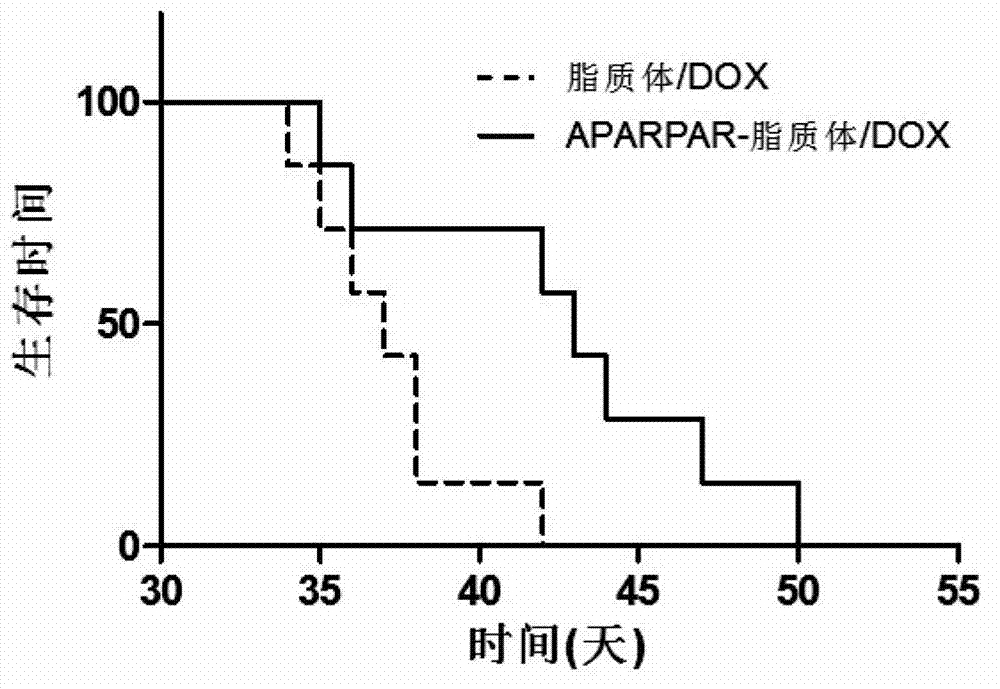
APARPAR polypeptide-modified invisible liposome and drug delivery system
A stealth liposome and polypeptide modification technology, which is applied in the direction of liposome delivery, antineoplastic drugs, drug combination, etc., can solve the problem of no expression and achieve the effect of improving the killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
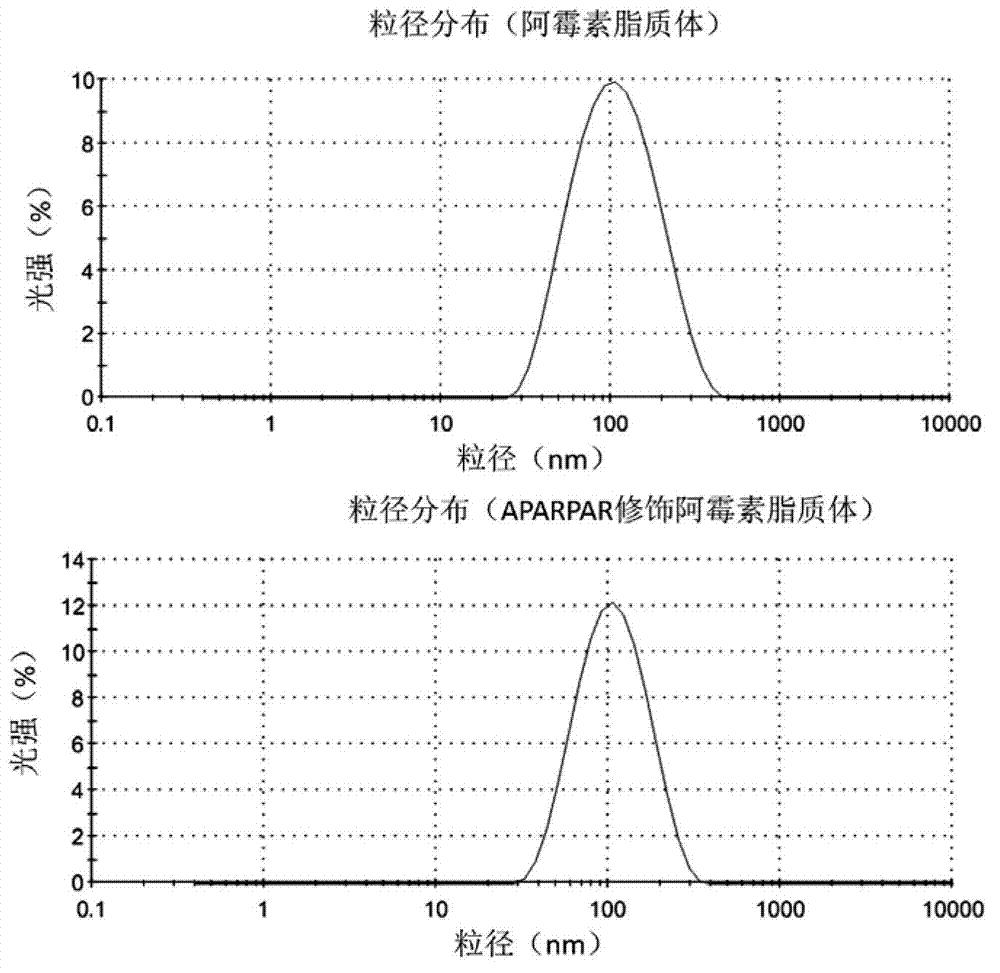
[0022] Embodiment 1, Preparation and Characterization of APARPAR-Liposome / DOX
[0023] The composition of the liposome membrane material formulation is HSPC (hydrogenated soybean lecithin) / Chol (cholesterol) / PEG-DSPE (polyethylene glycol-distearoylphosphatidylethanolamine complex) (14:6:2, mol / mol) , APARPAR modified PEG liposome membrane material formulation is HSPC / Chol / mPEG-DSPE / APARPAR-PEG-DSPE (14:6:2:1, mol / mol).
[0024] Each component was dissolved in chloroform, the solvent was removed by rotary evaporation, and the concentration of 0.1M (NH 4 ) 2 SO 4 Put the solution into the lipid film, ultrasonically disperse and rotate and shake in a water bath at 40°C to obtain a blank liposome suspension; then extrude through a carbonate film with a pore size of 50nm to obtain a blank liposome; Physiological saline is used as the mobile phase to pass through the Sephadex column, and the liposome part is collected, mixed with an appropriate amount of doxorubicin solution, l...
Embodiment 2
[0025] Embodiment 2, Preparation and Characterization of APARPAR-Liposome / DOX
[0026] The composition of the liposome membrane material formulation is HSPC (hydrogenated soybean lecithin) / Chol (cholesterol) / PEG-DSPE (polyethylene glycol-distearoylphosphatidylethanolamine complex) (16:6:4, mol / mol) , APARPAR modified PEG liposome membrane material formulation is HSPC / Chol / mPEG-DSPE / APARPAR-PEG-DSPE (16:6:4:1, mol / mol).
