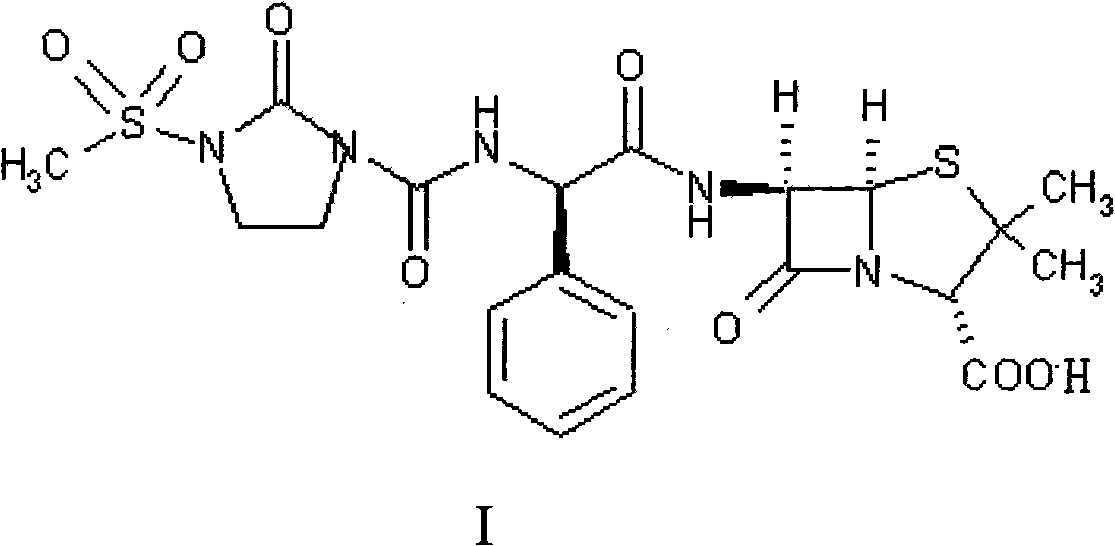
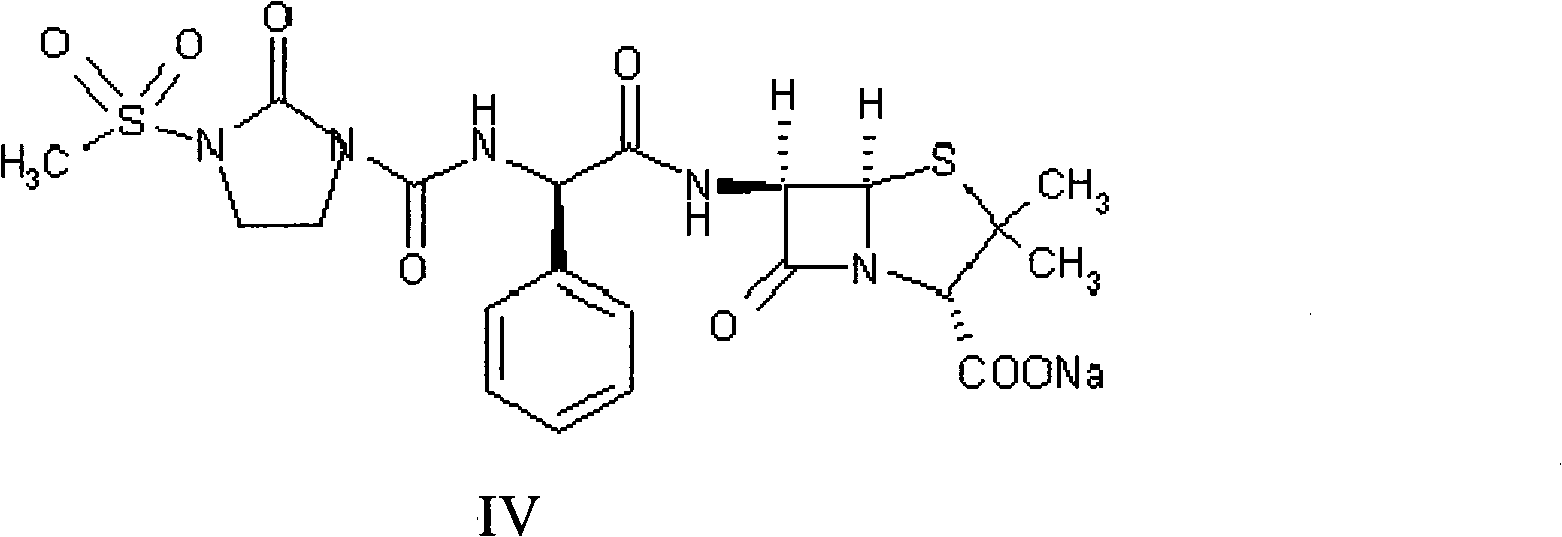
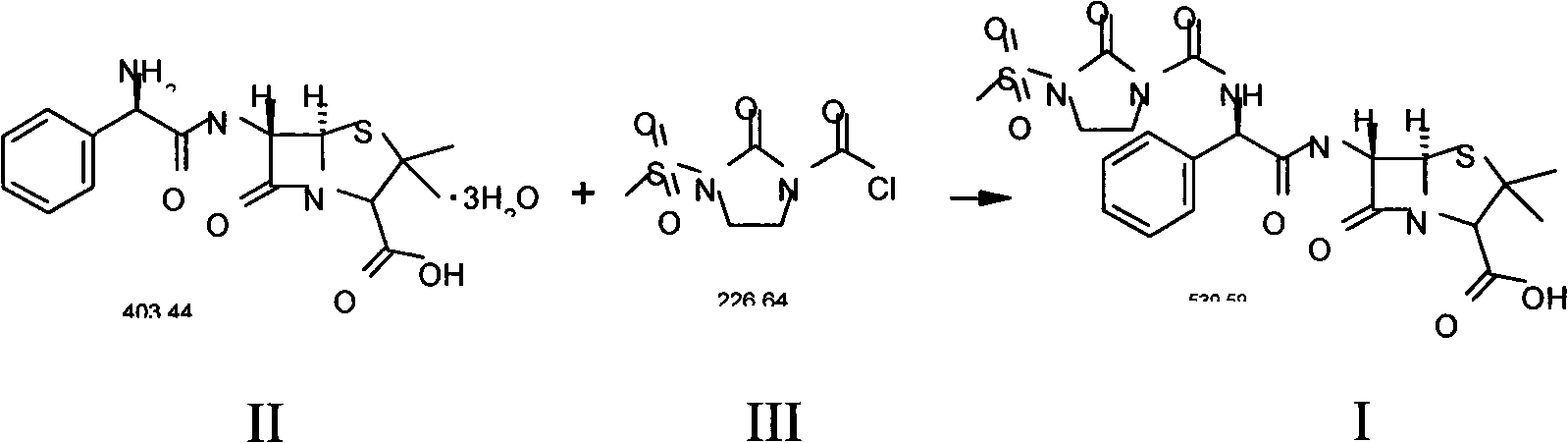
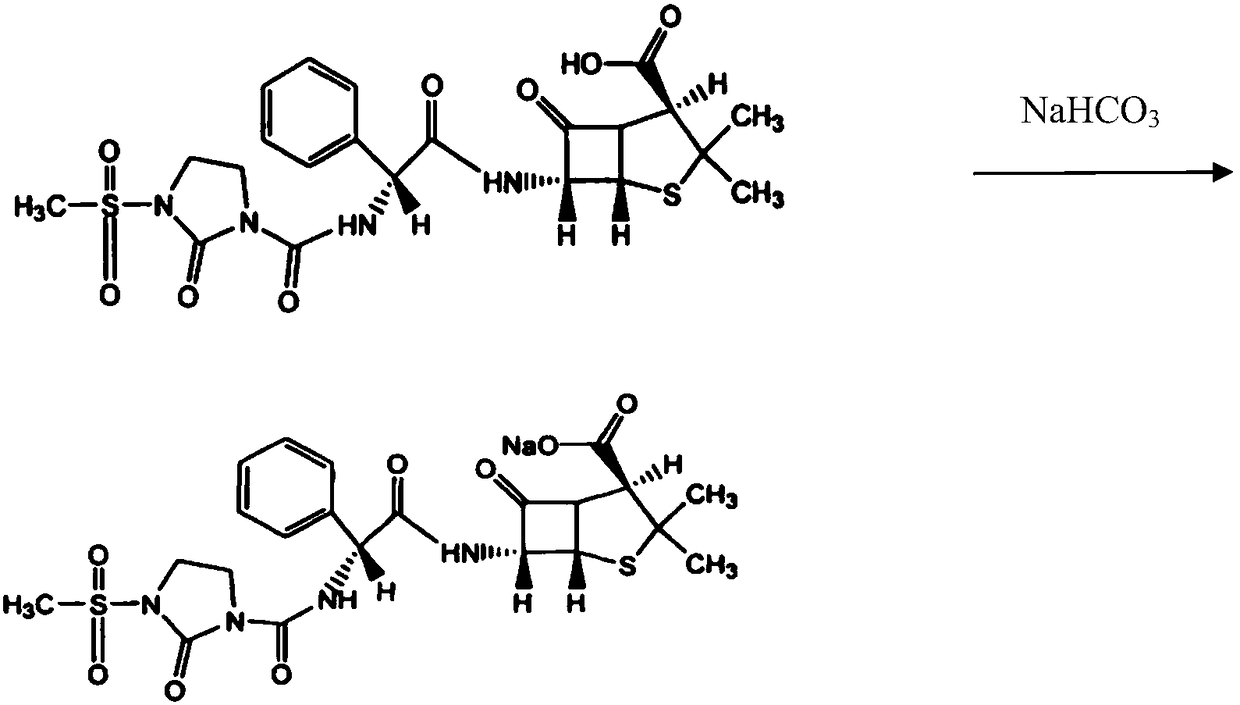
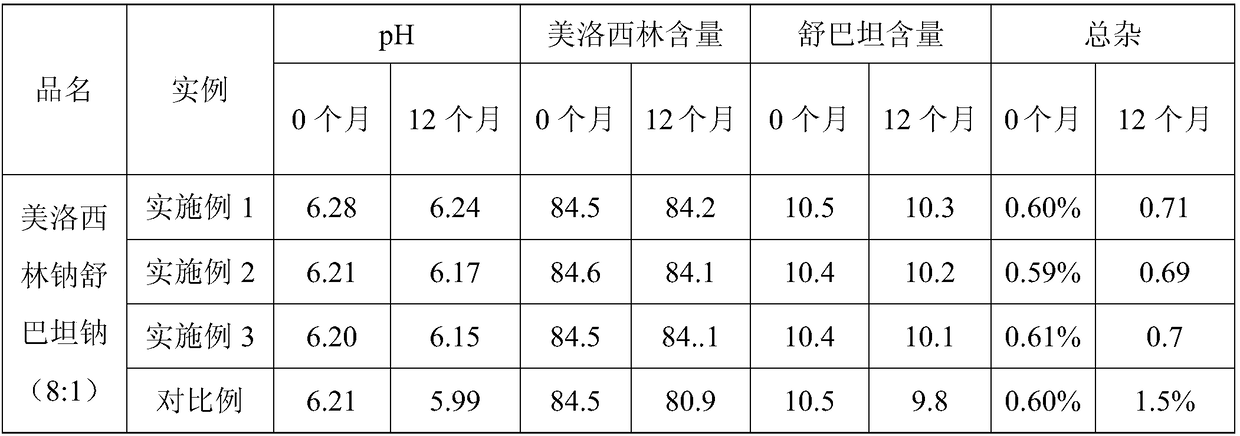
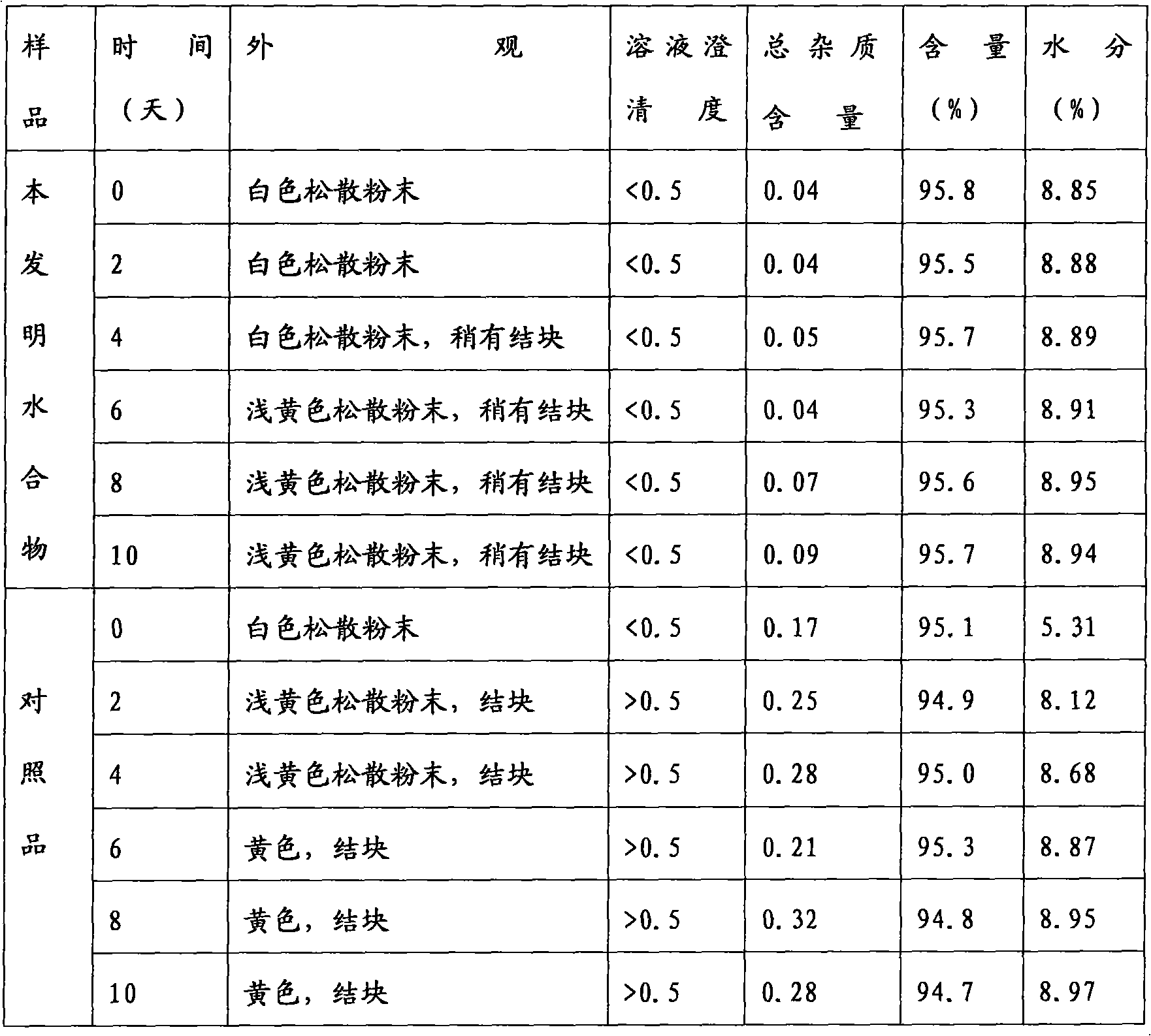
