Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
103results about How to "Short preparation route" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Polyimide foam and method of producing the same
The invention discloses polyimide foam and a preparation method thereof. Aromatic dianhydride and / or aromatic acid ester, alcohol with low molecular, a catalyst and a surface active agent are mixed proportionally in a polar solvent and react so as to form foam precursor solution. The foam precursor solution and isocyanate react in a mould and freely foam into a foam intermediate. The intermediate is solidified by microwave radiation or / and oven heating to obtain solid polyimide foam. The preparation process has short path, simple technology, good stability for storage of the foam precursor solution, adaptation to processing technologies of pouring, spray coating, extrusion, and the like, no occurrence of defects of cracked foam, foam combination, subsidence and incomplete amination, low preparation cost as well as uniform hold diameter and stable performance of the solid polyimide form, and is convenient for popularization and application.
Owner:BEIJING RADIATION APPL RES CENT
Modified polyimides foam and preparation thereof
The invention discloses modified polyimide foam and a preparation method thereof. The preparation method comprises the following steps: aromatic dianhydride and / or aromatic acid ester, siloxane with active terminal group, low molecular alcohol, a catalyst and a surface active agent are mixed into a polar solvent according to proportion and react to form foaming precursor solution which reacts with isocyanate to form foaming intermediate solution; and the foaming intermediate solution carries out foaming freely in a mold and forms a modified foaming intermediate which is radiated by microwave or / and heated and cured in an oven, and then the solid polyimide foam is obtained. The method has short route of preparation process and simple technique; the foaming precursor solution has good stability of storage, is suitable for the processing techniques of casting, painting, extruding and the like, and the defects of broken foam, parallel foam, depression and incomplete imidizate of materials do not occur; the polyimide foam has even aperture, high stability, low rigidity, good flexibility and good processability; and the preparation cost is low, thus being convenient to promotion and application.
Owner:BEIJING RADIATION APPL RES CENT
Fluorine-removing lanthanum-supported fiber adsorbent and synthesis method thereof
InactiveCN102814169AEasy to shapeHigh strengthOther chemical processesWater contaminantsSorbentSynthesis methods
The invention discloses a fluorine-removing lanthanum-supported fiber adsorbent and a synthesis method thereof, belonging to the field of polymer materials. The fiber adsorbent comprises an active component and a matrix fiber, wherein the matrix fiber is an amidoxime fiber prepared through polyamine crosslinking reaction and amidoxime modification reaction of an acrylic fiber; and the active component is element lanthanum supported on the matrix fiber through in-situ reaction. The fiber adsorbent can be used for adsorbing and removing fluorine pollutants in water. Compared with the conventional fluorine-removing adsorbents, the novel fiber adsorbent disclosed by the invention has the characteristics that the adsorption speed is high, the adsorption capacity is as high as 34mg / g, the range of applicable pH value is as wide as 4-8 and the fiber adsorbent can be applied in various forms such as fiber bundles, non-woven fabrics and fabrics and can further meet the requirement of actual water treatment. The synthesis method has the advantages of available raw materials, simple process, mild reaction conditions and the like.
Owner:HENAN ACADEMY OF SCI CHEM RES INST CO LTD
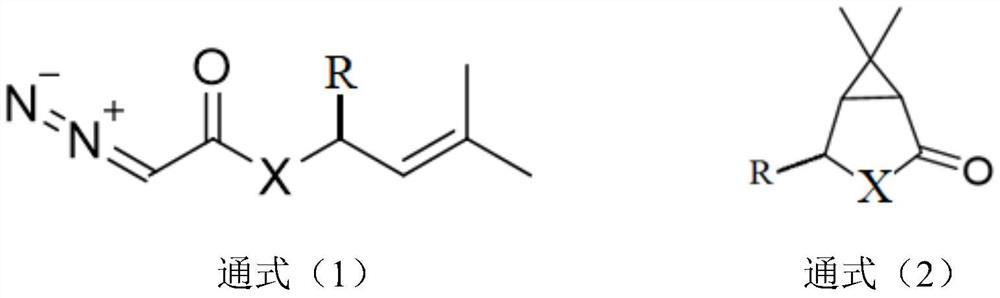
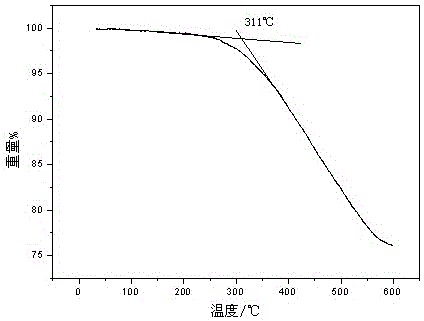
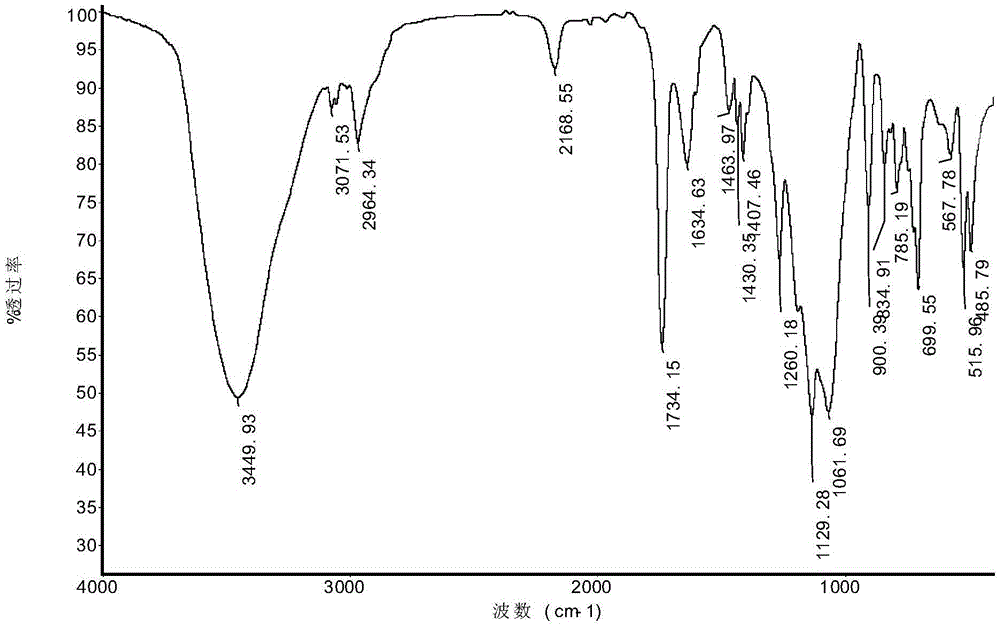
Novel preparation method of azabicyclo medical intermediate
ActiveCN114031542AReduce mass productionTo meet the huge demand of productionOrganic chemistryAntiviralsHeteroatomPharmaceutical drug
The invention relates to an azabicyclo medical intermediate, in particular to a synthesis method of 6,6-dimethyl-3-azabicyclo[3.1.0] hexane or a derivative thereof. A hetero-atom bicyclic compound is constructed through cyclization of an intra-molecular diazo group and an olefin bond, and a target product is finally obtained through an amination reaction and a reduction reaction.
