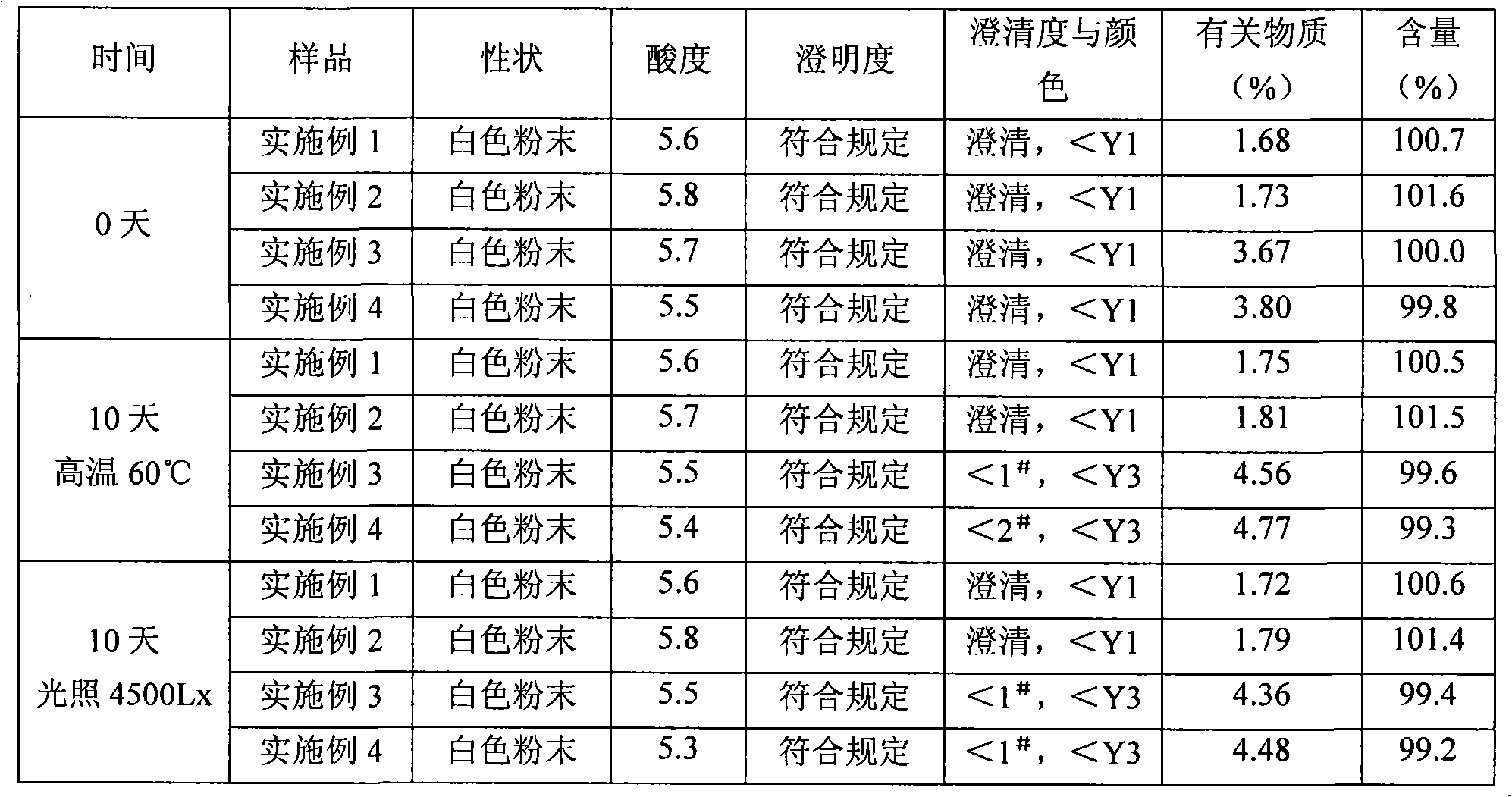
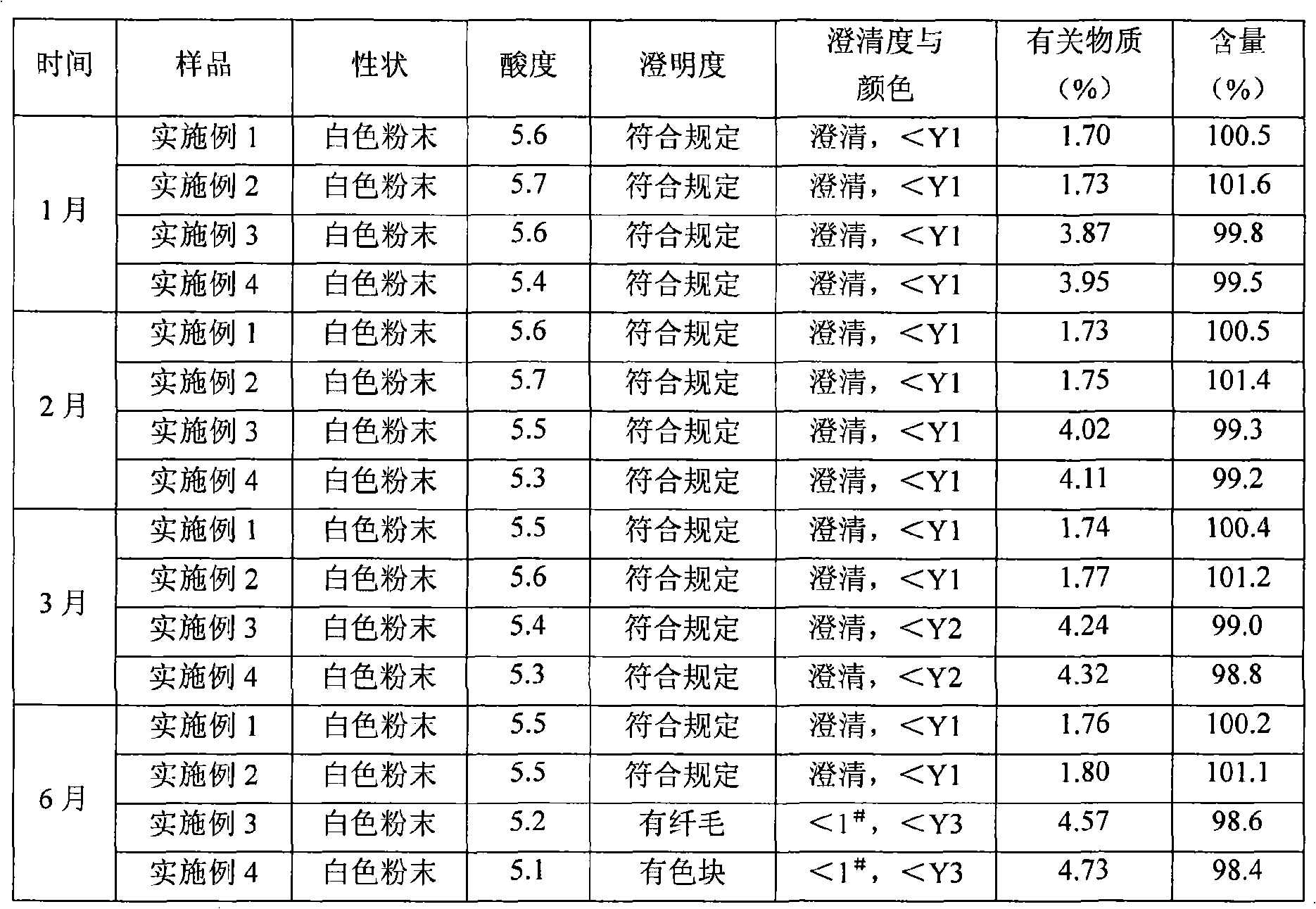
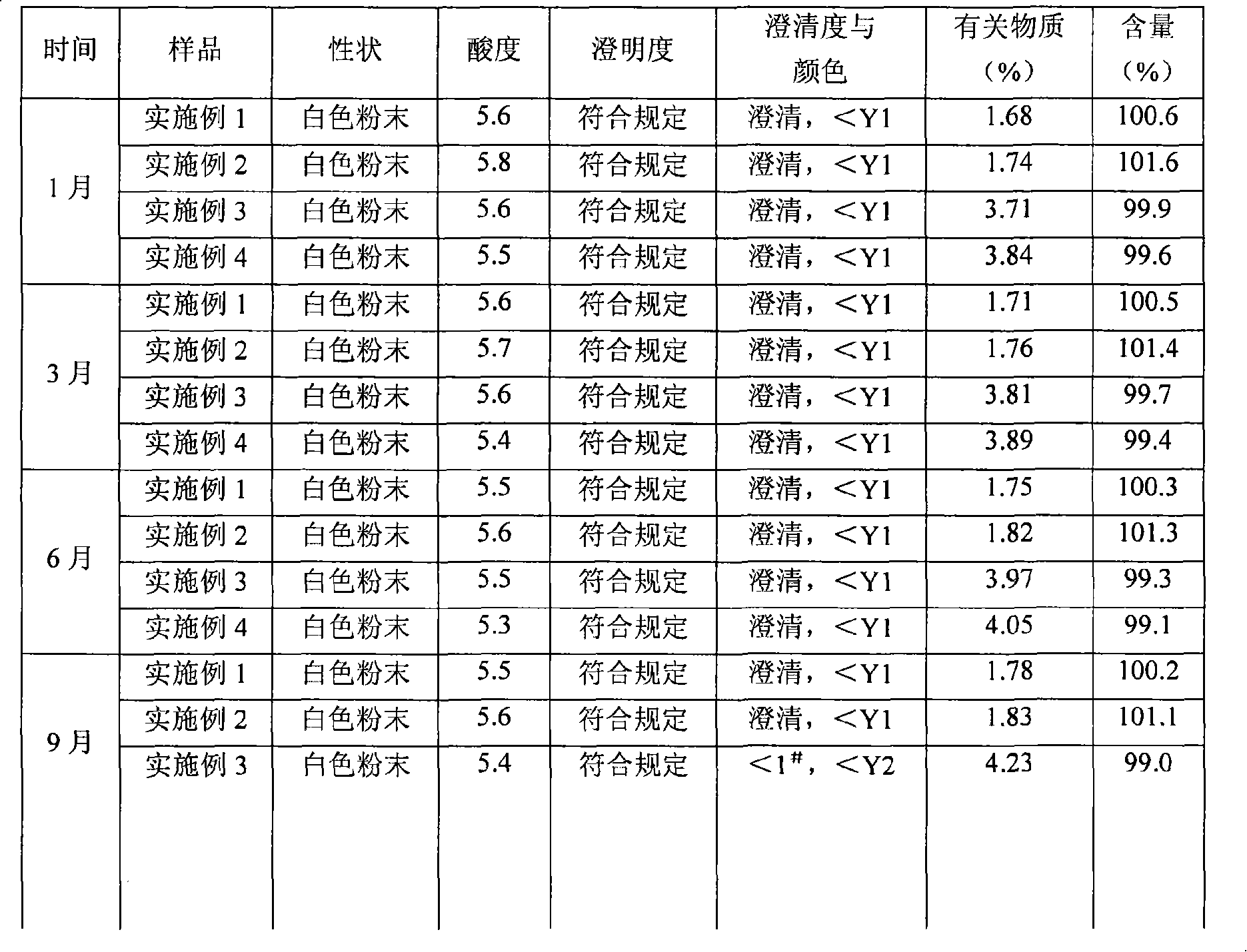
Mezlocillin sodium and sulbactam sodium for injection and freeze-dried injection preparation thereof
A technology of mezlocillin sodium sulbactam sodium and mezlocillin sodium, which is applied in the field of separation and purification of mezlocillin sodium and sulbactam sodium, can solve the problems of large side effects and low purity of active ingredients, and achieve high recovery rate , Avoid sample loss, improve the effect of stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A semi-preparative high-speed countercurrent chromatograph is used, equipped with a constant flow pump, a 15ml injection valve, a polytetrafluoroethylene column with a column volume of 200ml, and a UV ultraviolet detector. Mix chloroform with a volume ratio of 1.2:1.8:1: methanol: water in a separatory funnel, shake it up and let it stand to separate layers, take its upper layer solution (upper phase) as the stationary phase, and the lower layer solution (lower phase) ) as the mobile phase, after ultrasonic degassing, first fill the entire column with the stationary phase, then turn on the high-speed