Sophoridine nano liposome medicament and preparation of the same
A nano-liposome, sophoridine technology, applied in the directions of liposome delivery, drug combination, antipyretics, etc., can solve problems such as adverse reactions, and achieve the effects of avoiding phagocytosis, good quality and high curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] The present embodiment adopts sophoridine nano liposome drug formula as follows:
[0033] Hydrogenated Soy Lecithin and Vitamin E for Injection
[0034] 350g of a mixture with a weight ratio of 9.8:0.2;
[0035] Cholesterol and β-sitosterol weight ratio 1: 1 mixture 30g;
[0036] Sophoridine 10g;
[0037] 200g of a mixture of dextran-40 and mannitol in a weight ratio of 1:7;
[0038] Reduced Glutathione 5g;
[0039] Poloxamer F-68 40g;
[0040] Phosphate buffer (0.01M, PH6.0-7.0) 2000g.
[0041] The preparation method is as follows:
[0042] (1) Sophoridine, a mixture of hydrogenated soybean lecithin for injection and vitamin E in a weight ratio of 9.8:0.2, and a mixture of cholesterol and β-sitosterol in a weight ratio of 1-3:1 were dissolved in ether and ethanol at a volume ratio of 3- In the 5:1 mixed solution, diethyl ether and ethanol were evaporated under reduced pressure in a rotary evaporator at 30-40°C to form a liposome film on the bottle wall;
[0043...
Embodiment 2
[0049] The present embodiment adopts sophoridine nano liposome drug formula as follows:
[0050] Hydrogenated Soy Lecithin and Vitamin E for Injection
[0051] 500g of a mixture with a weight ratio of 9.8:0.2;
[0052] Cholesterol and β-sitosterol weight ratio 3: 1 mixture 20g;
[0053] Sophoridine 25g;
[0054] 125g of a mixture of dextran-40 and mannitol in a weight ratio of 1:9;
[0055] Reduced glutathione 10g;
[0056] Poloxamer F-68 35g;
[0057] Phosphate buffer (0.01M, PH6.0-7.0) 1750g.
[0058] The preparation method is the same as above.
[0059] Pharmacodynamic verification experiment:
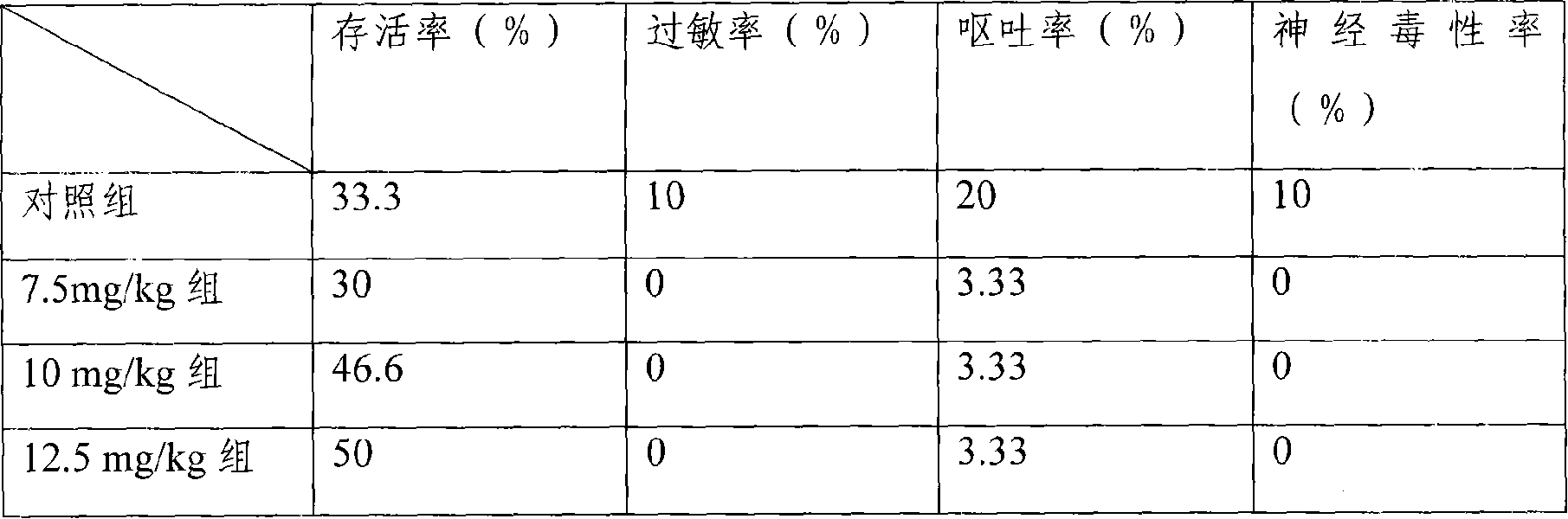
[0060] Mouse liver cancer model, the treatment group is the freeze-dried injection of the medicine of the present invention, divided into three dosage groups of 7.5mg / kg, 10mg / kg, and 12.5mg / kg, and the control group is the common water injection of 25mg / kg dosage, each group 30 mice. Carry out tail vein injection administration. Dosing once every three days, the statistic...
Embodiment 3
[0063] The present embodiment adopts sophoridine nano liposome drug formula as follows:
[0064] Hydrogenated Soy Lecithin and Vitamin E for Injection
[0065] 350g of a mixture with a weight ratio of 9.8:0.2;
[0066] Cholesterol and β-sitosterol weight ratio 1: 1 mixture 20g;
[0067] Sophoridine 10g;
[0068] 125g of a mixture of dextran-40 and mannitol in a weight ratio of 1:7;
[0069] Reduced Glutathione 5g;
[0070] Poloxamer F-68 35g;
[0071] Phosphate buffer (0.01M, PH6.0-7.0) 1750g.