[0027] Each component was dissolved in methanol, the solvent was removed by rotary evaporation, and the concentration of 0.5M (NH 4 ) 2 SO 4 Put the solution into the lipid film, ultrasonically disperse and rotate and shake in a water bath at 70°C to obtain a blank liposome suspension; then extrude through a carbonate film with a pore size of 50nm to obtain a blank liposome; Physiological saline is used as the mobile phase to pass through the dextran gel column, the liposome part is collected, mixed with an appropriate amount of doxorubicin solution, left...
Embodiment 3
[0028] Embodiment 3, Preparation and Characterization of APARPAR-Liposome / DOX
[0029] The composition of the liposome membrane material formulation is HSPC (hydrogenated soybean lecithin) / Chol (cholesterol) / PEG-DSPE (polyethylene glycol-distearoylphosphatidylethanolamine complex) (14:6:2, mol / mol) , APARPAR modified PEG liposome membrane material formulation is HSPC / Chol / mPEG-DSPE / APARPAR-PEG-DSPE (14:6:2:1, mol / mol).
[0030] Each component was dissolved in chloroform, the solvent was removed by rotary evaporation, and a concentration of 0.3M (NH 4 ) 2 SO 4 Put the solution into the lipid film, ultrasonically disperse and rotate and shake in a water bath at 50°C to obtain a blank liposome suspension; then extrude through a carbonate film with a pore size of 50nm to obtain a blank liposome; Physiological saline is used as the mobile phase to pass through the dextran gel column, and the liposome part is collected, mixed with an appropriate amount of doxorubicin solution, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com