Owner:ZHEJIANG NHU CO LTD +2
Preparation method of brivaracetam
The invention discloses a preparation method of brivaracetam (formula IV). The preparation method comprises the following steps: carrying out ammonolysis on (R)-4-propyl-dihydrofuran-2-one and (S)-2-amino butyric acid as raw materials to obtain an intermediate II, then carrying out steps of esterification, chlorination and cyclization to obtain an intermediate III, and finally, carrying out an ammonolysis step to obtain the brivaracetam (IV), wherein the steps of esterification, chlorination and cyclization are operated in a combination mode. The preparation method provided by the invention ismild in condition, is simple and convenient to operate, has easily obtained raw materials, and is suitable for large-scale industrial production.
Owner:BEIJING VENTUREPHARM BIOTECH
A kind of method for preparing formate by catalytic hydrogenation of carbon dioxide
ActiveCN105367404BShort preparation routeMild conditionsCarboxylic acid salt preparationHydrogenFormate
The invention belongs to the technical field of carbon dioxide resource utilization and relevant chemistry, and relates to a method for preparing formate through carbon dioxide catalytic hydrogenation. The method is characterized by comprising the steps that carbon dioxide is used as a raw material and reacts with hydrogen under the catalysis of nano-porous palladium catalyst and the alkaline condition to obtain formate. By means of the method for preparing the formate through carbon dioxide catalytic hydrogenation, the preparation route is short, raw materials are cheap and easy to obtain, conditions are mild, operation is simple and convenient, and the reaction yield is high.
Owner:DALIAN UNIV OF TECH
Self-crosslinking type LED package adhesive resin and preparing method thereof
The invention discloses self-crosslinking type LED package adhesive resin and a preparing method thereof. According to the preparing method, 100-105 weight parts of phenyltrimethoxysilane, 100-120 weight parts of diphenyldimethoxysilane, 10-20 weight parts of methyl-vinyl-cyclosiloxane, 30-50 weight parts of disiloxane, 20-60 weight parts of distilled water, 100-200 weight parts of solvent and 0.03-0.05 weight part of catalyst are sequentially added to a container, constant-temperature stirring is conducted for 3-5 h at 60-80 DEG C, stirring reaction is conducted for 4-8 h after temperature is increased to 100-120 DEG C, the pH value of obtained reaction liquid is adjusted to be 7, then reduced pressure distillation is conducted so as to remove the solvent and residual water, and finally the self-crosslinking type LED package adhesive resin high in light transmittance and refraction index is obtained. The resin can achieve self-crosslinking curing reaction under a certain condition, and the thermal decomposition temperature of a cured product is quite high.
Owner:浙江安贝新材料股份有限公司
Preparation method of carboxymethyl guar gum
The invention relates to a novel preparation method of carboxymethyl guar gum, which comprises the following three steps: alkalization reaction, etherification reaction and after-treatment. The carboxymethyl guar gum is prepared from the raw material guar gum raw powder by using ethanol as a reaction solvent and sodium hydroxide as a catalyst; and the viscosity of the prepared carboxymethyl guar gum is 3600 mPa.s, and the substitution degree is 0.68. The carboxymethylation reaction of the guar gum is performed in the ethanol solution, so that the carboxymethyl guar gum prepared by the reaction between the chloroacetic acid and guar gum has the advantages of high substitution degree, high viscosity, high solubility and low content of water-insoluble substances. The raw material and process route related in the reaction process conform to the green chemical requirements, and can not generate pollutants, and thus, the reaction process is economic and environment-friendly and has high adaptability. The method has the advantages of short preparation route and simple technique, and is convenient to operate and suitable for large-scale industrial production.
Owner:LANZHOU UNIVERSITY
Full-spectrum selective reflection and photoluminescence Janus oligomer and preparation method thereof
ActiveCN112142770AImprove securityHigh yieldLiquid crystal compositionsSilicon organic compoundsPhotoluminescenceSelective reflection
The invention discloses a full-spectrum selective reflection and photoluminescence Janus oligomer and a preparation method thereof, wherein the full-spectrum selective reflection and photoluminescenceJanus oligomer is constructed by graft copolymerization of a chiral liquid crystal monomer, a nematic liquid crystal monomer, fluorescent molecules and polymethyl hydrogen-containing siloxane, and the structure is shown as a formula I in the specification. According to the invention, the Janus oligomer can realize full-spectrum selective reflection in heating and cooling processes, and has high reversibility and accuracy; under the excitation of ultraviolet light, the Janus oligomer can emit fluorescence, wherein when the temperature rises to the thermal decomposition temperature of the Janusoligomer from the room temperature, the fluorescence intensity is monotonically reduced, and the fluorescence intensity still has high reversibility along with the change of the temperature; and theJanus oligomer prepared by the method has dual characteristics of dynamic reversible full-spectrum selective reflection and photoluminescence, has a wide application prospect in the anti-counterfeiting field, and is short in preparation route, safe and simple to operate and mild in reaction condition.