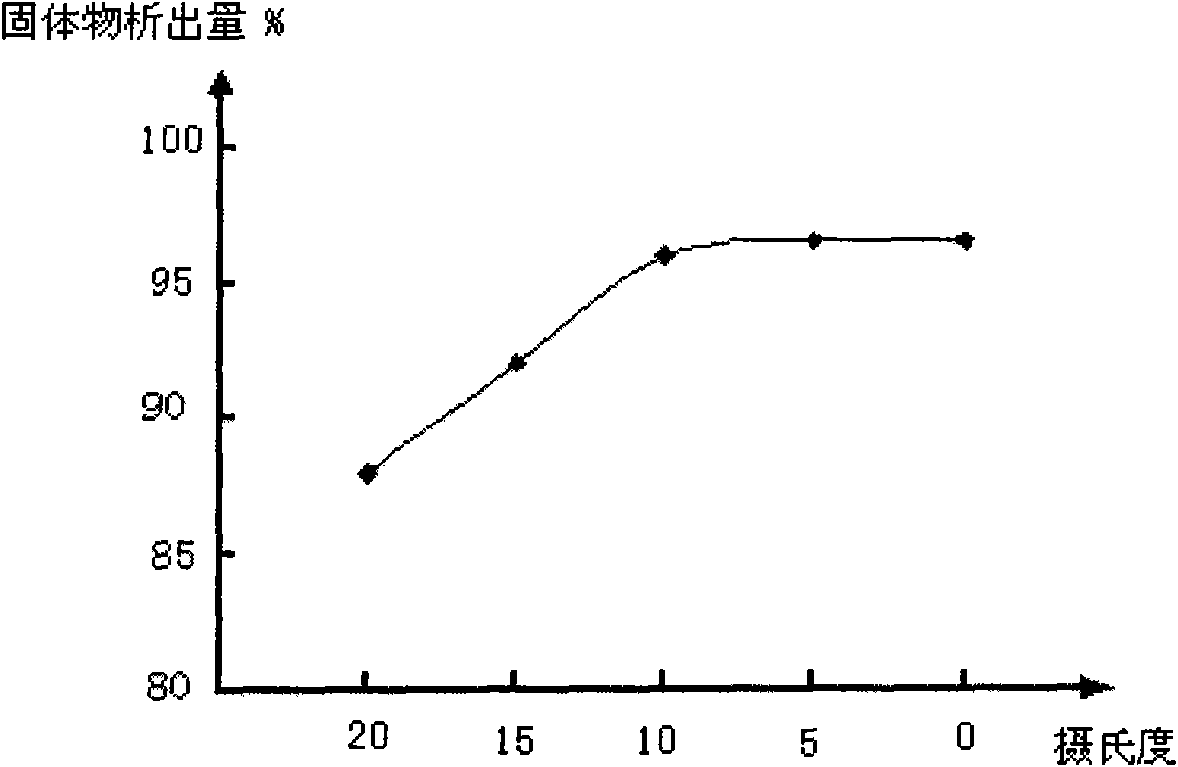
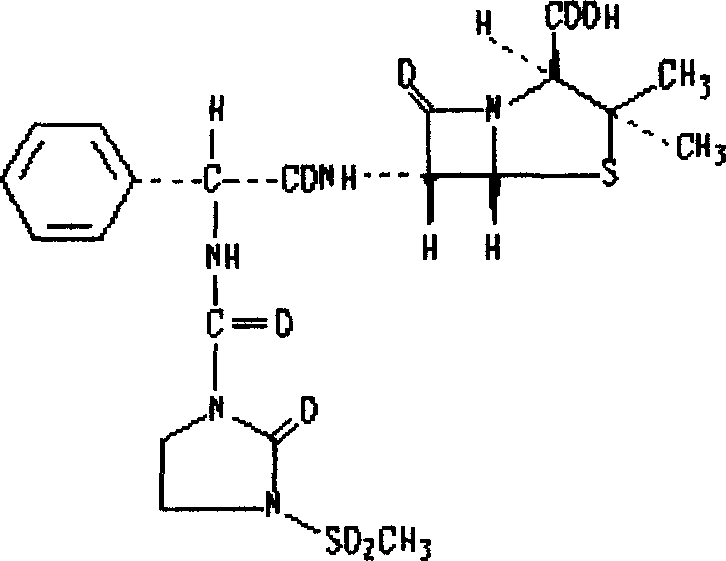
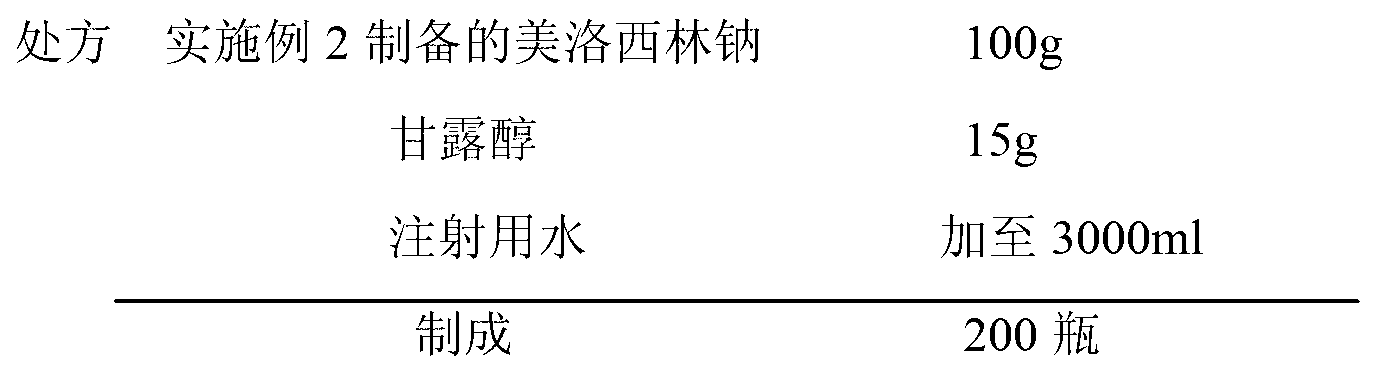
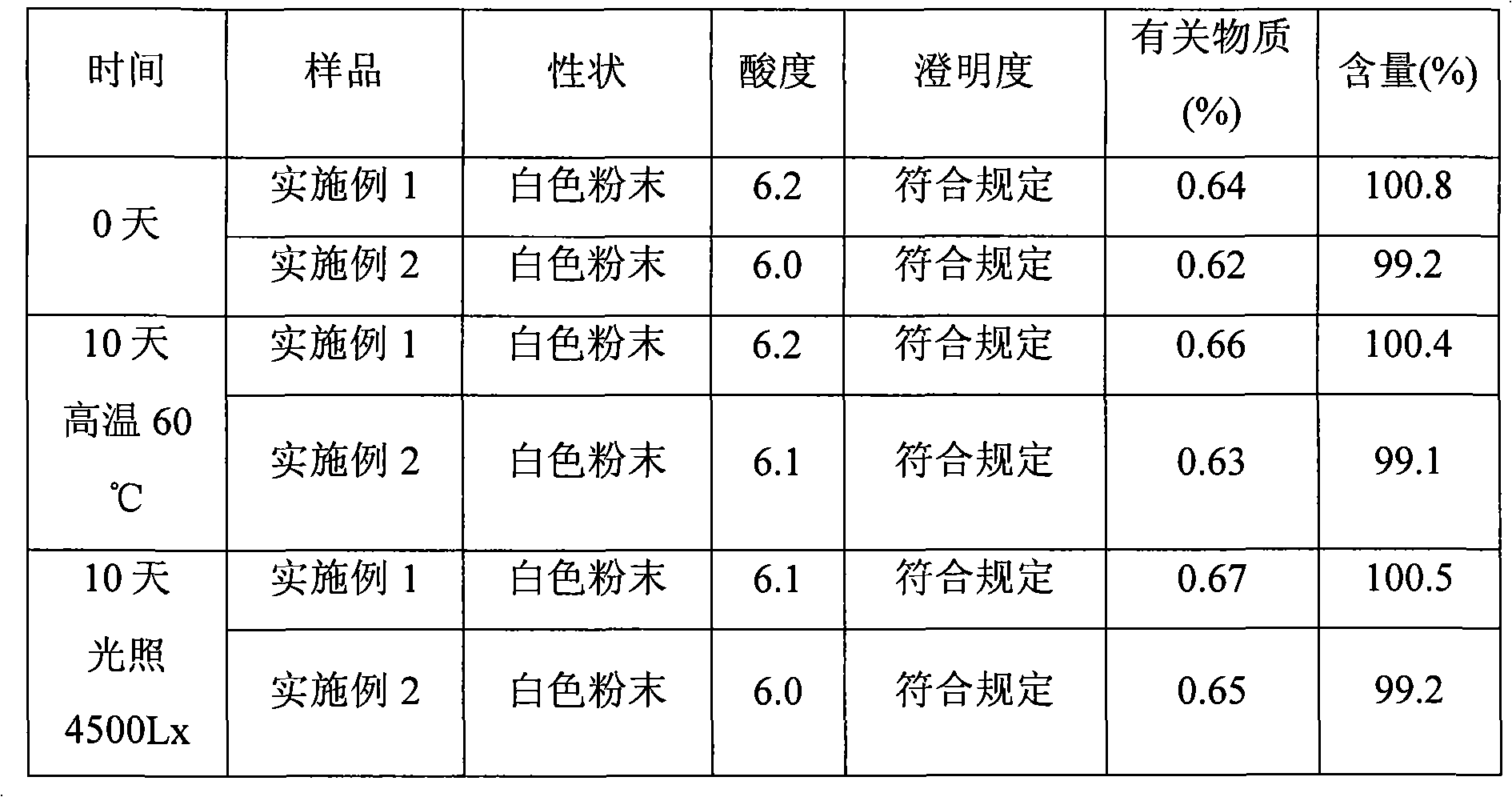
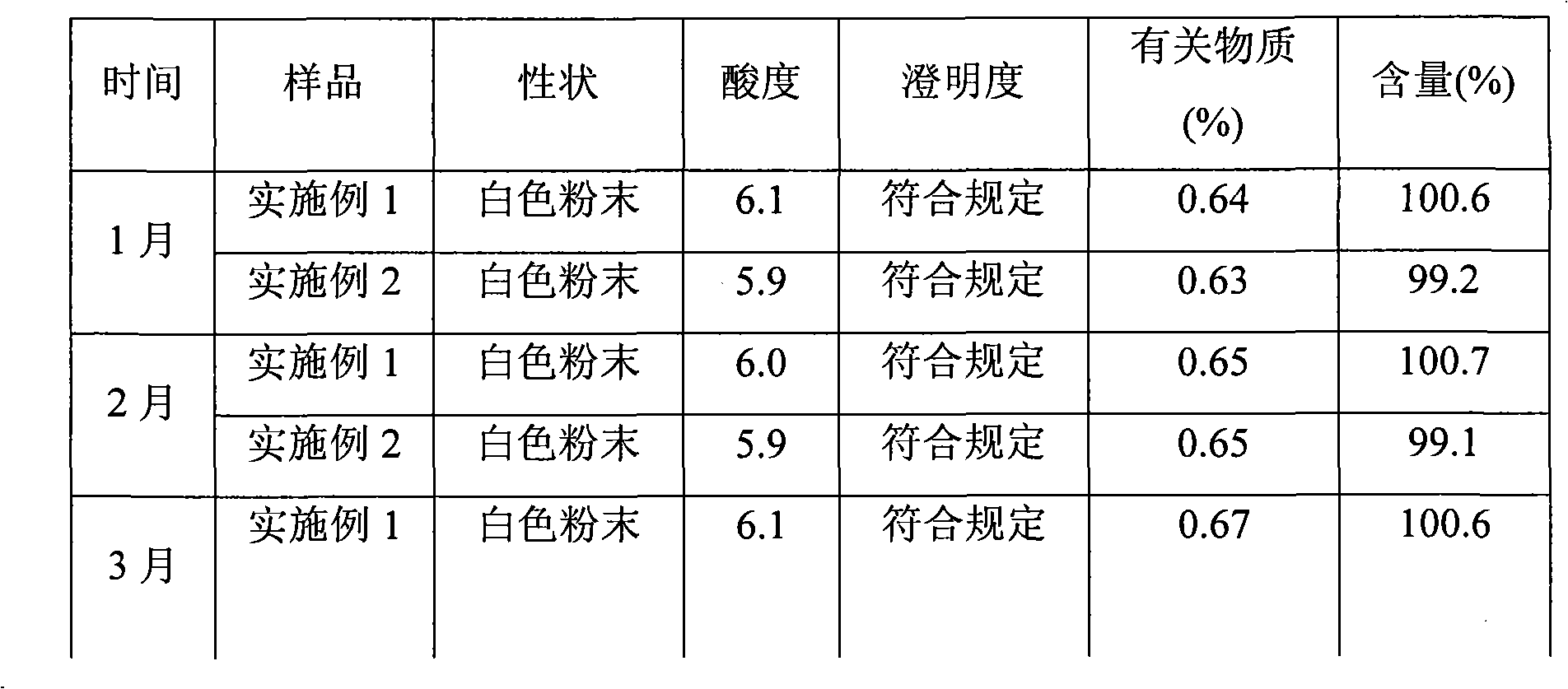
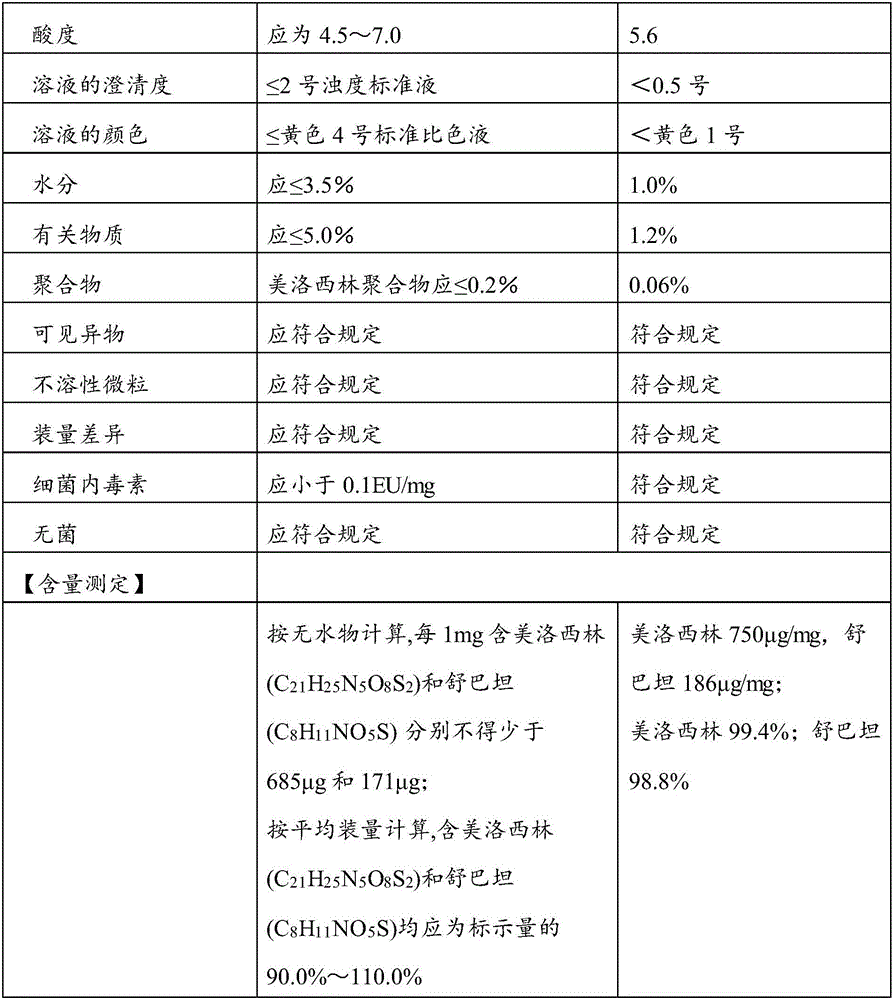
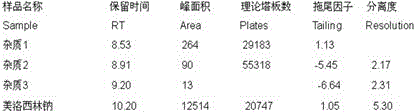
