Preparation technique for mezlocillin
A preparation technology of mezlocillin acid, which is applied in the field of drug synthesis, can solve problems such as poor quality stability, high solvent residue, and poor crystal form of the product, and achieve good stability, low solvent residue, and good crystal shape.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
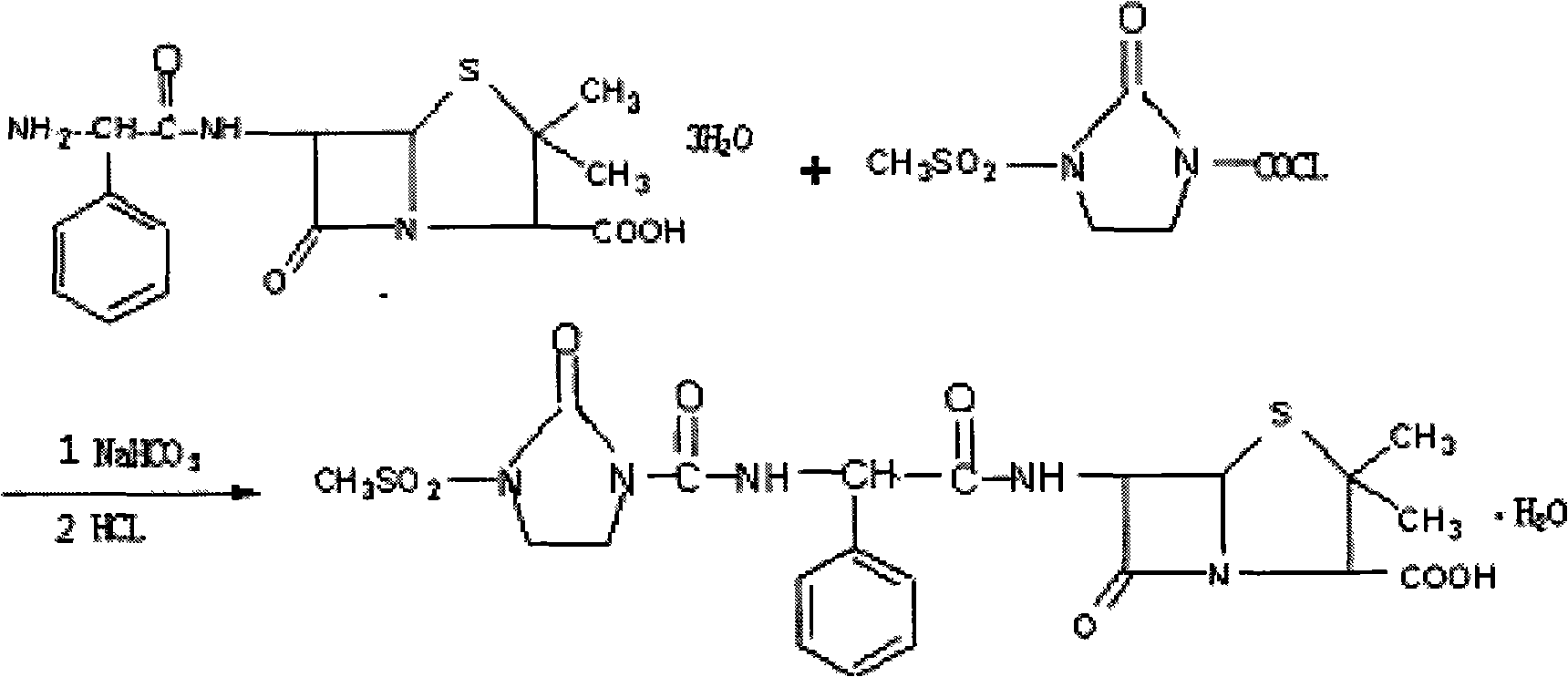
[0022] At 20°C, add 100mL of water, 6g of sodium bicarbonate and 40mL of acetone into a 250mL four-neck flask, add 12.5g of ampicillin in sequence while stirring, cool down to 16°C, and slowly add 1-chloroformyl-3- 6.6 g of methylsulfonyl-2-imidazolidinone was added, and the reaction was continued for another 30 minutes. After complete reaction, 60 mL of isopropyl ether was added for extraction, and after separation, the aqueous phase was filtered to a crystallization kettle.
[0023] Add 50mL of methyl acetate, cool down to 20°C and slowly add dilute hydrochloric acid dropwise to carry out acidification and crystallization, adjust the pH to 2.0, and stir for 30 minutes under temperature control. . Vacuum -0.08, 40 ° C under reduced pressure drying for 4 hours to obtain 16.0 g of needle crystals.
[0024] Quality index: content 99.5%, single impurity 0.09%, total impurity 0.25%, solvent residue 0.28%.
Embodiment 2
[0026] At 25°C, add 110mL of water, 6g of sodium bicarbonate and 45mL of acetone into a 250mL four-neck flask, add 12.5g of ampicillin in sequence while stirring, cool down to 17°C, and slowly add 1-chloroformyl-3- 6.5 g of methylsulfonyl-2-imidazolidinone, and the reaction was continued for another 30 minutes. After complete reaction, 50 mL of isopropyl ether was added for extraction, and the aqueous phase was filtered to a crystallization tank after separation.
[0027] Add 55 mL of methyl acetate, cool down to 22°C and slowly add dilute hydrochloric acid dropwise to carry out acidification and crystallization, adjust the pH to 2.2, and stir for 40 minutes under temperature control. . Vacuum -0.09, 35 ° C under reduced pressure drying for 5 hours to obtain 16.3 g of needle crystals.
[0028] Quality index: content 99.56%, single impurity 0.1%, total impurity 0.26%, solvent residue 0.25%.
Embodiment 3
[0030] At 25°C, add 120mL of water, 6g of sodium bicarbonate and 50mL of acetone into a 250mL four-neck flask, add 12.5g of ampicillin in sequence while stirring, cool down to 16°C, and slowly add 1-chloroformyl-3- 6.8 g of methylsulfonyl-2-imidazolidinone was added, and the reaction was continued for another 30 minutes. After complete reaction, 50 mL of isopropyl ether was added for extraction, and the aqueous phase was filtered to a crystallization tank after separation.
[0031] Add 40mL of methyl acetate, cool down to 23°C and slowly add dilute hydrochloric acid dropwise to carry out acidification and crystallization, adjust the pH to 2.2, and stir for 30 minutes under temperature control. . Vacuum -0.08, 50 ° C under reduced pressure drying for 3 hours to obtain 16.0 g of needle crystals.
[0032] Quality index: content 99.2%, single impurity 0.08%, total impurity 0.27%, solvent residue 0.29%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com