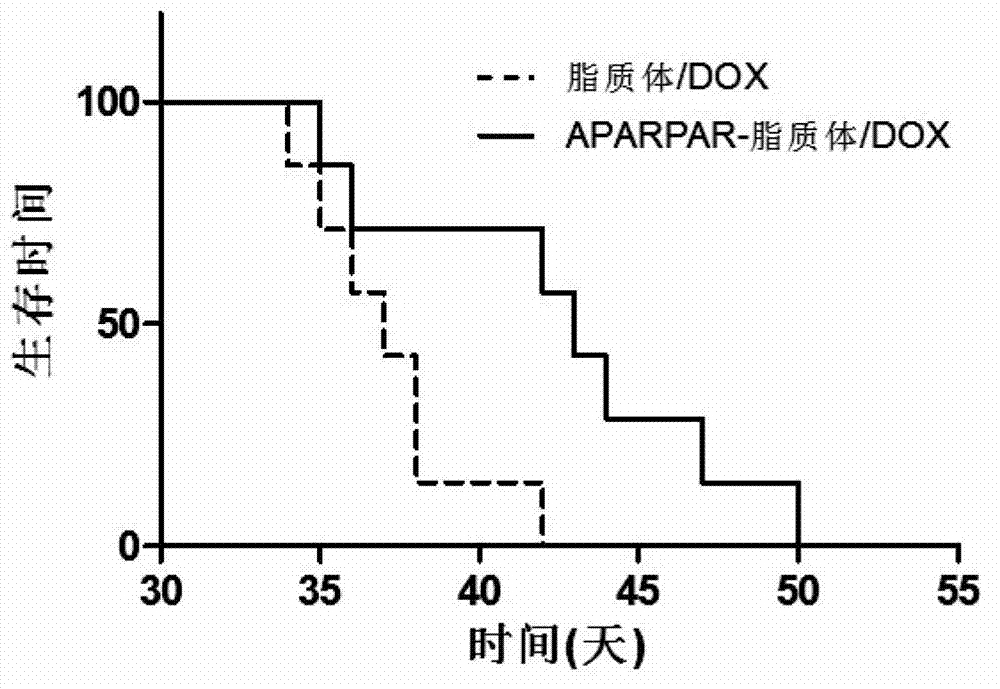
APARPAR polypeptide-modified invisible liposome and drug delivery system
InactiveCN103040755AIncrease lethalityOrganic active ingredientsMacromolecular non-active ingredientsCholesterolPolyethylene glycol
The invention discloses an APARPAR polypeptide-modified invisible liposome and a drug delivery system. The APARPAR polypeptide is modified on the surface of an invisible liposome. The membrane of the invisible liposome contains hydrogenated soybean lecithin, cholesterol, polyethylene glycol-stearoyl phosphatidyl ethanolamine and tearoyl phosphatidyl ethanolamine-polyethylene glycol-APARPAR. The invention further relates to the drug delivery system. The drug delivery system is formed by encapsulating an anti-tumor drug encapsulated with the APARPAR polypeptide-modified invisible liposome. The liposome drug delivery system can be used for targeted therapy of glioma and can go through the walls of blood vessels in a tumor mass under the mediating action of the APARPAR polypeptide to penetrate the inside of the whole tumor mass, so that the effect of killing the tumor cells is greatly improved.
Owner:SHANGHAI JIAO TONG UNIV +1
Chinese and western anti-fungal liposome and preparation method thereof
ActiveCN102631544AImprove solubilityPlay a protective effectOrganic active ingredientsAntimycoticsCholesterolAnti fungal
The invention discloses a Chinese and western anti-fungal liposome and a preparation method of the Chinese and western anti-fungal liposome. The Chinese and western anti-fungal liposome mainly consists of radix sophorae flavescentis and rhizoma polygonati extractive, voriconazole powder, hydrogenated soya bean lecithin, cholesterol and the like. The preparation method of the Chinese and western anti-fungal liposome comprises the following steps of: coating the radix sophorae flavescentis and rhizoma polygonati extractive in water phase of liposome, leading the voriconazole to be positioned on a phospholipid film, and combining the Chinese medicinal herb with the western medicine due to the preparation of the liposome. The Chinese and western anti-fungal liposome can play a part in slow release, wherein the Chinese medicinal herb immediately-dissolved medicine voriconazole is exposed after effecting to continuously play the medicine effect, and the slow-release effect is more obvious, and the Chinese medicine rhizoma polygonati has a certain repair function in vivo, so that the effects of treating and repairing can be achieved. The voriconazole is prepared into the liposome, and can be embedded with the phospholipid, so that the dissolubility of the voriconazole can be increased, and the Chinese and western anti-fungal liposome has a certain protection function, therefore, the stability of the voriconazole can be greatly improved, and the Chinese and western anti-fungal liposome plays a certain part in slow release.