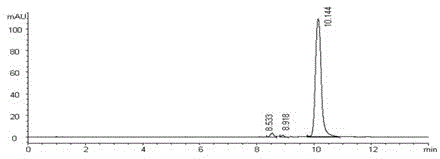
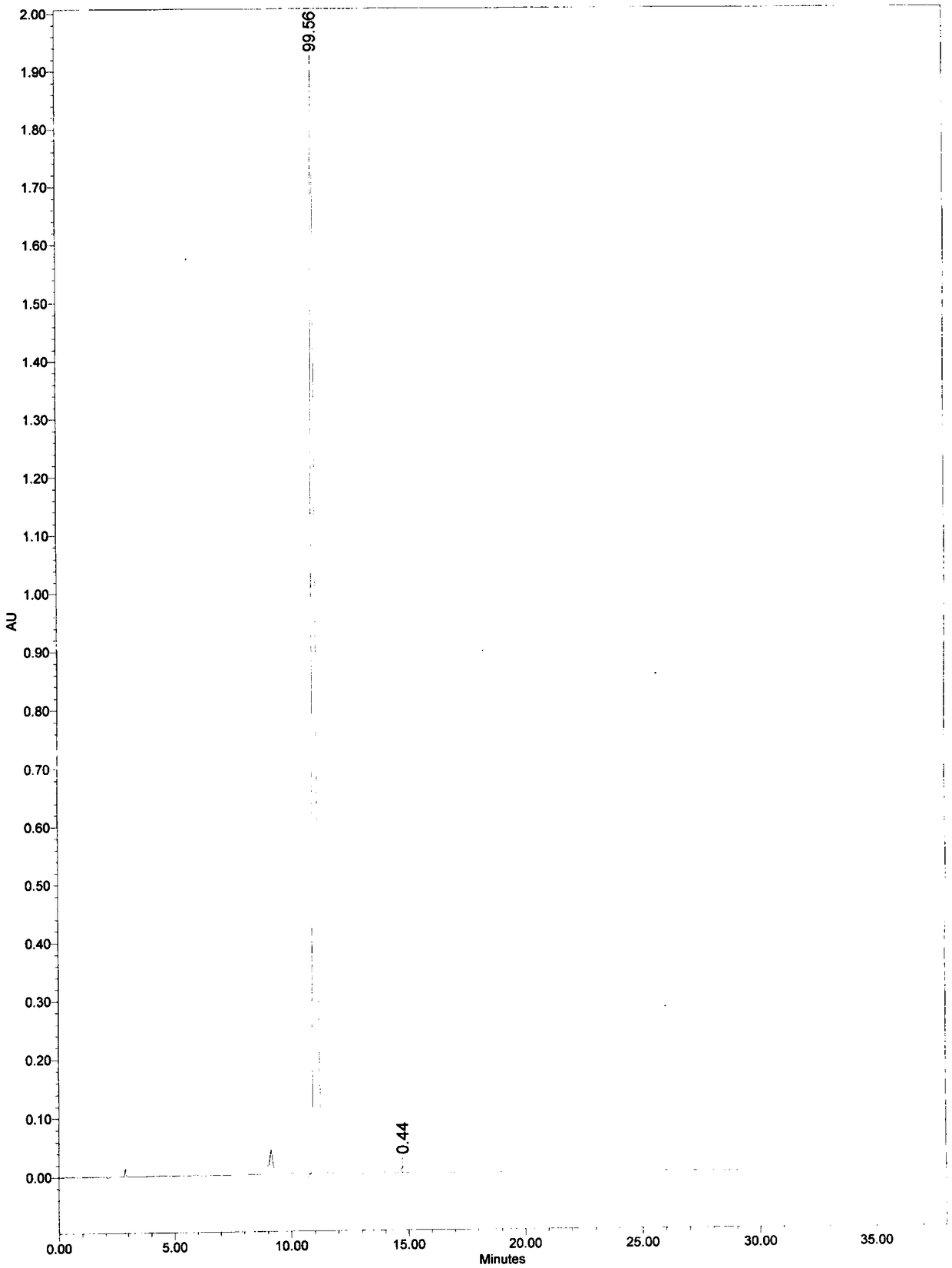
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
87 results about "MEZLOCILLIN SODIUM" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
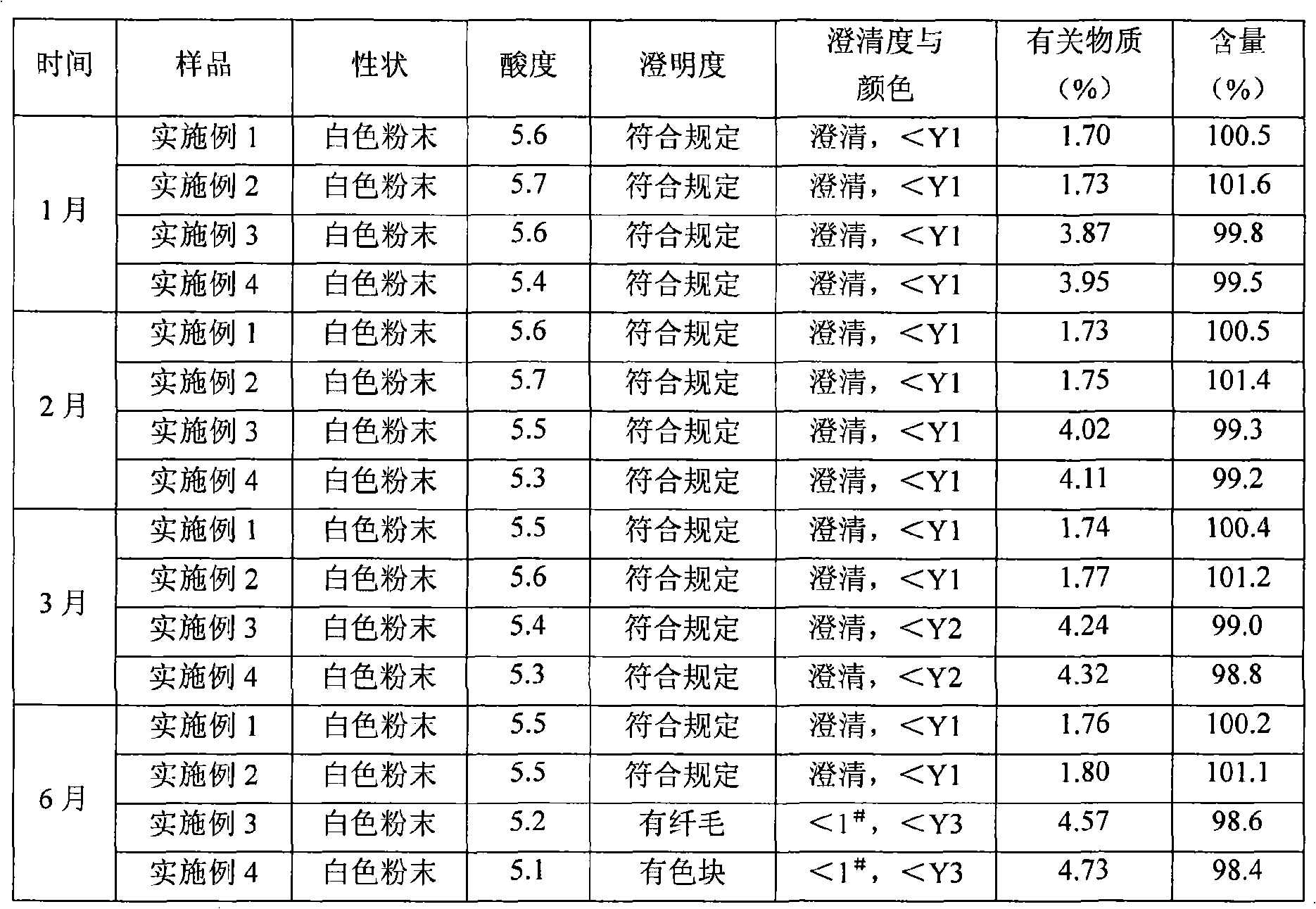
