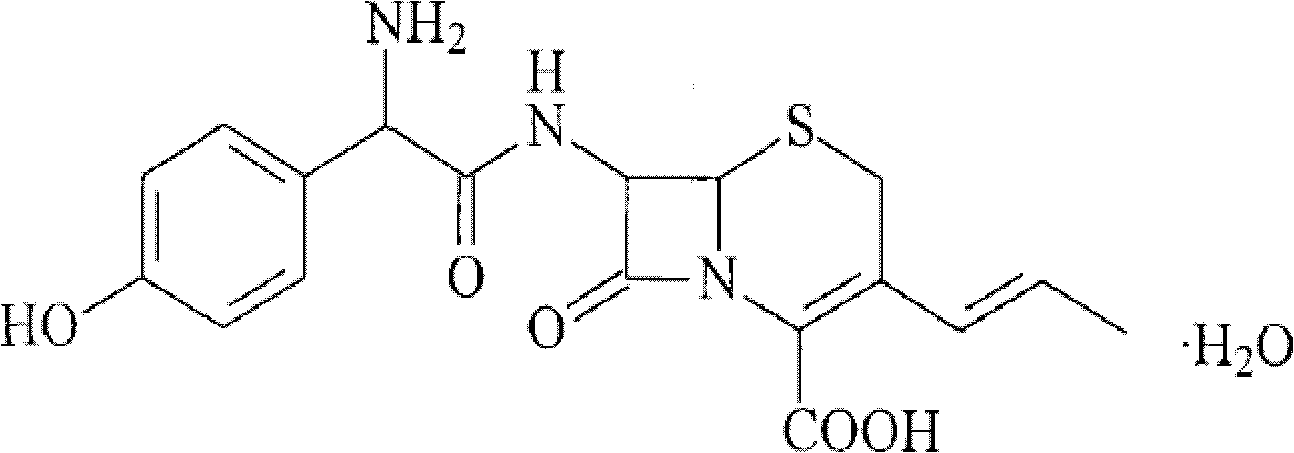
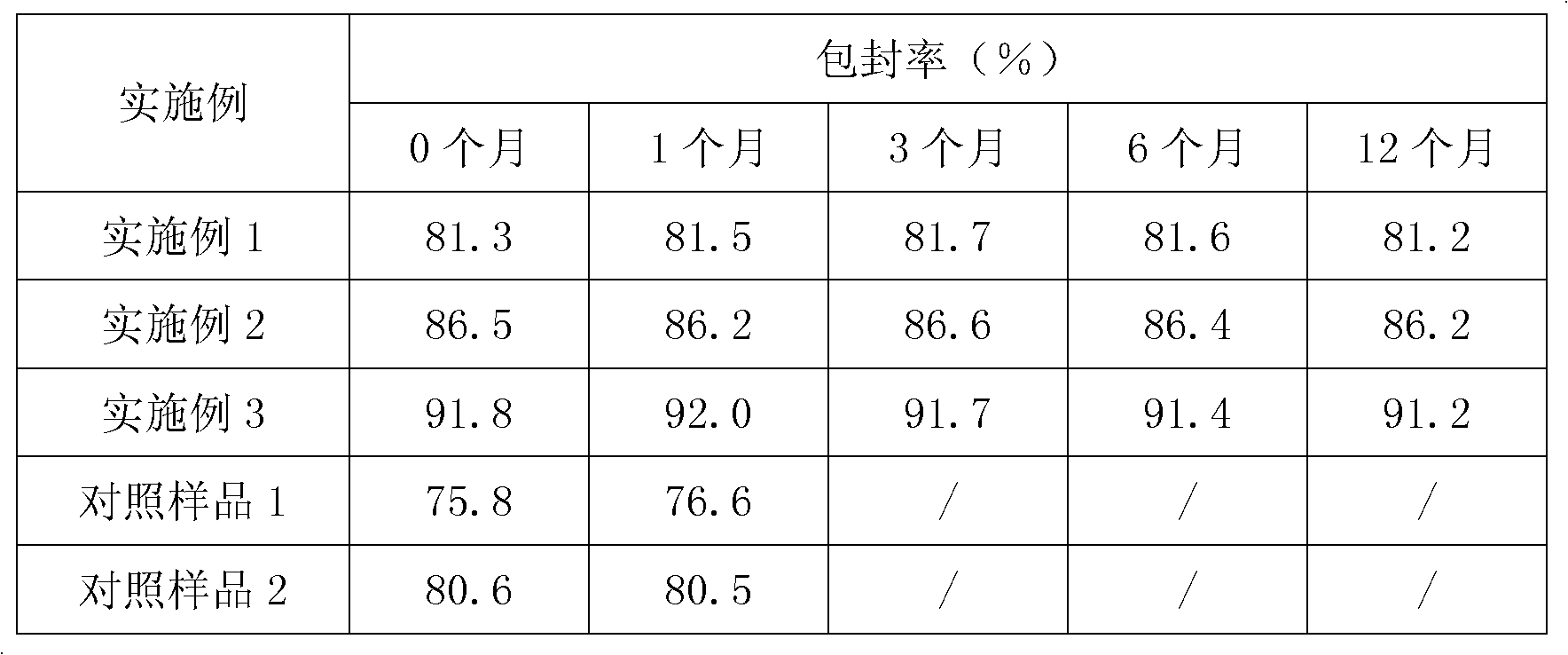
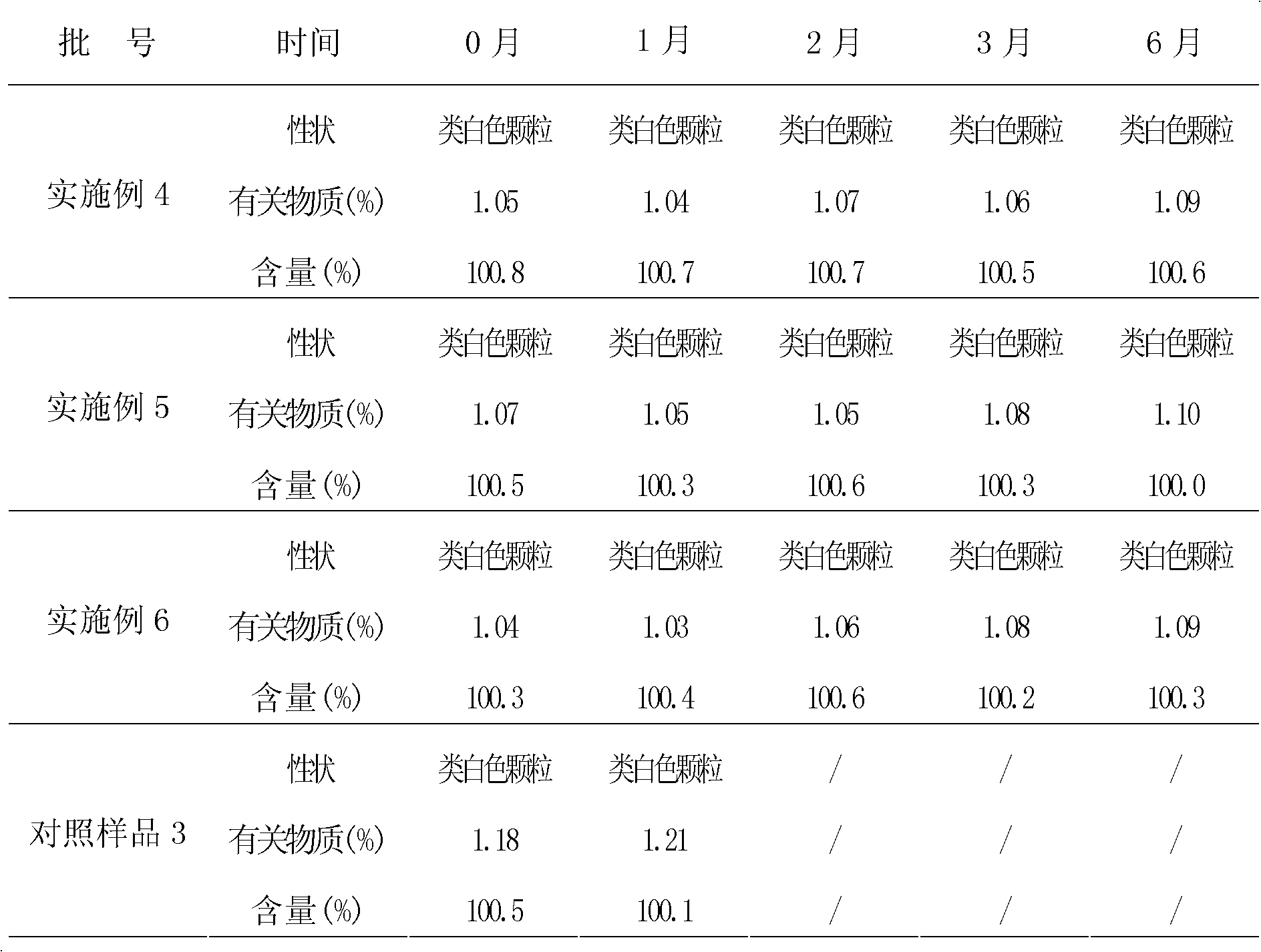
Granules comprising cefprozil lipidosome and preparation method thereof
A technology of cefprozil and acrylic ester, which is applied in the field of medicine, can solve the problems of poor solubility, stability and bioavailability of preparations, and low encapsulation efficiency of cefprozil submicroemulsion, so as to avoid peak and valley phenomena and improve bioavailability degree, storage stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1: the preparation of cefprozil liposome
[0071] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
cefprozil
62.50
125.00
250.00
Hydrogenated Soy Lecithin
156.25
312.50
625.00
156.25
312.50
625.00
312.50
625.00
1250.00
Vitamin E
50.00
100.00
200.00
total
737.50
1475.00
2950.00
[0072] Preparation:
