Azithromycin liposome composition medicine and its prepn process
A kind of technology of azithromycin lipid and azithromycin, which is applied in the field of azithromycin liposome combination medicine and preparation thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
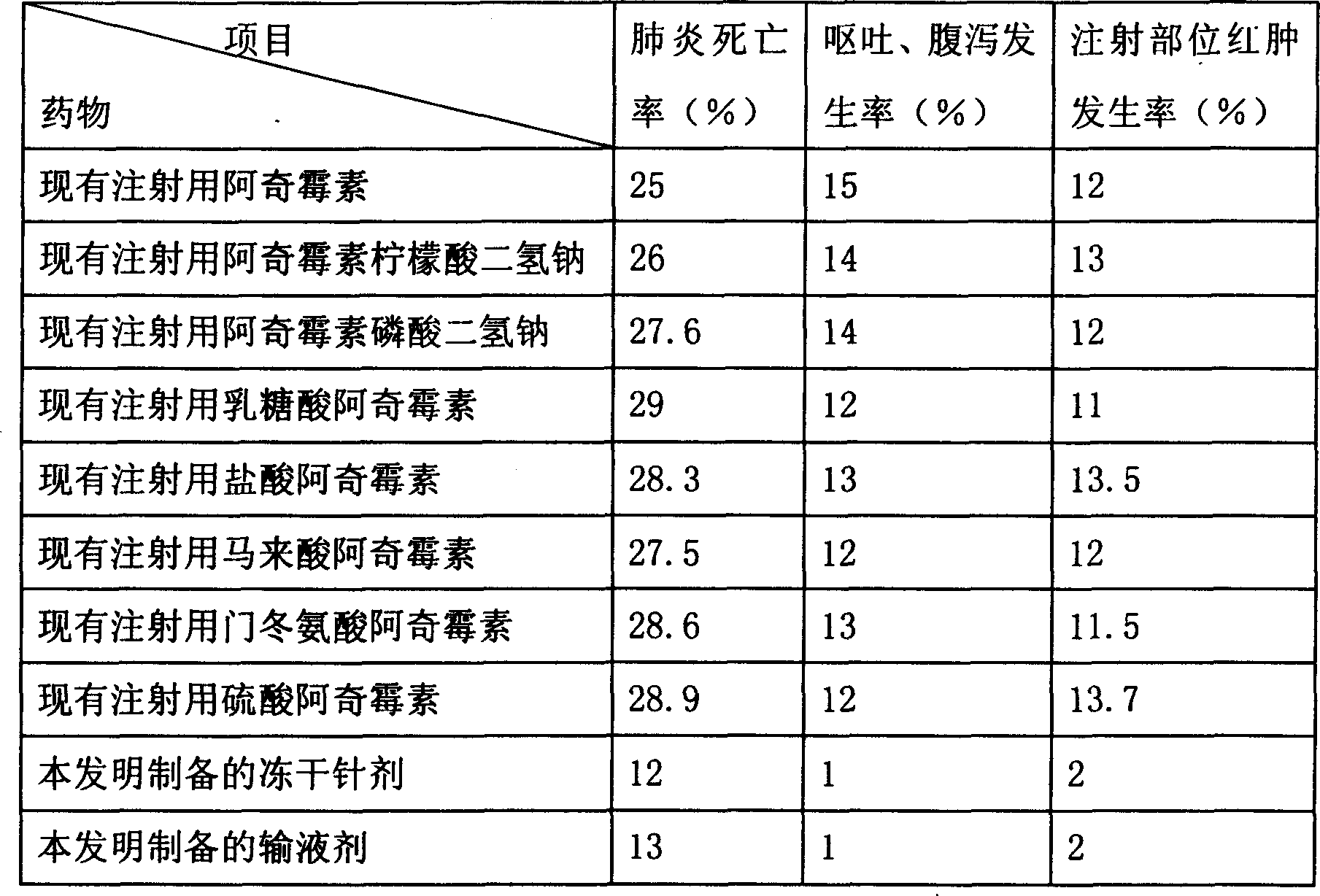
Examples
Embodiment 1
[0040] The formula used in this embodiment is as follows:
[0041] Azithromycin 1;
[0042] Any ratio mixture of hydrogenated soybean lecithin and egg yolk lecithin
[0043] Mixture 10 with a molar ratio of 8:1 to cephalin;
[0044] A mixture of soybean sterol and cholesterol at a molar ratio of 1:1 10;
[0045] Vitamin E 1;
[0046] PEG2000 0.1;
[0047] α-Mercaptopropionylglycine 0.1;
[0048] The above-mentioned combination medicine uses vitamin C as an excipient, and its added amount is 4% of the total formula.
[0049] Wherein, the azithromycin can be one of azithromycin dihydrogen citrate sodium citrate, azithromycin dihydrogen phosphate, azithromycin lactobionate, azithromycin hydrochloride, azithromycin maleate, azithromycin aspartate, azithromycin sulfate or any combination thereof, which is in the formula The moles of azithromycin are all based on azithromycin.