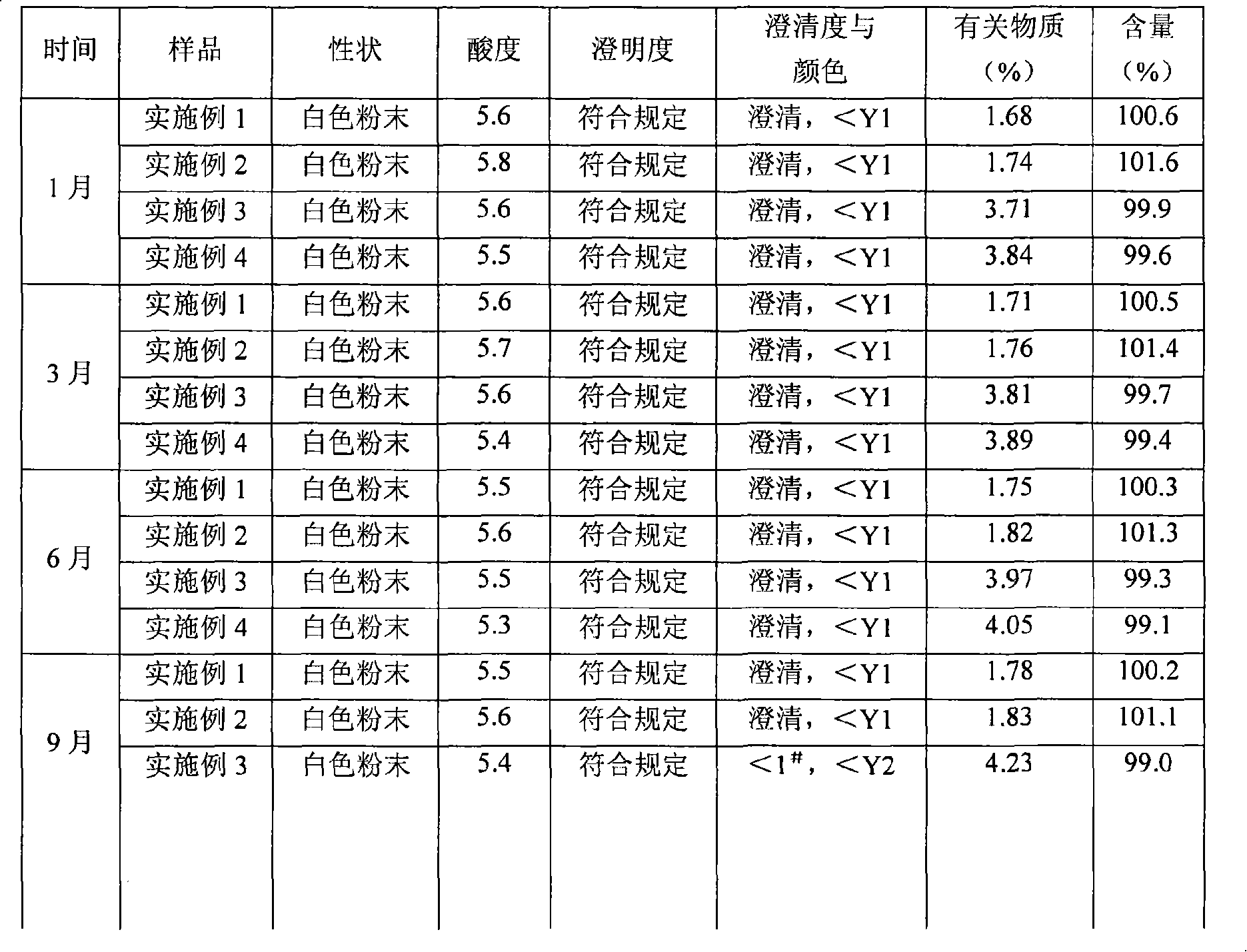
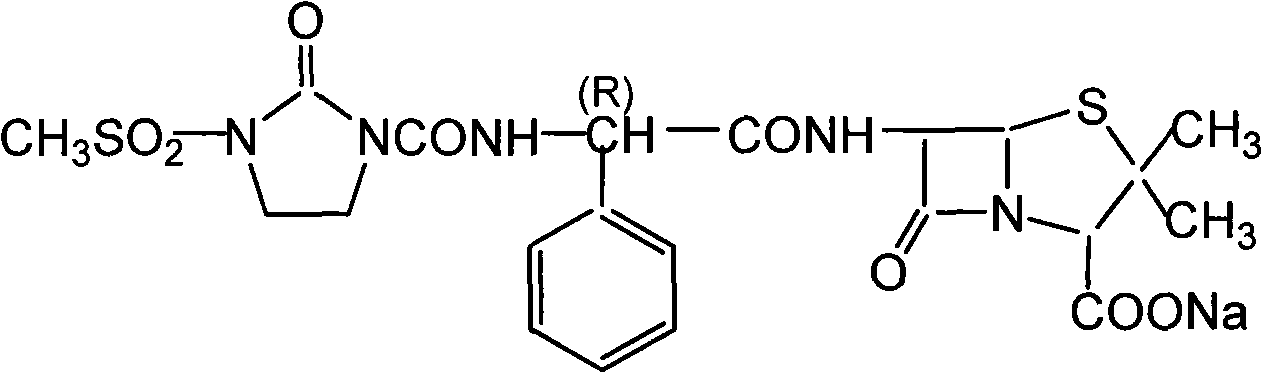
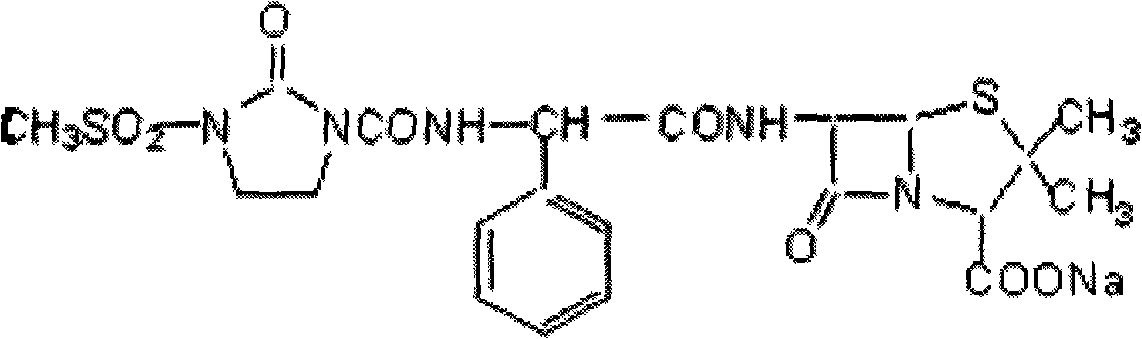
Mezlocillin sodium is an extended spectrum β-lactam antibiotic and is sparingly soluble in aqueous solution 0.47 mg/mL.