Owner:ZHUHAI COLLEGE OF JILIN UNIV
Tablet containing cefprozi liposome and preparation method thereof
InactiveCN101953787AHigh dissolution rateThe drug works quicklyAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefprozi liposome, a preparation method thereof and a tablet containing the cefprozi liposome. The cefprozi liposome comprises cefprozi, hydrogenated soybean phosphatidylcholine, hydrogenated egg yolk lecithin and cholesterol, wherein the weight ratio of the cefprozi, the hydrogenated soybean phosphatidylcholine, the hydrogenated egg yolk lecithin and the cholesterol is 1:1.25-5:1.25-5:2.5-10. The cefprozi tablet meets the requirements of Chinese Pharmacopoeia and also has the advantages of higher dissolution rate, faster effect and greatly enhanced bioavailability compared with the ordinary cefprozi drug composite.
Owner:王丽燕
Nilvadipine liposome tablets
InactiveCN101912372AImprove stabilityHigh encapsulation efficiencyOrganic active ingredientsPill deliverySolubilitySide effect
The invention provides nilvadipine liposome tablets, which are characterized by consisting of the following components in part by weight: 1 part of nilvadipine, 1.2 to 10 parts of hydrogenated soybean phosphatidylcholine, 0.3 to 6 parts of cholesterol, 0.2 to 5 parts of Tween 80 and 30 to 60 parts of other pharmaceutically acceptable excipients. The prepared nilvadipine liposome has the advantages of high entrapment rate, small side effect, great improvement on the stability and water solubility and facilitating to dissolution of the medicinal preparations.
Owner:HAINAN SHU ER PHARMA RES
Antioxidant for baked food
InactiveCN104082363AInhibitory activityReduce browningDough treatmentFruit and vegetables preservationBiotechnologyOxidative enzyme
The invention discloses an antioxidant for baked food. The antioxidant is prepared by mixing the following components by weight percent, 0.001 to 5% of glutathione, 0.003 to 2% of hydrogenated soybean lecithin, 0.001 to 5% of soybean protein polypeptide, 0.001 to 20% of food-grade bamboo vinegar, 0.003 to 4% of alpha-lipoic acid, 0.002 to 6% of Vitamin E, 0.005 to 3% of xanthan gum, and the balance of water. Through the effects of restraining the activity of polyphenol oxidase in the food, removing free radicals, preventing oxidization and the like, the browning of fruits and vegetables is slowed down, and the shelf life of products is prolonged.
Owner:NANTONG HAOYOU FOOD ADDITIVES
Production method of high-purity hydrogenated soybean lecithin
InactiveCN108822146AHigh purityLow hydrogen pressureGroup 5/15 element organic compoundsPhosphatide foodstuff compositionsHydrogen pressureSolvent
The invention provides a preparation method applied to catalytic hydrogenation and purification of soybean lecithin. The method comprises the following specific steps: (1) adding soybean lecithin, a catalyst and solvent into a stainless steel reactor, sealing the reactor, filling the reactor with nitrogen to replace the air in the reactor, then filling the reactor with hydrogen to replace the nitrogen in the reactor, and finally maintaining certain hydrogen pressure in the reactor and carrying out constant-temperature reaction; and (2) separating the product from the catalyst after the reaction is completed, adding active carbon and decolorizing, filtering then removing the solvent, re-crystallizing and pulping to obtain high-purity hydrogenated soybean lecithin. The method is capable of preparing the high-purity hydrogenated soybean lecithin; the high-purity hydrogenated soybean lecithin can be used as accessory materials of injection medicines in the pharmaceutical industry; the method is mild in reaction condition and easy to control, and is suitable for industrial large-scale production.
Owner:CHINA PHARM UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com