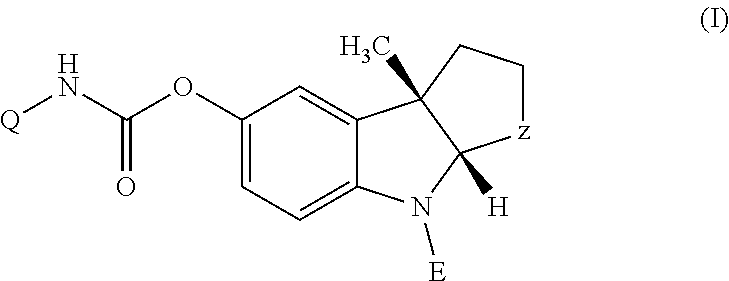
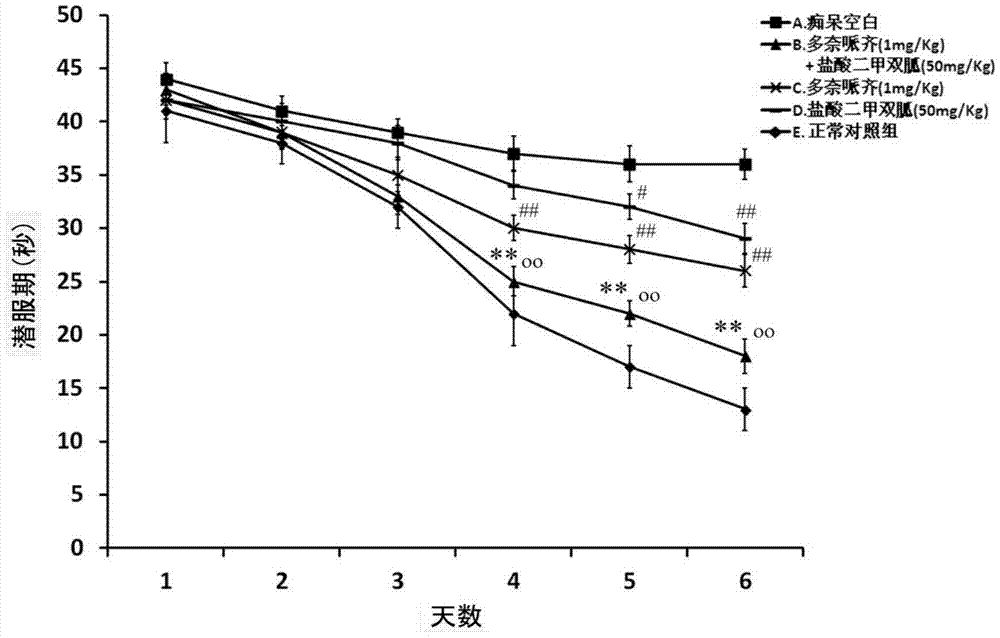
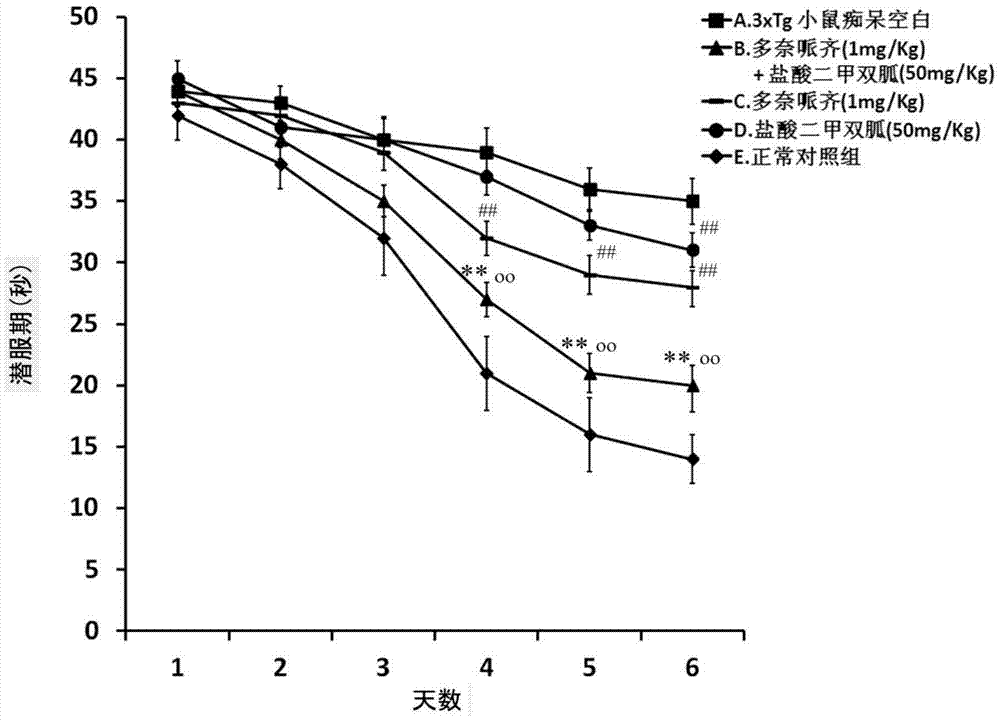
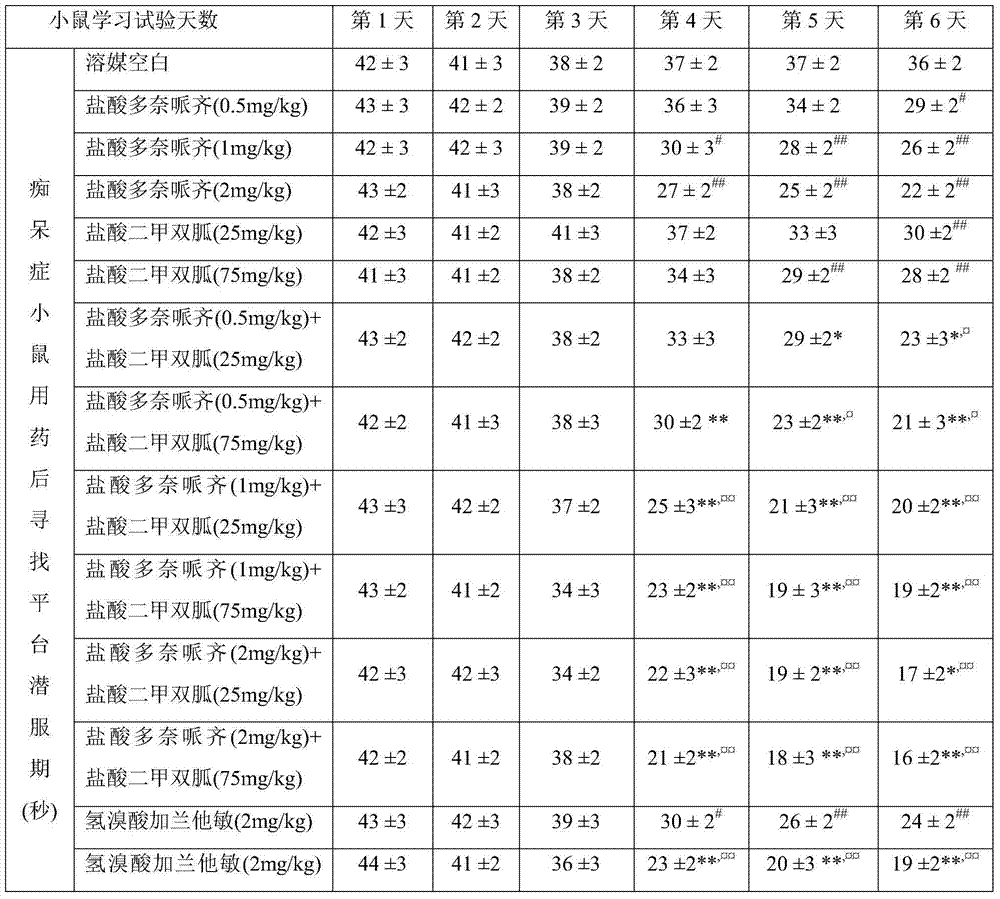
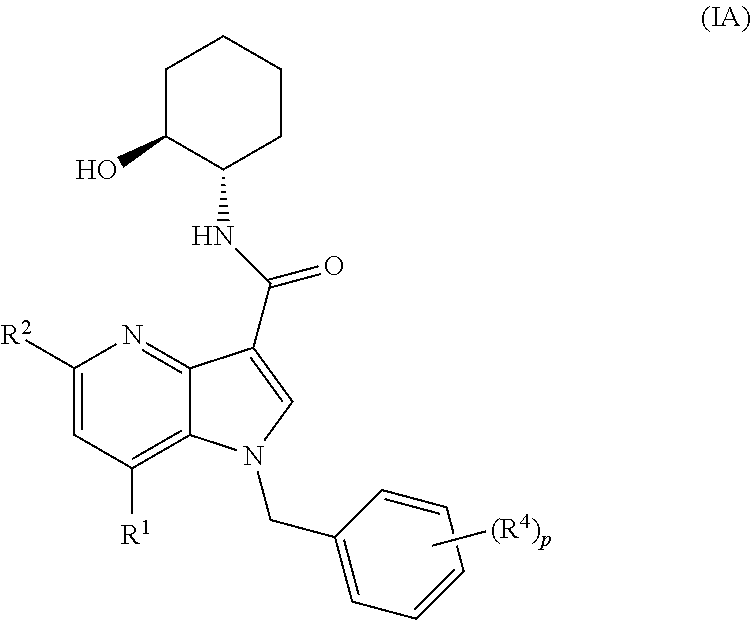
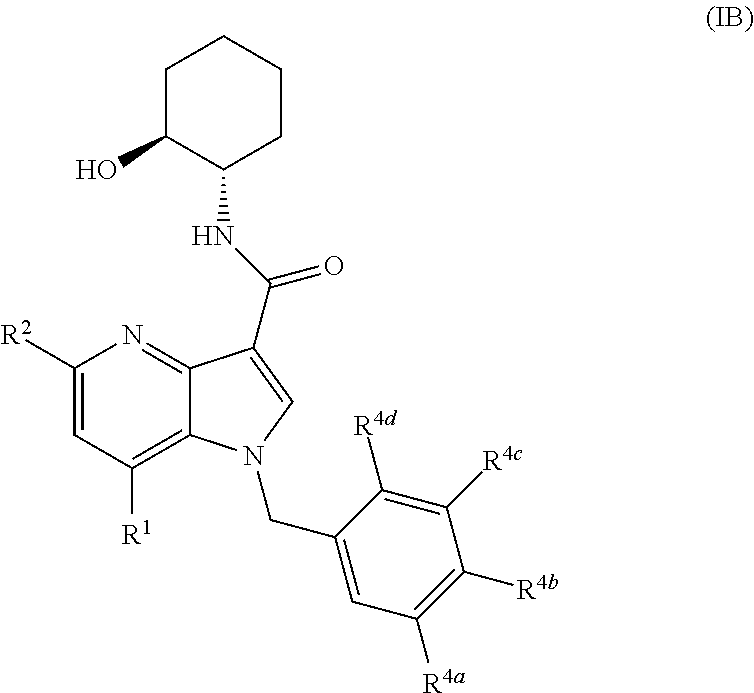
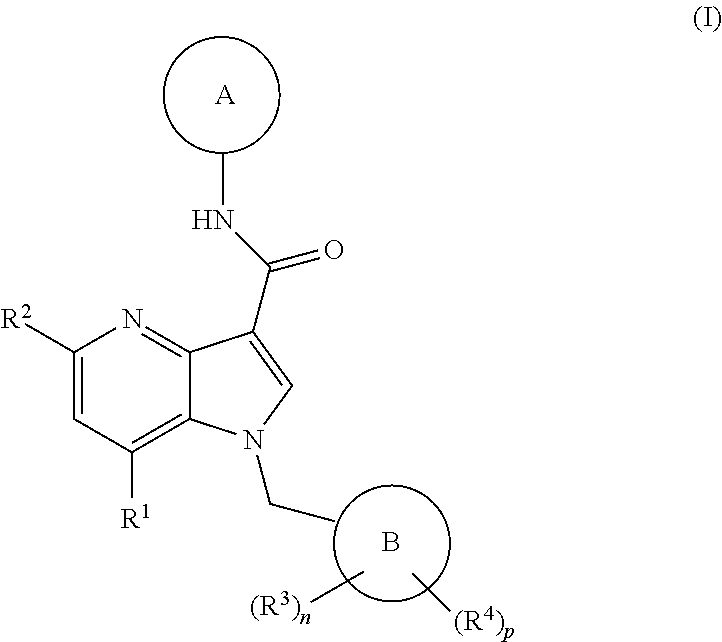
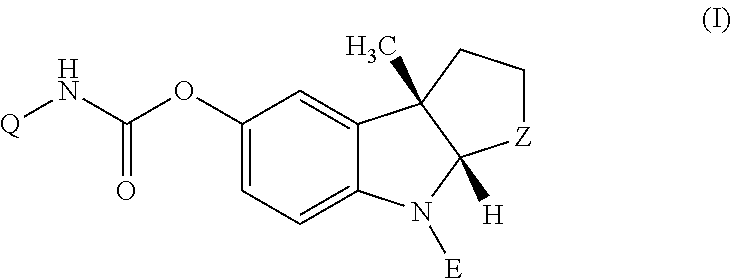
Patents
Literature
Hiro is an intelligent assistant for R&D personnel, combined with Patent DNA, to facilitate innovative research.