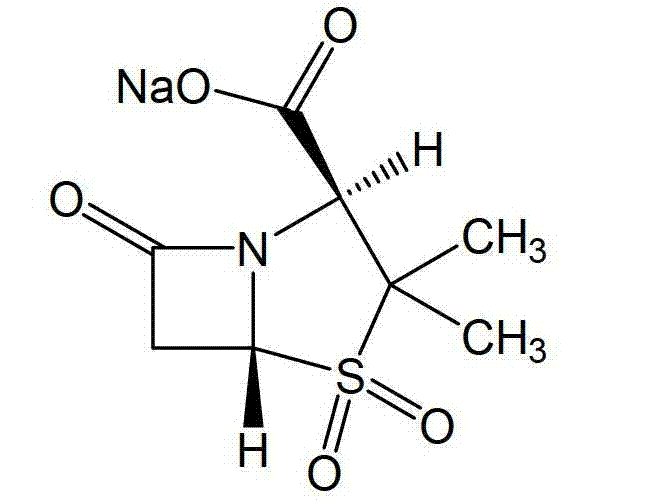
Mezlocillin sodium compound and medicine composition thereof
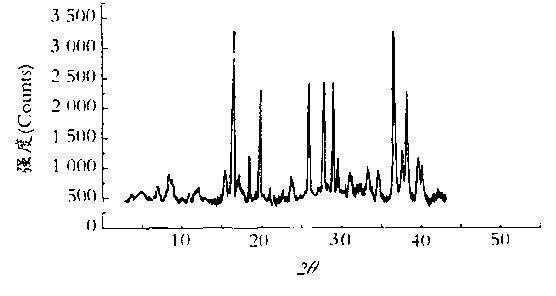
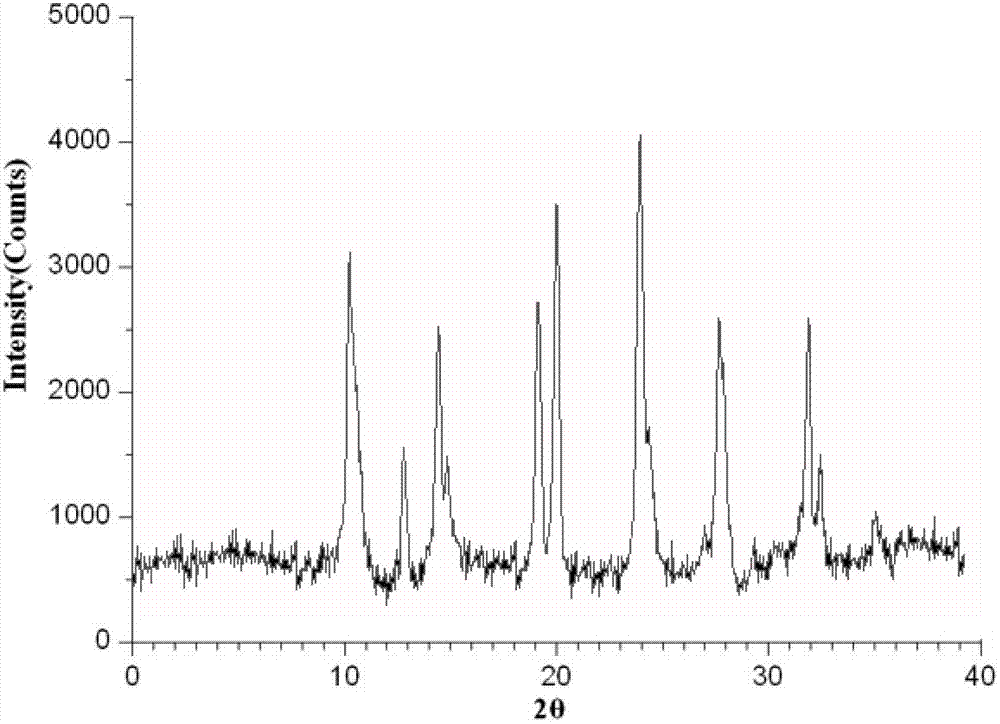
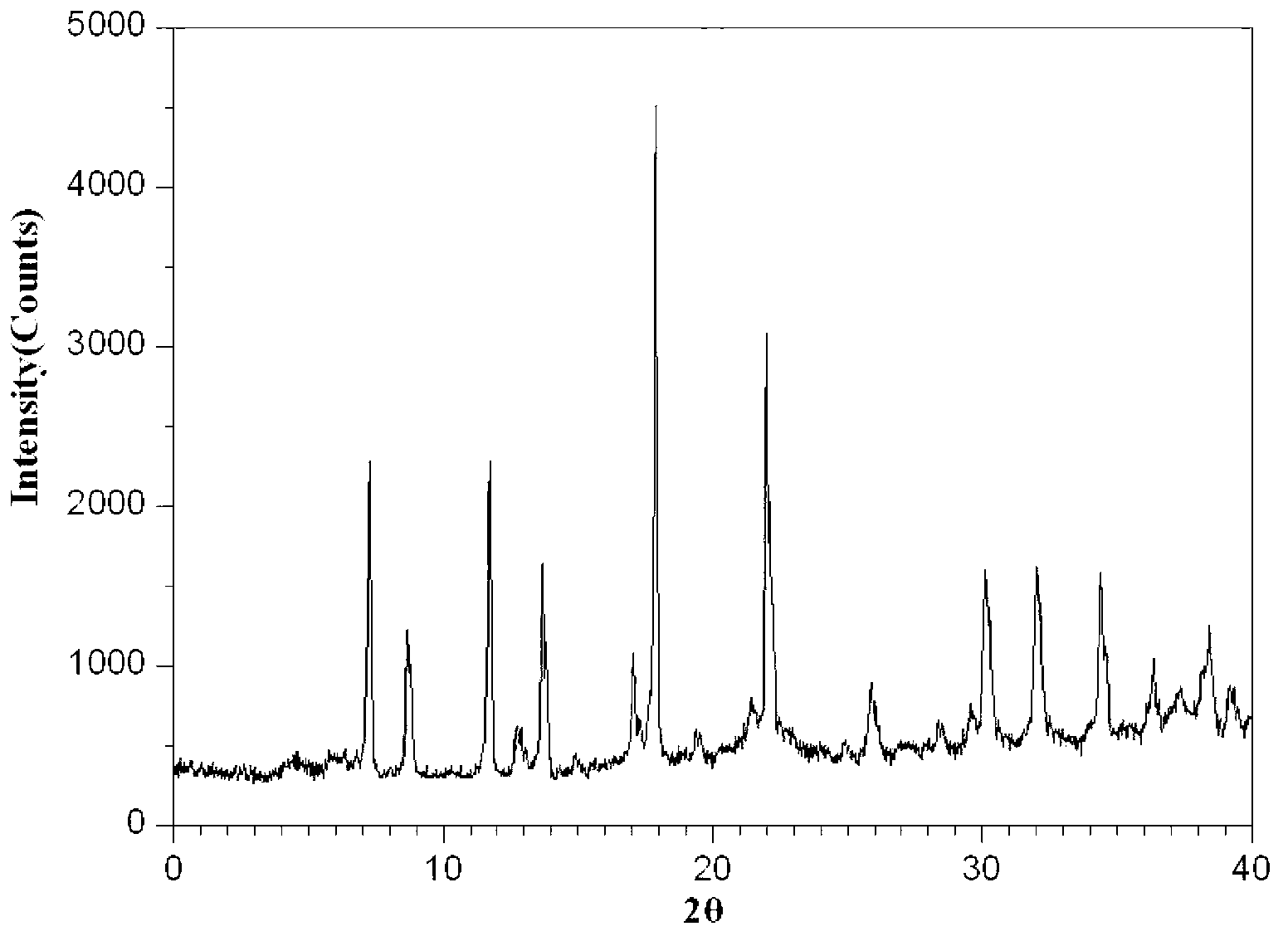
The invention relates to a mezlocillin sodium compound which is determined by adopting X-ray powder diffraction and has characteristic peaks shown in 2theta of 8.9, 15.7, 16.5, 18.9, 19.8, 24.6, 26.4, 27.8, 29.0, 29.7, 31.8, 33.2, 34.7, 36.8, 37.5, 38.9 and 40.1 in a map. The invention also relates to a mezlocillin sodium compound and a medicine composition with a medicine active component of the mezlocillin sodium compound or the mezlocillin sodium compound and sulbactam sodium or tazobactam sodium. The medicine composition is a powder injection of the mezlocillin sodium compound, or a medicine mixture powder injection of the mezlocillin sodium compound and the sulbactam sodium or tazobactam sodium. The mezlocillin sodium compound has the advantages of difficulty in absorbing mixture, good flowability, high dissolving speed, kept extremely high stability, and greatly improved convenience and safety of the mezlocillin sodium.
Owner:HUNAN KELUN PHARMA
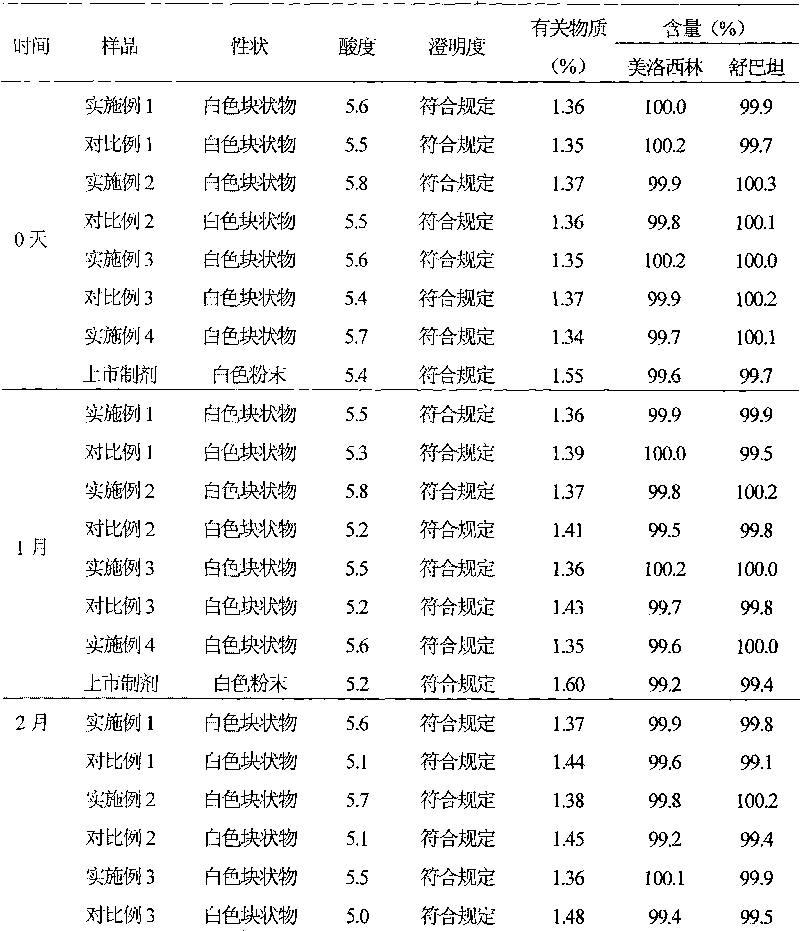
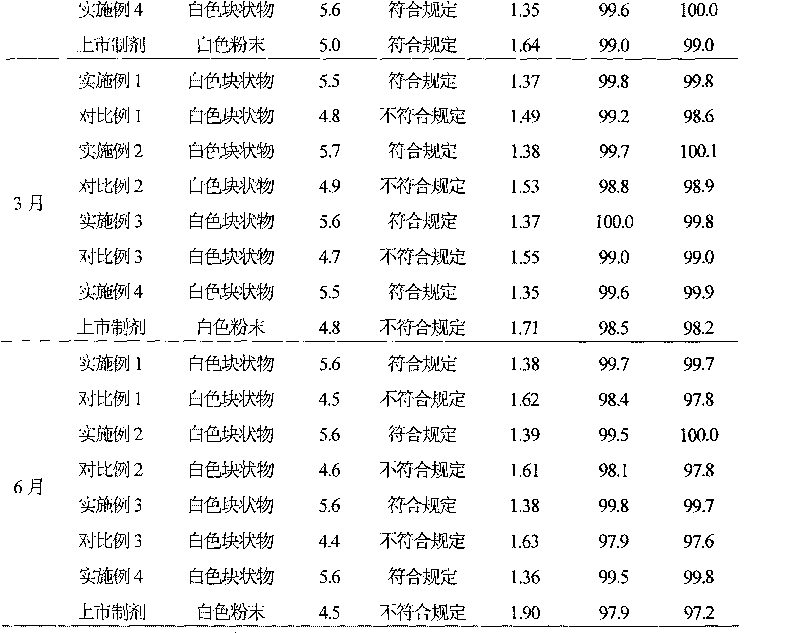
Mezlocillin sodium and sulbactam sodium for injection and freeze-dried injection preparation thereof
InactiveCN101322685AImprove stabilityAvoid lostAntibacterial agentsPowder deliveryCountercurrent chromatographyFreeze-drying
The invention provides a preparation method of mezlocillin sodium-sulbactam sodium for injection and the freeze-dried powder injection thereof as well as a separating and purifying method of the mezlocillin sodium and sulbactam sodium for injection. By adopting high speed countercurrent chromatography, the invention prepares trichloromethane, methanol and water to form a solvent system with an immobile phase and a mobile phase and separates and purifies mezlocillin sodium and sulbactam sodium to obtain the mezlocillin sodium and sulbactam sodium for injection; the purity of the obtained product can reach more than 99% and the prepared injection has improved stability.
Owner:海南华旗药业销售有限公司
Medicinal-composition suspension powder injection with mezlocillin sodium and sulbactam sodium, and novel application thereof
InactiveCN101703506AUnexpected effectImprove stabilityAntibacterial agentsPowder deliveryActive componentCholesterol
The invention belongs to the technical field of medicine, and discloses a medicinal-composition suspension powder injection taking mezlocillin sodium and sulbactam sodium as active components. The injection comprises 4 parts of mezlocillin sodium, 1 part of sulbactam sodium, 5 to 20 parts of Tween 80, 1 to 10 parts of cholesterol, 0.5 to 15 parts of deoxysodium cholate, and 2 to 30 parts of frozen-dried supporting agent. The invention further discloses novel application of the injection in the preparation of medicaments for preventing postoperative infection of appendicitis.