Owner:XUZHOU UNIV OF TECH
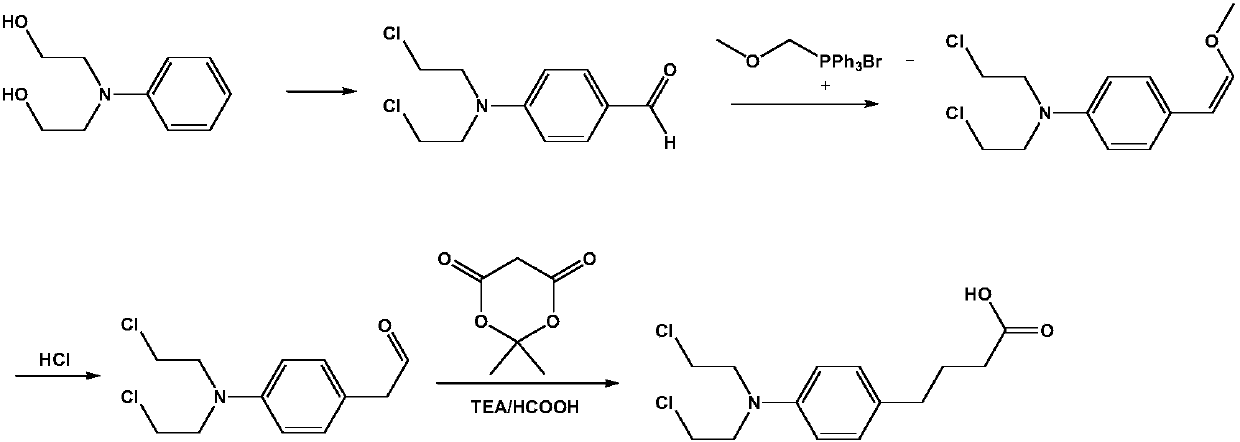
Preparation method of anti-tumor medicine chlorambucil
InactiveCN107628962ALow equipment requirementsReduce manufacturing costOrganic compound preparationAmino-carboxyl compound preparationAbnormal tissue growthBenzaldehyde
The invention belongs to the field of compound preparation, and specifically discloses a preparation method of an anti-tumor medicine chlorambucil. The preparation method comprises the steps of performing a Vilsmeier reaction on a raw material N,N-dihydroxyethylaniline and phosphorus oxychloride and DMF so as to prepare 4-[bi(2-chloroethyl)amino]benzaldehyde, then performing a witting reaction on4-[bi(2-chloroethyl)amino]benzaldehyde and methoxymethyl triphenylphosphonium chloride so as to prepare 4-[bi(2-chloroethyl)amino]-BETA-methoxystyrene, reacting under an acid condition so as to obtain4-[bi(2-chloroethyl)amino]phenylacetaldehyde, and finally, reacting with Meldrum's acid in triethylamine and formic acid systems so as to prepare a target product. The raw material N,N-dihydroxyethylaniline is cheap, has a wide source and is easy to obtain; the whole reaction process has high yield, production conditions are mild, the steps are short, and post treatment and purification are easyto operate, so that the preparation method is applicable to commercial large scale production, and meets the rapidly growing market demands.
Owner:TIANJIN DERCHEMIST SCI TECH
Resin for UV-curing LED packaging adhesive and preparation method
The invention discloses resin for an UV-curing LED packaging adhesive and a preparation method. The resin is prepared from phenyl hydrogen-containing silicone oil and a urethane acrylate compound through reaction under the action of a platinum catalyst; the light transmittance of the resin ranges from 81% to 90%, the transmissivity of the resin at the room temperature ranges from 1.5011 to 1.5455, and the curing time of the resin under the UV light irradiation ranges from 10 s to 60 s. The resin for the UV-curing LED packaging adhesive has the characteristics of high light transmittance, high reflective index, UV-curing capability and the like, can be used in the LED packaging field, and can be used in the technical fields of industries such as instruments, meters, household appliances, machinery, automobiles, electric products and the like.
Owner:SHANGHAI INST OF TECH
Preparation method of boric acid functional group resin
ActiveCN111777699AHigh reactivityImprove stabilityGroup 3/13 element organic compoundsBulk chemical productionBoronic acidPerylene derivatives
The invention discloses a preparation method of boric acid functional group resin, belongs to the field of resin, and aims to solve the technical problems of poor adsorption material selectivity, lowadsorption capacity, high price, lack of accurate regulation and control in the material modification process and difficulty in efficient introduction of specific boric acid functional groups in the existing extraction and separation process of polyhydric alcohols, polyhydric phenols, saccharides and other high-added-value derivatives. The method comprises the following steps: selecting an anilineboric acid monomer with higher reaction activity and aniline and benzylamine boronic acid pinacol ester monomers, and loading the monomers on a polystyrene resin skeleton through an efficient and mild nucleophilic substitution reaction or loading the monomers on a polyacrylic resin skeleton through a condensation reaction to prepare the boric acid functional group resin with high functional grouploading capacity and high selectivity. The method overcomes the defects of low boric acid functional group loading rate, poor stability, poor recyclability and the like of the existing material, canefficiently and mildly load the boric acid function based on the resin carrier, and can effectively enrich and separate the polyhydroxy compound with the cis-vicinal diol or m-diol structure.