53 results about "Alzheimer type dementia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Alzheimer's is a type of dementia that typically causes problems with memory, thinking and behavior. Alzheimer's disease is the most common form of dementia. Alzheimer's disease affects memory, thinking, concentration, and judgment. Alzheimer's ultimately impedes a person’s ability to perform normal daily activities.
Method and composition for treating alzheimer-type dementia
ActiveUS20110071135A1Extended durationFunction increaseBiocideNervous disorderTolerabilityH1 Receptor Antagonist
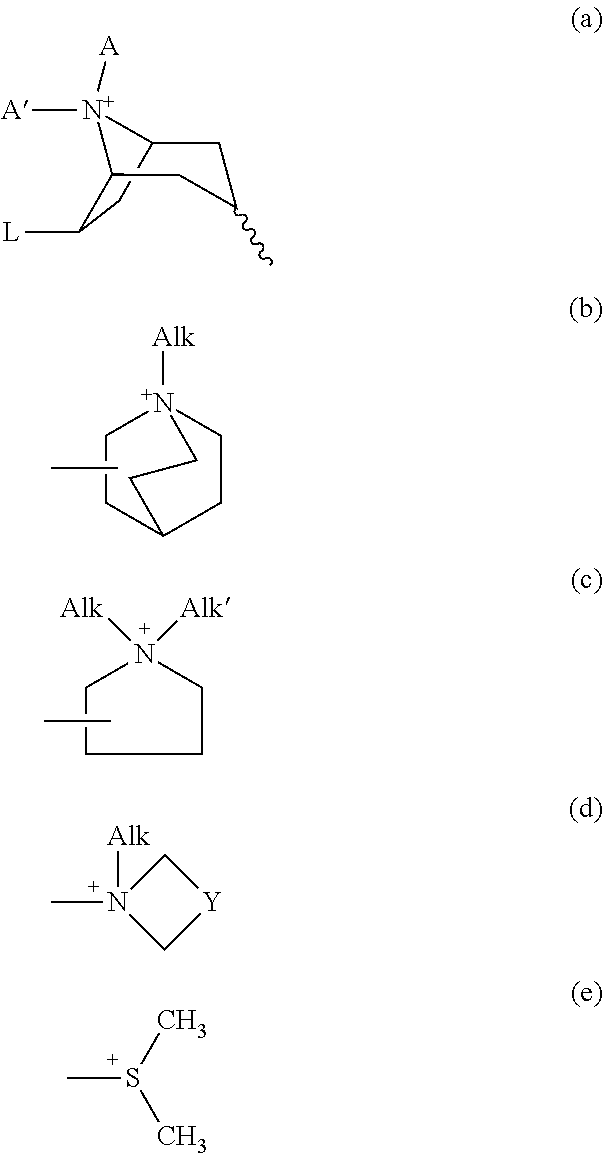
There is described a method for increasing the maximal tolerated close and thus the efficacy of an acetyl choline esterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetyl choline esterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetyl choline esterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholine esterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Use and composition for treating dementia
ActiveUS8404701B2Maximize the effectSymptoms improvedBiocideNervous disorderMaximum tolerated doseAnti cholinergic
Owner:CHASE PHARMA CORP
4-Azaindole Derivatives
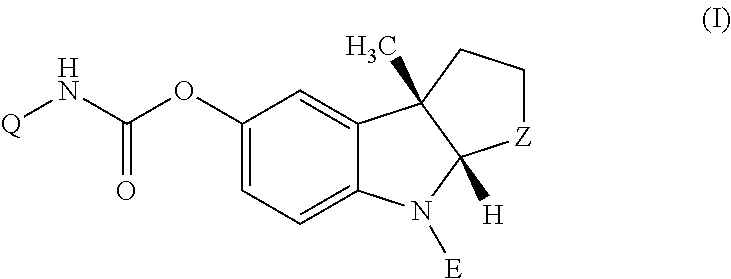
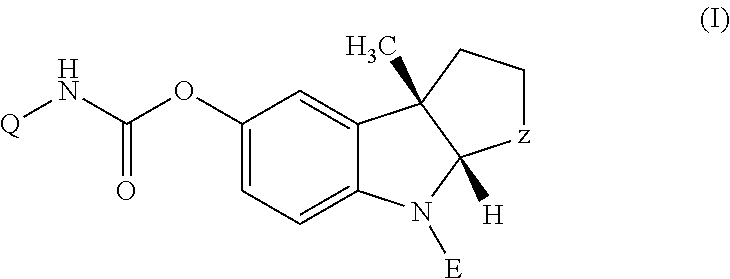
4-Azaindole derivatives which are modulators of muscarinic acetylcholine receptor (mAChR) M1 and which may be effective for the prevention or disease modifying or symptomatic treatment of cognitive deficits associated with neurological disorders such as Alzheimer-type dementia (AD) or dementia with Lewy bodies (DLB), and a pharmaceutical composition comprising a 4-azaindole derivative as an active ingredient.
Owner:EISIA R&D MANAGEMENT CO LTD
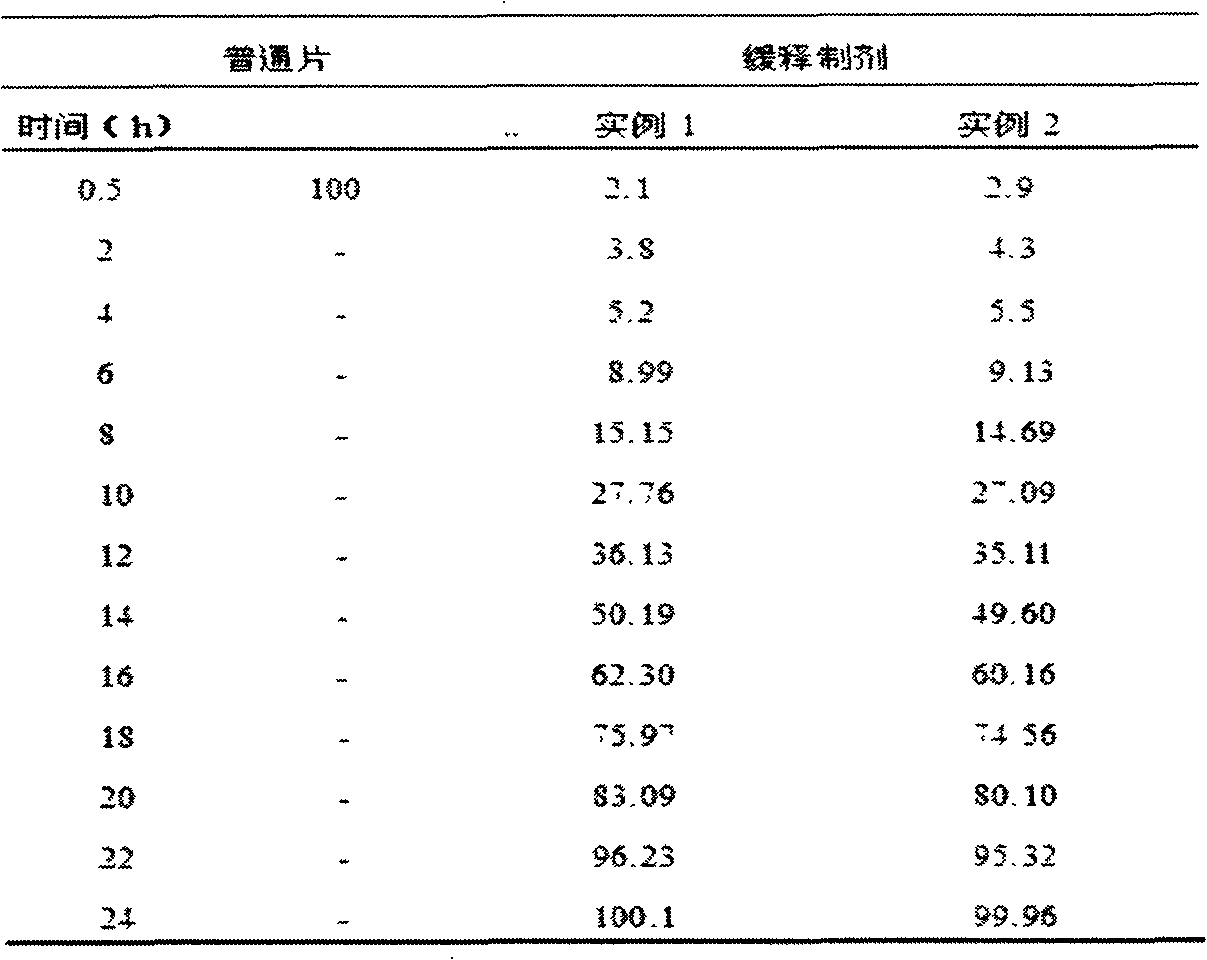
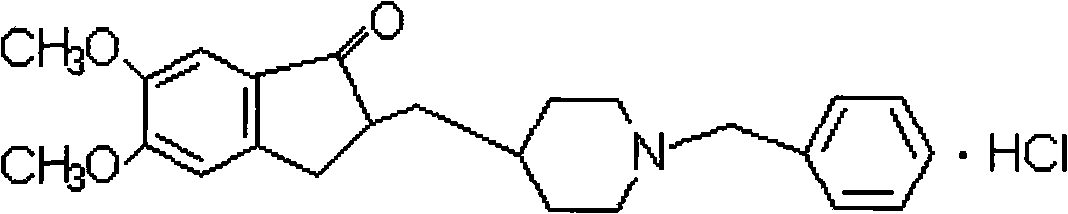
Memantine hydrochloride capsule sustained-release preparation and preparation method for same
InactiveCN102552218ALarge distribution areaImprove bioavailabilityNervous disorderPharmaceutical delivery mechanismControl layerSide effect
The invention discloses a memantine hydrochloride capsule sustained-release preparation, wherein the preparation is composed of two parts, namely, immediate-release grains and sustained-release grains; the sustained-release part is composed of a blank pellet core, a medicine layer and a release control layer; and the immediate-release part is composed of a blank pellet core and a medicine layer. The capsule is uniform in content, good in release effect, stable in blood concentration, good for reducing the toxic and side effects of medicines, and is capable of being used for treating moderate-to-severe Alzheimer-type dementia, and the medicine-taking times of the patient are reduced,.