[0072] The preparation method is the same as above.
[0073] Pharmacodynamic verification experiment:
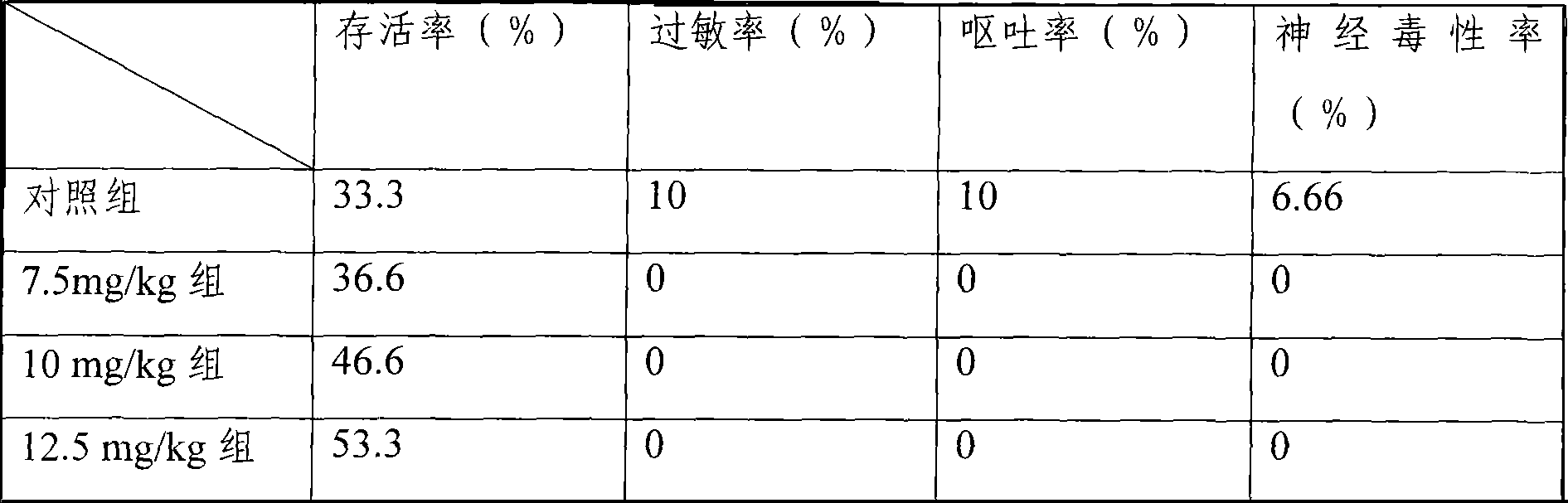
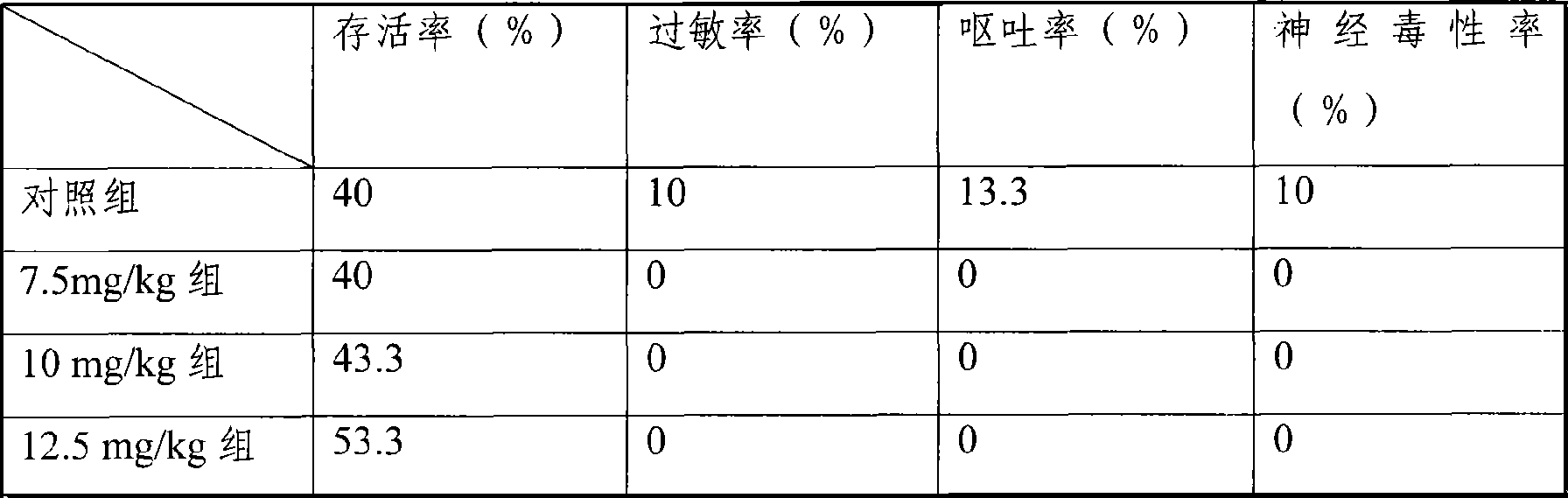
[0074] Mouse liver cancer model, the treatment group is the freeze-dried injection of the medicine of the present invention, divided into three dosage groups of 7.5mg / kg, 10mg / kg, and 12.5mg / kg, and the control group is the common water injection of 25mg / kg dosage, each group 30 mice. Carry out tail vein injection administration. Dosing once every three days, the statistical ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com