Owner:HAINAN YONGTIAN PHARMA INST
Preparation method of mezlocillin sodium and mezlocillin sodium for injection
The invention provides a preparation method of mezlocillin sodium and mezlocillin sodium for injection. The preparation method comprises the following steps of: (a) adding mezlocillin acid into water for injection, and cooling material liquor at 12-15 DEG C; (b) dropwise adding a NaHCO3 solution, adjusting the pH value at 5.8-6.5, and stirring until the material liquor is clean and the pH value is stable; (c) raising the temperature of the material liquor to be 20 DGE C+ / -2 DEG C, and stirring for 30-60 minutes; (d) adding active carbon for injections, stirring for 15-30 minutes, and decarburizing by a plate frame filter; (e) degerming and filtering the decarburized filtrate by a 0.45mu m filter and a 0.22mu m filter; and (f) freeze-drying to obtain the mezlocillin sodium finished product. By improving the conventional freeze-drying technology and combining with the improved preparation method of the mezlocillin acid, the method prepares the mezlocillin sodium product with low polymer content, so that the product quality is obviously improved, and the possibility of allergy caused by the product is greatly reduced.
Owner:SUZHOU ERYE PHARMA CO LTD
Preparation process of mezlocillin sodium
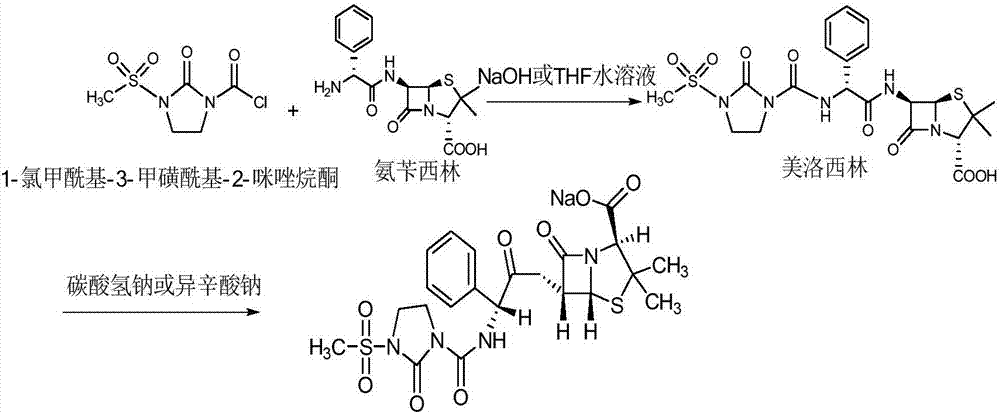
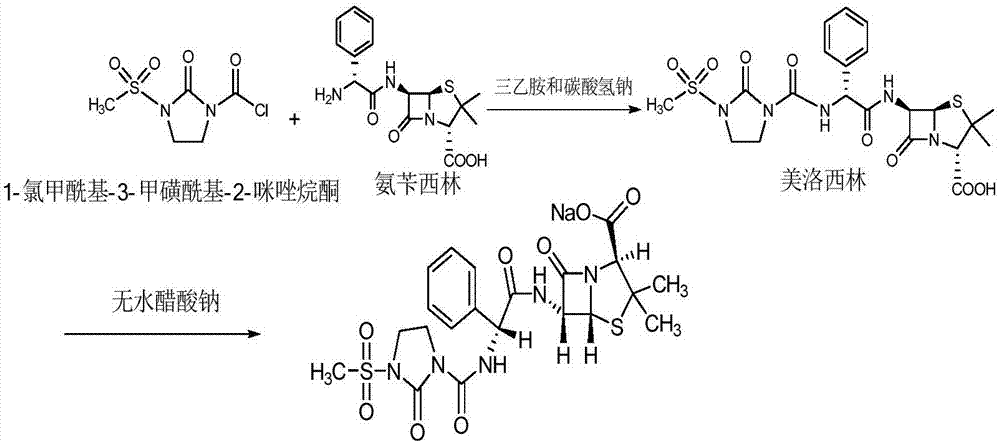
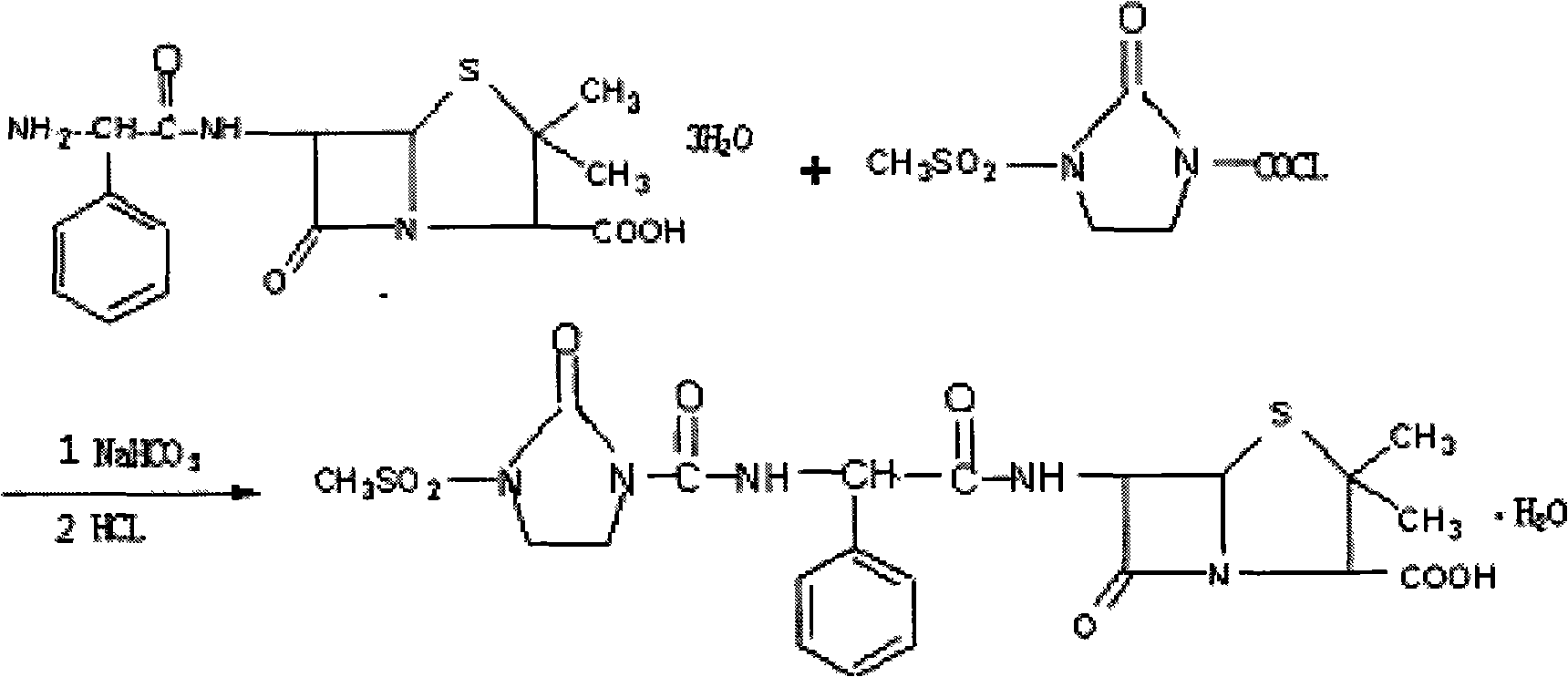
Disclosed is a process for preparing mezlocillin sodium belonging to compound preparation technical field. By acylation reaction of aminobenzyl triaqua acid with 1-chloroformyl-3-mesyl-2-imidazolidinone, acidizing after an ethyl acetate solvent being added, esterified layer removing, salifying reaction after a sodium salt forming agent being added, and seedout, the mezlocillin sodium is obtained. In the invention, the mixed solvent is adopted and the speed of adding the sodium salt forming agent is adjusted. There is recrystallization buffer, so the crystal grain is big and the purity is high.
Owner:REYOUNG PHARMA
Preparation method of mezlocillin sodium solvent crystal
ActiveCN101570543AImprove product qualitySolubility problem solvingAntibacterial agentsOrganic chemistryEthyl acetateSodium hexanoate
The invention discloses a preparation method of mezlocillin sodium solvent crystal, comprising the steps of (1) dissolving crude mezlocillin acid in a reaction solvent A to obtain solution A; (2) dissolving organic salt forming agent in a reaction solvent B to obtain solution B; (3) after adding the solution A into the solution B for sufficient reaction, slowly adding a poor solvent, separating out sodium solvent crystal, filtering, obtain filtrate and filter mass, drying the filter mass; the organic salt forming agent is sodium iso-octoate, ethyl acetoacetate or 2-ethyl sodium hexanoate; the reaction solvent A or B is independently acetone or ethyl acetate; the poor solvent is methanol, ethanol, propanol, isopropanol, aether, isopropyl ether, tetrahydrofuran, ethyl formate orbutyl acetate. The method is simple to operate, and has high stability, large yield, excellent product quality and few impurity contents; the reaction solvents and crystallizing solvent can be recycled, thus having low production cost, high reaction yield, excellent implementing value, and social and economic benefits.
Owner:ZHEJIANG UNIV OF TECH
Method for preparing mezlocillin sodium for injection
ActiveCN102895181AImprove drug stabilityImprove injection safetyAntibacterial agentsPharmaceutical delivery mechanismMicrometerNitrogen
The invention relates to a method for preparing mezlocillin sodium for injection, comprising the following steps of introducing nitrogen filtered by a 0.22 micrometer membrane filter to an injection bottle subjected to sterilization pretreatment, separately loading mezlocillin sodium with the specific rotation of +190-(+195) DEG into the injection bottle, introducing nitrogen to the separately-loaded drug, covering the bottle with a pretreated rubber plug, finally putting into a bottle capping chamber, capping the bottle by using an aluminium cap and obtaining mezlocillin sodium for the injection. According to the invention, the injection preparation is prepared by selecting mezlocillin sodium with the specific rotation of +190-(+195) DEG and the pH value of 7.0-7.5, therefore, mezlocillin sodium for the injection is better in drug stability and safer when serving as the injection preparation.
Owner:SICHUAN PHARMA
Preparation technique of mezlocillin sodium freeze-dried powder
InactiveCN102008452ASimple stepsHigh yieldAntibacterial agentsPowder deliverySodium bicarbonateActivated carbon
The invention relates to a preparation technique of mezlocillin sodium freeze-dried powder, which comprises the following steps of: (1) mixing and stirring mezlocillin and distilled water; (2) adding saturated sodium bicarbonate solution until the pH value of the reaction liquid reaches 4.5-7.5, and continuing stirring; (3) adding activated carbon, which accounts for 0.3% of the reaction liquid by volume, to the reaction liquid, and stirring to decoloring the reaction liquid; (4) carrying out rough filtration by using filter paper, and carrying out fine filtration on the rough filtration liquid by using a 0.22 mum filter membrane; (5) sublimating water in the fine filtration liquid by freeze-drying; and (6) collecting the material, pulverizing and screening to obtain the mezlocillin sodium aseptic powder. The preparation technique has the advantages of simple procedure, high yield and low processing cost, and is suitable for mass production.
Owner:JIANGSU HI STONE PHARMA
Antibiotics medicine for injection
InactiveCN1432361AHigh antibacterial activityBroad spectrum antibacterialAntibacterial agentsOrganic active ingredientsEscherichia coliUpper urinary tract infection
The present invention relates to medicine technology and is improved mezlocillin sodium injection. The injection has one medicine weight ratio of mezlocillin sodium / Sulbactam sodium 1-16 to 1. It hasstrong antibacterial activity, wide antibacterial spectrum and thus high curative effect, and may be used in treating serious urinary tract infection, respiratory tract infection, intestinal tract, otorbinolaryngological infection, abdominal cavity infection, etc. caused by zymogenic staphylococcus, pneumococcus, enterococcus, purulent streptococcus, Hemophilus infuenzae, etc.