[0050] The preparation method is as follows:
[0051] 1) A mixture composed of a mixture of hydrogenated soyb...
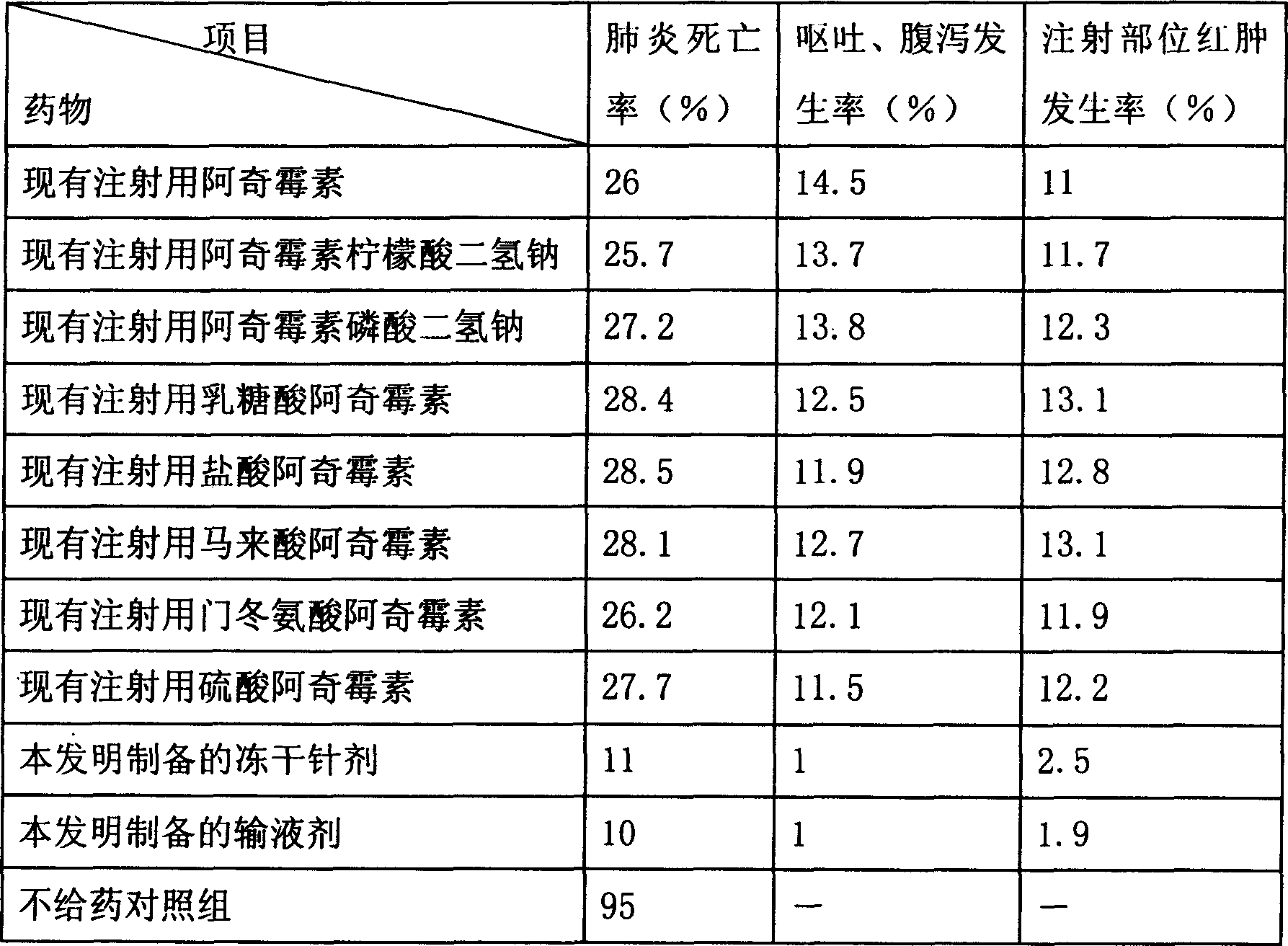
Embodiment 2
[0061] The formula used in this embodiment is as follows:
[0062] Azithromycin 0.8;
[0063] Any ratio mixture of hydrogenated soybean lecithin and egg yolk lecithin
[0064] Mixture 12 with a molar ratio of 8:1 to cephalin;
[0065] A mixture of soybean sterol and cholesterol at a molar ratio of 1:2 8;
[0066] Vitamin E 1.2;
[0067] PEG2000 0.2;
[0068] α-Mercaptopropionylglycine 0.2;
[0069] The above-mentioned combination medicine uses vitamin C as an excipient, and its addition amount is 8% of the total formula.
[0070] Wherein the azithromycin can be one or any combination of azithromycin dihydrogen citrate sodium citrate, azithromycin dihydrogen phosphate, azithromycin lactobionate, azithromycin hydrochloride, azithromycin maleate, azithromycin aspartate, azithromycin sulfate, and any combination thereof. The number of moles is based on azithromycin.