[0073] (A) Dissolve hydrogenated soybean lecithin, egg yolk lecithin, cholesterol and vitamin E in an appropriate amount of absolute ethanol according to the prescription amount, mix evenly, filter, evaporate and remove absolute ethanol on a rotary evaporator to obtain a phospholipid film, and vacuum reduce Press dry. The amount of dehydrated alcohol used is 30 times of the amount of cefprozil.
[0074] (B) Weigh the prescribed amount of cefprozil...
Embodiment 2
[0079] Embodiment 2: the preparation of cefprozil liposome
[0080] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
[0081] cefprozil
62.50
125.00
250.00
Hydrogenated Soy Lecithin
243.75
487.50
975.00
egg yolk lecithin
243.75
487.50
975.00
487.50
975.00
1950.00
Vitamin E
50.00
100.00
200.00
total
1087.50
2175.00
4350.00
[0082] Preparation:
[0083] (A) Dissolve hydrogenated soybean lecithin, egg yolk lecithin, cholesterol and vitamin E in an appropriate amount of absolute ethanol according to the prescription amount, mix evenly, filter, evaporate and remove absolute ethanol on a rotary evaporator to obtain a phospholipid film, and vacuum reduce Press dry. The amount of dehydrated alcohol used is 30 times of the amount of cefprozil.
[0084] (B) Weigh the prescribed...
Embodiment 3
[0089] Embodiment 3: the preparation of cefprozil liposome
[0090] Name of raw material
Dose 1 (mg)
Dose 2 (mg)
Dose 3 (mg)
cefprozil
62.50
125.00
250.00
Hydrogenated Soy Lecithin
312.50
625.00
1250.00
egg yolk lecithin
312.50
625.00
1250.00
cholesterol
625.00
1250.00
2500.00
Vitamin E
50.00
100.00
200.00
[0091] total
1362.50
2725.00
5450.00
[0092] Preparation:
[0093] (A) Dissolve hydrogenated soybean lecithin, egg yolk lecithin, cholesterol and vitamin E in an appropriate amount of absolute ethanol according to the prescription amount, mix evenly, filter, evaporate and remove absolute ethanol on a rotary evaporator to obtain a phospholipid film, and vacuum reduce Press dry. The amount of dehydrated alcohol used is 30 times of the amount of cefprozil.
[0094] (B) Weigh the prescri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com