Owner:无锡万全医药技术有限公司
Method and composition for treating alzheimer-type dementia
ActiveUS8877768B2Maximize the effectSymptoms improvedBiocideNervous disorderTolerabilityH1 Receptor Antagonist
There is described a method for increasing the maximal tolerated close and thus the efficacy of an acetyl choline esterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetyl choline esterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetyl choline esterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholine esterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Medicament for prophylactic and/or therapeutic treatment of alzheimer-type dementia
InactiveUS20100035927A1Prolonged therapeutic periodAvoid symptomsBiocideNervous disorderValproic AcidTherapeutic treatment
A medicament for prophylactic and / or therapeutic treatment of Alzheimer-type dementia, which comprises donepezil hydrochloride and valproic acid or a salt thereof in combination.
Owner:NAGOYA CITY UNIVERSITY +1
Method and composition for treating alzheimer-type dementia
InactiveUS20110201597A1Extended durationFunction increaseBiocideNervous disorderNK1 receptor antagonistMaximum tolerated dose
There is described a method for increasing the maximal tolerated dose and thus the efficacy of an acetylcholinesterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetylcholinesterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetylcholinesterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholinesterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Memantine combinations and use
ActiveUS20180116979A1Simplify the management processNervous disorderPharmaceutical delivery mechanismDonepezilSolifenacin
A pharmaceutical combination of memantine and a non-anticholinergic antiemetic agent for the treatment of hypocholinergic disorders in further combination with high doses of donepezil and with solifenacin, and kits comprising said combination. A pharmaceutical combination of memantine and solifenacin for the treatment of hypocholinergic disorders, including Alzheimer type dementia, in further combination with high doses of donepezil, and kits comprising said combination.
Owner:CHASE PHARMA CORP
Anticholinergic neuroprotective composition and methods
The present invention relates to a pharmaceutical composition comprising propiverine, trospium or glycopyrrolate; and a non-anticholinergic antiemetic agent. It is also related to a pharmaceutical composition comprising a high dose of solifenacin or a pharmaceutically acceptable salts thereof; and a non-anticholinergic antiemetic agent. Pharmaceutical compositions containing high dose of nsPAChA for use for increasing the AChEI blood concentrations and for combating neurodegeneration are also described. The invention also relates to a method for inducing neuroprotection and combating neurodegeneration in a patient suffering from Alzheimer type dementia as well as to a method for increasing the blood levels of an acetyl choline esterase inhibitor (AChEI) in a human subject treated with an AChEI dose.