Owner:QINGDAO INST OF BIOENERGY & BIOPROCESS TECH CHINESE ACADEMY OF SCI
UV (ultraviolet) cured LED packaging adhesive resin and method for preparing same
The invention discloses UV (ultraviolet) cured LED packaging adhesive resin and a method for preparing the same. The method includes sequentially adding 100-105 parts of diethoxysilane, 10-20 parts of hydrogen-containing mixed rings, 30-50 parts of hydrogen-containing double-seal heads, 20-60 parts of distilled water, 100-200 parts of solvents and 0.03-0.05 part of catalysts into a container, carrying out constant-temperature reaction at the temperature of 60-90 DEG C, increasing the temperature until the temperature reaches 100-120 DEG C, carrying out constant-temperature reaction, treating reaction and carrying out post-treatment to obtain phenyl hydrogen-containing silicone oil; enabling the phenyl hydrogen-containing silicone oil and ethoxylation trimethylolpropane triacrylate to react to each other under the effect of the platinum catalysts to obtain the UV cured LED packaging adhesive resin. The UV cured LED packaging adhesive resin and the method have the advantages that the UV cured LED packaging adhesive resin is high in light transmittance, has a high refractive index and can be used in the field of LED packaging and the technical fields of industries of instruments, apparatuses, household appliances, machinery, automobiles, electronic and electrical appliances and the like.
Owner:SHANGHAI INST OF TECH
Cationic curing epoxy organic silicon resin and preparation method thereof
InactiveCN104672432AShort preparation routeMild reaction conditionsSilicon organic compoundsEpoxyNitrogen
The invention provides a cationic curing epoxy organic silicon resin of which the structural formula is disclosed in the specification. The invention also provides a preparation method of the resin, which comprises the following steps: adding 1,3,5,7-tetramethylcyclotetrasiloxane and allyl glycidyl ether into a reaction vessel, and adding a solvent, a catalyst and a polymerization inhibitor; heating to react, distilling the reaction mixture under reduced pressure to obtain epoxy-group-containing cyclotetrasiloxane, adding the epoxy-group-containing cyclotetrasiloxane, methylphenylcyclosiloxane and a blocking agent into another reaction vessel, heating to react in a nitrogen protective atmosphere, and distilling under reduced pressure, thereby obtaining the cationic curing epoxy organic silicon resin. The curing product of the cationic curing epoxy organic silicon resin has the characteristics of high crosslinking degree, high hardness and the like.
Owner:SHANGHAI INST OF TECH
Single-package organic silicon rubber packaging adhesive for high-power type white LED (light-emitting diode) and preparation method of single-package organic silicon rubber packaging adhesive
InactiveCN104232015AHigh refractive indexHigh light transmittanceNon-macromolecular adhesive additivesMacromolecular adhesive additivesDisplay boardPtru catalyst
The invention discloses a single-package organic silicon rubber packaging adhesive for a high-power type white LED (light-emitting diode) and a preparation method of the single-package organic silicon rubber packaging adhesive. The preparation method of the single-package organic silicon rubber packaging adhesive for the high-power type white LED comprises the following steps: uniformly stirring 100-120 parts by weight of high-viscosity self-crosslinked phenyl organic silicon rubber, 0.001-0.005 part by weight of a catalyst, 0.002-0.005 part by weight of an inhibitor and 4-8 parts by weight of a reinforcing agent; and, then, deaerating the components in vacuum for 15-30 minutes to obtain the single-package organic silicon rubber packaging adhesive, with a high refractive index and high light transmittance, for the high-power type white LED, wherein the single-package organic silicon rubber packaging adhesive is transparent after being cured, and has shore A hardness of 53-77. The preparation method is simple, environment-friendly and efficient. The single-package organic silicon rubber packaging adhesive is used for high polymer material fields such as high-power LED packaging, is used for filling and sealing of electrical elements, filling and sealing of high-pressure parts and moisture-proof coating of circuit boards, packaging, insulation protection and the like of electronic LED indoor and outdoor display panels, LED traffic signals, and the like.