countercurrent chromatograph, adjust the host speed to 1500rpm, pump the mobile phase into the column at a flow rate of 1.5ml / min, and wait for the entire system to After the dynamic equilibrium is established, the mezlocillin sodium raw material is dissolved in the lower phase, and the sample is injected through the injection valve, and then according to the ultraviolet spe...
Embodiment 2
[0033] A semi-preparative high-speed countercurrent chromatograph is used, equipped with a constant flow pump, a 15ml injection valve, a polytetrafluoroethylene column with a column volume of 200ml, and a UV ultraviolet detector. Mix chloroform:methanol:water with a volume ratio of 0.8:2.5:1 in a separatory funnel, shake it up and let it stand for layering, take its upper layer solution (upper phase) as the stationary phase, and the lower layer solution (lower phase) ) as the mobile phase, after ultrasonic degassing, first fill the entire column with the stationary phase, then turn on the high-speed countercurrent chromatograph, adjust the speed of the main engine to 2000rpm, and pump the mobile phase into it at a flow rate of 1.2ml / min, and wait until the entire system is established After dynamic equilibrium, dissolve the raw material of mezlocillin sodium in the lower phase, inject the sample through the injection valve, and then collect the part above 9% according to the ul...
Embodiment 3
[0037] Embodiment 3 (comparison)
[0038]A semi-preparative high-speed countercurrent chromatograph is used, equipped with a constant flow pump, a 15ml injection valve, a polytetrafluoroethylene column with a column volume of 200ml, and a UV ultraviolet detector. Mix chloroform:methanol:water with a volume ratio of 0.7:2.6:1 in a separatory funnel, shake it up and let it stand for layering, take its upper layer solution (upper phase) as the stationary phase, and the lower layer solution (lower phase) ) as the mobile phase, after ultrasonic degassing, first fill the entire column with the stationary phase, then turn on the high-speed countercurrent chromatograph, adjust the host speed to 1000rpm, pump the mobile phase into the column at a flow rate of 1.5ml / min, and wait until the entire system After the dynamic equilibrium is established, the mezlocillin sodium raw material is dissolved in the lower phase, and the sample is injected through the injection valve, and then accord...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com