Owner:CHASE PHARMA CORP
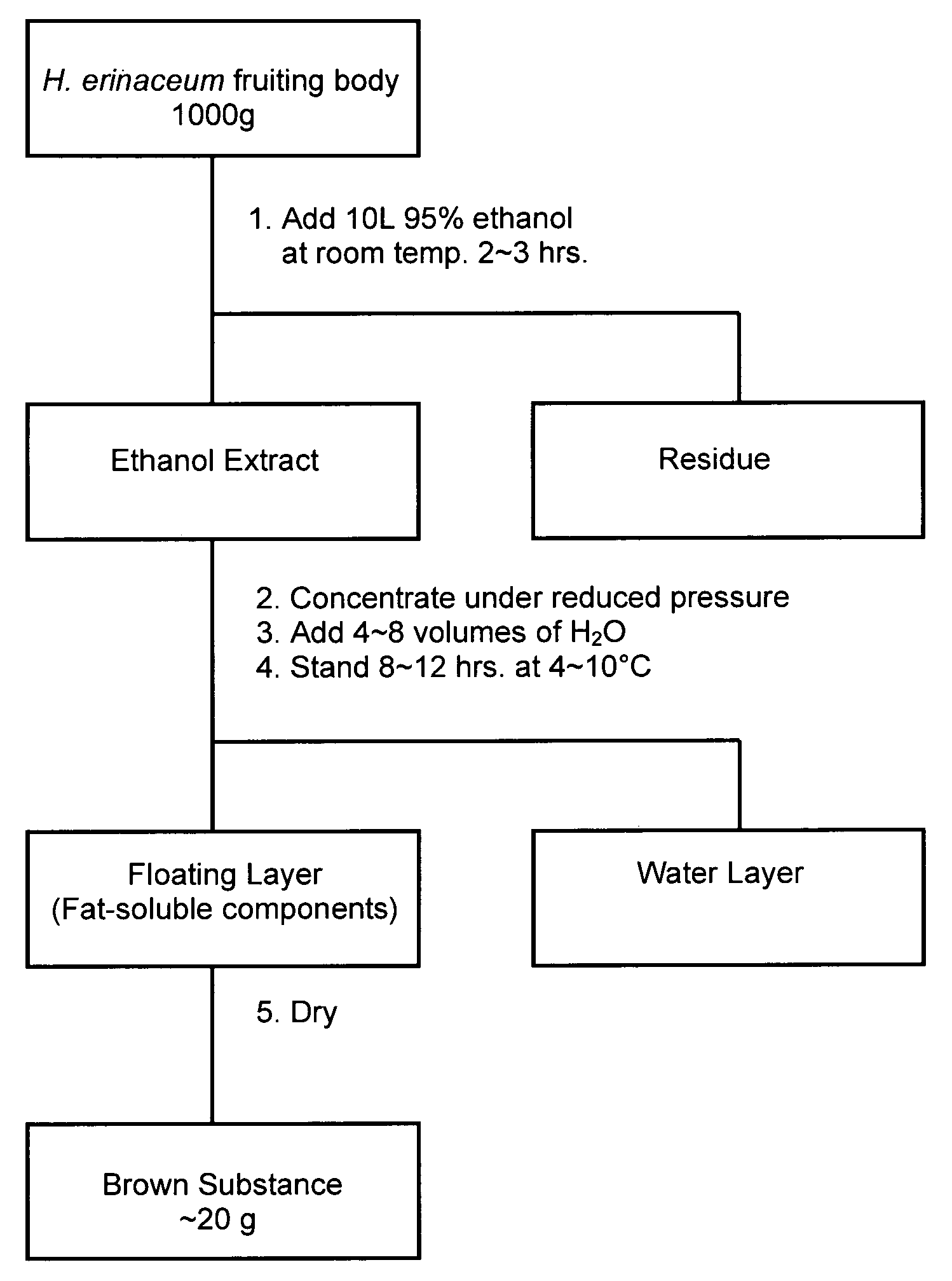
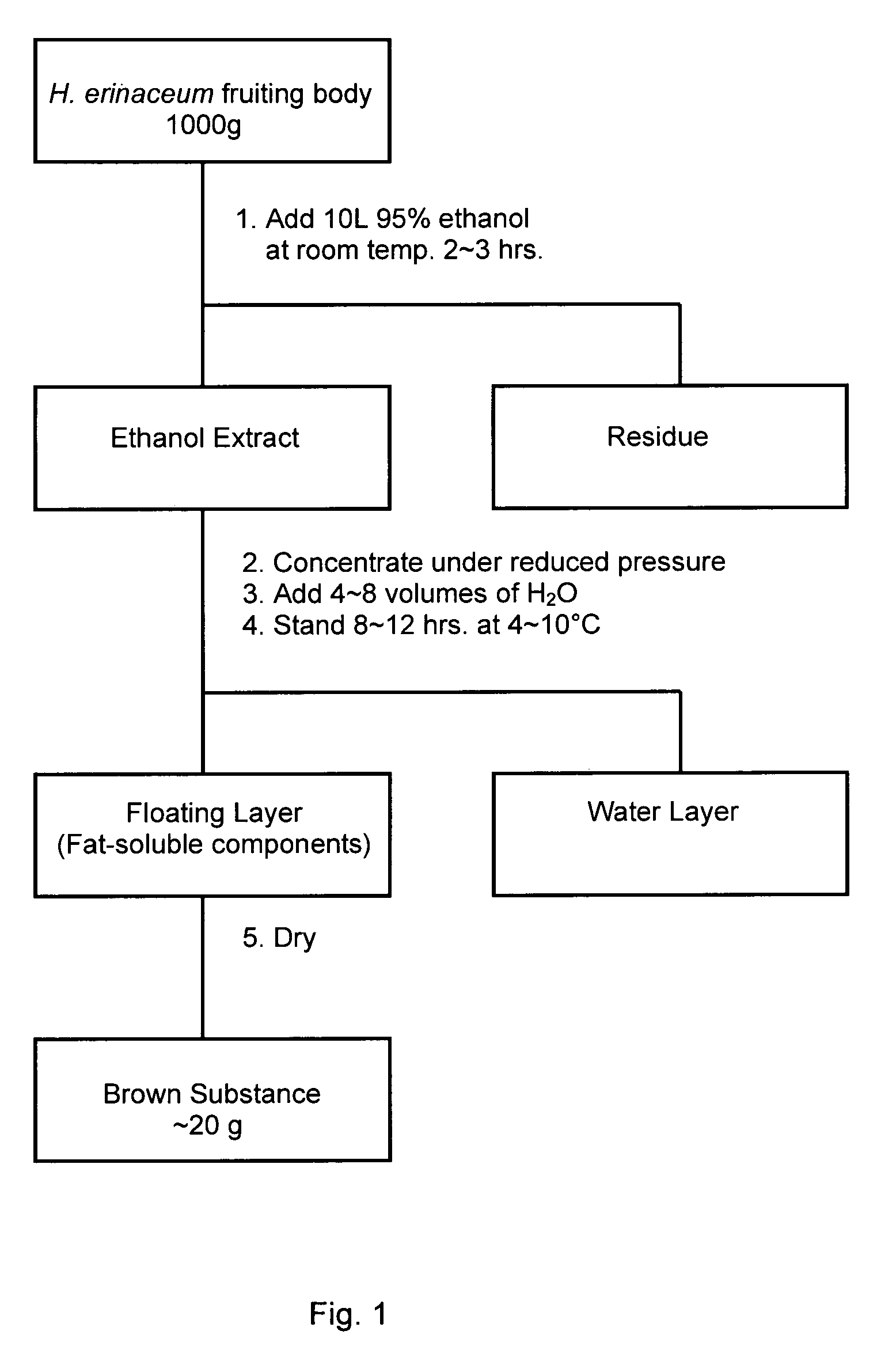
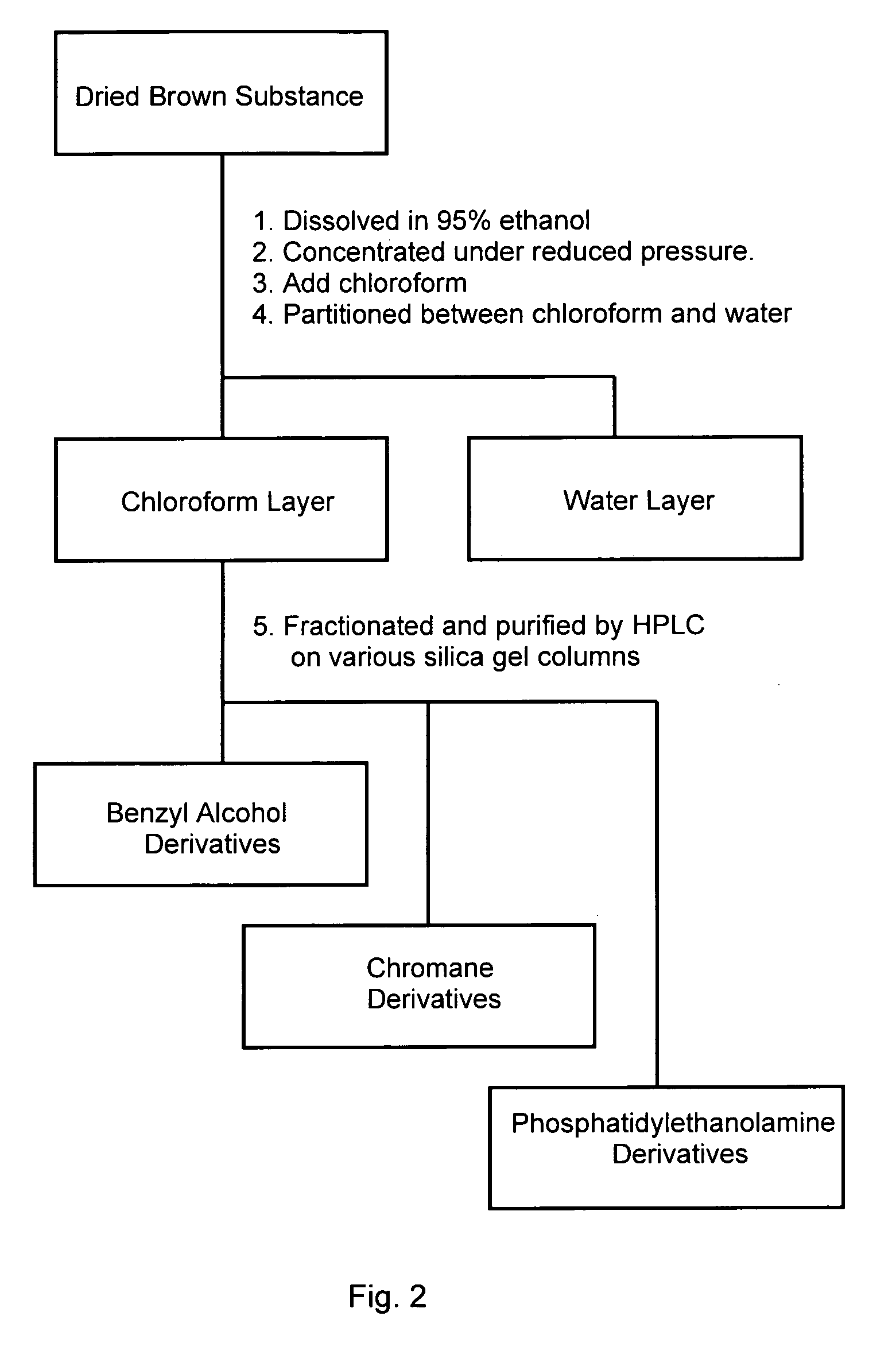
Anti-dementia substance from hericium erinaceum and method of extraction
ActiveUS20090274720A1Increase synthesisReduction and elimination of toxicityBiocideNervous disorderAmyloid betaBULK ACTIVE INGREDIENT
A fat-soluble fraction extracted from the fruiting body of Hericium erinaceum is demonstrated to inhibit the neuronal toxicity of amyloid beta-peptide (Aβ) and induce the synthesis of nerve growth factor (NGF), and has great potential as an active ingredient for pharmaceutical products, health food products, food products and / or beverages to prevent and / or treat dementia, especially Alzheimer-type dementia. This invention is to provide the bioactive fraction and its preparation method.
Owner:MUSHROOM WISDOM INC
Sustained release tablet containing donepezil hydrochloride active component as well as preparation method and application thereof
InactiveCN102309465AGood reproducibilityImprove consistencyNervous disorderPharmaceutical delivery mechanismSustained Release TabletSide effect
The invention discloses a sustained release tablet containing a donepezil hydrochloride serving as an active component. The sustained release tablet provided by the invention comprises the donepezil hydrochloride serving as an active component, a sustained-release material and other auxiliary materials, wherein the weight ratio of the donepezil hydrochloride serving as an active component to the sustained-release material is 1:(0.2-20), preferably 1:(0.5-10) and further preferably 1:(0.7-8). The sustained release tablet containing donepezil hydrochloride prepared by the invention is released steadily, which is favorable for the reduction of the fluctuation of the in-vivo medicament, thus the side effect is reduced, and the sustained release tablet is more suitable for treating patients suffering from Alzheimer-type dementia.
Owner:TIANJIN INSTITUTE OF PHARMA RESEARCH
Anticholinergic neuroprotective composition and methods
The present invention relates to a pharmaceutical composition comprising propiverine, trospium or glycopyrrolate; and a non-anticholinergic antiemetic agent. It is also related to a pharmaceutical composition comprising a high dose of solifenacin or a pharmaceutically acceptable salts thereof; and a non-anticholinergic antiemetic agent. Pharmaceutical compositions containing high dose of nsPAChA for use for increasing the AChEI blood concentrations and for combating neurodegeneration are also described. The invention also relates to a method for inducing neuroprotection and combating neurodegeneration in a patient suffering from Alzheimer type dementia as well as to a method for increasing the blood levels of an acetyl choline esterase inhibitor (AChEI) in a human subject treated with an AChEI dose.