Owner:SHANGHAI INST OF TECH
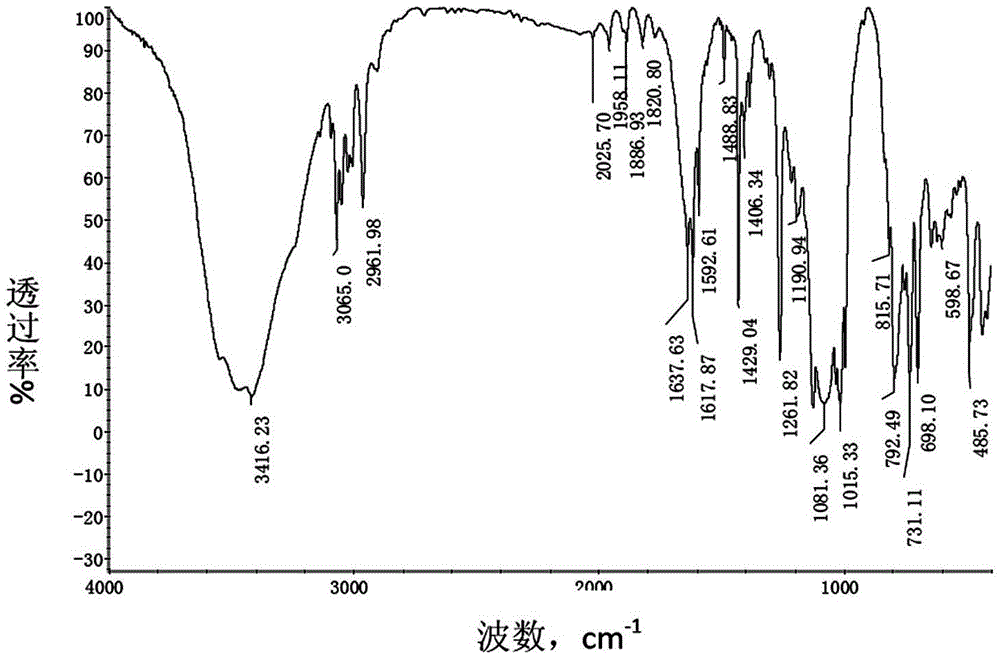
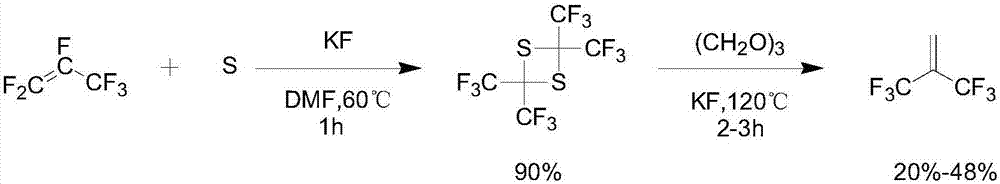
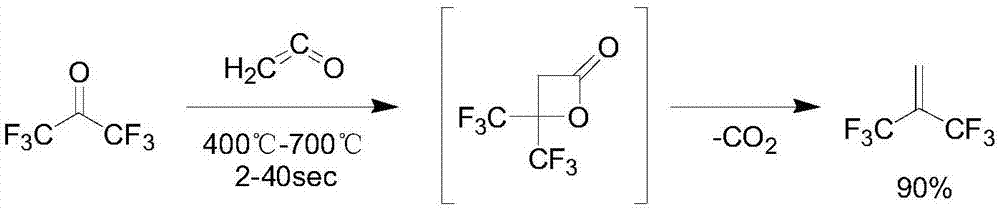
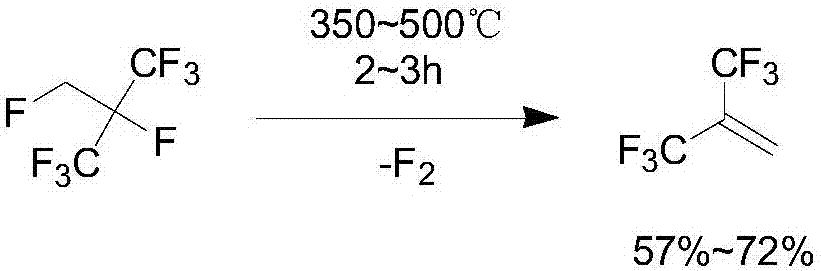
Preparation method of hexafluoroisobutene
InactiveCN107151198ASimple processEasy to separate and purifyOrganic compound preparationCarboxylic acid esters preparationEtherSolvent
The invention discloses a preparation method of hexafluoroisobutene, comprising the steps of (1) reacting heptafluoroisobutene methyl ether, methanol and a halide in type I solvent, cooling after reacting, filtering, rectifying the filtrate to obtain methyl hexafluoroisobutyrate; (2) allowing the methyl hexafluoroisobutyrate of step (1) to react with a reducing agent in type II solvent, quenching with hydrochloric acid after reacting, filtering, rectifying the filtrate to obtain hexafluoroisobutanol; (3) allowing the hexafluoroisobutanol of step (2) to react with an alkali in a molar ratio of 1:(1-10) in type III solvent, collecting the reaction product, and rectifying to obtain finished hexafluoroisobutene. The preparation method has the advantages that the process is simple, the yield is high, the raw materials are low in price and easy to obtain and the preparation method is suitable for industrialization.
Owner:JUHUA GROUP TECH CENT
Chiral fluorescent liquid crystal with three primary colors at room temperature and with dual anti-counterfeiting mechanisms and preparation method thereof
ActiveCN113150056AImprove securityHigh yieldLiquid crystal compositionsSteroidsLiquid crystallineSelective reflection
The invention discloses a chiral fluorescent liquid crystal with three primary colors at room temperature and with dual anti-counterfeiting mechanisms and a preparation method thereof. The chiral fluorescent liquid crystal is constructed by grafting and copolymerizing a chiral liquid crystal monomer, a nematic fluorine-containing liquid crystal monomer, a fluorescent monomer and polymethylhydrosiloxane. The structure of the chiral fluorescent liquid crystal is shown as a formula I which is described in the specification. According to the invention, at a room temperature (0-25 DEG C) and under visible light of a bright field, a color reflected by the chiral fluorescent liquid crystal is gradually changed from red to green along with the change of an angle between a sight line and a planar film of the chiral fluorescent liquid crystal from 90-30 degrees due to Bragg selective reflection of the chiral fluorescent liquid crystal; and in a dark field and under the irradiation of ultraviolet light with a length of 310 nm, the chiral fluorescent liquid crystal can emit blue characteristic fluorescence. The chiral fluorescent liquid crystal prepared by the invention can realize three primary colors, namely red, green and blue in a room temperature range (0-25 DEG C), and has wide application prospects in an anti-counterfeiting field.
Owner:XUZHOU UNIV OF TECH
Aqueous fluorine-containing resin and preparation method thereof
InactiveCN109912770AImprove mechanical propertiesGood film-forming performancePolyurea/polyurethane coatingsAirplaneDimethylolpropionic acid
The invention provides aqueous fluorine-containing resin which is characterized by comprising the following preparation raw materials in parts by weight: 30-60 parts of isophorone diisocyanate, 50-100parts of polyether polyol, 5-10 parts of dimethylolpropionic acid, 10-20 parts of a fluorine-containing chain extender, 5-10 parts of a neutralizing agent, 10-20 parts of ethanediamine and 230-460 parts of deionized water, wherein polyether polyol comprises one or a mixture of two of PTMG1000 and N210,and the neutralizing agent is triethylamine. A preparation method is mild in experiment conditions, simple to operate, and suitable for industrial production, and the aqueous fluorine-containing resin can be widely used for protecting airplanes, ships, buildings, traffic and various mechanical equipment.
