Memantine hydrochloride capsule sustained-release preparation and preparation method for same
A technology of memantine hydrochloride and sustained-release capsules is applied in the directions of pharmaceutical formulation, drug delivery, amine active ingredients, etc., which can solve the problems of inability to effectively control the drug release amount, poor safety, and poor effectiveness, and achieves good content uniformity, Short coating time and less irritating effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030]
[0031] Raw material name content
[0032]
[0033] Memantine Hydrochloride 2.0g
[0034] Sucrose spherical particles (15-30 mesh) 250g
[0035] Eudragit RS 100 25g
[0036] Eudragit RL 100 9g
[0037] Triethyl citrate 2g
[0038] Povidone K30 2g
[0040] Sucrose 15g
[0041]
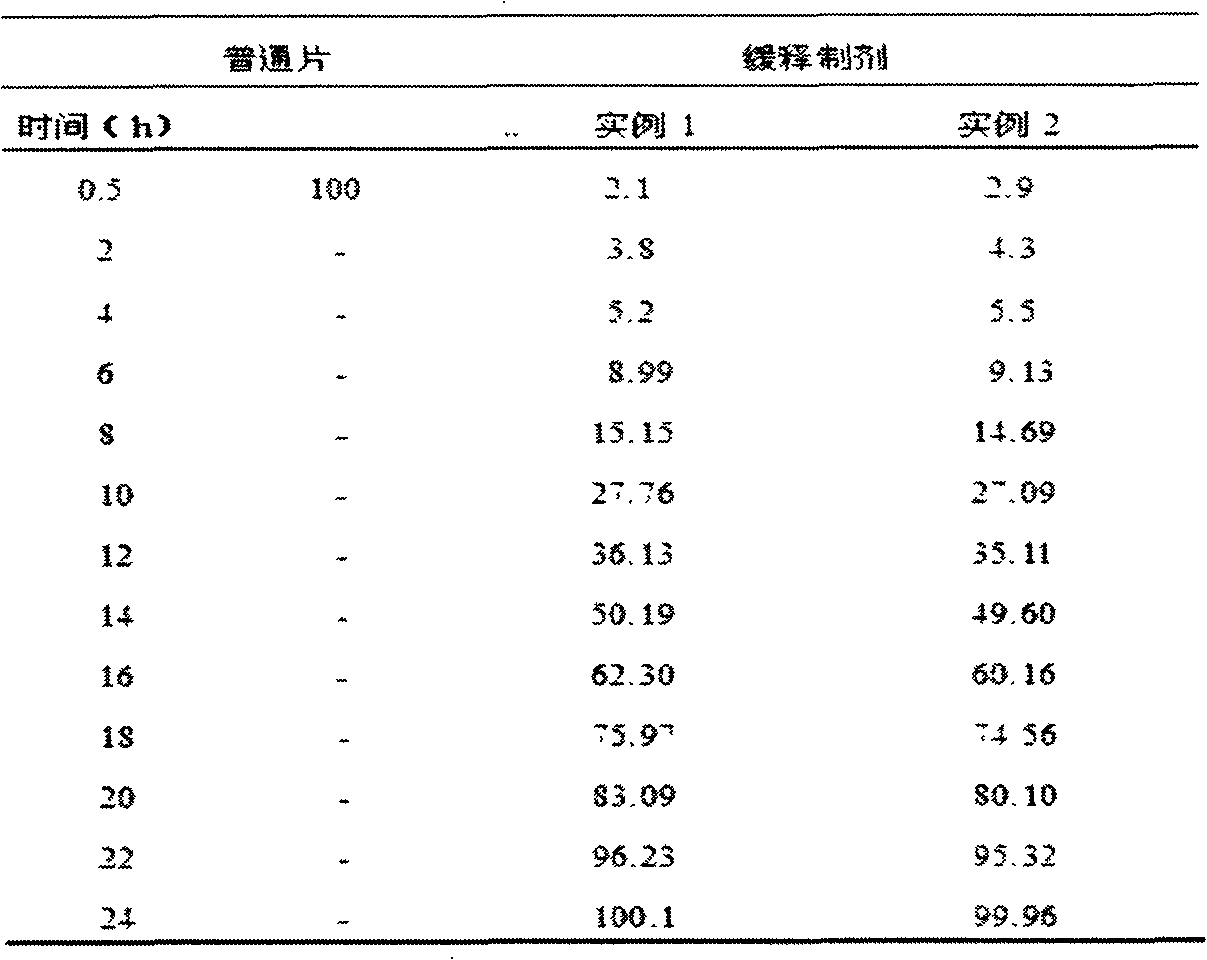
[0042] Preparation: Take the prescribed amount of sucrose, add the same amount of water to make 50% syrup, add the prescribed amount of memantine hydrochloride, mix well, and form a suspension for later use; take another prescribed amount of sucrose spherical particles, put it in a high-efficiency coating pan, adjust The temperature of the air inlet is 30-40°C, and the above suspension is evenly sprayed on the spherical sucrose particles according to the coating process, dried at 50-60°C for 8 hours, and passed through a 20-mesh sieve to obtain ...
Embodiment 2
[0044]
[0045] Preparation: Take the prescribed amount of sucrose, add the same amount of water to make 50% syrup, add the prescribed amount of memantine hydrochloride, mix well, and form a suspension for later use; take another prescribed amount of microcrystalline cellulose spherical particles, and put it in a high-efficiency coating pan In the process, adjust the air inlet temperature to 30-40°C, spray the above-mentioned suspension evenly on the microcrystalline cellulose spherical particles according to the coating process, dry at 50-60°C for 8 hours, and pass through a 20-mesh sieve to obtain drug-containing spherical particles , stand-by; take the prescription amount of Eudragit RS 100, Eudragit RL 100, polyethylene glycol 6000 plus the prescription amount of ethanol to dissolve, spray evenly on the above-mentioned drug-containing spherical particles according to the coating process, dry at 50-60 ° C for 8 hours, pass Sieve through a 15-mesh sieve to obtain sustained...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com