Owner:CHASE PHARMA CORP
Donepezil hydrochloride orally disintegrating tablet and preparation method thereof
InactiveCN102038653AOvercome the disadvantages of single-actingGood disintegrationNervous disorderPill deliverySodium bicarbonateOrally disintegrating tablet
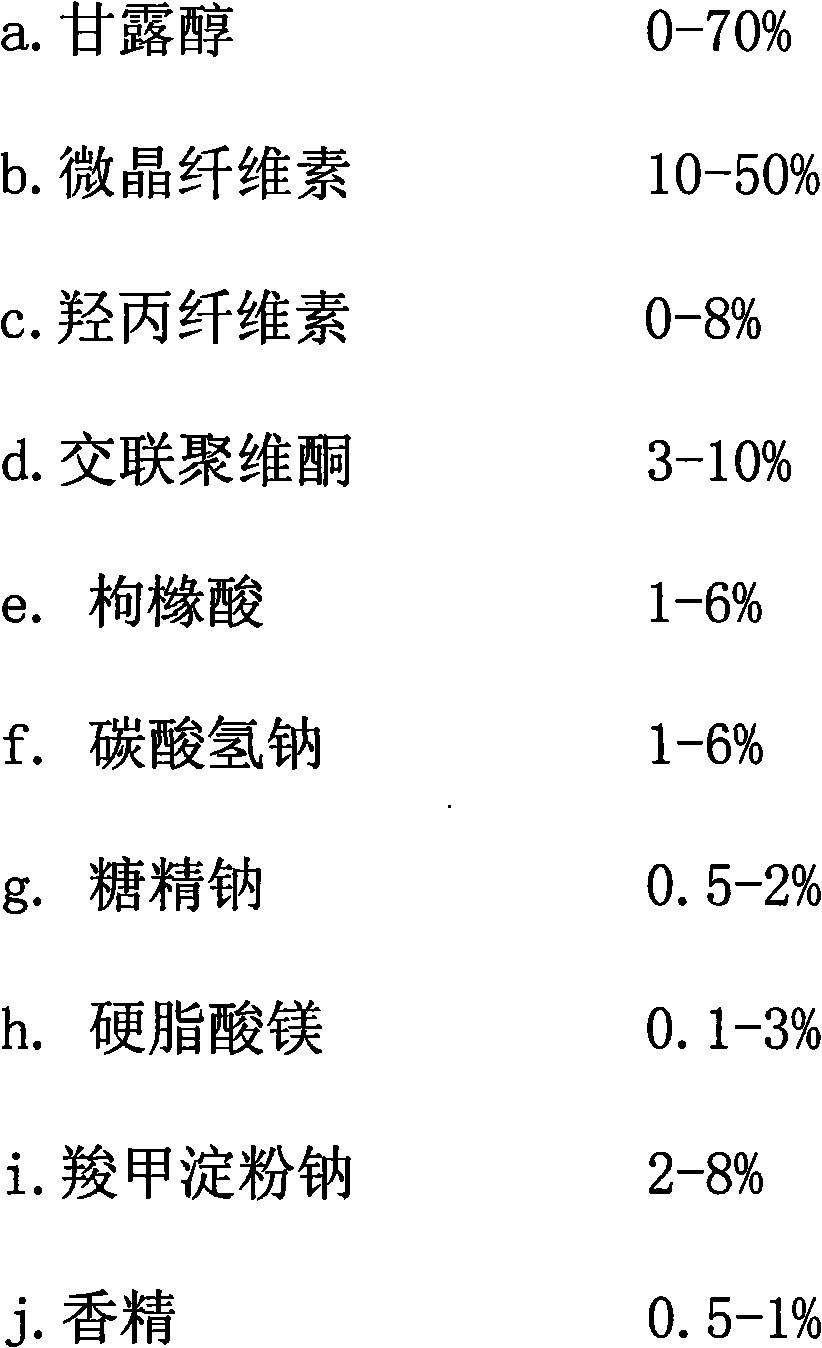
The invention discloses a donepezil hydrochloride orally disintegrating tablet. The main medicine of the donepezil hydrochloride orally disintegrating tablet is donepezil hydrochloride; and the auxiliary medicines of the donepezil hydrochloride orally disintegrating tablet comprise mannitol, microcrystalline cellulose, hyprolose, crospovidone, sodium bicarbonate, citric acid, soluble saccharin, carboxymethyl starch sodium, magnesium stearate and essence. The invention can preferably treat the low-degree or moderate-degree alzheimer type dementia, can preferably improve the cognitive dysfunction, the mental and behavioral abnormalities and the daily self-care ability of the patient who suffers from the alzheimer type dementia (AD), can alleviate the dementia degree, is convenient to take, can fast disintegrate, and is convenient for the old or the patient who has medication obstacles or is hard to take water.
Owner:SHANDONG FANGMING PHARMACEUTICAL CO LTD
Traditional Chinese medicine composition for treating Alzheimer type dementia and preparation method thereof
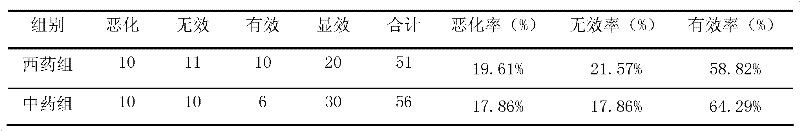
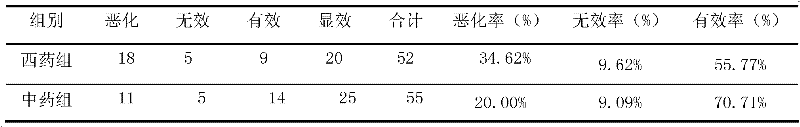
ActiveCN102406778ANot easy to absorb moistureReduce volumeNervous disorderInanimate material medical ingredientsTreatment effectDonepezil
The invention provides a traditional Chinese medicine composition for treating Alzheimer type dementia. The active ingredients of the traditional Chinese medicine composition are prepared from the following traditional Chinese medicine raw materials according to a weight ratio: 7.5 g of codonopsis, 5 g of cassia twig, 5 g of radix paeoniae alba, 5 g of licorice, 4.5 g of polygala, 5 g of acorus gramineus and 5 g of dragon's bone. After 107 light and medium-degree AD (Alzheimer's disease) patients undergo treatment for 24 weeks and 48 weeks, the mini-mental state examination (MMSE) treatment effect comparison result is as follows: after treatment for 24 weeks, the effective rate of a western medicine control group is 55.77% and the effective rate of a traditional Chinese medicine group is 52.73%; and after treatment for 48 weeks, the effective rate of the western medicine control group is 44.23% and the effective rate of the traditional Chinese medicine group is 71.71%. The result shows that the traditional Chinese medicine composition for treating Alzheimer type dementia is superior to donepezil when used for treating Alzheimer type dementia. The traditional Chinese medicine composition is clinically applied by direct taking, and is simple for use. The traditional Chinese medicine granules are packaged by aluminum foil bags,, has small volume and is convenient for carrying, and moisture absorption is difficult to occur. The traditional Chinese medicine composition provides a new traditional Chinese medicine treatment direction for treating Alzheimer type dementia, and has greater clinical application value.
Owner:SHANGHAI GERIATRIC INST OF CHINESE MEDICINE
Application of composition to preparation of medicines for preventing and treating senile dementia
ActiveCN105287985AConvenience to workOptimize spaceNervous disorderAmine active ingredientsSalvia miltiorrhizaNiacin
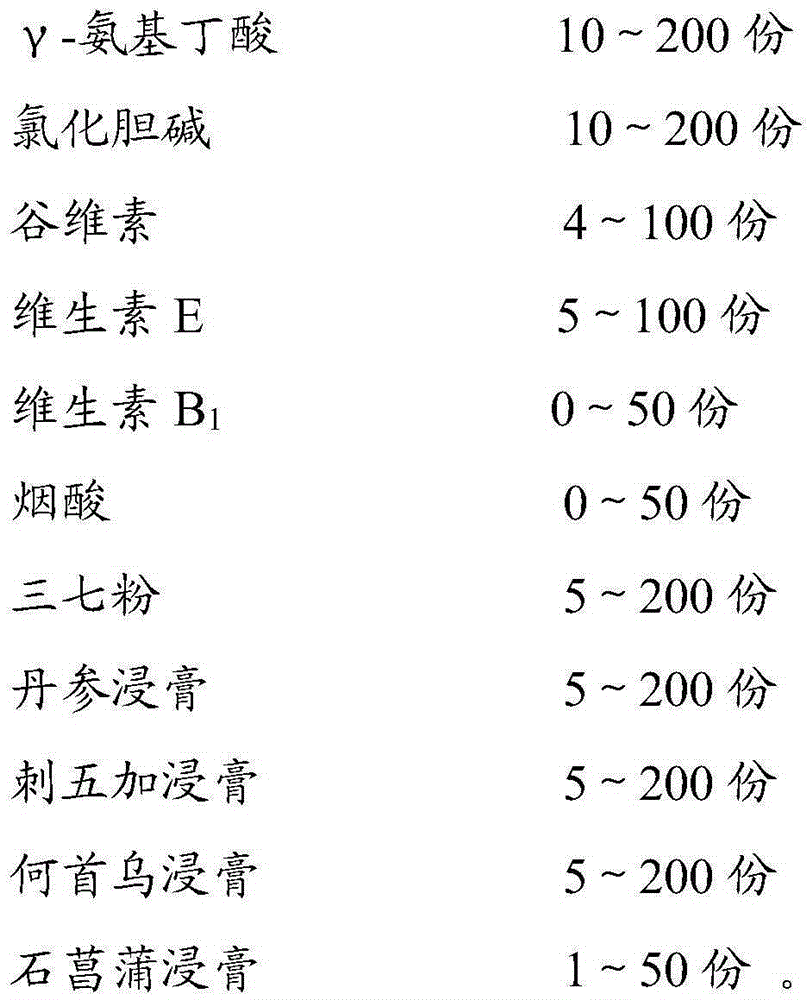
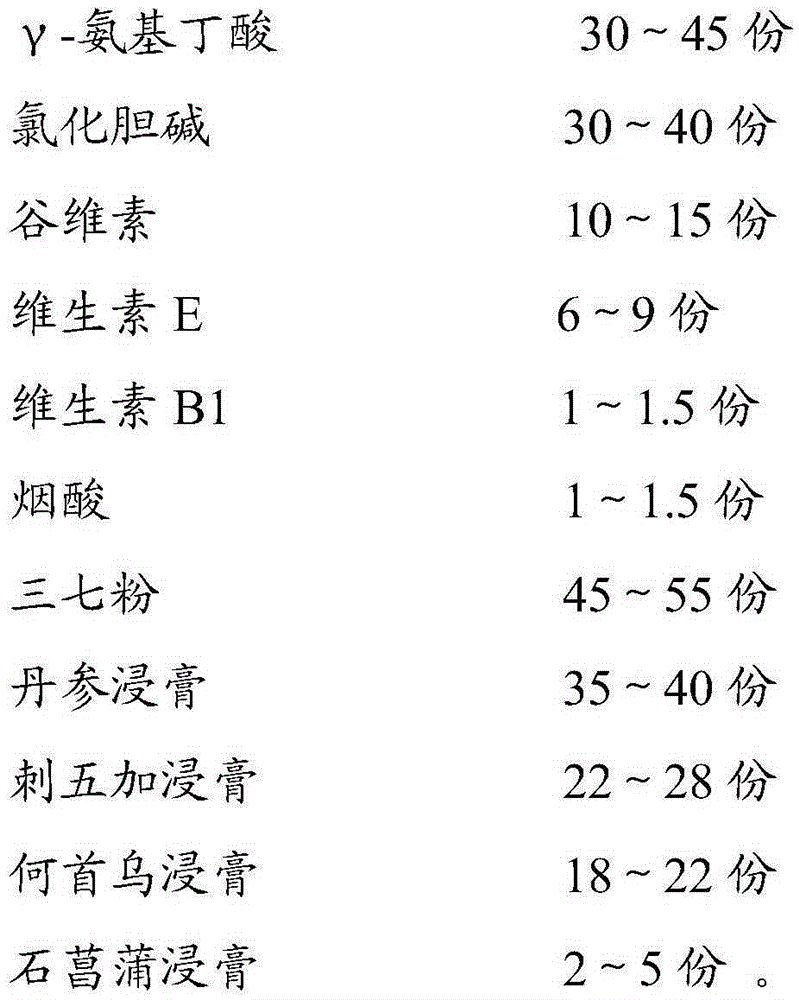
The invention relates to application of a composition to preparation of medicines for preventing and treating senile dementia. The composition comprises the following components: gamma-aminobutyric acid, choline chloride, oryzanol, vitamin E, vitamin B1, niacin, pseudo-ginseng powder, a salvia miltiorrhiza extract, an acanthopanax extract, a polygonum multiflorum extract and an acorus gramineus extract. The composition can obviously improve alzheimer type dementia, vascular dementia model rat working dysmnesia and spatial discrimination studying dysmnesia and the like, can relieve oxygen radical damage, and has an excellent curative effect of preventing and treating senile dementia.