Owner:REYOUNG PHARMA
Preparation method of mezlocillin sodium silica gel surface molecularly imprinted polymer
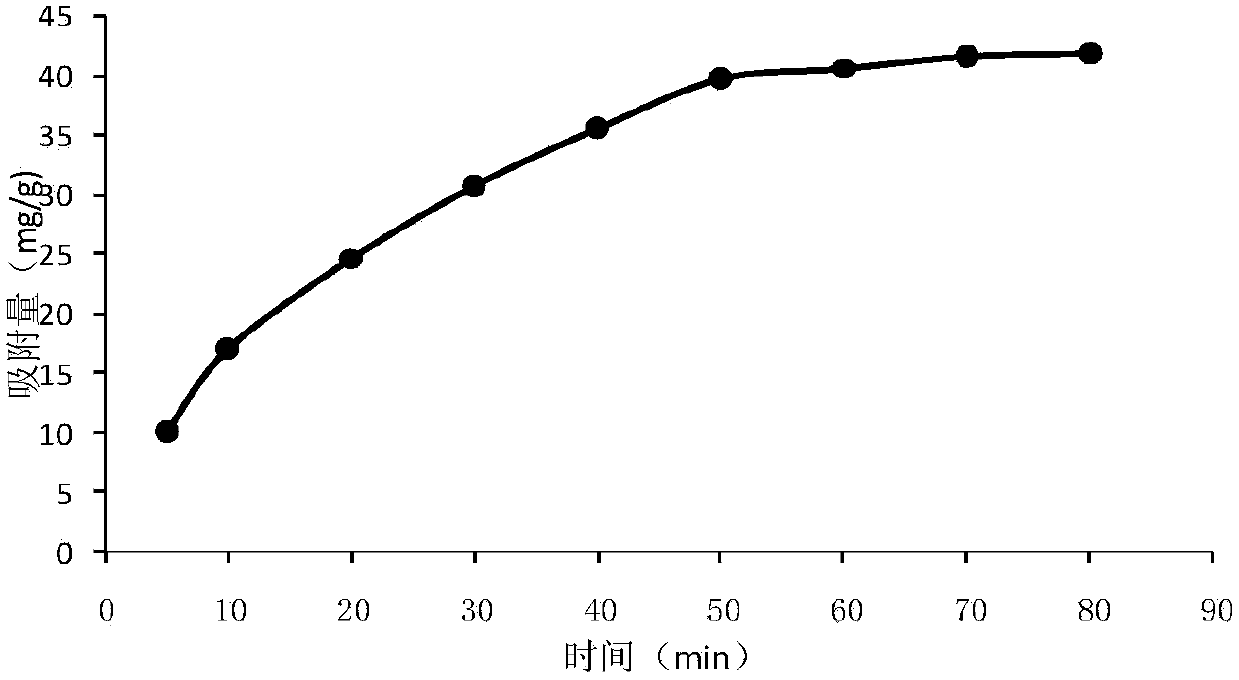
InactiveCN103626938AAdsorption equilibrium time is shortShort elution timeOther chemical processesSilica gelMolecularly imprinted polymer
The invention discloses a preparation method of a mezlocillin sodium silica gel surface molecularly imprinted polymer, which comprises the following steps: activating silica gel by heating under reflux in hydrochloric acid, carrying out surface modification on the activated silica gel, and finally, synthesizing the mezlocillin sodium molecularly imprinted polymer on the silica gel surface. The mezlocillin sodium molecularly imprinted polymer is a porous structure, contains a structure complementary with template molecules inside, has a memory function, and thus, has very high specificity and selectivity for the template molecules.
Owner:XI AN JIAOTONG UNIV
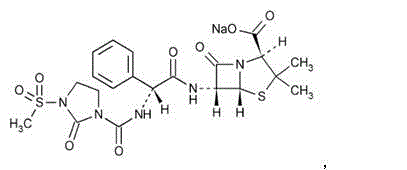
Sulbactam sodium compound and medical composition of sulbactam sodium compound and mezlocillin sodium
ActiveCN103113390ALess impurity content in storageGood storage stabilityAntibacterial agentsOrganic chemistryChemical compositionPowder diffraction
The invention belongs to the technical field of medicines, and in particular relates to a sulbactam sodium compound and a medical composition of the sulbactam sodium compound and mezlocillin sodium. The sulbactam sodium compound is shown as by an X-ray powder diffraction spectrogram I measured by a Cu-K alpha ray. The sulbactam sodium compound provided by the invention has better storage stability. The invention further provides a preparation method of the sulbactam sodium compound as well as a medical composition of the sulbactam sodium compound and mezlocillin sodium. The medical composition has better storage stability and higher using safety performance.
Owner:SHANXI C&Y PHARMACEUTICAL GROUP CO LTD
Liposome injection based on drug combination of mezlocillin sodium and sulbactam sodium
InactiveCN101804052AImprove stabilityHigh encapsulation efficiencyAntibacterial agentsPharmaceutical non-active ingredientsAntioxidantDissolution
The invention provides a liposome injection based on a drug combination of mezlocillin sodium and sulbactam sodium. The liposome injection comprises the following components: mezlocillin sodium, sulbactam sodium, liposome carriers, frozen and dried supporting agent and optional existing antioxidant, wherein the liposome carriers are particularly hydrogenated soybean phosphatidylcholine and octadecylamine. The liposome injection of the invention has good preparation stability, prevents the liposome from being cracked under the action of dehydration, fusion and ice crystal generation in the freezing-drying process, and keeps good entrapment rate of the liposome after re-dissolution through hydration.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Preparation process of Mezlocillin
The invention relates to a preparation process of Mezlocillin, which reacts Ampicillin trihydrate with 1-chlorocarbonyl-3-methylsulfonyl-2-imidazolidinone in water phase under alkaline condition and then proceeds acidified crystallization, filtration, washing and drying in acetone / water, or alcohol / water, or isopropanol / water system to obtain Mezlocillin. The Mezlocillin prepared by the inventive method can reach 95%-97% of molar yield, 0.06%-0.4% of organic residual quantity, more than 98.5% of dry basis content, less than 0.4% of single impurity, less than 1.5% of total related substances and good quality, is particularly suitable for direct lyophilization in water-phase salt formation to obtain high-quality Mezlocillin sodium for injection of medicinal product, and has prominent economical and social benefits.
Owner:ZHEJIANG JINHUA CONBA BIO PHARM CO LTD
Method for producing high-purity azlocillin sodium and powder injection thereof
InactiveCN101265265AHigh purityNo pollution in the processAntibacterial agentsOrganic active ingredientsFreeze-dryingChromatography column
The invention provided a method for preparing high-purity Mezlocillin sodium, and a method for preparing the Mezlocillin sodium powder injection. The method includes (1) dissolving Mezlocillin sodium crude product in purified water, and adjusting the pH value to less than 7 with the pH regulator; (2) extracting with organic solvent, separating the organic phase, drying with desiccant, and vacuum recovering to dry to obtain the product; (3) dissolving the product with eluting agent of mixed solution of hydrocarbon solvent and chlorine-containing solvent, eluting with alumina as filler of medium-pressure chromatography column, and collecting the fraction in a sectional manner; (4) mixing fractions with the Mezlocillin acid content of no smaller than 80%, vacuum concentrating, dissolving into ethanol, and basifying with base to separate precipitate; and (5) re-crystallizing the precipitate with ethanol, and freeze drying to obtain the high-purity Mezlocillin sodium.
Owner:海南华旗药业销售有限公司
Powder mixing technology for compound antibiotic
ActiveCN103040768AReduce pollutionStable chemical qualityPowder deliveryAntiinfectivesChemical qualityOrganic solvent
The invention relates to a powder mixing technology for a compound antibiotic. The compound antibiotic is obtained by cold drying after uniform mixing of antibiotic A and antibiotic B in advance, wherein the antibiotic A is mezlocillin sodium, amoxicillin sodium, cefoperazone sodium, and piperacillin sodium, and the antibiotic B is sulbactam sodium. According to the powder mixing technology, organic solvent is omitted, the environment is less polluted, the freeze-drying technology has stable yield, and the cost is low; the freeze-drying mixed powder has good uniformity, fast dissolution rate, and stable chemical quality, so that the powder mixing technology is applicable to preparation of various of compound antibiotics, and is suitable for mass production.
Owner:山东二叶制药有限公司
Injection mezlocillin sodium liposome and preparation method
ActiveCN102579351ASafety proofGood technical effectAntibacterial agentsPowder deliverySolubilitySide effect
The invention relates to the technical field of medicine and discloses injection mezlocillin sodium liposome and a preparation method. The injection mezlocillin sodium liposome is powder-injection. Mezlocillin sodium is packed with liposome carriers formed by liposome framework materials containing antioxygen and a stabilizing agent so as to obtain freezing-dried preparation. The injection mezlocillin sodium liposome is composed of 1 part of the mezlocillin sodium, 2 parts to 13 parts of the liposome framework materials, 0.3 part to 5 parts of the stabilizing agent, 4 parts to 10 parts of a freezing-dried protection agent and 0.1 part to 2 parts of the antioxygen. The injection mezlocillin sodium liposome has the obvious advantages that the mezlocillin sodium is wrapped in the liposome, thereby resolving the problem of slight solubility and guaranteeing quality of products; and medicine carrier liposome is degraded in bodies, thereby having no toxicity and immunogenicity, effectively improving therapeutic indexes of medicine, reducing toxicity of the medicine and reducing side effects of allergy rate, drug resistance, cardiotoxicity and the like.
Owner:SHANGHAI JINCHENG PHARMACEUTICAL CO LTD
Preparation method of mezlocillin sodium-sulbactam sodium for injection
InactiveCN108096196AIncreased acidity stabilityGood storage stabilityAntibacterial agentsPowder deliverySodium bicarbonateFreeze-drying
The invention discloses a preparation method of mezlocillin sodium-sulbactam sodium for injection. The method comprises the following steps: firstly, adding mezlocillin into a mixing tank, and then dropwise adding a sodium hydrogen carbonate solution; after the addition is finished, adding sulbactam, and dropwise adding the sodium hydrogen carbonate solution again; after material feeding is finished, vacuumizing to remove carbon dioxide gas, and then adjusting the pH value to 6.0-6.5; then, adding an ethylene diamine tetraacetic acid (EDTA) solution, stirring for 15-30min, then vacuumizing again, and maintaining the pH value to be 6.0-6.5; sterilizing and filtering, and freeze-drying to obtain mezlocillin sodium-sulbactam sodium freeze-dried preparation raw powder for injection. Accordingto the method, mezlocillin sodium-sulbactam sodium is generated by using the mezlocillin, the sulbactam and sodium hydrogen carbonate as raw materials for carrying out a reaction, and the acidity stability of the product is improved by adding EDTA into reaction liquid, so that the mezlocillin sodium-sulbactam sodium, which has mezlocillin sodium content and sulbactam content meeting the requirements and is good in storage stability, for injection is finally obtained.
Owner:QILU TIANHE PHARMA
Mezlocillin sodium trihydrate and preparation method thereof
InactiveCN101863905AFlat surfaceImprove liquidityAntibacterial agentsOrganic chemistryMedication injectionElution
The invention relates to a mezlocillin sodium trihydrate, wherein the molecular formula thereof is C21H24N5NaO8S2.3H2O. The preparation method of the mezlocillin sodium trihydrate comprises the following steps: dissolving 5-10 parts of a mezlocillin sodium bulk drug with 10-20 parts of water according to parts by weight; placing the obtained dissolving solution into a crystallizer and then adding 60-90 parts of an elution agent by weight to cool to 2-8 DEG C; keeping a constant temperature for 2-5 hours; separating out a solid and then filtering to obtain the solid; and finally washing the solid with 3-5 parts of the elution agent, and then drying under a ventilating or vacuum condition for 3-5 hours at the temperature of 10-50 DEG C to obtain the mezlocillin sodium trihydrate. The invention further provides mezlocillin sodium for medicine injection which is prepared by crushing the mezlocillin sodium trihydrate, sieving the crushed mezlocillin sodium trihydrate with a 100 mesh sieve and then carrying out aseptic-packaging. The mezlocillin sodium trihydrate and the mezlocillin sodium for medicine injection which are obtained by the invention have clean surface, good product mobility, good stability and fast dissolution velocity.