Owner:SHANGHAI APPLIED TECHNOLOGIES COLLEGE
Preparation method of polysubstituted pyrroloquinoline derivate
InactiveCN106749235AReduce consumptionRaw materials are easy to getOrganic chemistryChalconeEnantio selectivity
The invention discloses a preparation method of a polysubstituted pyrroloquinoline derivate. The preparation method is characterized in that aminochalcone and aldehyde are condensed to obtain chalcone imine; the chalcone imine and glycinate imine are subjected to series cyclizing through Michael / Mannich and then are subjected to acidizing, and thus the polysubstituted pyrroloquinoline derivate containing four chiral centers, as shown in formula (I), can be generated based on high diastereoselectivity. The preparation method is on the basis of series reaction, and has the advantages that the environmental protection is achieved; the synthesizing steps are simple and convenient; the atom economy is ensured; the diastereoselectivity is high; in addition, the reaction conditions are mild; the substance is wide in applicable scope; the operation is simple and safe; the preparation method is applicable to industrial production.
Owner:SOUTHWEST UNIVERSITY
Synthesis method of hexafluoroisobutene
ActiveCN107032950ASimple processEasy to separate and purifyOrganic compound preparationHydroxy compound preparationHydrogenEther
The invention discloses a synthesis method of hexafluoroisobutene. The synthesis method comprises the following steps of 1, making heptafluoroisobutenyl methyl ether react with borohydride in an I type solvent, conducting filtering and rectification after the reaction finishes to obtain hexafluoroisobutenyl methyl ether; 2, making hexafluoroisobutenyl methyl ether obtained in the step 1 react with acid, and conducting rectification after the reaction finishes to obtain hexafluoroisobutyraldehyde; 3, under the action of a catalyst, leading hydrogen into hexafluoroisobutyraldehyde obtained in the step 2 for a reaction, and conducting filtering after the reaction finishes to obtain hexafluoroisobutanol; 4, making hexafluoroisobutanol obtained in the step 3 react with alkali in an II type solvent, and collecting and rectifying the reaction product to obtain the hexafluoroisobutene product. The synthesis method of hexafluoroisobutene has the advantages of being simple in technology, high in yield, easy to operate and low in cost.
Owner:JUHUA GROUP TECH CENT
Silicone sealant for daylighting board and preparation method thereof
InactiveCN104357002AMeet the needs of useAvoid disadvantagesNon-macromolecular adhesive additivesOther chemical processesPolymer sciencePtru catalyst
The invention discloses a silicone sealant for a daylighting board and a preparation method thereof. The preparation method comprises the following steps: adding the silicone rubber 107, the reactive reinforcing agent and the active filler into a vacuum kneading machine in sequence according to the fact that the weight ratio of silicone rubber 107 to a reactive reinforcing agent to an active filler is (100-120): (3-10): (3-10), kneading for 10-30 minutes at a room temperature, then stirring and heating to 150 DEG C, carrying out heat preservation and dehydrating for 2-4 hours, cooling to a room temperature and then discharging to obtain a base material; adding the base material into a planetary stirrer, stirring and vacuumizing for 1 hour, then adding a cross-linking agent, a tackifier, a plasticizer, a flame retardant and a catalyst in sequence according to the fact that the weight ratio of the base material to the cross-linking agent to the tackifier to the plasticizer to the flame retardant to the catalyst is 100: (3-15): (1-10): (5-25): (30-50): (0.1-1), uniformly stirring under vacuum and discharging to obtain the silicone sealant with high elongation rate, low modulus and high light transmittance for the daylighting board.
Owner:SHANGHAI INST OF TECH
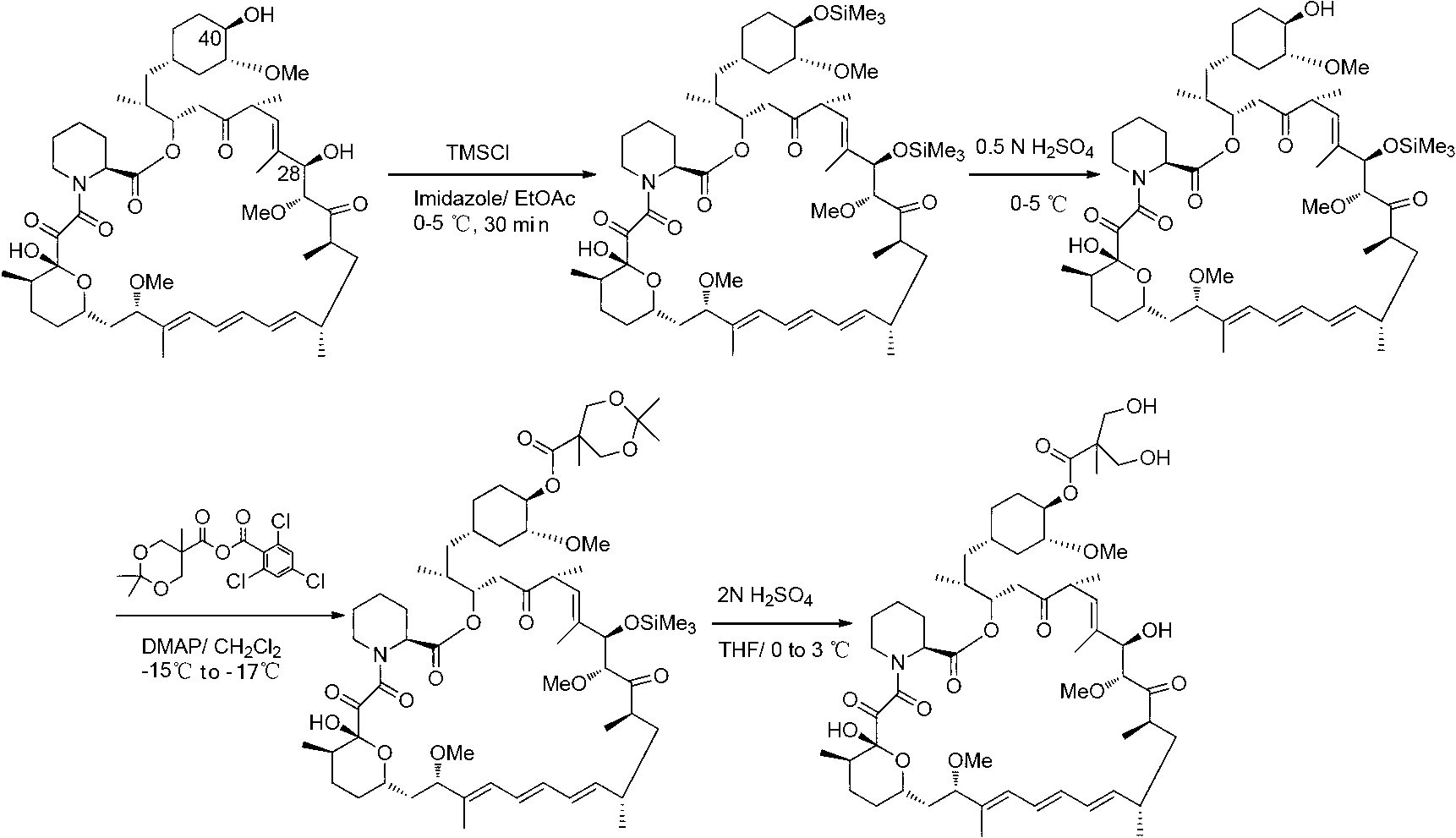
Preparation methods of himbacine analogue and intermediate thereof
ActiveCN105777681AStable in natureEasy to purifyGroup 4/14 element organic compoundsOrganic compound preparationPtru catalystPalladium catalyst