Owner:GUANGZHOU BAIYUNSHAN PHARMA HLDG CO LTD BAIYUNSHAN PHARMA GENERAL FACTORY
Method and composition for treating alzheimer-type dementia
There is described a method for increasing the maximal tolerated dose and thus the efficacy of an acetylcholinesterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetylcholinesterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetylcholinesterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholinesterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Compound medicinal composition containing acetylcholinesterase inhibitor and metformin
ActiveCN104623671BNervous disorderHeterocyclic compound active ingredientsCholinesterase inhibitionBULK ACTIVE INGREDIENT
The invention belongs to the technical field of medicine, and specifically relates to a compound medicinal composition with acetylcholinesterase inhibitors such as donepezil, galantamine or huperzine A and metformin as active ingredients, a preparation method thereof and a therapeutic use thereof. Pharmacodynamic tests show that the compound pharmaceutical composition of the present invention can be used for the prevention and treatment of Alzheimer's dementia, and the combined use of the compound medicine has a synergistic effect in curative effect.
Owner:南京千手共创生物科技有限公司 +1
Oxybutynin transdermal therapeutic system combination
ActiveUS20160243070A1Useful in treatmentLarge doseNervous disorderEster active ingredientsOxybutyninCholinesterase inhibition
There is described a pharmaceutical combination comprising oxybutynin or a pharmaceutically acceptable addition salt thereof, in a transdermal therapeutic system, and an acetylcholinesterase inhibitor, useful for safely treating hypocholinergic disorders of the central nervous system such as Alzheimer type dementia. In this combination, the acetylcholinesterase inhibitor (AChEI) is present at a dose that is higher than the maximal recommended dose, per unit form. In particular, the transdermal therapeutic system comprising oxybutynin is in combination with rivastigmine in a transdermal formulation or oral form.
Owner:CHASE PHARMA CORP
Method and composition for treating alzheimer-type dementia
ActiveUS20140288057A1Maximize the effectSymptoms improvedBiocideNervous disorderNK1 receptor antagonistMaximum tolerated dose
There is described a method for increasing the maximal tolerated dose and thus the efficacy of an acetylcholinesterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetylcholinesterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetylcholinesterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholinesterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Muscarinic combination of a selective m2-antagonist and a peripheral non-selective antagonist for treating hypocholinergic disorders
InactiveUS20180360845A1Nervous disorderAmine active ingredientsSchizo-affective typeNK1 receptor antagonist
A combination of a muscarinic receptor antagonist consisting of a M2-receptor antagonist and of a non-selective, peripheral anticholinergic agent, and optionally an anticholinesterase inhibitor, and use of the same for treatment of hypocholinergic type disorders such as Alzheimer type dementia, schizophrenia, schizophrenia associated dementia, and schizoaffective disorders.
Owner:CHASE PHARMA CORP
Radioactive halogen-labeled phenyloxyaniline derivatives
InactiveCN101133017AHigh affinityHigh selectivityRadioactive preparation carriersIsotope introduction to acyclic/carbocyclic compoundsDiseaseHalogen
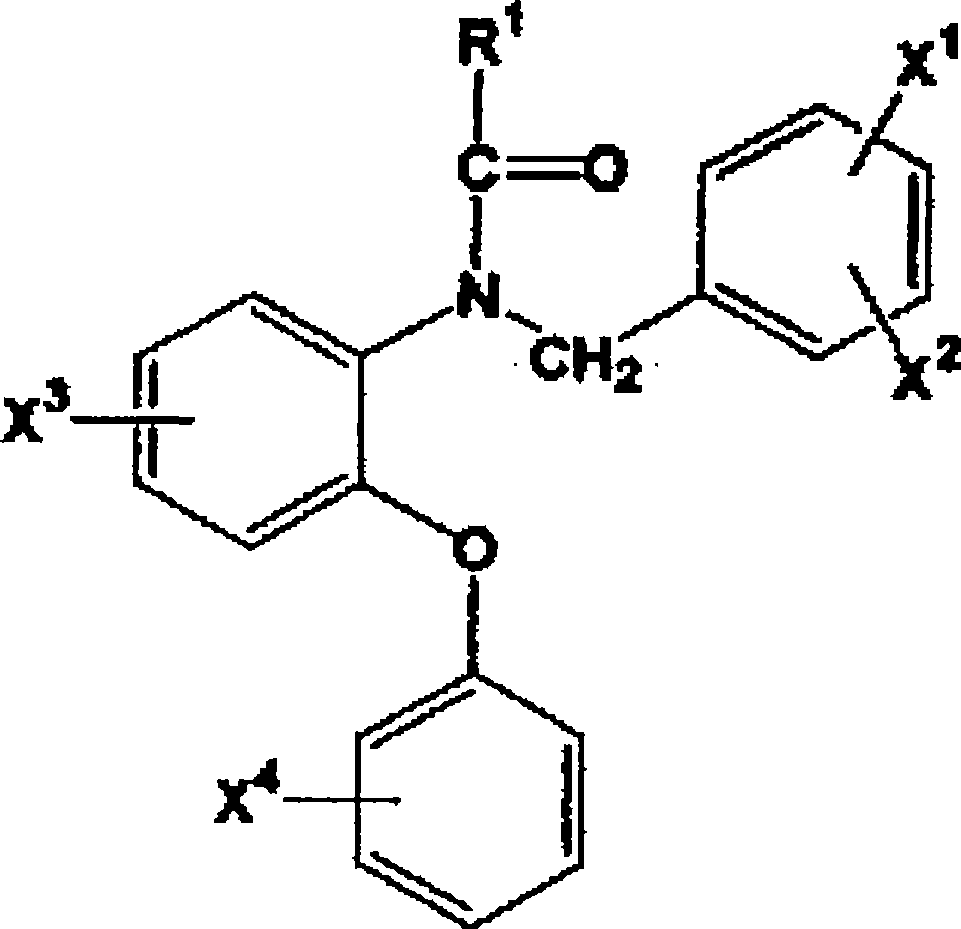
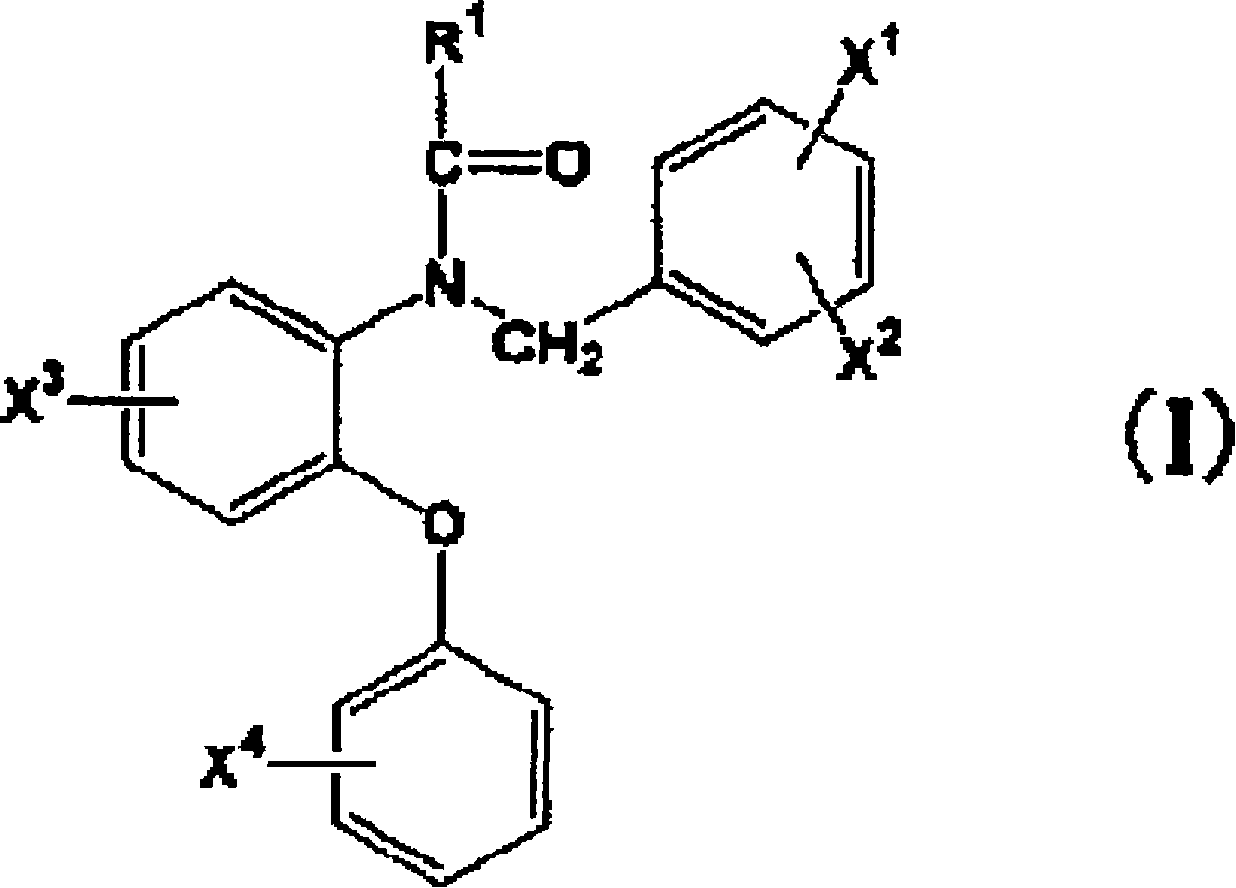
A radioactive halogen-labeled phenyoxyaniline derivative represented by the following formula: wherein R<1> represents a group such as an alkyl group; X<1>, X<2>, X<3> and X<4> represent each a hydrogen atom, an alkyl group, an alkoxy group, an alkoxy group carrying <11>C introduced thereinto or a radioactive halogen atom, provided that at least one of X<1>, X<2>, X<3> and X<4> represents an alkoxy group carrying <11>C introduced thereinto or a radioactive halogen atom; which is a compound useful as a PBR ligand having a high affinity and a high selectivity. In in vitro measurement of PBR, a PBR ligand having a high affinity and a high selectivity is labeled with a radioactive halogen nuclear species so as to enable the measurement of PBRin vivo with the use of means including not only PET but also SPECT. Thus, a compound which is useful in early diagnosing, preventing and treating diseases such as Alzheimer type dementia can be obtained.