Owner:湖北美林药业有限公司
Stable antibiotic powder injection
InactiveCN101045050APowder deliveryHeterocyclic compound active ingredientsSodium bicarbonateArginine
Owner:GUANGDONG QIFANG MEDICINES CO LTD
Preparation technology of mezlocillin sodium
The invention relates to a preparation technology of mezlocillin sodium, and belongs to the technical field of drug synthesis. The preparation technology comprises the steps of performing an acylation reaction on ampicillin trihydrate and 1-chloroformyl-3-mesyl-2-imidazolidinone under a low-temperature alkaline condition, performing decoloration, filtering, acidification, washing and dehydration, adding sodium salt, and then performing crystallization to obtain mezlocillin sodium, wherein ampicillin needs to be dissolved and clarified by triethylamine prior to the acylation reaction; and 1-chloroformyl-3-mesyl-2-imidazolidinone and an ethyl acetate solvent are mixed and then added to a reaction system twice. According to the preparation technology, a dissolution manner of ampicillin trihydrate is changed and an addition manner of 1-chloroformyl-3-mesyl-2-imidazolidinone is changed, so that a side reaction in a reaction process is reduced; the washing is performed by purified water after the acidification and anhydrous sodium acetate is used during the crystallization, so that impurities are further reduced; compared with an original drug, a prepared product has the advantages in the quantity and levels of the impurities.
Owner:REYOUNG PHARMA
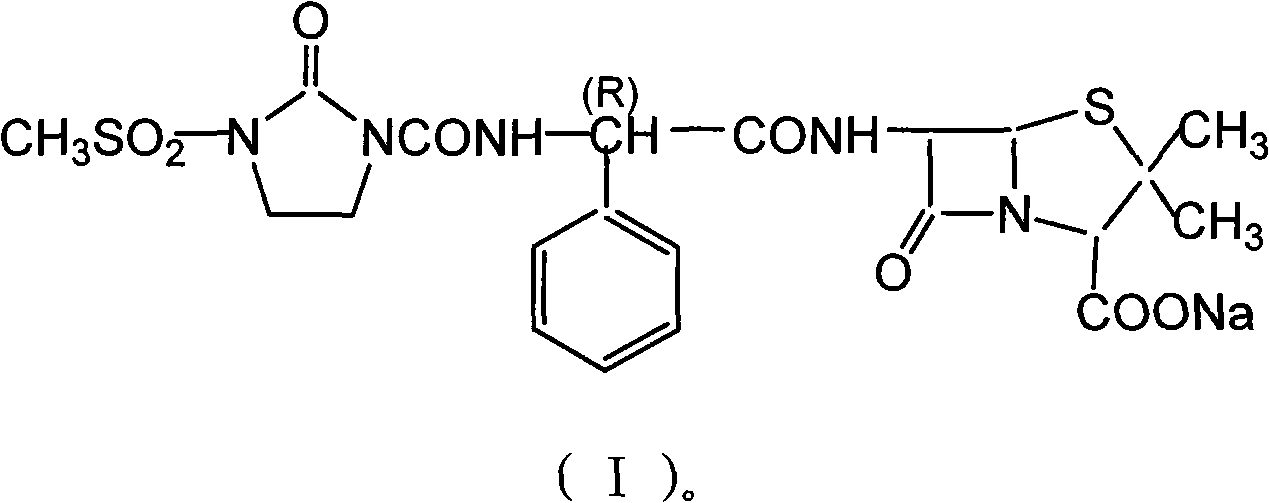
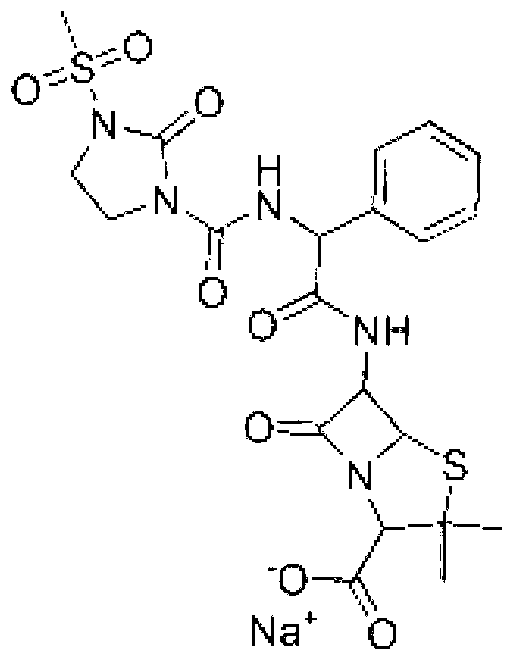
Mezlocillin sodium compound as well as preparation method and pharmaceutical composition thereof
InactiveCN103275100AFast dissolution rateImprove liquidityOrganic chemistryHeterocyclic compound active ingredientsFreeze-dryingX-ray
The invention belongs to the technical field of medicines, and particularly relates to a mezlocillin sodium compound. The structural formula of the mezlocillin sodium compound is as follows: with the adoption of Cu-K alpha ray measurement, the mezlocillin sodium compound obtains an X-ray powder diffraction spectrogram as shown in figure 1. The invention further provides a preparation method of the mezlocillin sodium compound, a pharmaceutical composition containing the mezlocillin sodium compound and a preparation method of the mezlocillin sodium compound. The dosage form of a mezlocillin sodium drug is a sterile powdered injection and a freeze-dried powder injection. The mezlocillin sodium compound is high in dissolution rate and good in fluidity, thereby facilitating subpackage and improvement on accuracy of packaging quantity.
Owner:四川省惠达药业有限公司
Method for synthesizing mezlocillin sodium through solvent method
The invention belongs to the preparation of penicillin pharmaceuticals and particularly relates to a method for synthesizing mezlocillin sodium through solvent method. The preparation method comprisesthe following steps: dissolving mezlocillin in ketone solvent, dissolving sodium isooctanoate in a mixed solvent of ether and ketone, dropwise adding both reactants in a reaction container simultaneously for synthesis, precipitating crystals, filtrating and drying to obtain the finished product. The invention has the advantage of mild reaction, high production yield, good stability, high content,low residual solvent, fast water dissolution rate and the like; in addition, wastewater can not be generated in the production process and the solvent can be recovered for repeated use.
Owner:SHANDONG RUNZE PHARMA
Method for producing high-purity mezlocillin sodium and powder injection thereof
InactiveCN101265264AHigh purityNo pollution in the processAntibacterial agentsPowder deliveryActivated carbonFreeze-drying
The invention provides a method for preparing high-purity Mezlocillin sodium, and a method for preparing Mezlocillin sodium powder for injection. The method includes the following steps: (1) absorbing Mezlocillin sodium or Mezlocillin sodium made from Mezlocillin acid crude drug and alkaline sodium salt with macroporous resin, eluting with purified water, and collecting the eluent; (2) purifying the primary pure product with a gel column, eluting with salt solution having certain concentration to obtain refined product with the Mezlocillin sodium content of greater than 99%, decoloring with activated carbon, filtering with 0.22 Mum microporous filter membrane, sterilizing, freeze drying to obtain the high-purity Mezlocillin sodium, pulverizing, and sterile packaging to obtain Mezlocillin sodium powder for injection.
Owner:海南华旗药业销售有限公司
Preparation technique for mezlocillin
The invention pertains to the technical field of drug combination preparation, more particularly relates to a preparation technique for mezlocillin. The preparation technique for mezlocillin comprises the following steps: ampicillin trihydrate and 1-chloroformyl-3-methylsulfonyl-2-imidazolidinone carry out acylation reaction under alkaline condition and then extraction, acidification and crystallization are conducted to obtain the mezlocillin. The invention is characterized in that when acylation reaction is conducted, reaction menstruum is water and acetone; after acylation reaction is finished, an ether extractant is added for carrying out extraction; and menthyl acetate is added before acidification so as to carry out acidified crystallization. The synthetic technique has obvious advantages, provides powerful guarantee for synthesizing the mezlocillin with high quality and has relatively great implementation value and long-term social and economic benefit.
Owner:SHANDONG RUIYING PIONEER PHARMA
Method for producing high-purity azlocillin sodium and powder injection thereof
InactiveCN101265265BHigh purityNo pollution in the processAntibacterial agentsPowder deliveryFreeze-dryingChromatography column
Owner:海南华旗药业销售有限公司
Production process of mezlocillin sodium
The invention discloses a production process of mezlocillin sodium. The production process comprises the following steps: (1), cleaning, sterilizing and checking reaction equipment and a working place; (2), preparing a sodium bicarbonate suspension; (3), performing a salt forming reaction; (4), decoloring; (5), filtering and removing bacteria; (6), freeze-drying; (7), crushing; (8), packaging, wherein a salt forming reaction equation is described in the specification. According to the production process, in accordance with the provision of the new-edition Chinese pharmacopoeia, through improving a salt forming procedure and strictly controlling filtering, bacterium-removing, and freeze drying operations, the yield of drugs is increased; prepared mezlocillin sodium is low in content of polymer and impurities, and better in applicability and medicinal property.
Owner:JIANGSU HI STONE PHARMA
Mezlocillin sodium and sulbactam sodium compound drug composition for injection
ActiveCN105963263AImprove solubilityLight colorAntibacterial agentsPowder deliverySolubilityCompounding drugs
The invention relates to the technical field of medicines and discloses a mezlocillin sodium and sulbactam sodium compound drug composition for injection. The mezlocillin sodium and sulbactam sodium compound drug composition comprises mezlocillin sodium, sulbactam sodium and sodium benzoate. The mezlocillin sodium and sulbactam sodium compound drug composition realizes higher product performance by less auxiliary materials, has the advantages of being good in solubility, shallow in color, uniform in main drug mixing, low in related substance, low in polymer content, low in incidence rate of adverse reactions and the like and ensures that patients are safer in the clinical use, and the treatment effect is more reliable.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Detection method for determining polymer impurities in mezlocillin sodium
InactiveCN103063751AAccurate Qualitative and Quantitative AnalysisEasy to operateComponent separationPhosphateColumn temperature
The invention provides a detection method for determining polymer impurities in mezlocillin sodium. The detection method employs a high performance liquid chromatography size exclusion chromatography. The chromatography conditions are as follows: a chromatographic column employs hydrophilic silica for globular protein as a stationary phase and 50 mmol of a phosphate buffered solution as a mobile phase; a flow velocity of the mobile phase is 0.5-2.0 mL / min; a detection wavelength is 230-254 nm; a column temperature is a room temperature; a sample volume is 10-80 [mu]L; granularity of the chromatographic column stationary phase is 5 [mu]m, aperture of the chromatographic column stationary phase is 10 nm; the inner diameter of the chromatographic column is 7.8 mm; and the column length is 300 mm. he detection method can perform rapidly qualitative and quantitative determination on the polymer impurities in mezlocillin sodium, simultaneously simplifies operation steps, increases detection precision and shortens detection time.
Owner:SEPAX TECH
Separation preparation method of mezlocillin sodium impurity A
InactiveCN109081837AShort preparation routePost-processing is simpleOrganic chemistryInorganic saltsDrug compound
The invention belongs to the technical field of medicine and relates to a refining method of antibiotic drug compound impurities, in particular to a separation preparation method of mezlocillin sodiumimpurity A, comprising the steps of subjecting mezlocillin sodium as a raw material to reaction with an alkaline reagent to obtain mezlocillin sodium impurity A. The mezlocillin sodium impurity A ismade with mezlocillin sodium, an alkaline solution, an acid solution and an inorganic salt at room temperature by filtering, washing and drying. The invention also provides a refining method for separating and purifying mezlocillin sodium impurity A; the mezlocillin sodium impurity A prepared herein has purity of greater than 95% and is applicable to control researches; the invention belongs to the field of medicine.
Owner:REYOUNG PHARMA
Mezlocillin sodium freeze-dried powder injection and preparation method thereof
InactiveCN106176628AAdvantages and Notable ImprovementsImprove stabilityAntibacterial agentsPowder deliveryFreeze-dryingPowder injection
The invention discloses a mezlocillin sodium freeze-dried powder injection. The freeze-dried powder injection is prepared from mezlocillin sodium, isonicotinamide and propylgallate according to a weight ratio of 10:(0.1-0.4):(0.01-0.05). The mezlocillin sodium freeze-dried powder injection is high in stability, high in safety due to few types of auxiliary materials, simple in process and suitable for mass production.
Owner:NANJING ZHENGKUAN MEDICAL TECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com