The invention provides preparation methods of a himbacine analogue and intermediates thereof. The invention specifically provides novel preparation methods of a compound A, a compound 4, a compound D and a compound C. According to the invention, a scheme for preparing the compound A does not require a process for reducing carboxylic acid into aldehyde, but directly oxidizing corresponding alcohol into corresponding aldehyde. The operation is safe. In prior arts, carboxylic acid is first converted into acyl chloride, and acyl chloride is catalytically hydrogenated into aldehyde with a palladium catalyst. In the above process, anhydrous palladium-carbon is needed. With the methods provided by the invention, the above harsh condition is avoided. The preparation methods are simple, and operation steps are easy. Especially, when a hydroxyl group protection group P is benzyl group, nitro group can be reduced into amino group when the protection group P is removed. Therefore, the reaction steps are greatly simplified, and raw materials and agents are saved. The entire route has the advantages of low production cost and high yield, and is suitable for industrialized production.
Owner:BRIGHTGENE BIO MEDICAL TECH (SUZHOU) CO LTD
Macrocyclic amide metal complex and preparation method and application thereof
InactiveCN106083843AChoose diversityImprove stabilityOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsAlcoholOrganic matter
The invention provides a macrocyclic amide metal complex and a preparation method and an application thereof. The metal complex has the structural formula represented by the formula I; the formula I is defined in the specification, wherein R is CH3 or F, M is one of Fe, Co, Mn or Cu, and M' is K, Na or Li. The invention also provides the method for preparing the metal complex, wherein the method comprises a first-stage reaction, a second-stage reaction and a third-stage reaction. The invention further provides the application of the metal complex, wherein the metal complex is applied in oxidation reaction of alcohols organic matters and aldehydes organic matters. The metal complex provided by the invention can be prepared by a variety of routes, the preparation process is short, operations are simple, sources of used raw materials are rich, properties are stable, a central metal does not belong to precious metals, is low in price and is non-toxic or is low in toxicity, and the complex has excellent application properties in the oxidation reaction.
Owner:BEIJING INSTITUTE OF CLOTHING TECHNOLOGY
High-viscosity self-crosslinking LED package gum resin and preparation method thereof
The invention discloses high-viscosity self-crosslinking LED package gum resin and a preparation method thereof. The preparation method comprises the following steps: sequentially adding the following components in parts by weight into a container: 100-105 parts of phenyltrimethoxysilane, 100-120 parts of diphenyldimethoxysilane, 10-20 parts of hydrogenous annulus, 20-60 parts of distilled water, 30-50 parts of divinyl tetramethyl disiloxane, 100-200 parts of a solvent and 3-5 parts of a catalyst; heating to 40-60 DEG C, and stirring at a constant temperature for 4-8 hours; heating to 100-130 DEG C, and stirring and reacting at a constant temperature for 4-8 hours; rinsing the reaction liquid to be neutral by using distilled water; distilling at reduced pressure of -0.096MPa at 200 DEG C to remove the solvent and residual moisture so as to obtain the high-viscosity self-crosslinking LED package gum resin which has the characteristics of high light transmittance and high refractive index.
Owner:浙江广胶新材料有限公司
Functional emulsion and preparation method thereof
InactiveCN112480304AImprove mechanical propertiesImprove surface propertiesCoatingsPolymer scienceAcrylate ester
The invention discloses a functional emulsion which is characterized by comprising the following raw materials: a fluorine-containing acrylate monomer, an acrylate monomer, a crosslinking monomer 1, acrosslinking monomer 2, an emulsifier, an initiator and deionized water. The preparation method comprises the following steps: adding deionized water and the emulsifier into a reaction container, dropwise adding a mixture of the fluorine-containing acrylate monomer, the acrylate monomer, the crosslinking monomer 1 and the crosslinking monomer 2 into the reaction container, heating to 60-75 DEG C,adding the initiator into the reaction container, reacting for 3-5 hours while stirring, heating to 85-90 DEG C, and reacting for 1-2 hours while stirring, thereby obtaining the functional emulsion.The functional emulsion is good in film-forming property and high in contact angle with water, and can be widely applied to protection of airplanes, ships, buildings, traffic and various machines.