Owner:TAISHO PHARMACEUTICAL CO LTD +1
Memantine combinations and use
InactiveUS20190269627A1Simplify the management processNervous disorderPharmaceutical delivery mechanismDonepezilSolifenacin
A pharmaceutical combination of memantine and a non-anticholinergic antiemetic agent for the treatment of hypocholinergic disorders in further combination with high doses of donepezil and with solifenacin, and kits comprising said combination. A pharmaceutical combination of memantine and solifenacin for the treatment of hypocholinergic disorders, including Alzheimer type dementia, in further combination with high doses of donepezil, and kits comprising said combination.
Owner:CHASE PHARMA CORP
Preventive or Therapeutic Drug for Alzheimer-Type Dementia
InactiveUS20090286879A1Highly safe preventiveHigh therapeutic effectBiocideNervous disorderTreatment effectThyroid hormones
It is intended to provide a highly safe preventive or therapeutic drug for Alzheimer-type dementia which can replace the conventional therapies currently used for Alzheimer-type dementia or which can be used together with the conventional therapy to realize high therapeutic effects, characterized in that a ω-3 polyunsaturated fatty acid and thyroid hormone are used in combination.
Owner:MOCHIDA PHARM CO LTD
Pharmaceutical composition containing memantine hydrochloride and huperzine A and preparation thereof
The invention relates to a medicinal composition containing memantine hydrochloride and huperzine A and a preparation method and purposes thereof. Memantine hydrochloride and huperzine A are used as active components to prepare the medicinal composition by mixing with an excipient acceptable in pharmacy. Using memantine hydrochloride and huperzine A as raw materials and mixing with a plurality of excipients with a specific category and proportion, the medicinal composition of the invention is developed into various oral preparations like tablets, capsules, dispersible tablets, chewable tablets, orally disintegrating tablets, buccal tablets, dropping pills and so on according to a technical means disclosed in the invention. The medicinal composition of the invention can be used for treating moderate or severe Alzheimer type dementia.
Owner:BEIJING RUNDEKANG MEDICAL TECH CO LTD
Oxybutynin transdermal therapeutic system combination
ActiveUS10149828B2Large doseMaximum efficacyNervous disorderEster active ingredientsOxybutyninCholinesterase inhibition
There is described a pharmaceutical combination comprising oxybutynin or a pharmaceutically acceptable addition salt thereof, in a transdermal therapeutic system, and an acetylcholinesterase inhibitor, useful for safely treating hypocholinergic disorders of the central nervous system such as Alzheimer type dementia. In this combination, the acetylcholinesterase inhibitor (AChEI) is present at a dose that is higher than the maximal recommended dose, per unit form. In particular, the transdermal therapeutic system comprising oxybutynin is in combination with rivastigmine in a transdermal formulation or oral form.
Owner:CHASE PHARMA CORP
Inhibitor of tau protein phosphorylation
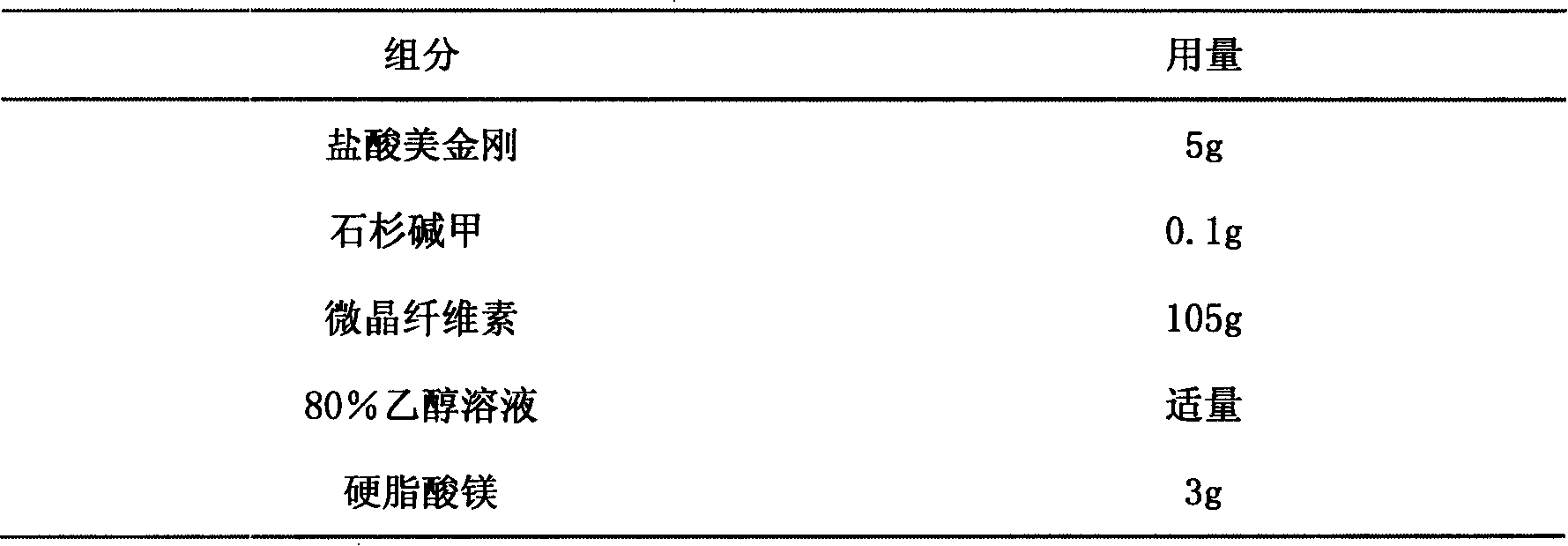
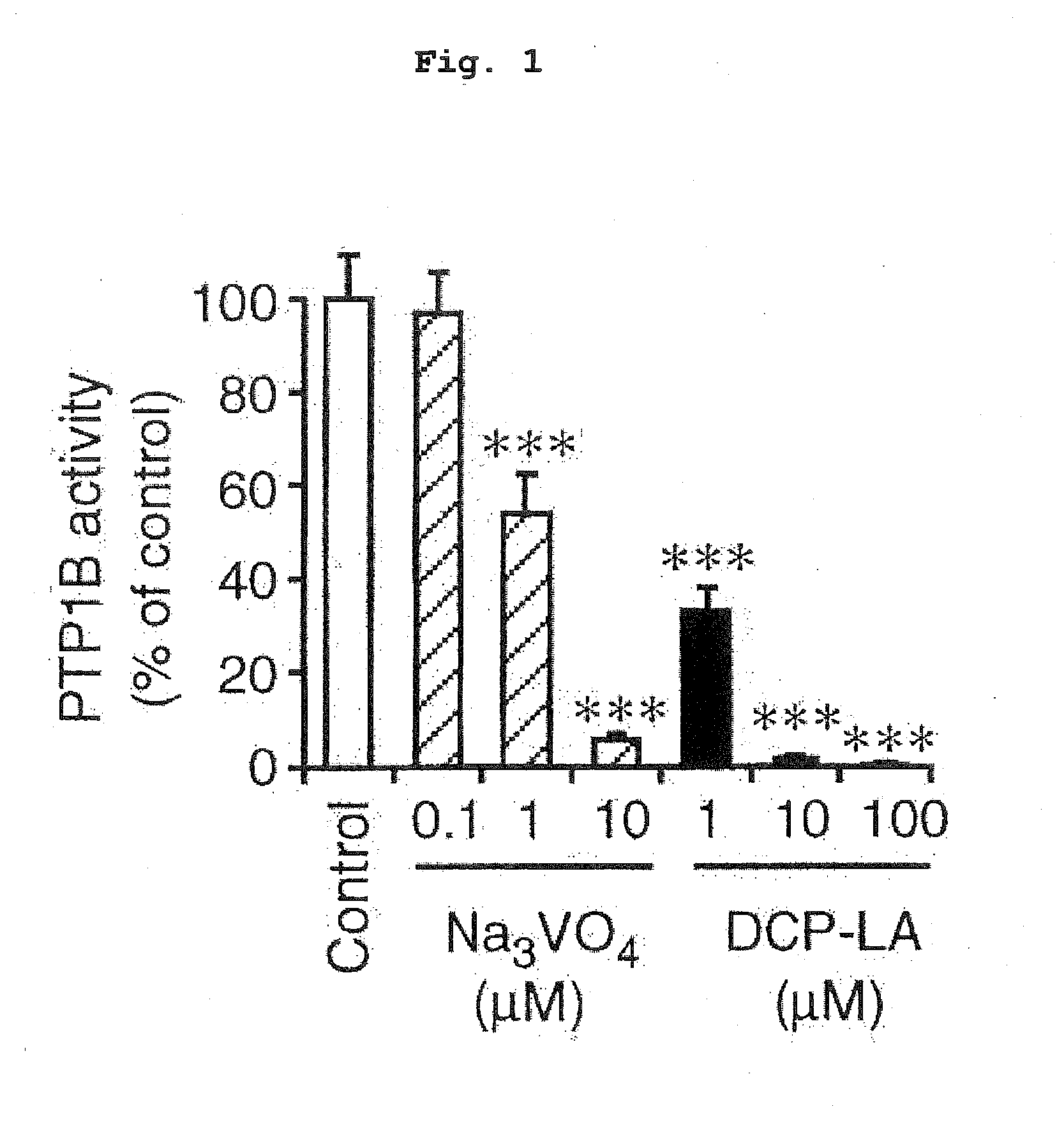
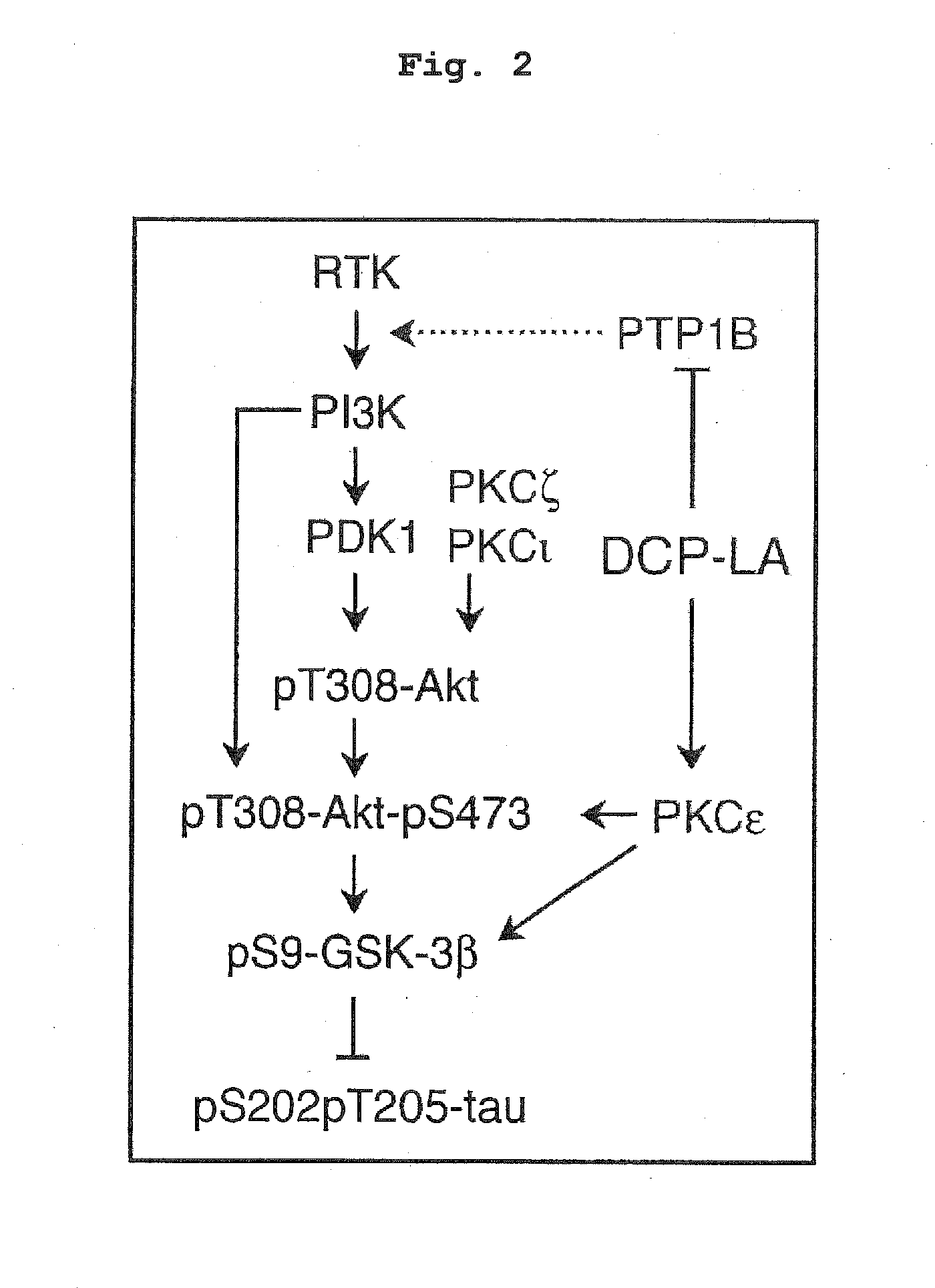
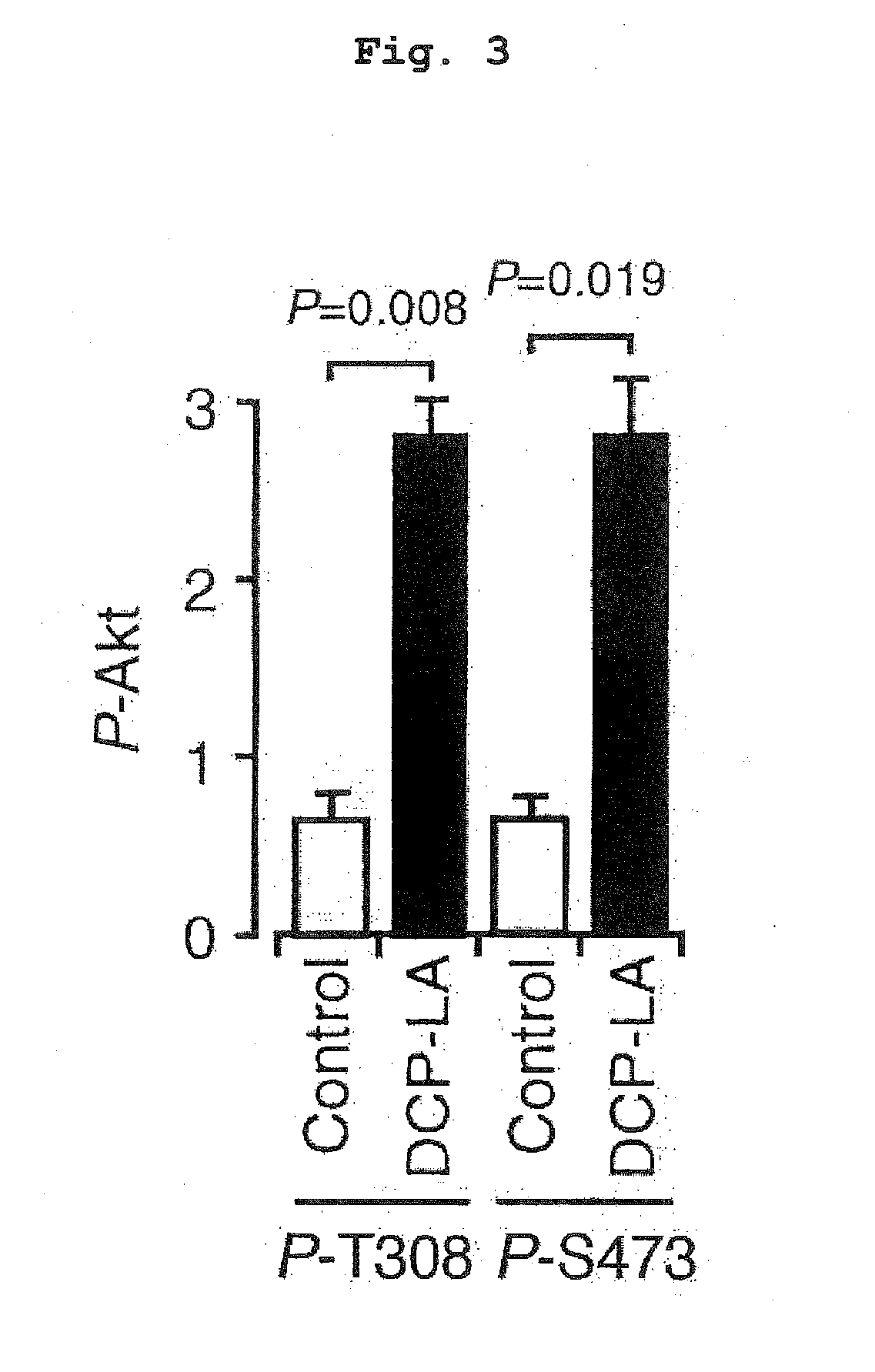
ActiveUS20160008308A1Improve cognitive functionAvoid problemsNervous disorderOrganic chemistryOctanoic AcidsProtein Tyrosine Phosphatase 1B
8-[2-(2-pentyl-cyclopropylmethyl)-cyclopropyl]-octanoic acid has a protein tyrosine phosphatase 1B (PTP1B) inhibitory action, an Akt activating action, a GSK-3β phosphorylation promoting action, and a suppressive action on τ protein phosphorylation induced by Aβ1-42, and is useful as a therapeutic drug for Alzheimer-type dementia, an antidepressant and / or an anti-aging drug.