[0071] The preparation method is as follows:
[0072] 1) A mixture composed of a mixture of hydrogenated soybean lecit...
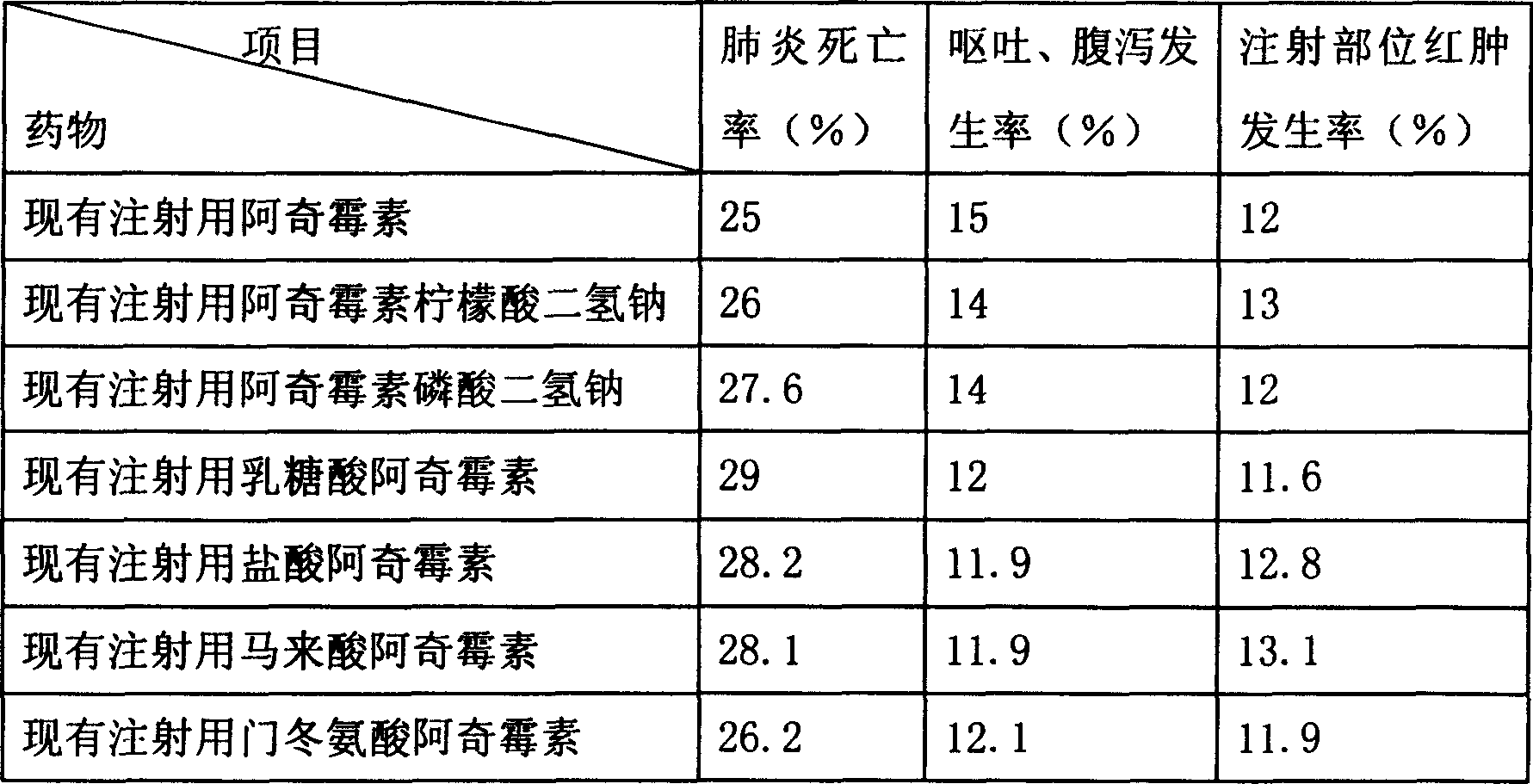
Embodiment 3
[0081] The formula used in this embodiment is as follows:
[0082] Azithromycin 1.2;
[0083] Any ratio mixture of hydrogenated soybean lecithin and egg yolk lecithin
[0084] Mixture 8 with cephalin 8:1 molar ratio;
[0085] A mixture of soybean sterol and cholesterol at a molar ratio of 1:2 12;
[0086] Vitamin E 0.8;
[0087] PEG2000 0.1;
[0088] α-Mercaptopropionylglycine 0.2;
[0089] The above-mentioned combination medicine uses vitamin C as an excipient, and its added amount is 5% of the total formula.
[0090] Wherein the azithromycin can be one or any combination of azithromycin dihydrogen citrate sodium citrate, azithromycin dihydrogen phosphate, azithromycin lactobionate, azithromycin hydrochloride, azithromycin maleate, azithromycin aspartate, azithromycin sulfate, and any combination thereof. The number of moles is based on azithromycin.
[0091] The preparation method is as follows:
[0092] 6) The mixture of hydrogenated soy lecithin and egg yolk lecithin in any ra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com