Owner:SHANGHAI INST OF TECH
Method for preparing temsirolimus
ActiveCN102796115AShort preparation routeHigh yieldOrganic chemistryBulk chemical productionChemistryChlorosilane
The invention belongs to the technical field of methods for preparing temsirolimus, and relates to a method for preparing temsirolimus. The method comprises the following steps of: 1) adding 2,2-bis(hydroxymethyl) propionic acid, organic alkali and chlorosilane into an organic solvent, and reacting to obtain a compound I; 2) dissolving the compound I and the alkali in the organic solvent, adding 2,4,6-trichlorobenzene formyl chloride, reacting, adding reaction liquid into an organic solvent of a compound A and 4-(N,N-dimethylamino) pyridine, and reacting to generate a compound B; and 3) reacting the compound B and acid to obtain the temsirolimus. Compared with the prior art, the method has the advantages of short preparation route, high yield, simplicity of operation and low cost, and is suitable for industrial production.
Owner:SHANGHAI SHYNDEC PHARMA CO LTD
Tetrahydrofuran benzodihydropyran polycyclic compound and application thereof
InactiveCN103554118ALower synthesis costShort preparation routeOrganic chemistryAntineoplastic agentsBenzaldehydeBenzene
The invention discloses a tetrahydrofuran benzodihydropyran polycyclic compound shown as a formula (I). The tetrahydrofuran benzodihydropyran polycyclic compound is prepared by the following steps: performing 3+2 cycloaddition on isatin diazo, ortho-substituted benzaldehyde and nitrophenyl alkene under the catalysis of rhodium acetate to construct a multi-substituted tetrahydrofuran intermediate; adding a base and performing intramolecular Michael addition to further cyclize so as to synthesize the tetrahydrofuran benzodihydropyran polycyclic compound. The compound has excellent inhibitory activity on histone deacetylase.
Owner:EAST CHINA NORMAL UNIVERSITY
A kind of aqueous resin emulsion and preparation method thereof
The invention provides waterborne resin emulsion and a method for preparing the same. The waterborne resin emulsion is characterized by comprising, by weight, 20-60 parts of functional acrylate monomers, 100-150 parts of acrylate monomers, 1-10 parts of cross-linking monomers, 4-20 parts of emulsifiers, 0.3-1 part of initiators and 250-350 parts of deionized water. Raw materials for the functionalacrylate monomers include hydroxyethyl acrylate, isophorone diisocyanate, antioxidants CA, solvents and a catalyst, a weight ratio of the hydroxyethyl acrylate to the isophorone diisocyanate to the antioxidants CA to the solvents to the catalyst is 50-60:110-150:160-200:100-200:0.01-1, the solvents are mixtures of more than one or two types of acetone and ethyl acetate, and the catalyst is dibulytin dilaurate. The waterborne resin emulsion and the method have the advantages that the waterborne resin emulsion is good in film-forming property and wide in damping temperature range, and can be widely used for vibration attenuation and noise reduction for aircrafts, ships, buildings, transportation and diversified machinery, and good high and low-temperature damping effects can be realized.
Owner:SHANGHAI INST OF TECH
Method for preparing mycophenolate sodium from mycophenolic acid strains
The invention provides a method for preparingmycophenolate sodium from mycophenolic acid strains, comprising the following steps: selecting the mycophenolic acid strains to produce strain spore suspension liquid, inoculating the strain spore suspension liquid in a seed medium and cultivating for 2-4 days at a temperature of 26-28 DEG C; inoculating the seed medium in a fermentation tank, adding a fermentation medium, and fermenting for 9-11 days at a temperature of 26-28 DEG C; adding acid into fermentation liquid in such a manner that pH value of the fermentation liquid is 3-4, adding a sodium hydroxide solution into the fermentation liquid in such a manner that pH value of the fermentation liquid is 8-9, decolorizing and recrystallizing to obtain mycophenolic acid; and synthesizing the mycophenolic acid and sodium hydroxide or sodium methylate in alcohols solvent to obtain the mycophenolate sodium. According to the method provided by the invention, the fermentation liquid obtained by cultivating the mycophenolic acid strains is subjected to acid and alkali treatment, and the sodium hydroxide or the sodium methylate is adopted to synthesize the mycophenolate sodium; and therefore the method has the advantages of short preparation route, simple operating procedure and lower production cost; meanwhile, the application of ketones organic solvent is avoided, and harm to the environment and human body is reduced.
Owner:WUXI FORTUNE PHARMA
Modified polyimides foam and preparation thereof
The invention discloses modified polyimide foam and a preparation method thereof. The preparation method comprises the following steps: aromatic dianhydride and / or aromatic acid ester, siloxane with active terminal group, low molecular alcohol, a catalyst and a surface active agent are mixed into a polar solvent according to proportion and react to form foaming precursor solution which reacts with isocyanate to form foaming intermediate solution; and the foaming intermediate solution carries out foaming freely in a mold and forms a modified foaming intermediate which is radiated by microwave or / and heated and cured in an oven, and then the solid polyimide foam is obtained. The method has short route of preparation process and simple technique; the foaming precursor solution has good stability of storage, is suitable for the processing techniques of casting, painting, extruding and the like, and the defects of broken foam, parallel foam, depression and incomplete imidizate of materialsdo not occur; the polyimide foam has even aperture, high stability, low rigidity, good flexibility and good processability; and the preparation cost is low, thus being convenient to promotion and application.
Owner:BEIJING RADIATION APPL RES CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com