Owner:NISHIZAKI BIOINFORMATION RES INST
4-azaindole derivatives
ActiveUS9926312B2Organic active ingredientsNervous disorderDementia with Lewy bodiesActive ingredient
4-Azaindole derivatives which are modulators of muscarinic acetylcholine receptor (mAChR) M1 and which may be effective for the prevention or disease modifying or symptomatic treatment of cognitive deficits associated with neurological disorders such as Alzheimer-type dementia (AD) or dementia with Lewy bodies (DLB), and a pharmaceutical composition comprising a 4-azaindole derivative as an active ingredient.
Owner:EISIA R&D MANAGEMENT CO LTD
Method and composition for treating alzheimer-type dementia
ActiveUS20150005292A1Maximize the effectSymptoms improvedBiocideNervous disorderTolerabilityAnti cholinergic
There is described a method for increasing the maximal tolerated close and thus the efficacy of an acetyl choline esterase inhibitor (AChEI) in a patient suffering from an Alzheimer type dementia by decreasing concomitant adverse effects by administration of said AChEI in combination with a non-anticholinergic antiemetic agent, whereby an enhanced acetyl choline esterase inhibition in the CNS of said patient is achieved and alleviation of the symptoms of Alzheimer type dementia in said patient is thereby improved to a greater extent. The use of a non-anticholinergic antiemetic agent for the preparation of a pharmaceutical composition for the treatment of Alzheimer type dementia in combination with an acetyl choline esterase inhibitor (AChEI) and pharmaceutical compositions comprising (a) a 5HT3 receptor antagonist, a dopamine antagonist, a H1-receptor antagonist, a cannabinoid agonist, aprepitant or casopitant as an antiemetic agent and (b) an acetylcholine esterase inhibitor are also described.
Owner:CHASE PHARMA CORP
Oxybutynin-xanomeline transdermal therapeutic system combinations
Transdermal therapeutic system and method of using the same for safely treating hypocholinergic disorders of the central nervous system such as Alzheimer type dementia. The transdermal therapeutic system comprises oxybutynin in combination with a cholinergic receptor agonist (CRA) such as xanomeline.
Owner:CHASE PHARMA CORP
Memantine hydrochloride/donepezil slow-release resin composition and preparation method thereof
InactiveCN106581682ASmall particle sizeNo grittinessNervous disorderPharmaceutical non-active ingredientsDonepezilMemantine Hydrochloride
The invention belongs to the field of pharmacy, and concretely relates to a memantine hydrochloride / donepezil slow-release resin composition and a preparation method thereof. The composition is composed of drug-loaded resin and a dressing layer, and the drug-loaded resin comprises the following raw auxiliary materials by weight percentage: 10-60% of memantine hydrochloride, 5-30% of donepezil, 20-70% of cation resin, 5-50% of an impregnating agent and the pharmaceutically acceptable auxiliary materials. The preparation method comprises the following steps: 1) preparing the cation resin; 2) preparing a drug-loaded solution; 3) preparing the drug-loaded resin; and 4) preparing slow-release resin. The composition has the characteristic of strong medication compliance for patients with moderate and severe alzheimer-type dementia, and can prepare a dry suspension and suspension with a corrigent, a suspending agent and a lubricant, the composition is convenient for administration by the patient, only one time administration is required for each day, administration frequency and administration dosage are reduced, patient compliance is increased, and the medicine combination effect of the medicine can be performed by using the technical advantage of the dosage form.
Owner:ZHONGSHUAI PHARMA SCI & TECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com